Abstract
It is becoming increasingly clear that the worldwide outbreak of severe acute respiratory syndrome coronavirus 2 will have long-term negative consequences. Some patients report functional complaints long after recovery from coronavirus disease-2019 (COVID-19), which include fatigue, breathlessness, heart palpitations, loss or alteration of taste and smell, and problems with attention, memory, and cognition. However, the long-term complications for those patients who had severe symptoms and prolonged hypoxia during their course of their hospital stay is still unknown. We report 2 patients with confirmed diagnoses of COVID-19 who experienced prolonged infection and developed rapid progressive dementia following COVID-19 pneumonia after a follow-up period of 5 to 10 months. As these cases may become more prevalent over time, we should learn to recognize the early signs of long-term COVID-19 complications in those who are especially vulnerable to neurocognitive decline.
Key Words: COVID-19, Alzheimer disease, neuropsychiatry, pet functional imaging, frontal lobe
CASE REPORT
Case 1
Patient 1 is a 79-year-old female with no significant past medical history or neuropsychiatric history. She had complained of developing mild short-term memory loss and word finding difficulty over several years, but it was not pronounced nor interfering with her daily life until she was diagnosed with coronavirus disease-2019 (COVID-19), confirmed by nasopharyngeal swab. The pneumonia was complicated with significant metabolic and respiratory syndromes and required 20 days of hospitalization. Her oxygen saturation was below 94% for nearly half of her stay, with the lowest being 78% on a nonrebreather. She also experienced extended periods of hypoxia spanning 2 to 3 days where her oxygen saturation was below 90%, and she was not intubated.
She presented to the neuropsychiatric clinic 10 months after discharge, and she has had significant difficulty in word retrieval and word finding, as well as feeling like she has “brain fog,” poor attention, and inability to execute planned actions. Thus, she has been suffering from rapid progressive dementia worsening mostly in the domain of episodic memory and word finding difficulties since her COVID-19 infection. She was started on bupropion 75 mg to rule out treatable etiologies including attention deficit/mood-related cognitive impairment/reversible dementia because of the mood and attention disorder, but the patient is no longer able to track her medication and requires aid from her daughter. Her activities of daily living are severely impaired.
A computed tomography (CT) angio of the head and neck, brain magnetic resonance imaging (MRI), and 18fluorodeoxyglucose-positron emission tomography (F-FDG-PET) were done as part of the neurocognitive work-up to rule out vascular and neurodegenerative etiologies. The CT angio showed no signs of occlusion or aneurysms. There were no abnormalities on the brain MRI. Images were processed and analyzed as per Franceschi and colleagues. In brief, images were obtained with an integrated 3-T PET/MRI system. PET surface maps, fused T1-weighted magnetization-prepared rapid acquisition gradient echo and axial FLAIR/PET images were generated with postprocessing software. MIMneuro 6.9.5 was used to provide a z score analysis of brain hypometabolism, which compares patients to selected age-matched set of healthy control subjects. PET MRI exhibited hypometabolism involving the left temporal lobe, in particular superior temporal gyrus, mild hypometabolism in the adjacent left frontal and parietal regions with mildly asymmetric decreased tracer activity in the left basal ganglia and thalamus (Fig. 1).
FIGURE 1.
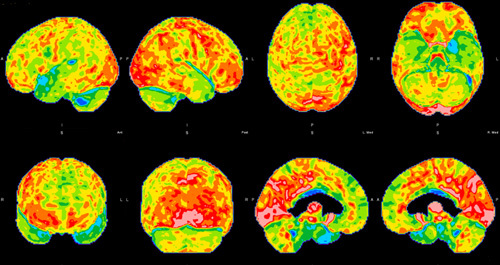
Brain 18fluorodeoxyglucose-positron emission tomography hypometabolism of the first patient. Hypometabolism can be seen in the left temporal lobe, in particular superior temporal gyrus, and mild hypometabolism in the adjacent left frontal and parietal regions with mildly asymmetric decreased tracer activity in the left basal ganglia and thalamus.
Case 2
Patient 2 is a 63-year-old female with a past medical history of type 2 diabetes, thyroid cancer, hyperlipidemia, fibromyalgia, hypertension, and obesity. She had no previous neuropsychiatric history. She was diagnosed with COVID-19 by nasopharyngeal swab, and was on home quarantine for 32 days. Her daily symptoms included fevers (maximum temperature 100.8° F), myalgias, and severe nonproductive cough. Her oxygen saturation was monitored by a home pulse oximeter, and it ranged from 95% to 98% on room air. She reported that she was walking around her home and denies shortness of breath with physical activity.
She presented to have a neuropsychiatric evaluation 5 months after her initial SARS-CoV-2 infection, complaining about “brain fog,” increasing word finding difficulties, word retrieval deficits, deficits with memory and attention, and executive function with planning and organizing day activities. She states that she sometimes cannot remember what she is talking about or find the right words to continue the conversation. She was also having increasing depressive symptoms with poor motivation and focus since recovering from COVID-19, and cannot remember what she read a few minutes ago. The new onset personality, mood, behavioral, and neurocognitive symptoms in the domain of expressive language, memory, and mood regulation was interfering significantly with her quality of life, and she now requires help from her daughter to manage medications. She was started on bupropion 300 mg which has had no effect. She was also on gabapentin 1800 mg and Percocet for her chronic pain.
A structural brain MRI was done as part of the neurocognitive work-up with a CT scan to assess any structural abnormalities which showed no cortical focal mass or any stroke or infarct or normal pressure hydrocephalus but did show significant scattered subcortical white matter microvascular disease consisting of chronic small vessel diseases. 18F-FDG-PET showed hypometabolism in parietal and mesial temporal lobe consistent with neurodegenerative dementia and clinically correlates with current neurocognitive examination findings suggestive of Alzheimer disease.
DISCUSSION
The long-term impacts of the COVID-19 pandemic are just beginning to be elucidated. Many neuropsychiatric manifestations of COVID-19 have been reported, and on imaging, bilateral hypometabolism in the olfactory gyrus, right temporal lobe, pons/medulla brainstem, and cerebellum have been seen.1 In addition, the possible neurotropism of COVID-19 has been hypothesized to cause a delayed onset and drastic progression on neuropsychiatric and neurodegenerative disease.2 A recent large systematic review assessing the psychiatric and neuropsychiatric presentations of SARS, MERS, and COVID-19 confirmed the high prevalence of post-illness disease.3 However, these presentations have appeared to persist and/or develop long after the initial infection, and this clinical presentation of delayed symptoms postinfection has been termed “long COVID,” with unknown implications and prevalence because of ongoing research. Notable studies performed PET scanning on patients during the recovery phase of COVID-19 found hypometabolism in the parahippocampal, fusiform gyri, and insula, with no change in brain volumes.4
It has been suggested that 18F-FDG-PET hypometabolism in the post-infection context could be used as a cerebral quantitative biomarker of long COVID. Case control PET studies on active COVID-19 patients with neurological symptoms have already shown decreased metabolism in parietal and frontal association cortices compared with control patients.5 We presented 2 cases, 5 months and 10 months initial COVID-19 infection, with a sharp decline in neurocognitive function following the initial infection. 18F-FDG-PET of both patients showed prominent hypometabolism that are consistent with their functional complaints. Frontal-temporal hypoperfusion has been commonly seen in COVID-19 patients, and the frontal cortex has been suggested to be the primary target of COVID-19.6 Both patients had deficits in executive and working memory function that progressed at an extraordinary pace following COVID-19 infection, suggesting that the infection might have been a trigger to their decline.
In addition, one patient had an extended hospital stay with prolonged hypoxia. While ischemic hypoxia should result in MRI brain changes, this patient had no appreciable structural changes. Previous studies have recorded that memory disturbances are the most common sequalae of cerebral hypoxia.7 However, our patient did not appreciate large changes until months after her initial infection. It is also possible that prolonged infection with COVID-19, such as our second patient, could cause these changes from long-term neuroinflammation. While we did not perform a CT angio for the second patient, she also had risk factors for vascular dementia and infection with COVID-19 may have exacerbated ischemic hypoxia, though her MRI brain did not show any evidence of infarcts.
Lastly, hypometabolism seen on the 18F-FDG-PET scans can possibly be because of hypoperfusion of the affected brain regions. “Brain fog” secondary to COVID-19 is also highly prevalent and has been associated with orthostatic cerebral hypoperfusion syndrome post COVID-19 infection. There have also been reports of COVID-19 patients having lowered gray matter volume.8 However, there were no changes in the MRI of our patients; the brain is structurally intact. Prolonged periods of hypoperfusion/hypoxia without progressing to infarct may recover function if eventually reperfused. It has been shown that neural activation increases blood flow,9 and thus mental and cognitive exercise may prove to help stimulate perfusion. This includes stress management, meditation and mindfulness, cognitive stimulation, and brain exercises including brain games or crossword puzzles and daily reading books or newspaper on a regular basis.
In summary, we reported 2 patients who presented with long-term neuropsychiatric sequalae of COVID-19 after 5 and 10 months of initial infection. These patients did not have MRI changes, but showed hypometabolism in certain brain regions as assayed by 18F-FDG-PET. In addition, it is possible that COVID-19 may have acted as a trigger for emergence of underlying cognitive symptoms in those with unknown previous pathologies, as we do not have prior PET imaging from the patients. We believe that maintaining neurocognitive health, which includes nutrition, physical, mental, and cognitive exercise can help prevent or delay long-term consequences of COVID-19. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet may prove to be beneficial as well, as it has efficacy in other neurodegenerative diseases.10 Physical exercise, such as daily walking, would improve cerebral perfusion and increase blood flow to those regions that may be hypoperfused. Cognitive health should be practiced and implemented in addition to pharmacological and psychotherapeutic intervention in order to target each domains of cognition to improve the overall physical and mental functioning and quality of life.
Thus, it is important to be aware of the possibilities following COVID-19 infection so that possible intervention and rehabilitation can be started once symptoms are recognized, which may be important to perhaps delay and reduce the progression of neurodegenerative disease.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Allen T. Yu, Email: allen.yu@mountsinai.org.
Nicole M. Absar, Email: Nicole.absar@stonybrookmedicine.edu.
REFERENCES
- 1. Guedj E, Campion JY, Dudouet P, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serrano-Castro PJ, Estivill-Torrus G, Cabezudo-Garcia P, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia. 2020;35:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donegani MI, Miceli A, Pardini M, et al. Brain metabolic correlates of persistent olfactory dysfunction after SARS-Cov2 infection. Biomedicines. 2021;9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toniolo S, Di Lorenzo F, Scarioni M, et al. Is the frontal lobe the primary target of SARS-CoV-2? J Alzheimers Dis. 2021;81:75–81. [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Molina A, Roig-Rovira T, Ensenat-Cantallops A, et al. Neuropsychological profile of persons with anoxic brain injury: differences regarding physiopathological mechanism. Brain Inj. 2006;20:1139–1145. [DOI] [PubMed] [Google Scholar]
- 8. Duan K, Premi E, Pilotto A, et al. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol Stress. 2021;14:100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen J, Wang D, Wang X, et al. Neurovascular coupling in the dentate gyrus regulates adult hippocampal neurogenesis. Neuron. 2019;103:878.e3–890.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]


