Abstract
Objective:
Examine the impact of COVID-19 pandemic on the outcomes in patients with CLTI or DFI.
Background:
Patients with CLTI and/or DFI are at risk of amputations if not treated in a timely manner.
Methods:
We compared the outcomes in patients with CLTI or DFI during 2 periods; Period 1[P1] (15/03/2019-31/05/2019) and period 2[P2] (15/03/ 2020-31/05/2020- corresponding to COVID-19 pandemic).
Results:
One hundred thirty-nine patients were treated in P1 [mean age 70 years (±11), Male:Female = 102:37] whereas 95 patients were treated in P2 [mean age 67 (±12), Male:Female = 64:31]. The 2 cohorts were matched regarding Rutherford category (P = 0.25) and GLASS classification (P = 0.38). Notably, the time from onset of symptom to clinical presentation was significantly longer [31 (1-105) days vs 27 (0–78) days, (P = 0.017)], whereas the time from presentation to first intervention was significantly shorter [3 (0–61) days vs 5 (0–65) days, (P = 0.013)] in P2 compared to P1. There was a significantly higher white cell count (P = 0.014) and CRP (P = 0.004) on admission in P2. Having treatment for CLTI or DFI in P2 was an independent predictor of worse primary patency rate and freedom from major adverse limb events. At 90 days, amputation-free survival and limb salvage were noticeably worse in P2 compared to P1 (amputation-free survival was 80% and 87% whereas limb salvage was 64% and 72% in P2 and P1, respectively).
Conclusions:
Patients with CLTI and DFI experienced a significantly delayed presentation with features of sepsis on admission in P2. Treatment in P2 was a predictor of worse primary patency and freedom from major adverse limb events and therefore close and long follow-up is advisable.
Keywords: COVID-19, critical limb-threatening ischemia, diabetic foot infection, interventions
Abbreviations: AFS, Amputation free survival, CLTI, Critical limb threatening ischemia, COVID 19, Coronavirus 2019, CRP, Creactive protein, DFI, Diabetic foot infection, F–Mace, Freedom from major cardiovascular events, F-Male, Freedom from major adverse limb events, F-CDR, Freedom from clinically driven reintervention, LS, Limb salvage, WCC, white cell count
Critical limb-threatening ischaemia (CLTI) and diabetic foot infection (DFI), with or without ischaemic element, are leading causes of urgent hospital admissions and limb loss, especially in elderly patients. Easy access to clinical assessment, hospital and community-based multidisciplinary diabetic foot clinics and timely interventions have been shown to improve amputation-free survival (AFS) and limb salvage (LS) rates in this cohort of high-risk patients.1,2
Coronavirus disease 2019 (COVID-19) pandemic has represented a huge challenge to healthcare systems worldwide.3,4,5,6 A rising number of acute aortic, carotid, and peripheral vascular complications associated with laboratory findings of coagulopathy and antiphospholipid antibodies, often suggestive of underlying COVID-19 infection, have been reported worldwide.7 To cope with the high demand to treat patients with COVID-19, healthcare systems had to implement various strategies to create the required capacity. The Vascular Society of Great Britain and Ireland and National Health Services (NHS) issued guidance for clinicians and hospitals recommending the deferral of elective procedures, especially in asymptomatic patients.8,9 Consequently, healthcare organizations took various measures such as deferral of all elective procedures,10,11 repurposing of theatres so they can potentially be used as a back-up for critical care units (CCUs) if these get overwhelmed12 and advising the public to avoid hospital encounters unless absolutely necessary.13 Also to ensure enough staffing for the CCUs, augmentation of staff with colleagues from other CCUs or even non-CCU areas, including vascular surgeons and anesthetists, was advocated.14,15
Various changes to the usual working patterns in both hospital and community-based settings had to be implemented in response to the pandemic. These included cancellation of nonurgent face-to-face clinic appointments and moving towards telephonic consultations whenever possible.16,17 Collectively, the above measures might have impacted the patients’ access to community and hospital services.
The aim of this study was to examine the impact of COVID-19 pandemic on the outcomes in patients with CLTI or DFI.
METHODS
Vascular Services in the United Kingdom (UK) are structured using a “hub and spoke” model, with arterial surgery conducted at the hub site only. Our unit forms the hub of the Southeast London Vascular Network with a catchment population of 3.5 million. Our hospital is also a designated regional center for high consequences infectious disease in South London.
Reorganization of Vascular Services
In line with published recommendations, our unit began a rapid restructuring of services at the beginning of March 2020. The purpose of the restructuring was to redeploy resources, including staff, theatre space, inpatients beds and CCU beds, so that they were available to care for patients with COVID-19. It was also used to maintain a structured pathway for the assessment and management of urgent and emergency referrals. These changes aimed at reducing unnecessary exposure to hospitals by deferring elective arterial and venous surgery as well as switching to telephonic clinic appointments whenever possible.
Meanwhile, the full emergency service was maintained at the hub site which included 24-hour, 7-day access to a Vascular Surgeon, Vascular Interventional Radiology service, and the emergency department.
Diabetic foot clinics (DFCs) were maintained during the weekdays in the hub and spoke hospitals. Our unit benefits from a 1-stop Emergency Vascular Clinic from Monday to Friday. The scope of this clinic was expanded during the COVID-19 pandemic, offering rapid triage, assessment, management, and subsequent follow-up of urgent patients. Urgent patients were referred via the on-call team, the spoke clinics, or primary care using a generic email which was attended 12 hours a day.
Data Collection
To assess the specific impact of the COVID-19 pandemic on the outcomes of patients with CLTI and DFIs, we used 2 time frames for comparison. Period 1 (from March 15, 2019 till May 30, 2019 -P1) and Period 2 (from March 15, 2020 till May 30, 2020 - P2). A prospectively-collected database including patients’ demographics, co-morbidities, medications, nature of presentation, time between onset of symptoms to presentation, time from presentation to intervention, type of interventions performed, complications as well as major and minor amputations was analyzed. We also collected data on the laboratory results on admission and discharge. Procedural and anatomical data were also collected including Rutherford classification (pre and postintervention) and GLASS classification.18 All angiographic images were reviewed and reported by an experienced consultant interventional radiologist (NT) to ensure consistency. All patients were discussed in a dedicated multidisciplinary team meeting where the most appropriate intervention was recommended after careful consideration of patients’ clinical picture and co-morbidities whenever practically possible.
Outcome Measures
Primary outcomes: AFS and LS rates in the 2 time periods Secondary outcomes included:
Time between onset of symptoms to presentation to healthcare facilities
Time between presentation to intervention
Freedom from clinically-driven Reintervention (F-CDR) in those who had revascularization procedures.
Freedom from major adverse limb events and freedom from major adverse cardiovascular event as defined by the society of vascular surgery objective performance goals.19,20
Statistical Analysis
Continuous variables are expressed as mean (s.d.) for normally distributed data and median (range) for those without a normal distribution, and compared using the independent samples t test and Mann-Whitney U test respectively. Categorical variables were compared using the x 2 test or Fischer exact test. Data was analyzed as total numbers and compared using contingency tables or divided into numbers per month and analyzed using either parametric or nonparametric tests as above. All analyses were carried out using GraphPad Prism 6 (GraphPad Software, San Diego, California, USA) and SPSS version 22 (IBM, Armonk, New York, USA). P value <0.05 was considered significant. As per NHS Research and Ethics definitions (institutional review board equivalent; available from www.nres.nhs.uk/), this analysis is not classified as research requiring formal ethics approval and was approved locally as an audit project
RESULTS
Patterns of Presentation and Management
During the 2 periods of the study, a total of 234 patients were admitted for urgent or emergency intervention (139 in P 1 and 95 in P 2). Patient demographics and perioperative anticoagulation and antiplatelet regimens are detailed in Table 1. The two cohorts were comparable for medical comorbidities, but differed significantly from each other with respect to the incidence of smoking (81% vs 60% P = 0.02) and hypercholesterolemia. Preoperatively patients were more likely to be taking DOACs (8% vs 17%, P = 0.09) as well as dual antiplatelet agents (6% vs 16%, P = 0.001) in P 2. Postoperatively there was a significant increase in the use of therapeutic anticoagulation (P = 0.001) as well as a concomitant decrease in the use of antiplatelets (P = 0.001) in P 2 compared to P 1.
TABLE 1.
Patient Demographics and Medication
| Period 1 (N = 139) | Period 2 (N = 95) | P Value | |
| Age (mean (SD) (yrs) | 70 (±11) | 67 (±12) | 0.10† |
| Sex (M:F) | 102:37 | 64:31 | 0.38∗ |
| IHD n (%) | 46 (33) | 31 (32) | 0.52∗ |
| Stoke/TIA n (%) | 18 (13) | 8 (8) | 0.12∗ |
| DM n (%) | 84 (60) | 57 (60) | 0.52∗ |
| Smoker n (%) | 112 (81) | 57 (60) | 0.02∗ |
| Hypertension n (%) | 104 (75) | 82 (86) | 0.034∗ |
| Hypercholesterolaemia n (%) | 72 (52) | 63 (64) | 0.06∗ |
| eGFR (mean (SD) (ml/min/1.73 m2) | 75 (±36) | 74 (±50) | 0.90† |
| Renal replacement therapy | 4 (3) | 8 (8) | 0.072∗ |
| Preoperative anticoagulation | |||
| Warfarin | 8 (6) | 6 (6) | |
| Direct oral anticoagulant (DOAC) | 11 (8) | 16 (17) | 0.09∗ |
| Low molecular weight heparin | 5 (4) | 7 (7) | |
| Preoperative antiplatelets | |||
| Aspirin | 63 (45) | 33 (35) | |
| Clopidogrel | 18 (13) | 12 (13) | 0.001∗ |
| Dual antiplatelets | 8 (6) | 15 (16) | |
| Postoperative anticoagulation | |||
| Warfarin | 9 (6) | 9 (6) | |
| Direct oral anticoagulant (DOAC) | 15 (11) | 18 (19) | 0.001∗ |
| Low molecular weight heparin | 6 (4) | 16 (17) | |
| Postoperative antiplatelets | |||
| Aspirin | 29 (21) | 31 (33) | |
| Clopidogrel | 41 (29) | 19 (20) | 0.001∗ |
| Dual antiplatelets | 59 (42) | 26 (27) | |
Chi-Square.
Unpaired t-test.
DM indicates diabetes mellitus; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; TASC, Transatlantic Inter-Society Consensus; TIA, transient ischaemic attack.
Patients presenting in both treatment periods were well matched in terms of the spectrum of clinical presentations (Table 2). Patients presenting with CLTI and DFI or tissues loss (N = 113 in P 1 and N = 73 in P 2) were further sub categorized to determine if severity of disease was different in the 2 treatment periods. Patients in both periods were well matched with regards to Rutherford category (P = 0.25), anatomical level of the critical arterial disease (P = 0.19) as well as overall GLASS classification (P = 0.38). For the whole cohort, there was no significant difference in those presenting with radiographic evidence of osteomyelitis (11% in P1 vs 8% in P2, P = 0.12) or gas in the soft tissues (4% vs 5%, P = 0.9) between the time periods, however, there was a significantly higher WCC [9.5 (±3.9) vs 11 (±4.5), P = 0.014] and CRP [44 (1–354) vs 73 (1–321), P = 0.004] on admission in P2. In addition, the time from onset of symptom to clinical presentation was significantly longer [31 (1–105) days vs 27 (0–78) days, (P = 0.017)], whereas the time from presentation to first intervention was significantly shorter [3 (0–61) days vs 5 (0–65) days, (P = 0.013)] in P2 compared to P1.
TABLE 2.
Presentation
| Period 1 (N = 139) | Period 2 (N = 95) | P Value | |
| Clinical presentation n (%) | |||
| Critical limb ischemia | 76 (55) | 47 (49) | |
| Neuro-ischemic infection/tissue loss | 37 (27) | 26 (27) | 0.26∗ |
| Diabetic foot infection/tissue loss | 17 (12) | 16 (17) | |
| Acute limb ischaemia | 8 (6) | 4 (4) | |
| Aneurysmal disease | 1 (1) | 2 (2) | |
| Rutherford classification n (%) | N=113 | N=73 | |
| 4 | 30 (27) | 28 (38) | |
| 5 | 41 (36) | 24 (32) | 0.25∗ |
| 6 | 46 (40) | 21 (29) | |
| Level of critical lesion (s) n (%) | |||
| Iliac | 13 (12) | 6 (8) | |
| Femoro-popliteal | 31 (28) | 17 (23) | 0.19∗ |
| Infra-popliteal | 26 (23) | 14 (19) | |
| Multi-level | 43 (38) | 36 (49) | |
| Overall GLASS classification n (%) | |||
| I | 22 (19) | 19 (26) | |
| II | 11 (10) | 9 (12) | 0.38∗ |
| III | 80 (71) | 45 (62) | 0.014‡ |
| WCC on admission - mean (±SD) | 9.5 (±3.9) | 11 (±4.5) | |
| CRP on admission - median (range) | 44 (1–354) | 73 (1–321) | 0.004† |
Chi-square.
Man-Whitney U test.
Unpaired t-test.
Over the 2 treatment periods, 199 patients went on to have urgent or emergency revascularisation, and a further 45 went on to have primary major or minor amputation with no revascularisation (Table 3). When comparing the 2 treatment periods, there was no significant difference in the pattern of open (P = 0.66), endovascular (P = 0.45), and hybrid (P = 0.58) revascularisation approaches. Furthermore, there was no significant increase in the proportion of patients having primary major (P = 0.62) or minor amputations (P = 0.09).
TABLE 3.
Procedural Details
| Period 1 (N = 139) | Period 2 (N = 95) | P Value | |
| Surgical revascularisation | |||
| Bypass | 18 (13) | 14 (14) | |
| Embolectomy | 3 (2) | 2 (2) | 0.66∗ |
| Endovascular revascularisation | |||
| Percutaneous PTA+/_stent | 72 (52) | 42 (44) | |
| Arterial lysis | 5 (4) | 2 (2) | 0.45∗ |
| Hybrid revascularisation (CFA endarterectomy + PTA+/_stent) | 17 (12) | 14 (14) | 0.58∗ |
| Primary major amputation | 10 (7) | 5 (5) | 0.62∗ |
| Primary minor amputation/debridement | 14 (10) | 16 (17) | 0.09∗ |
| Median time from revascularisation to amputation (Range) | 13 (1–84) days | 13 (1–90) days | NS |
Chi-Square.
Predictors of Outcome
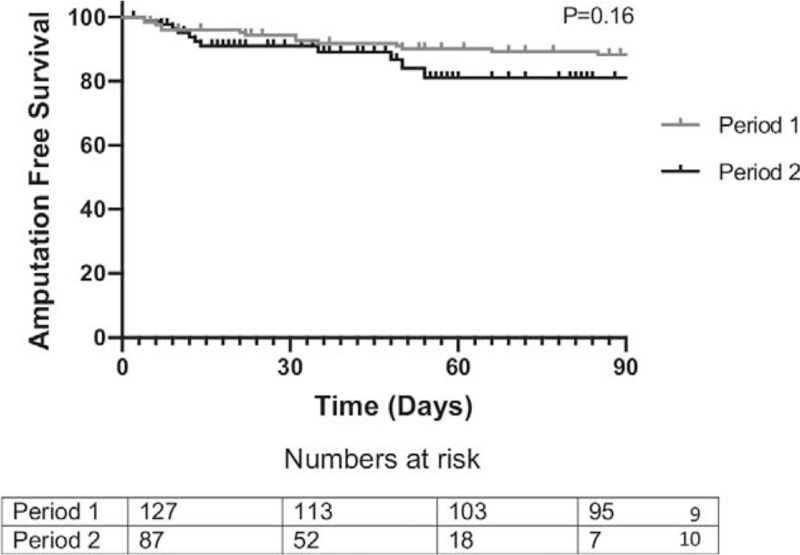
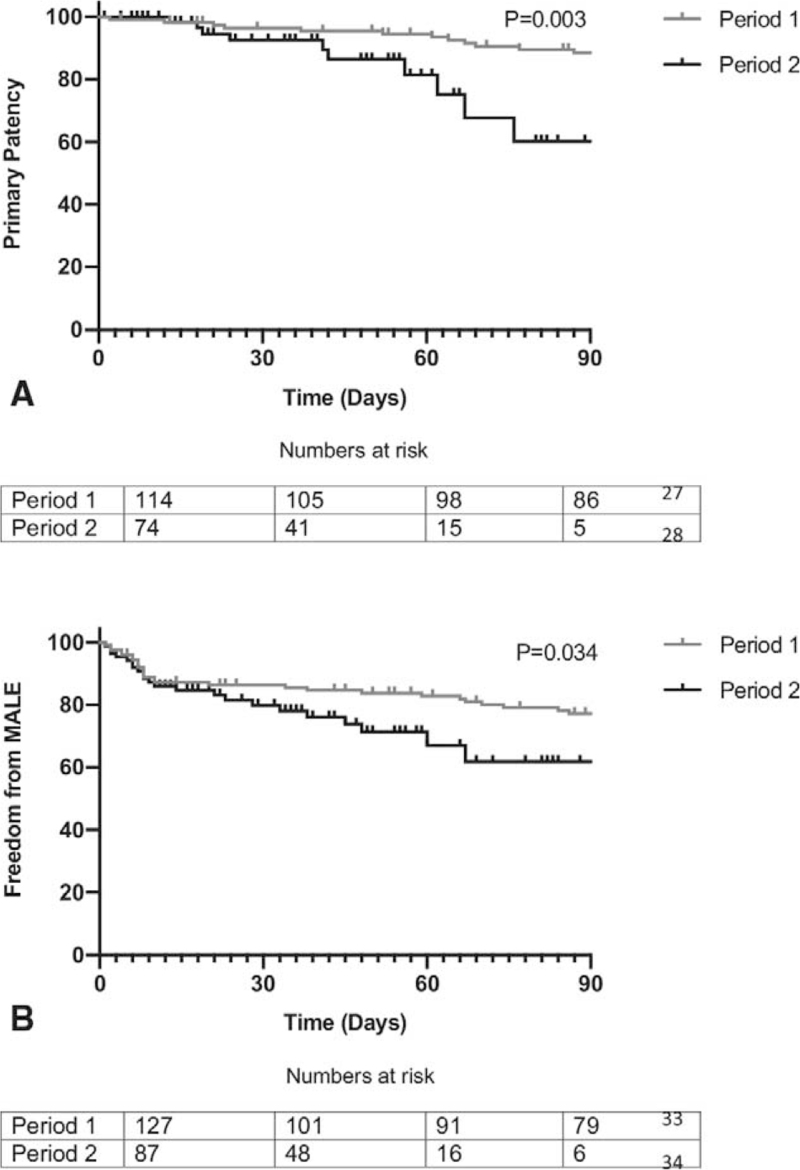
Comparing outcomes in the 2 time periods, in patients who underwent revascularisation AFS at 90 days by Kaplan Meier was 87% in P1 and 80% in P2 (P = 0.16, Fig. 1), and LS was 72% vs 64% (P = 0.41). Primary patency was significantly better in P 1 (88% vs 60%, P1 vs P2, P = 0.003, Fig. 2A), and although assisted primary patency (90% vs 71%, P = 0.15) and secondary patency (91% vs 84%, P = 0.24) were also better in P1, this did not reach statistical significance. F-CDR was significantly worse in P2 (83% vs 67%, P = 0.037) as was F-MALE (60% vs 46% P = 0.034, Fig. 2B). The overall length of hospital stay was significantly better in P 2 (13(0–176) in P1 vs 9 (0–90) in P2, P = 0.01).
FIGURE 1.
Amputation free survival by Kaplan Meier analysis. Log rank test.
FIGURE 2.
A, Primary patency. B, Freedom from (major adverse limb event) MALE - by Kaplan Meier analysis. Log Rank test.
To adjust for differences in the 2 cohorts, we carried out a multivariate Cox regression analysis to look for independent factors which may predict primary patency (Fig. 2A) and F-MALE (Fig. 2B). A higher WCC on admission (P = 0.018) and treatment during P 2 (P = 0.003) were independent factors predicting worse primary patency. In addition, a lower eGFR (P = 0.038) and treatment during P 2 (P = 0.009) independently predicted worse freedom from major adverse limb events (Table 4).
TABLE 4.
Multivariate Cox Regression Analysis Showing Factors Affecting Primary Patency and Freedom From MALE
| Predictors of Outcome | Hazard Ratio (95% CI) | P | |
| Primary patency | WCC on admission | 0.83 (0.81–0.98) | 0.018 |
| Presentation period | 0.19 (0.06–0.56) | 0.003 | |
| f-MALE | eGFR | 1.01 (1.0–1.014) | 0.038 |
| Presentation period | .41 (0.21–0.79) | 0.009 | |
| Postprocedural DAPT | 0.44 (0.27–0.74) | 0.002 |
Complications and Survival
Overall survival of the entire cohort at 90 days was 85% in P1 vs 92% in P2 (P = 0.41). Thirty-day mortality was 2% in both cohorts. Of note, only 5 patients in P2 tested positive for COVID-19. During the first 30 days, we recorded MACE in 5% of patients in P1 and 3% in P2 (P = 0.12). In addition to this, the most common perioperative/procedural complications were wound infection (7%), groin or wound bleeding/hematoma (7%) Pneumonia (6%), acute kidney injury (6%), and CVA (3%). When the morbidity was classified using the Clavien-Dindo grade (Table 5) and compared in the 2 time periods, there was no significant difference, though there was a trend towards less overall complications in P 2.
TABLE 5.
Thirty Day Morbidity (I-IV) and Mortality (V) Stratified Using the Clavien-Dindo Classification
| Complication Grade | Period 1 N = 139 (%) | Period 2 N = 95 (%) | P Value |
| I | 7 (5) | 5 (5) | 0.08 |
| II | 10 (7) | 4 (4) | |
| III | 9 (6) | 4 (4) | 0.07 |
| IV | 8 (6) | 3 (3) | |
| V | 3 (2) | 2 (2) | 0.91 |
Chi square based on minor- Grade I and II or major - Grade III and IV complications.
DISCUSSION
To our knowledge, this is the largest series of CLTI and DFI undergoing vascular interventions during the COVID-19 pandemic. This study showed that patients with CLTI and DFI were more likely to experience significantly delayed presentation with raised inflammatory markers on admission in P2 compared to P1. Having treatment for CLTI or DFI in P2 was an independent predictor of both worse primary patency rate and F-MALE. AFS and LS were noticeably worse in P2 compared to P1; however, this did not reach statistical significance.
Restructuring the NHS services and patients’ pathways was required to allow for flexibility and resilience in response to COVID-19 pandemic.21,22 Different measures have been put in place to slow the rate of infection and reduce the strain on the NHS resources. Advice was therefore given to the public to only seek help from the NHS when it is absolutely necessary to free capacity to treat sicker COVID-19 patients and also reduce risk of spreading the infection to the general population.11 In addition, various measures were implemented in both hospital and community settings including moving away from face-to-face appointments whenever possible. Changes in various aspects of the delivery of vascular services have also been reported in other studies .23,24
Our study highlighted few key observations in patients with CLTI and DFI including the significantly longer duration from the onset of symptoms to presentation to healthcare facilities and the higher likelihood of presenting with features of sepsis. Arguably, these observations may be a consequence of delayed access to healthcare facilities as well as strict adherence to the government's advice to avoid hospital encounters whenever possible.11 As expected, due to delayed presentation and sepsis, patients with CLTI and DFI had a significantly worse F-CDR and F-MALE in P2 compared to P1. It is therefore paramount to address the causes which led to these outcomes in the future if the healthcare systems were to face further COVID-19 infection waves or any other similar pandemics.
CLTI represents a significant health problem amongst elderly populations. If not treated in a timely manner, CLTI could lead to major amputation and even death.25,26,27 It has been shown that availability of clearly established patients’ pathways allowing rapid access to clinical assessment and timely interventions is necessary to achieve satisfactory AFS and LS rates.28 In addition, diabetes mellitus is a major cardiovascular risk factor with DFI representing a significant risk of limb loss if untreated in a timely manner. Multidisciplinary DFCs have been proven essential to minimize limb loss in these high-risk patients.29 Some authors even advocated treating DFI as a “foot attack” where “time is tissue” to emphasize the importance of timely assessment and management of this emergency to avoid major amputations.30 Although our findings showed a significant delay in presentations with CLTI and DFI in P2, we also noted that these patients received their treatment significantly quicker than in P1. This could be attributed to the freeing up of capacity due to cancellation of elective procedures and would explain the similar LS rates in the 2 periods despite the alarming delay in presentation. The cancellation of the elective procedures was one of a pack of measures taken by the healthcare institutions based on governmental and professional body guidance to reduce the burden on the NHS resources.8,9,22 Of note, the LS rate was still worse in P2 compared to P1, however, this was not statistically significant.
In this study, the AFS was noticeably worse in P2 compared to P1, however, this did not reach statistical significance. This could be explained by the short follow-up duration in this study and highlights the need for a longer follow-up of these patients who were treated during P2. Also, although primary patency was significantly worse in P2, timely reinterventions were needed to achieve similar assisted primary and secondary patency rates; which would also explain the significantly worse F-CDR in P2 compared to P1. The higher CDR could also explain the significant increase of anticoagulation after interventions in P2 to maintain patency and should therefore be viewed in light of the reports highlighting the vascular complications in patients with COVID-19. The evidence of COVID-19-related hypercoagulability, disseminated intravascular coagulation and increased risk of multi-territorial, venous, and arterial thrombotic events has been extensively reported.31,32,33 Several hypotheses have been raised to explain the physiopathology of COVID-19 associated cardiovascular events including ACE-2 receptor-mediated CV endo-thelial injury, microvascular dysfunction, and thrombosis, IL-6 mediated cytokine “storm.”34,35,36
Although only 5 patients in this study have tested positive for COVID-19, there have been reports on false-negative results.37 This is an important factor to consider as it could potentially lead to increased reintervention or even amputations in infected patients who have tested negative to COVID-19, despite being challenging to prove. Being aware of these thrombotic risks associated with COVID-19 might explain the high likelihood of being on anticoagulation after revascularisation in P2 compared to P1.
We did not notice significant differences between the 2 time periods in terms of the overall number of patients presenting with CLI/DFI, their demographics, spectrum of clinical presentations, severity of disease, anatomical distribution, or GLASS classification. There was also no difference in the treatment modality they received or in the proportion of those who went on to have primary major or minor amputation. These findings reflect the fact that the workload generated by these patients who usually need urgent/emergency interventions has not diminished in P2. Therefore, it is important to take these findings into consideration when planning the response to future infection waves to ensure enough capacity is available to treat these patients in a timely manner.38
Recent results from an international multicenter study showed 50% risk of postoperative pulmonary complications in patients with perioperative COVID-19, with associated high mortality. Postoperative outcomes seemed to be worse than prepandemic baseline across all patients’ subgroups, including elective patients who had minor surgery. The study identified male sex, age 70 years or older, American Society of Anaesthesiology (ASA) grades 3 to 5 and emergency surgery as negative predicting factors of perioperative mortality.39 Such risk factors are commonly encountered in vascular patients, especially those who undergo emergency/urgent procedures as shown in Table 1 of our study.
Although it might seem that deferring elective arterial and venous procedures could be done without significant consequences to patients, the results of our study show this not to be the case in patients who present with CLTI and DFI. It would therefore be reasonable to expect any future plan to manage vascular patients during the second wave, or in fact during any future pandemics, to establish COVID-protected pathways with the view of reducing perioperative complications in this high-risk cohort of patients who would not be able to self-isolate for prolonged periods due to their critical symptoms. In our study, there was no difference in mortality between the 2 time periods; however, we managed to significantly reduce the waiting times for intervention and the overall hospital stay in P2 which might have contributed to reducing the risk of perioperative COVID-19 infection.
A major concern in the healthcare systems worldwide is the possibility of a second peak of COVID-19 infection which could put further pressure on the healthcare resources.40,41 The mortality rate from the second wave of the Spanish flu was the highest, followed by the third wave as compared to the initial one.42 If this pattern was to be replicated in the current COVID-19 pandemic, this will constitute a huge challenge to the various NHS services, including the vascular services. Therefore, based on our observations and in the event of future pandemics, the authors recommend that vascular networks should establish clear, rapid-access and COVID-protected pathways for this cohort of high-risk patients with CLTI and DFI where they could be reviewed and assessed with the infrastructure required to treat them in a timely manner. These patients seem to have a significantly worse F-CDR and F-MALE and therefore longer follow-up is advisable. In our institution, all aspects of inpatient care remained the same in both periods, including multidisciplinary input from various teams such as Vascular Surgery, Interventional Radiology, Diabetes/ Endocrine Diseases, Vascular Medicine, Podiatry, Infectious Diseases, Physiotherapy, and Tissue Viability Nurses. However outpatient facilities were affected to varying degrees. For example, we used to benefit from an amputation rehabilitation unit dedicated to intensive rehabilitation of new amputees after hospital discharge. This facility would no longer accept referrals during P2 due to infection control recommendations. Similarly, outpatient intravenous antibiotics services were suspended during P2 which meant that patients who needed intravenous antibiotics for prolonged periods had to remain in hospital until treatment is completed if no suitable oral alternative was available. In addition to the delays in presentation noted in P2, this restricted access to outpatient support after discharge might have contributed to the worse F-MALE observed in P2 compared to P1.
In the event of a second wave and based on our observations, we will continue running the community and spoke foot clinics with multi-disciplinary support using virtual platforms. We will also maintain our daily one-stop emergency vascular clinics and DFCs to identify patients who need urgent interventions in a timely manner. We plan to disseminate a clear message to patients to contact us if they experience any deterioration in their condition to avoid delayed presentations. We have also developed Covid-protected pathways in our hospital to allow those urgent patients to be admitted and treated with the least risk of getting infected. Testing capacity is also currently much larger compared to P2 which allows for timely testing of patients and staff to further reduce the risk of spreading the infection and avoid treatment delays.
The limitations of this study include its retrospective nature and the shorter duration of follow-up in P2 considering the recent pandemic. The small number of patients limited the ability to perform more in-depth analysis. Also, our hospital is a designated center for high consequences infectious diseases which might have produced a more significant impact on our vascular services than in other hospitals.
CONCLUSIONS
Our study showed that patients with CLTI and DFI experienced a significantly delayed presentation with features of sepsis in P2. Having treatment in P2 was a predictor of worse primary patency and F-MALE and therefore close and longer follow-up is advisable. Strategies to manage vascular patients during future pandemic should ensure easy access with timely interventions for patients with CLI and DFI.
PERSPECTIVES
COVID-19 pandemic has presented a huge challenge to the healthcare systems. There is a delayed presentation and worse outcomes in patients with CLTI and DFI treated during the pandemic. Strategies to manage vascular patients during future pandemics should ensure easy access with timely interventions for patients with CLTI and DFI.
CONTRIBUTORS
H Zayed: Study design, Literature search, Data analysis, Data interpretation, writing, manuscript review. M Musajee: Literature search, Study design, Data collection, Data analysis, writing, manuscript review. N Thulasidasan: Literature search, Study design, Data collection, writing, manuscript review. M Sayed: Data collection, data analysis, data interpretation. M Green: Data collection, data analysis, data interpretation. F Francia: Data collection, data analysis, data interpretation. M Arissol: Data collection, data analysis, data interpretation. A Lakhani: Data collection, data analysis, data interpretation. L. Biasi: Literature search, Data analysis, Figures, Data interpretation, writing, manuscript review. S. Patel: Literature search, Data collection, writing, manuscript review.
Acknowledgment
The authors would like to acknowledge the contribution by the members of the Guy's and St. Thomas’ Multidisciplinary Diabetic Foot Research Collaborative: Dr Stephen Thomas, Mr Tommaso Donati, Mr Prakash Saha, Mr Said Abisi, Mr Morad Sallam, Miss Rebecca Sandford, Mr Ashish Patel, Dr Athanasios Diamantopoulos, Dr Irfan Ahmed, Dr Tarun Sabharwal, Dr Panos Gkoutzios, Dr Leo Monzon, Miss Talia Lea.
REFERENCES
- 1. Rogers LC, Lavery LA, Joseph WS, et al. All feet on deck-the role of podiatry duringtheCOVID-19 pandemic: preventing hospitalizations inanoverburdened healthcare system, reducing amputation and death in people with diabetes. J Am Podiatr Med Assoc 2020; doi: 10.7547/20-051. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. Shin L, Bowling FL, Armstrong DG, et al. Saving the diabetic foot during the COVID-19 pandemic: a tale of two cities. Diabetes Care 2020; 43:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Lancet Planetary Health. Post-COVID-19 spending. Lancet Planet Health 2020; 4:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cash R, Patel V. Has COVID-19 subverted global health? Lancet 2020; 395:1687–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Masic I, Naser N, Zildzic M. Public health aspects of COVID-19 infection with focus on cardiovascular diseases. Mater Sociomed 2020; 32:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai CC, Wang CY, Wang YH, et al. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents 2020; 55:105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020; 382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vascular Society of Great Britain and Ireland, COVID-19 virus and vascular surgery, 2020. Available at: https://www.vascularsociety.org.uk/professio-nals/news/113/covid19_virus_and_vascular_surgery. [Google Scholar]
- 9. NHS England, Clinical guide for the management of vascular surgery patients during the Coronavirus pandemic, 2020. Available at: https://www.england.nh-s.uk/coronavirus/wpcontent/uploads/sites/52/2020/03/specialty-guidemanage-ment-of-vascular-surgerypatients-v1-20-march-2020.pdf. Accessed July, 2020. [Google Scholar]
- 10. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; 8:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American College of Surgeons. Available at: https://www.facs.org/-/media/files/covid19/recommendations_for_management_of_elective_surgical_pro-cedures.ashx. Accessed July, 2020. [Google Scholar]
- 12. Qiu H, Tong Z, Ma P, et al. China Critical Care Clinical Trials Group (CCCCTG). Intensive care during the coronavirus epidemic. Intensive Care Med 2020; 46:576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scally G, Jacobson B, Abbasi K. The UK's public health response to covid-19. BMJ 2020; 369:1–3. [DOI] [PubMed] [Google Scholar]
- 14. Myles PS, Maswime S. Mitigating the risks of surgery during the COVID-19 pandemic. Lancet 2020; 396:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn M, Sheehan M, Hordern J, et al. ‘Your country needs you’: the ethics of allocating staff to high-risk clinical roles in the management of patients with COVID-19. J Med Ethics 2020; 46:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anirudh Kumar, Divyang R, Patel, et al. Never let a crisis go to waste: implementing virtual innovations during the COVID-19 pandemic for a better tomorrow in health care. JACC Case Rep 2020; 2:1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert AW, Billany JCT, Adam R, et al. Rapid implementation of virtual clinics due to COVID-19: reportand early evaluation of a quality improvement initiative. BMJ Open Qual 2020; 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019; 69 (6S):3S–125S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Behrendt CA, Bertges D, Eldrup N, et al. International consortium ofvascular registries consensus recommendations for peripheral revascularisation registry data collection. Eur J Vasc Endovasc Surg 2018; 56:217–237. [DOI] [PubMed] [Google Scholar]
- 20. Stoner MC, Calligaro KD, Chaer RA, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg 2016; 64:e1–e21. [DOI] [PubMed] [Google Scholar]
- 21. Jee Y. WHO International Health Regulations Emergency Committee for the COVID-19 outbreak. Epidemiol Health 2020; 42:e2020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Jabir A, Kerwan A, Nicola M, et al. Impactofthe Coronavirus (COVID-19) pandemic on surgical practice - Part 2 (surgical prioritisation). Int J Surg 2020; 79:233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Vascular And Endovascular Research Network Vern Executive Committee. The COvid-19 Vascular sERvice (COVER) Study: An International Vascular and Endovascular Research Network (VERN) collaborative study assessing the provision, practice, and outcomes of vascular surgery during the COVID-19 pandemic. Eur J Vasc Endovasc Surg 2020; 60:156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020; 107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg 2016; 151:1070–1077. [DOI] [PubMed] [Google Scholar]
- 26. Patel SD, Biasi L, Paraskevopoulos I, et al. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg 2016; 103:1815–1822. [DOI] [PubMed] [Google Scholar]
- 27. Biasi L, Patel SD, Lea T, et al. Complex infrapopliteal revascularization in elderly patients with critical limb ischemia: impact of multidisciplinary integrated care on mid-term outcome. J Cardiovasc Surg (Torino) 2017; 58:665–673. [DOI] [PubMed] [Google Scholar]
- 28. Zayed H, Halawa M, Maillardet L, et al. Improving limb salvage rate in diabetic patients with critical leg ischaemia using a multidisciplinary approach. Int J Clin Pract 2009; 63:855–858. [DOI] [PubMed] [Google Scholar]
- 29. Jeffcoate WJ, Vileikyte L, Boyko EJ, et al. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018; 41:645–652. [DOI] [PubMed] [Google Scholar]
- 30. Vas PRJ, Edmonds M, Kavarthapu V, et al. The diabetic foot attack: “’tis too late to retreat! ”. Int J Low Extrem Wounds 2018; 17:7–13. [DOI] [PubMed] [Google Scholar]
- 31. Behnood Bikdeli, Mahesh V, Madhavan, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am College Cardiol 2020; 75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nabil K, Thalji, Prakash A, et al. Hematological consequences of the Coronavirus crisis-focus on relevant clues and complications for the perioperative cardiothoracic and vascular community. J Cardiothorac Vasc Anesth 2020; 34:3189–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ganatra S, Dani SS, Shah S, et al. Management of cardiovascular disease during Coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med 2020; 30:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nature Reviews Cardiology 2020; 17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou F, Yu T, Du R, et al. Clinical course and riskfactors for mortality ofadult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020; 173:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher D, Teo YY, Nabarro D. Assessing national performance in response to COVID-19. Lancet 2020; 396:653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020; 396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ali I. COVID-19: are we ready for the second wave? Disaster Med Public Health Prep 2020; 14:e16–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Lancet. COVID-19: the worst may be yet to come. Lancet 2020; 396:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simonsen L, Chowell G, Andreasen V, et al. A review of the 1918 herald pandemic wave: importance for contemporary pandemic response strategies. Ann Epidemiol 2018; 28:281–288. [DOI] [PubMed] [Google Scholar]