Abstract
Pirfenidone (PFD) was the first approved drug by FDA for the treatment of idiopathic pulmonary fibrosis (IPF). However, the rapid metabolism of 5-methyl of PFD increases the risk of side effects in clinics. Thus, in this paper, a common practice that a stable amide bond linking various groups used to replace 5-methyl of PFD in medicinal chemistry was applied, and total 18 PFD derivatives were synthesized. All compounds were investigated for their antiproliferation activities against NIH3T3 cells and the structure–activity relationships of the target compounds were also discussed. Among them, YZQ17 possessed potent antiproliferation activity compared to PFD and better potency in inhibiting TGF-β-induced migration of NIH3T3 cells at a much lower concentration than that of PFD. In addition, YZQ17 dramatically inhibited the expression levels of fibrotic markers α-SMA, collagen I, and fibronectin. Moreover, further mechanistic studies confirmed that YZQ17 exhibited this considerable anti-fibrosis activity via the TGF-β/Smad2/3 dependent pathway. Finally, the results of human and rat liver microsomes assay in vitro and pharmacokinetic assay in rats confirmed that YZQ17 showed better pharmacokinetics than that of PFD. Collectively, the preliminary study of PFD derivatives modified by the amide group indicated that YZQ17 could be regarded as a lead compound for further investigation and optimization.
Total 18 PFD derivatives with the amide group replacing 5-methyl were synthesized and evaluated. YZQ17 possessed considerable antifibrosis activity in vitro via TGF-β/Smad2/3 pathway and was regarded as a lead compound for further optimization.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a typical chronic, progressive, and fatal lung disease, and also a serious complication of COVID-19.1,2 The median survival time of IPF patients is just 2 to 4 years, suggesting that this disease is associated with higher mortality than most malignant cancers.3,4 Though the precise pathogenesis has not been clearly elucidated yet, extensive research has confirmed that transforming growth factor-β (TGF-β), a main profibrogenic factor, plays a vital role in the development of this disease.5 During the development, TGF-β not only induced the transformation of quiescent fibroblasts to myofibroblasts and stimulated myofibroblasts proliferation, migration, and anti-apoptosis6,7 but induced the deposition of extracellular matrix (ECM) formed by myofibroblasts-derived collagens I (CoL I), fibronectin (Fn), and α-smooth muscle actin (α-SMA), which are typical biomarkers of IPF, between the interstitial spaces of the lung.8
Pirfenidone (1, PFD) is the first approved antifibrotic agent against IPF,9 which delayed the development of IPF. Though there have been attractive benefits of PFD for IPF patients in clinic, some shortcomings cannot be ignored. Studies have showed that the 5-methyl of PFD was rapidly metabolized to a carboxylic acid group in vivo.10 As a result, in order to counteract the lessened bioactivity induced by rapid metabolism, high doses were applied in clinic. These high doses possibly increase the risk of side effects.11,12 Besides, some metabolites of PFD could induce liver toxicity due to idiosyncratic drug reactions.13 Thus, it is possible to prepare PFD derivatives with high antifibrosis activity and safety by avoiding their rapid mechanism in vivo.
Generally, replacing 5-methyl of PFD with stable groups in medicinal chemistry and more considerable efforts has been commonly reported.14–17 In this present work, the same strategy was applied, that is, a stable amide bond that linked various groups was introduced to replace the 5-methyl group and a total of 18 PFD derivatives were synthesized (Fig. 1). Firstly, the structure–activity relationships (SARs) of distinct substituents were systematically discussed, resulting in the discovery of a lead compound with potent antiproliferation activity against TGF-β-induced NIH3T3 cells, a widely recognized effective model for anti-fibrosis compounds in vitro.18 Besides, the activities of anti-migration and pro-apoptosis on TGF-β-induced NIH3T3 cells and the inhibition of biomarkers' expression were then evaluated. Next, the mechanism of anti-fibrosis activity was also investigated. Finally, the liver microsome assay in vitro and the pharmacokinetic assay in rats were used to evaluate the metabolic stability of the lead compound.
Fig. 1. The design concept for PFD derivatives with the amide bond at the 5-position.
Results and discussion
Chemistry
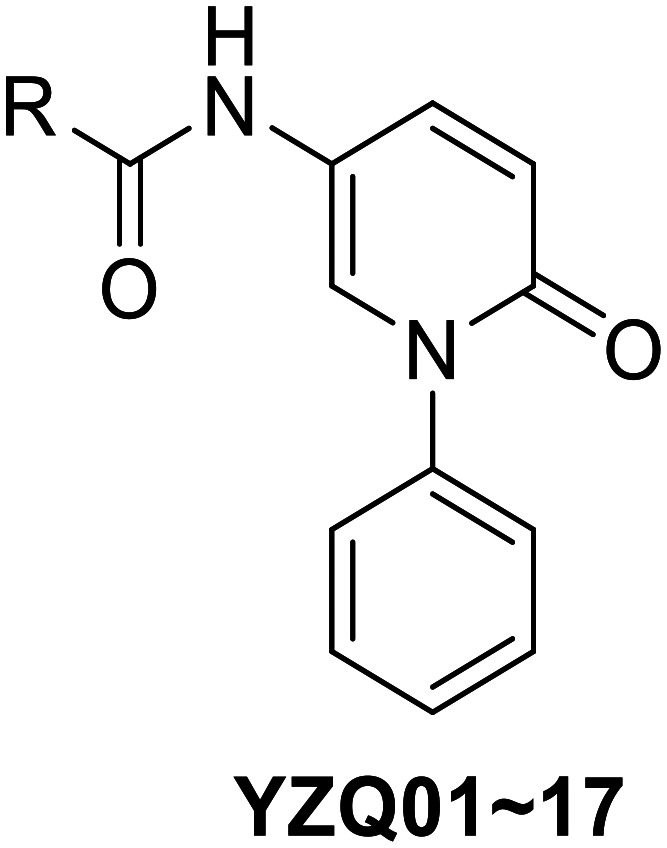
The synthetic route of the target compounds is shown in Scheme 1. Firstly, the key N-phenylpyridone scaffold (4) was easily obtained according to the reported literature.19,20 Iodobenzene (2) was easily transformed to the key diaryliodonium salt (3) in good yield by the oxidation of m-CPBA. Subsequently, the salt (3) was reacted with 2-hydroxy-5-nitropyridine to afford compound (4) at room temperature for 10 min with copper iodide as the catalyst. Then, the nitryl of compound 4 was reduced by Fe powder to get compound 5.21 Finally, compound 5 was reacted with various carboxylic acid derivatives using HATU or HOBT as the catalyst to obtain target compounds (YZQ01–18).
Scheme 1. Synthetic routes of YZQ01–18. Reaction conditions and reagents: (a) benzene, m-CPBA, TfOH, DCM, rt, 10 min; (b) 2-hydroxy-5-nitropyridine, triflate, CuI, TEA, DCM, rt, 10 min, 83%; (c) Fe, NH4Cl, EtOH : H2O = 4 : 1, reflux, 3 h, 94%; (d) carboxylic acid derivatives, HATU, DMF, rt, 43–82%.
Evaluation of the antiproliferation activity against NIH3T3 cells and SARs analysis
The mouse fibroblast cell line (NIH3T3 cells) is widely recognized as an effective screen model for anti-fibrosis compounds in vitro. Therefore, this model was chosen to evaluate the antiproliferation activity of the target compounds under TGF-β (5 ng mL−1) stimulation conditions. When the inhibition rate of the tested compound was more than 30% at the concentration of 1 mM, the IC50 value will be calculated. PFD was selected as the positive control.
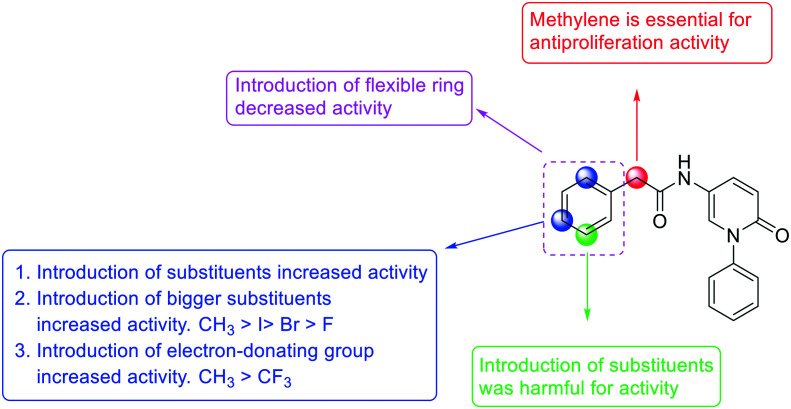
The influence of methylene on the antiproliferation activity was firstly investigated. As shown in Table 1, compared to YZQ01, introducing a methylene (YZQ02) between the amido bond and the phenyl dramatically improved the antiproliferation activity on the NIH3T3 cells, indicating that the existence of a methylene was beneficial for the antiproliferation activity. Based on this result, compounds YZQ03–15 were synthesized subsequently with this essential group maintained.
Antiproliferation activity of YZQ01–18.
| |||
|---|---|---|---|
| Compound | R | Inhibition% | IC50a (mM) |
| YZQ01 |

|
3.5 | —b |
| YZQ02 |
|
28.7 | — |
| YZQ03 |

|
35.2 | 1.98 ± 0.14 |
| YZQ04 |
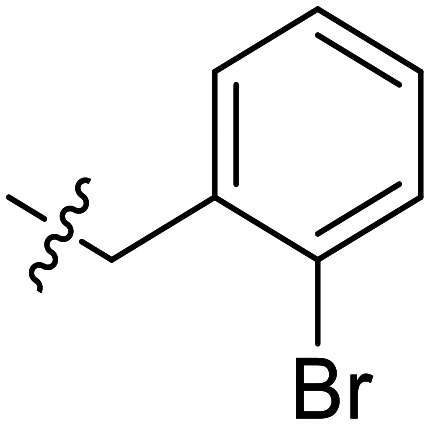
|
48.9 | 1.09 ± 0.21 |
| YZQ05 |

|
56.1 | 0.73 ± 0.13 |
| YZQ06 |

|
37.8 | 1.36 ± 0.21 |
| YZQ07 |

|
21.9 | — |
| YZQ08 |

|
27.6 | — |
| YZQ09 |
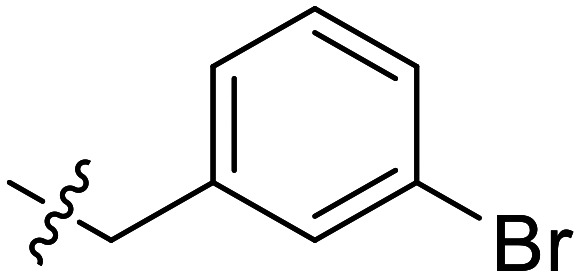
|
2.1 | — |
| YZQ10 |

|
19.8 | — |
| YZQ11 |

|
39.0 | 1.56 ± 0.51 |
| YZQ12 |
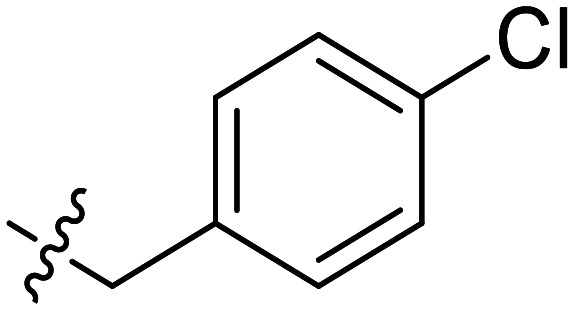
|
37.1 | 1.34 ± 0.04 |
| YZQ13 |

|
45.7 | 1.11 ± 0.15 |
| YZQ14 |
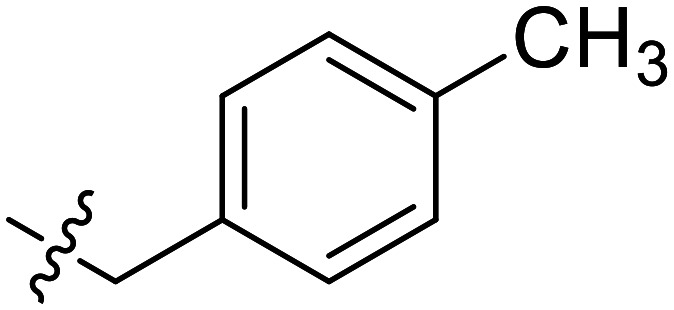
|
52.2 | 0.95 ± 0.20 |
| YZQ15 |
|
4.5 | — |
| YZQ16 |
|
68.9 | 0.24 ± 0.06 |
| YZQ17 |

|
74.4 | 0.14 ± 0.02 |
| YZQ18 |

|
28.1 | — |
| PFD | — | — | 13.72 ± 0.57 |
IC50 values are indicated as the mean ± SD of three independent experiments.
Stands for not calculated.
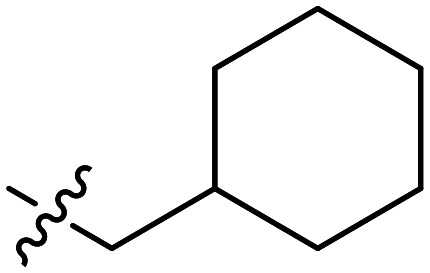
Further efforts were conducted to evaluate the antiproliferation activities of YZQ03–15, which consisted of distinct substituents and substituted-position on the aromatic ring. Introducing chlorine (YZQ03), bromine (YZQ04), and methyl (YZQ05) groups raised the activity compared to fluorine-substituted compound (YZQ02), indicating that a large volume substituent was beneficial for the antiproliferation activity. In addition, the activities of these compounds rose as the volume of the substituents increased. However, replacing the electron-donating group methyl (YZQ05) with the electron-withdrawing trifluoromethyl group (YZQ06) led to about a 2-fold decrease in the activity though both the groups possessed similar volumes. This result showed that introducing an electron-withdrawing group was harmful to its activity. Subsequently, it was observed that switching substituents from the ortho-position to the meta-position decreased the antiproliferation activity irrespective of the substituents, while the activities were restored with the substituents introduced at the para-position (YZQ07–10vs.YZQ11–14). Besides, the activity trend of the 4-position-substituted compounds were consistent with the 2-position-substituted compounds. Compound YZQ15, in which the cyclohexyl was introduced, showed almost no activity, showing that a flexible group was not tolerated in this structure.
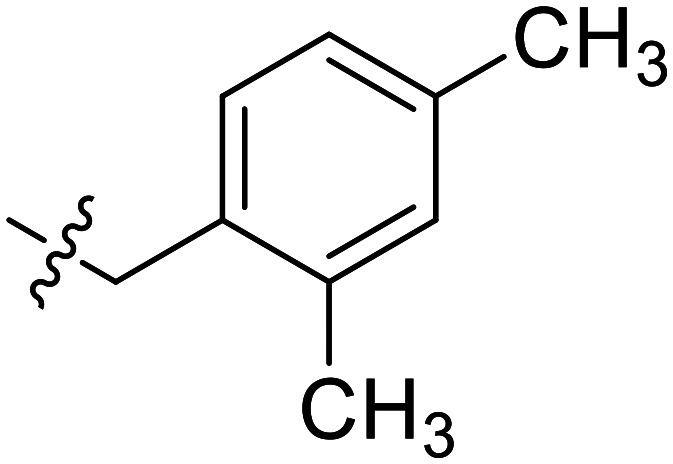
According to the results we obtained above, the monomethyl-substituted compounds (YZQ05, YZQ14) possessed gratifying activities with methyl at the 2- or 4-position of the phenyl ring. Thus, disubstituted and trisubstituted compounds (YZQ16, YZQ17) were then synthesized with this group at the 2,4-position or 2,4,6-position, respectively. In these two compounds, the trisubstituted compound (YZQ17) showed the most potent activity with an IC50 value of 0.14 mM, which was about 100 times that of PFD. However, replacing the methyl group of YZQ17 with an electron-withdrawing and small volume group, fluorine atom, led to a loss of activity, which was consistent with the above results (YZQ18vs.YZQ17). Therefore, based on the results obtained above, the most potent compound YZQ17 was selected for further biological evaluation. In addition, the obtained SARs of the target compounds are summarized in Fig. 2.
Fig. 2. The comprehensive SARs of newly designed PFD derivatives.
Antimigration activity against NIH3T3 cells (wound healing assay)
TGF-β-induced migration of fibroblasts to the fibrotic site is a vital pathological phenomenon, which plays a significant role in the fibrosis process.22 Thus, the inhibition of the TGF-β-induced fibroblast migration contributed to delaying the fibrosis process. The effect of the selected compound on migration was investigated by the wound healing assay. YZQ17 was tested at concentrations of 0.4 mM, 0.2 mM, and 0.1 mM. PFD was chosen as the positive control.
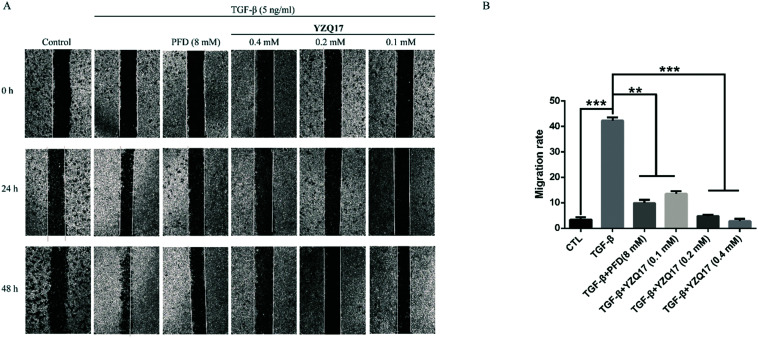
As shown in Fig. 3, the migration rate was sharply elevated by the stimulation of TGF-β compared to the control group at 48 h. After treatment with YZQ17 for 48 h, this compound reduced the migration rate by more than half at the concentration of 0.1 mM and possessed this anti-migration activity in a dose-dependent manner. Moreover, though the concentrations of YZQ17 were much lower than that of PFD, this compound showed better potency than PFD. Therefore, the results displayed that the selected compound possessed an excellent anti-migration activity and inhibited the TGF-β-induced migration of the fibroblasts in a dose-dependent manner.
Fig. 3. Anti-migration activities of YZQ17 and PFD were evaluated via the wound healing assay. (A) NIH3T3 cells were incubated with YZQ17 (0.4 mM, 0.2 mM, and 0.1 mM) or PFD (8 mM) with TGF-β (5 ng mL−1). Photographs were collected at 24 h and 48 h. (B) The migration rates for 48 h were calculated by ImageJ. Data are shown as the mean values ± SD (n = 3); **P < 0.01, ***P < 0.001.
Proapoptotic activity of NIH3T3 cells (Annexin-V/PI staining and flow cytometry assay)
Unlike normal physiological conditions, the activated-fibroblasts possess resistance to apoptosis in the pathological fibrosis process.23 Thus, inducing the apoptosis of the activated fibroblasts could be a benefit for the treatment of IPF. Then, YZQ17 was investigated for the ability of inducing apoptosis against NIH3T3 cells under TGF-β stimulation condition via Annexin-V/PI staining and flow cytometry. PFD was chosen as the positive control.
As shown in Fig. 4, the percentages of apoptotic cells induced by PFD increased to 31.9% at the concentration of 10 mM compared to that DMSO control (9.61%), while YZQ17 showed better potency than the positive control at a much lower concentration (34.5% at 0.05 mM). Besides, the apoptosis rates induced by YZQ17 rose as the concentrations increased. Therefore, these experimental data exhibited that compound YZQ17 could strongly induce the apoptosis of NIH3T3 cells in a dose-dependent manner.
Fig. 4. Flow cytometric analysis of NIH3T3 cells apoptosis of YZQ17 and PFD at the doses above using Annexin V-FITC/PI.
Inhibition expression activity of fibrotic biomarkers in NIH3T3 cells (Western blot assay)
CoL I, α-SMA, and Fn were overexpressed in myofibroblasts and regraded as significant biomarkers of fibrosis.24 Therefore, the inhibited expression activities of YZQ17 on these biomarkers were investigated via the Western blot assay. PFD was chosen as the positive control as well.
As shown in Fig. 5, the expression levels of the biomarkers were dramatically elevated in response to TGF-β stimulation compared to those of the control group. After treatment with YZQ17 or PFD, the expression levels of these biomarkers were sharply reduced. Notably, YZQ17 showed better activities than that of PFD at much lower concentrations and exerted this potency in a dose-dependent manner. These data illustrated that YZQ17 could effectively inhibit the expression of TGF-β-induced fibrotic markers.
Fig. 5. (A) The expression levels of biomarkers were detected via the Western blot assay. NIH3T3 cells were cultured with TGF-β (5 ng mL−1) or compounds at doses above for 48 h. (B) The α-SMA relative expression level of YZQ17 and PFD treatment. (C) The Fn relative expression level of YZQ17 and PFD treatment. (D) The CoL I relative expression level of YZQ17 and PFD treatment. The corresponding biomarker relative expression level of the DMSO group was set as 100%. GAPDH was used as the loading control. Data are shown as the mean values ± SD (n = 3); ***P < 0.001.
Mechanism of the antifibrotic activity (Western blot assay)
TGF-β/Smads pathway plays a primary role in fibrotic progression. Smad2/3 was phosphorylated due to the stimulation of TGF-β.25 Thus, the expression levels of phosphorylated Smad2/3 were detected via the Western blot assay as well.
It was obvious that the phosphorylation levels of Smad2/3 were greatly increased with the stimulation of TGF-β. Then, after co-incubation with YZQ17 for 48 h, the phosphorylation levels of these two proteins were significantly reduced in a dose-dependent manner. Moreover, the total expression levels of Smad2/3 were not influenced by TGF-β or YZQ17 treatment. According to the experimental data above, YZQ17 possessed antifibrotic activity by inhibiting the TGF-Smad2/3 pathway (Fig. 6).
Fig. 6. (A) Phosphorylation levels of Smad2/3 were detected via the Western blot assay. NIH3T3 cells were cultured with TGF-β (5 ng mL−1) or YZQ17 at doses above for 48 h. (B) The p-Smad2 relative expression level of YZQ17 treatment. (C) The p-Smad3 relative expression level of YZQ17 treatment. The corresponding protein relative expression level of the DMSO group was set as 100%. GAPDH was used as the loading control. Data are shown as the mean values ± SD (n = 3); **P < 0.01, ***P < 0.001.
Evaluation of the preliminary pharmacokinetics in vitro (human and rat liver microsomes assay)
Finally, the preliminary pharmacokinetics of YZQ17 were evaluated by the liver microsome assay in vitro. As shown in Table 2, YZQ17 both exhibited lower intrinsic clearances and longer half-life periods than those of PFD in human and rat liver microsomes, indicating that YZQ17 possibly had a better metabolic stability than that of PFD in vitro and was worth further investigation and modification.
Preliminary pharmacokinetics evaluation of compound YZQ17 in HLM and RLM.
| Comp. | HLM | RLM | ||
|---|---|---|---|---|
| Clint (mL min−1 kg−1) | T 1/2 (min) | Clint (mL min−1 kg−1) | T 1/2 (min) | |
| PFD | 1231.17 | 5.76 | 1732.10 | 1.46 |
| YZQ17 | 63.91 | 27.20 | 110.45 | 22.49 |
Evaluation of pharmacokinetics in rats
Finally, we then conducted a pharmacokinetic study with YZQ17 compared to PFD. As shown in Table 3, YZQ17 clearly showed a great improvement in the pharmacokinetic properties over PFD. For instance, YZQ17 exhibited a reasonable maximum concentration (Cmax = 712.46 ng mL−1), good bioavailability (F = 51%), and favorable half-life (T1/2 = 2.77 h), which is much better than the half-life of PFD (T1/2 = 0.4 h),26 indicating that YZQ17 was relatively metabolically stable in vivo and regarded as a lead compound for further modification.
Pharmacokinetic properties of PFD, compound YZQ17 in rats.
| Comp. | Mode | T 1/2 (h) | T max (h) | C max (ng mL−1) | AUC(0−t) (ng h mL−1) | AUC(0−∞) (ng h mL−1) | MRT(0−t) (h) | F% |
|---|---|---|---|---|---|---|---|---|
| YZQ17 | i.v. a | 0.694 | 0.083 | 712.46 | 700.647 | 701.170 | 0.856 | |
| p.o. b | 2.772 | 1.012 | 68.495 | 332.288 | 406.497 | 4.837 | 51% |
Dose: 2 mg kg−1.
Dose: 10 mg kg−1.
Conclusions
In summary, the common practice that an amide bond linked to various groups was used to take place of 5-methyl of PFD in medicinal chemistry was applied in this work. Total 18 compounds were synthesized and evaluated for their anti-fibrosis activity in vitro. Among them, YZQ17 exhibited more potent anti-fibrosis activity than that of PFD for inhibiting TGF-β-induced proliferation and migration, and for inducing the apoptosis of NIH3T3 cells. In addition, the Western blot assay showed that YZQ17 effectively suppressed the fibrotic biomarkers (α-SMA, CoL I, and Fn) expression in a dose-dependent manner in vitro and possessed the potent anti-fibrosis activity via the TGF-β/Smad2/3 pathway. Besides, the results of the liver microsome assay and pharmacokinetic assay in rats indicated that YZQ17 might possess a better metabolic stability than that of PFD in vitro and in vivo, which should be further investigated. Collectively, the obtained results have motivated our group to further investigate and optimize YZQ17, and relevant work is ongoing.
Experimental section
General methods and materials of chemistry
Most chemicals and solvents were of analytical grade and, when necessary, were purified and dried by standard methods. Reactions were monitored by thin-layer chromatography (TLC) using precoated silica gel plates (silica gel GF/UV 254), and spots were visualized under UV light (254 nm). Melting points (uncorrected) were determined on a XT5A melting point apparatus and were uncorrected. 1H NMR and 13C NMR spectra were recorded with a Bruker Avance 400 MHz spectrometer at 300 K using TMS as an internal standard. MS spectra were recorded on a Shimadzu GC-MS 2050 (ESI) or an Agilent 1946A-MSD (ESI) Mass Spectrum. Column chromatography was performed with silica gel (Qingdao Ocean Co., Ltd. 200–300 mesh). Chemical shifts (δ) are expressed in parts per million relative to tetramethylsilane, which was used as an internal standard, coupling constants (J) are in hertz (Hz), and the signals are designated as follows: s, singlet; d, doublet; t, triplet; m, multiplet.
2-Fluoro-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)benzamide (YZQ01)
Compound 5 (0.285 g, 1.53 mmol), 2-fluorobenzoic acid (0.236 g, 1.69 mmol), EDCI (0.325 g, 1.69 mmol), and HOBT (0.229 g, 1.69 mmol) were dissolved in anhydrous DCM (10 mL) and the mixture was stirred at room temperature for 5 h. After completion, the reaction mixture was extracted with water, and then the organic phase was washed with brine and concentrated to a crude product in vacuum. Compound YZQ01 was afforded by recrystallization through ethanol as a colorless solid (0.368 g, 78%); mp: 196.2–196.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.31 (s, 1H), 8.30 (d, J = 2.7 Hz, 1H), 7.71–7.64 (m, 2H), 7.63–7.52 (m, 3H), 7.51–7.43 (m, 3H), 7.40–7.30 (m, 2H), 6.57 (d, J = 9.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.45, 160.10, 159.42, 141.11, 136.37, 132.80, 129.90, 129.18, 129.01, 128.24, 126.66, 124.61, 124.16, 120.73, 119.74, 116.32. HRMS (ESI) for C18H13FN2O2 calculated 309.1039, found 309.1036.
2-(2-Fluorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ02)
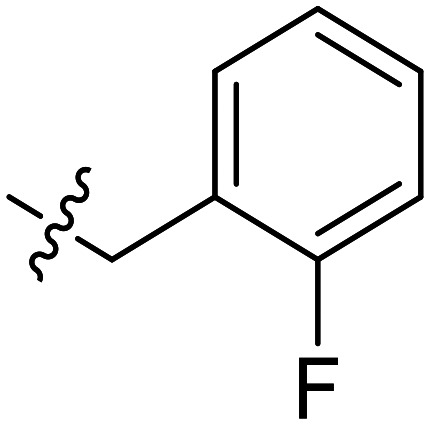
To a solution of compound 5 (0.3 g, 1.61 mmol), 2-(2-fluorophenyl) acetic acid (0.27 g, 1.77 mmol) and HATU (0.76 g, 2.00 mmol) in anhydrous DMF (10 mL) were slowly added to DIPEA (0.78 mL, 4.72 mmol) under nitrogen condition. Then, the mixture was stirred at room temperature for 5 h. After that, the reaction mixture was diluted with water and extracted with ethyl acetate (15 mL × 3). The organic layer was washed with brine and dried with sodium sulfate. Compound YZQ02 was obtained by recrystallization with methanol as a light green needle solid (0.35 g, 65%); mp: 242.8–243.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.53 (dd, J = 7.1, 2.7 Hz, 1H), 7.50 (dd, J = 5.4, 3.9 Hz, 2H), 7.45 (dt, J = 4.7, 1.9 Hz, 1H), 7.40 (dd, J = 5.3, 3.2 Hz, 2H), 7.36 (dd, J = 7.8, 1.6 Hz, 1H), 7.30 (dq, J = 5.5, 1.8 Hz, 1H), 7.19–7.12 (m, 2H), 6.54 (d, J = 9.8 Hz, 1H), 3.69 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.40, 162.29, 159.81, 141.61, 136.54, 132.42, 129.61, 129.37, 128.75, 127.07, 124.71, 123.18, 123.02, 121.29, 120.39, 115.58, 36.24. HRMS (ESI) for C19H15FN2O2 calculated 323.1196, found 323.1191.
The same process was used for the synthesis and characterization of YZQ03–18.
2-(2-Chlorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ03)
A dark green solid (0.3 g, 58%); mp: 200.2–200.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.55–7.48 (m, 3H), 7.42 (ddd, J = 18.0, 7.8, 5.7 Hz, 5H), 7.32–7.27 (m, 2H), 6.53 (d, J = 9.7 Hz, 1H), 3.79 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 167.77, 159.30, 141.12, 136.05, 133.59, 132.10, 129.10, 128.96, 128.87, 128.62, 128.22, 128.14, 127.03, 126.57, 120.80, 119.92, 40.24. HRMS (ESI) for C19H15ClN2O2 calculated 339.0900, found 339.0898.
Synthesis of 2-(2-bromophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ04)
A pale-yellow solid (0.43 g, 76%); mp: 164.7–165.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.14 (d, J = 2.7 Hz, 1H), 7.60 (d, J = 7.9 Hz, 1H), 7.55–7.48 (m, 3H), 7.44 (t, J = 4.8 Hz, 1H), 7.39 (d, J = 7.7 Hz, 3H), 7.34 (t, J = 7.4 Hz, 1H), 7.20 (td, J = 7.8, 1.8 Hz, 1H), 6.54 (d, J = 9.8 Hz, 1H), 3.80 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 167.72, 159.30, 141.12, 136.06, 135.35, 132.22, 132.15, 129.11, 128.82, 128.21, 128.14, 127.58, 126.57, 124.48, 120.81, 119.93, 42.70. HRMS (ESI) for C19H15BrN2O2 calculated 383.0395, found 383.0392.
Synthesis of N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)-2-(o-tolyl)acetamide (YZQ05)
A blue solid (0.46 g, 83%); mp: 204.2–204.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.03 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.54–7.47 (m, 3H), 7.43 (dd, J = 8.4, 5.9 Hz, 1H), 7.41–7.37 (m, 2H), 7.23–7.19 (m, 1H), 7.17–7.10 (m, 3H), 6.52 (d, J = 9.7 Hz, 1H), 3.63 (s, 2H), 2.27 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 168.90, 159.30, 141.13, 136.60, 136.12, 134.29, 129.91, 129.84, 129.10, 128.25, 128.13, 126.70, 126.58, 125.72, 120.74, 119.94, 40.38, 19.33. HRMS (ESI) for C20H18N2O2 calculated 319.1447, found 319.1443.
N-(6-Oxo-1-phenyl-1,6-dihydropyridin-3-yl)-2-(2-(trifluoromethyl) phenyl)acetamide (YZQ06)
A blue solid (0.39 g, 71%); mp: 185.6–185.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.13 (d, J = 2.7 Hz, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.54–7.47 (m, 5H), 7.46–7.42 (m, 1H), 7.41–7.37 (m, 2H), 6.54 (d, J = 9.8 Hz, 1H), 3.88 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 167.84, 159.30, 141.10, 136.04, 133.57, 133.29, 132.20, 128.25, 128.14, 127.62, 127.33, 126.56, 125.79, 125.59, 120.83, 119.85, 38.85. HRMS (ESI) for C20H15F3N2O2 calculated 373.1164, found 373.1161.
2-(3-Fluorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ07)
A pale green solid (0.44 g, 85%); mp: 214.5–214.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.54–7.48 (m, 3H), 7.47–7.42 (m, 1H), 7.41–7.38 (m, 2H), 7.37–7.32 (m, 1H), 7.14 (d, J = 7.5 Hz, 2H), 7.08 (ddd, J = 9.1, 2.8, 1.3 Hz, 1H), 6.53 (d, J = 9.8 Hz, 1H), 3.64 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.39, 163.21, 159.31, 141.11, 138.36, 136.10, 130.14, 129.11, 128.38, 128.15, 126.58, 125.27, 120.76, 119.81, 116.01, 113.48, 42.22. HRMS (ESI) for C19H15FN2O2, calculated 323.1196, found 323.1194.
2-(3-Chlorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ08)
A pale-yellow solid (0.35 g, 59%); mp: 223.5–223.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.14 (t, J = 6.6 Hz, 1H), 7.54–7.48 (m, 3H), 7.44 (t, J = 7.3 Hz, 1H), 7.39 (d, J = 7.8 Hz, 3H), 7.36–7.29 (m, 2H), 7.26 (d, J = 7.1 Hz, 1H), 6.52 (d, J = 9.8 Hz, 1H), 3.63 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.37, 159.31, 141.10, 138.02, 136.07, 132.78, 130.09, 129.11, 129.02, 128.36, 128.23, 128.16, 127.89, 126.58, 120.77, 119.80, 42.06. HRMS (ESI) for C19H15ClN2O2 calculated 339.0900, found 339.0900.
2-(3-Bromophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ09)
A brown solid (0.38 g, 64%); mp: 223.1–223.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.16 (d, J = 2.7 Hz, 1H), 7.54–7.48 (m, 4H), 7.44 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 7.3 Hz, 2H), 7.33–7.26 (m, 2H), 6.53 (d, J = 9.8 Hz, 1H), 3.63 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.37, 159.30, 141.10, 138.31, 136.06, 131.89, 130.39, 129.46, 129.11, 128.34, 128.27, 128.16, 126.59, 121.43, 120.78, 119.81, 42.03. HRMS (ESI) for C19H15BrN2O2 calculated 383.0395, found 383.0392.
N-(6-Oxo-1-phenyl-1,6-dihydropyridin-3-yl)-2-(m-tolyl)acetamide (YZQ10)
A light green solid (0.42 g, 68%); mp: 181.2–181.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.04 (s, 1H), 8.16 (d, J = 2.7 Hz, 1H), 7.54–7.48 (m, 3H), 7.43 (dd, J = 8.6, 6.0 Hz, 1H), 7.41–7.37 (m, 2H), 7.19 (t, J = 7.5 Hz, 1H), 7.12–7.03 (m, 3H), 6.52 (d, J = 9.8 Hz, 1H), 3.54 (s, 2H), 2.28 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 168.97, 159.29, 141.13, 137.32, 136.04, 135.51, 129.66, 129.11, 128.18, 128.16, 128.10, 127.19, 126.59, 126.09, 120.76, 119.97, 42.74, 20.93. HRMS (ESI) for C20H18N2O2 calculated 319.1447, found 319.1443.
2-(4-Fluorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ11)
A yellow solid (0.45 g, 74%); mp: 212.0–212.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.07 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.54–7.48 (m, 3H), 7.44 (t, J = 7.3 Hz, 1H), 7.39 (d, J = 7.3 Hz, 2H), 7.33 (dd, J = 8.4, 5.7 Hz, 2H), 7.14 (t, J = 8.9 Hz, 2H), 6.53 (d, J = 9.8 Hz, 1H), 3.60 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.85, 162.31, 159.30, 141.17, 136.08, 131.80, 130.98, 130.90, 129.11, 128.29, 128.15, 126.58, 120.76, 119.87, 115.07, 41.72. HRMS (ESI) for C19H15FN2O2 calculated 323.1196, found 323.1194.
2-(4-Chlorophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ12)
A white solid (0.44 g, 76%); mp: 209.2–210.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.54–7.49 (m, 3H), 7.44 (t, J = 7.3 Hz, 1H), 7.39 (dd, J = 7.6, 5.7 Hz, 4H), 7.32 (d, J = 8.4 Hz, 2H), 6.53 (d, J = 9.8 Hz, 1H), 3.61 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.57, 159.30, 141.10, 136.09, 134.63, 131.31, 130.97, 129.11, 128.34, 128.18, 128.16, 126.58, 120.76, 119.83, 41.86. HRMS (ESI) for C19H15ClN2O2 calculated 339.0895, found 339.0887.
2-(4-Bromophenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ13)
A yellow solid (0.39 g, 65%); mp: 222.9–223.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.14 (d, J = 2.7 Hz, 1H), 7.54–7.48 (m, 5H), 7.44 (t, J = 7.3 Hz, 1H), 7.39 (d, J = 7.4 Hz, 2H), 7.26 (d, J = 8.3 Hz, 2H), 6.53 (d, J = 9.8 Hz, 1H), 3.59 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.50, 159.30, 141.10, 136.08, 135.06, 131.36, 131.11, 129.12, 128.34, 128.16, 126.58, 120.76, 119.82, 119.77, 41.93. HRMS (ESI) for C19H15BrN2O2 calculated 383.0395, found 383.0391.
N-(6-Oxo-1-phenyl-1,6-dihydropyridin-3-yl)-2-(p-tolyl)acetamide (YZQ14)
A white solid (0.48 g, 82%); mp: 240.5–240.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.03 (s, 1H), 8.15 (d, J = 2.7 Hz, 1H), 7.55–7.43 (m, 4H), 7.39 (d, J = 7.2 Hz, 2H), 7.19 (d, J = 7.9 Hz, 2H), 7.12 (d, J = 7.9 Hz, 2H), 6.52 (d, J = 9.8 Hz, 1H), 3.54 (s, 2H), 2.27 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 169.12, 159.29, 141.13, 136.06, 135.59, 132.57, 129.11, 128.88, 128.83, 128.17, 128.15, 126.59, 120.74, 119.97, 42.39, 20.61. HRMS (ESI) for C20H18N2O2 calculated 319.1447, found 319.1443.
2-Cyclohexyl-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ15)
A yellow solid (0.42 g, 70%); mp: 217.7–217.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.75 (s, 1H), 8.18 (d, J = 2.7 Hz, 1H), 7.55–7.44 (m, 4H), 7.43–7.39 (m, 2H), 6.51 (d, J = 9.7 Hz, 1H), 2.13 (d, J = 6.9 Hz, 2H), 1.73–1.58 (m, 6H), 1.26–1.10 (m, 3H), 1.00–0.89 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 170.34, 159.31, 141.16, 136.13, 129.12, 128.14, 128.01, 126.61, 120.66, 120.04, 48.56, 43.74, 34.74, 32.43, 25.54. HRMS (ESI) for C19H22N2O2 calculated 311.1760, found 311.1755.
2-(2,4-Dimethylphenyl)-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ16)
A light green solid (0.27 g, 47%); mp: 192.7–193.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.01 (s, 1H), 8.16 (d, J = 2.7 Hz, 1H), 7.55–7.43 (m, 4H), 7.40 (d, J = 7.3 Hz, 2H), 7.04 (d, J = 7.5 Hz, 2H), 6.95 (d, J = 7.7 Hz, 1H), 6.53 (d, J = 9.8 Hz, 1H), 3.59 (s, 2H), 2.24 (s, 3H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 169.07, 159.29, 141.13, 136.33, 136.13, 135.62, 131.21, 130.55, 129.82, 129.10, 128.22, 128.13, 126.58, 126.25, 120.73, 119.97, 40.03, 20.52, 19.29. HRMS (ESI) for C21H20N2O2 calculated 333.1603, found 333.1599.
2-Mesityl-N-(6-oxo-1-phenyl-1,6-dihydropyridin-3-yl)acetamide (YZQ17)
A yellow solid (0.24 g, 43%); mp: 194.3–194.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.99 (s, 1H), 8.13 (d, J = 2.7 Hz, 1H), 7.56–7.42 (m, 4H), 7.39 (d, J = 7.3 Hz, 2H), 6.81 (s, 2H), 6.53 (d, J = 9.8 Hz, 1H), 3.64 (s, 2H), 2.22 (s, 6H), 2.19 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 168.76, 159.29, 141.13, 136.74, 136.22, 135.05, 129.89, 129.08, 128.30, 128.25, 128.11, 126.57, 120.68, 119.97, 36.09, 20.45, 19.96. HRMS (ESI+) for C22H22N2O2 calculated 347.1760, found 347.1756.
N-(6-Oxo-1-phenyl-1,6-dihydropyridin-3-yl)-2-(2,4,6-trifluorophenyl)acetamide (YZQ18)
A pale-yellow solid (0.40 g, 64%); mp: 233.4–233.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.24 (s, 1H), 8.17 (d, J = 2.7 Hz, 1H), 7.59–7.48 (m, 4H), 7.44 (d, J = 7.2 Hz, 2H), 7.23 (t, J = 8.5 Hz, 2H), 6.58 (d, J = 9.8 Hz, 1H), 3.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 166.60, 162.36, 159.87, 159.30, 141.08, 135.98, 129.09, 128.38, 128.15, 126.56, 120.82, 119.71, 108.10, 100.25, 28.98. HRMS (ESI) for C19H13F3N2O2 calculated 359.1007, found 359.1004.
Cell culture
NIH3T3 cells were obtained from the China Center for Type Culture Collection at Wuhan University (Wuhan, Hubei, China). NIH3T3 cells were cultured in Dulbecco's modified Eagle medium (DMEM, Cytiva) without sodium pyruvate, containing 10% fetal bovine serum (FBS, GIBCO), 1% penicillin–streptomycin, at 5% CO2, and under 37 °C in a humidified atmosphere.
CCK-8 assay
NIH3T3 cells (8 × 103 cells per well) were incubated in a 96-well plate in DMEM with TGF-β (5 ng mL−1) for 24 h. Then, the target compounds were dissolved in TGF-β-containing DMEM at doses (2, 1, 0.5, 0.1, and 0.05 mM), while PFD was tested at 40, 20, 10, 5, and 1 mM, respectively. PFD was dissolved in the same medium. All compounds were added to each well and co-incubated for 48 h. Equivalent volume medium containing 1% DMSO was deemed as the vehicle control. After 48 h, 5 μL CCK-8 (Dojindo, Japan) was added to each well in dark and the cell plates were incubated for another 4 h. After that, the plates were placed in a shaker for 10 min, followed by absorbance measurement at 450 nm using a microplate reader (BioTek, Synergy H1, USA). Finally, the inhibition rate and IC50 value of the tested compound were calculated via GraphPad Prism 7.0.
Wound healing assay
NIH3T3 cells were grown in 6-well plates for 24 h and scratched in a confluent monolayer using pipette tips. Then, the cells were co-incubated with YZQ17 (0.4 mM, 0.2 mM, and 0.1 mM) or PFD (8 mM) in the presence of TGF-β (5 ng mL−1). The cells that were cultured without compounds and TGF-β were regarded as the negative control. Cells were photographed using a light microscope (Nikon, Japan). Migration rates were calculated by ImageJ.
Cellular apoptosis study
The apoptosis study was performed according to the protocol of the apoptosis analysis kit (KeyGen, KGA106). NIH3T3 cells were treated with YZQ17 (0.05 mM, 0.1 mM, and 0.2 mM) or PFD (2.5 mM, 5 mM, and 10 mM) for 48 h in 6-well plates. Then, the cells were washed with PBS, collected, and stained with Annexin V fluorescein isothiocyanate (FITC, 5 μL) for 30 min at room temperature in dark and subsequently stained with PI (5 μL) for 30 min at room temperature in the dark. After that, the samples were analyzed by flow cytometry using LSRFortessa (Becton Dickinson, USA).
Western blot assay
NIH3T3 cells (2 × 104 cells per dish) were cultured in 60 mm dishes for 24 h. After that, the cells were incubated with YZQ17 (0.01 mM, 0.05 mM, 0.1 mM, and 0.2 mM) and PFD (0.5 mM, 2.5 mM, 5 mM, and 10 mM) in the presence of TGF-β (5 ng mL−1) for 48 h. Equivalent volume medium containing 0.1% DMSO was deemed as a vehicle control in the absence of TGF-β. Cells were washed twice with PBS (pH 7.4), then collected and lysed in a lysis buffer (14 μL, Salarbio) for 1 h in an ice bath. The lysates were then subjected to centrifugation (12 000 rpm) at 4 °C for 10 min. Protein concentration in the supernatants was detected by BCA protein assay (Salarbio, Lot No.: 20200903). Next, protein loading buffer (70 μL, WellBio, Shanghai) was added to the lysates. Then, an equal amount of protein was separated with a distinct percent of SDS-PAGE according to different primary antibodies and transferred to polyvinylidene difluoride (PVDF) membranes. Then, the PVDF membranes were blocked with 5% aqueous skim milk powder at 4 °C overnight and washed with 1 × TBST for three times (5 min × 3). Then, the PVDF membranes were incubated with aqueous primary antibodies, including α-SMA, collagen I, fibronectin, Smad2, Smad3, p-Smad2, p-Smad3, and GAPDH, which were diluted with primary antibody dilution buffer (Beyotime, 1 : 1000 for α-SMA, fibronectin, Smad2, Smad3, p-Smad2, and p-Smad3 antibody, 1 : 750 for collagen I antibody and 1 : 10 000 for GAPDH antibody) at room temperature for 2.5 h, and then were washed with 1 × TBST three times (5 min × 3). Next, the PVDF membranes were added with secondary antibody aqueous (Abcam, rabbit), which was diluted with 1 × TBST (1 : 10 000) and incubated for 1 h at room temperature. After that, the PVDF membranes were washed with 1 × TBST (5 min × 3). Finally, the PVDF membranes were reacted with previously prepared ECL reaction solution for 2 min and the visualized bands were captured by a UVP MultiSpectral Imaging System (Ultra-Violet company, USA), which was optimized for the bands color by Photoshop and analyzed by the ImageJ software for the optical density value of the target bonds. Collagen I antibody was purchased from Bioss, and others were purchased from Beyotime.
Liver microsomes assay
Firstly, three types of buffer solutions were prepared, namely, buffer A: 1.0 L of 0.1 M monobasic potassium phosphate buffer containing 1.0 mM EDTA; buffer B: 1.0 L of 0.1 M dibasic potassium phosphate buffer containing 1.0 mM EDTA; buffer C: 0.1 M potassium phosphate buffer, 1.0 mM EDTA, pH 7.4 by titrating 700 mL of buffer B with buffer A while monitoring with the pH meter. Next, 10 μL DMSO stock solution (10 mM) of YZQ17 or PFD were added to 190 μL acetonitrile. Then, this solution (1.5 μL) and liver microsomes (18.75 μL) solution were added into buffer C (479.75 μL) on ice. Subsequently, the obtained solution was dispensed to the assay plates designated for different time points (0, 5, 15, 30, and 45 min) on ice. At 0 min, 135 μL of acetonitrile containing ketanserin was added to the wells of 0 min plate, followed by the addition of 15 μL of previously prepared NADPH stock solution (6 mM). All other plates were pre-incubated for 5 min at 37 °C. Next, 15 μL NADPH stock solution (6 mM) was added to the plates to start the reaction and timing. At 5, 15, 30, and 45 min points, 135 μL of acetonitrile containing ketanserin was added to the wells of the corresponding plates to stop the reaction. After quenching, the plates were shaken on a shaker (IKA, MTS 2/4) for 10 min (600 rpm min−1) and then centrifuged for 15 min (Thermo Multifuge × 3R, 5594 r min−1). Finally, 50 μL of the supernatant was transferred from each well into a 96-well sample plate containing 50 μL of ultra-pure water (Millipore, ZMQS50F01) for LC/MS analysis. Ketanserin was selected as the reference compound.
Pharmacokinetics assay
YZQ17 was intravenously or orally administered at different dose levels. Rats were restricted from food overnight prior to dosing (approx. 12 h) and fed 2 h post-dose. Water was provided ad libitum. Approximately, 150 μL of whole blood were collected from the orbital plexus using a glass capillary and collected into a tube, which contained potassium EDTA. The plasma samples were stored at −70 °C prior to analysis. An aliquot of 10 μL of the supernatant was injected into the HPLC-UV system for analysis.
Additional statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhengzhou University and approved by the Animal Ethics Committee of Zhengzhou University.
The accreditation number for operating experimental animals is SCXK2019-0010 and was purchased from Sibeifu Biotechnology Co. Ltd., Beijing, China.
Author contributions
Xiufang Shi: experimental design and synthesis, data collections, data analysis, manuscript preparation; Zhenqiang Yu and Chaoran Zhu: design, synthesis, data collections, partial manuscript preparation; Linlin Jiang: CCK-8 assay; Nanqi Geng: wound healing assay and Annexin-V/PI staining and flow cytometry assay; Xingting Fan and Zhanghui Guan: Western blot assay. Xiang Lu: research design, supervision of whole project, manuscript preparation.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by grants from National College Students' innovation and entrepreneurship training program (2021cxcy304).
Electronic supplementary information (ESI) available: Synthetic procedures of compound 2–4 and 1H and 13C NMR spectra of YZQ01–18. See DOI: https://doi.org/10.1039/d1md00403d
Notes and references
- Bazdyrev E. Rusina P. Panova M. Novikov F. Grishagin I. Nebolsin V. Pharmaceuticals. 2021;14:807. doi: 10.3390/ph14080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. M. Wells A. U. Jenkins R. G. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer D. J. Martinez F. J. N. Engl. J. Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- Raghu G. Weycker D. Edelsberg J. Bradford W. Z. Oster G. Am. J. Respir. Crit. Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- King, Jr. T. E. Pardo A. Selman M. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- Kurita Y. Araya J. Minagawa S. Hara H. Ichikawa A. Saito N. Kadota T. Tsubouchi K. Sato N. Yoshida M. Kobayashi K. Ito S. Fujita Y. Utsumi H. Yanagisawa H. Hashimoto M. Wakui H. Yoshii Y. Ishikawa T. Numata T. Kaneko Y. Asano H. Yamashita M. Odaka M. Morikawa T. Nakayama K. Kuwano K. Respir. Res. 2017;18:114. doi: 10.1186/s12931-017-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N. Parekh T. V. O'Connor R. Antman N. Kepron W. Yehaulaeshet T. Xu Y. D. Gold L. I. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall R. T. Feghali-Bostwick C. A. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S. Keating G. M. Drugs. 2015;75:219–230. doi: 10.1007/s40265-015-0350-9. [DOI] [PubMed] [Google Scholar]
- Bruss M. L. Stanley S. D. Margolin S. B. Giri S. N. Biopharm. Drug Dispos. 2008;29:119–126. doi: 10.1002/bdd.595. [DOI] [PubMed] [Google Scholar]
- King, Jr. T. E. Bradford W. Z. Castro-Bernardini S. Fagan E. A. Glaspole I. Glassberg M. K. Gorina E. Hopkins P. M. Kardatzke D. Lancaster L. Lederer D. J. Nathan S. D. Pereira C. A. Sahn S. A. Sussman R. Swigris J. J. Noble P. W. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- Giri S. N. Wang Q. Xie Y. Lango J. Morin D. Margolin S. B. Buckpitt A. R. Biopharm. Drug Dispos. 2002;23:203–211. doi: 10.1002/bdd.311. [DOI] [PubMed] [Google Scholar]
- Zhou S. Li W. Tian M. Zhang N. Yang X. Li W. Peng Y. Zheng J. J. Med. Chem. 2020;63:8059–8068. doi: 10.1021/acs.jmedchem.9b02073. [DOI] [PubMed] [Google Scholar]
- Zhu W. Shen J. Li Q. Pei Q. Chen J. Chen Z. Liu Z. Hu G. Arch. Pharm. 2013;346:654–666. doi: 10.1002/ardp.201300138. [DOI] [PubMed] [Google Scholar]
- Wu L. Liu B. Li Q. Chen J. Tao L. Hu G. Molecules. 2012;17:1373–1387. doi: 10.3390/molecules17021373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Q. Meng X. Lao Z. Xuan L. Bai J. Hou Q. Hu G. Luo R. Tao L. Li Z. Molecules. 2012;17:884–896. doi: 10.3390/molecules17010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Lu M.-M. Liu B. Chen Z. Li Q.-B. Tao L.-J. Hu G.-Y. Bioorg. Med. Chem. Lett. 2012;22:2300–2302. doi: 10.1016/j.bmcl.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Liu C. H. Hu Y. Y. Wang X. L. Liu P. Xu L. M. World J. Gastroenterol. 2000;6:361–364. doi: 10.3748/wjg.v6.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.-H. Sung D.-B. Park C.-H. Kim W.-S. J. Org. Chem. 2016;81:7717–7724. doi: 10.1021/acs.joc.6b01415. [DOI] [PubMed] [Google Scholar]
- Bielawski M. Olofsson B. Chem. Commun. 2007:2521–2523. doi: 10.1039/B701864A. [DOI] [PubMed] [Google Scholar]
- Lu X. Huang A. Xiao M. Sun L. Mao J. Luo G. Xiang H. Bioorg. Chem. 2020;97:103666. doi: 10.1016/j.bioorg.2020.103666. [DOI] [PubMed] [Google Scholar]
- Dhainaut J. F. Charpentier J. Chiche J. D. Crit. Care Med. 2003;31:S258–S264. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- Liu F. Mih J. D. Shea B. S. Kho A. T. Sharif A. S. Tager A. M. Tschumperlin D. J. J. Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. J. Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Venkatesan N. Pini L. Ludwig M. S. Am. J. Physiol. 2004;287:L1342–L1347. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- Lin R. Zhang Z. Cao S. Yang W. Zuo Y. Yang X. Zhang J. Xu J. Li J. Wang X. RSC Med. Chem. 2021;12:1222–1231. doi: 10.1039/D1MD00023C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.