Abstract
Homologous recombination over large genomic regions is difficult to achieve due to low efficiencies. Here, we report the successful engineering of a humanized mTert allele, hmTert, in the mouse genome by replacing an 18.1-kb genomic region around the mTert gene with a recombinant fragment of over 45.5-kb, using homologous recombination facilitated by the Crispr/Cas9 technology, in mouse embryonic stem cells (mESCs). In our experiments, with DNA double strand breaks (DSBs) generated by Crispr/Cas9 system, the homologous recombination efficiency was up to 11% and 16% in two mESC lines TC1 and v6.5, respectively. Overall, we obtained a total of 27 mESC clones with heterozygous hmTert/mTert alleles and 3 clones with homozygous hmTert alleles. DSBs induced by Crispr/Cas9 cleavages also caused high rates of genomic DNA deletions and mutations at single guide RNA (sgRNA) target sites. Our results indicated the Crispr/Cas9 system significantly increased the efficiency of homologous recombination-mediated gene editing over a large genomic region in mammalian cells, and also caused frequent mutations at unedited target sites. Overall, this strategy provides an efficient and feasible way for manipulating large chromosomal regions.
Keywords: Crispr/Cas9, mESC, knock-in, TERT
INTRODUCTION
Homologous recombination involving large DNA fragments is often necessary in genome editing. Cis-regulatory elements for many genes spread over a large genomic region, including the intergenic region between two genes. For example, human telomerase reverse transcriptase gene, hTERT, is controlled by multiple cis-regulatory elements over a large genomic regions, including the intergenic region between hTERT and its upstream gene cisplatin resistance-related protein 9 (CRR9) in somatic cells (Zhu et al., 2010). To study these elements in their native context, it was necessary to edit the genomic region over 45-kb. However, the traditional replacement strategy, mediated by homologous recombination, has been very inefficient (1 in 106–109 cells), and therefore labor- and time-consuming (Hsu et al., 2014).
Crispr/Cas9 is a new genome editing technique and has been developed recently. Cas9, a nuclease from S. pyogenes, facilitates DNA cleavages at genomic sequences specified by 20-bp single guide RNAs (sgRNAs) in vitro and in vivo (Cong et al., 2013; Jinek et al., 2012). The target sequence is followed by an NGG protospacer adjacent motif (PAM) at the 3’ end, which can be recognized by Cas9. The site-specific alteration is subsequently created by repairing the DNA double strand breaks (DSBs), through non-homologous end-joining (NHEJ) or homologous directed repair (HDR) mechanisms in vivo (Chu et al., 2015). The Crispr/Cas9 system significantly increases genomic editing efficiencies in variety of organisms (Khatodia et al., 2016; Li et al., 2016; Platt et al., 2014). Researchers successfully exchanged genome segments combining Crispr/Cas9 and a donor vector containing homologous sequences with targeted DNA region. A 7-kb DNA fragment was integrated into E. coli chromosome with efficiency exceeding 60% when facilitated by the Crispr/Ca9 system (Chung et al., 2017). 7 out of 60 mouse embryonic stem cell (ESC) colonies were inserted with 7.4-kb segment upon Crispr/Cas9-mediated cleavage (B. Wang et al., 2015). In spite of these successes, replacement of larger fragments (>10-kb) still occurred with very low efficiencies. In addition, it is difficult to create chimeric genomic regions, even with a modified Crispr/Cas9 system, such as Tild-CRISPR or HMEJ-based method (Yao et al., 2017; Yao, Zhang, et al., 2018; Zhang et al., 2017). Therefore, it is necessary to develop a new strategy to precisely integrate large DNA segments into chromosomes with high efficiency.
In this study, we modified a large genomic region in mouse ESCs and generated a humanized mTert gene, hmTert, using a Crispr/Cas9-assisted homologous recombination strategy. In this strategy, an 18.1-kb mouse genomic region at the mTert locus was cleaved by Cas9 guided by two sgRNAs, and precisely replaced by a 45.5-kb chimeric human/mouse genomic DNA fragment from a bacterial artificial chromosome (BAC). The efficiency of homologous recombination was increased from 0.05% to over 10% in mouse ESCs. Our data indicated that Crispr/Cas9-mediated homologous recombination provided a feasible method of genomic editing involving large chromosomal regions.
MATERIAL AND METHODS
1. Bacterial artificial chromosomes (BACs)
BAC clones containing human and mouse genomic loci, hTERT and mTert, were reported previously (S. Wang et al., 2009a). The 5’ intergenic region (5’IR), introns 2 and 6 (In2 & In6) of the hTERT gene were inserted into the mTert gene, replacing their mouse counterparts in a BAC containing the mTert gene. Two selection markers, puromycin and TKneo, were also added into the construct, resulting in the donor BAC. All modifications were performed using the BAC recombineering technique (Lee et al., 2001; Zhao et al., 2011).
2. Single guide RNA (sgRNA) design
sgRNAs were designed to target sequences near 5’ and 3’ ends of the replacement region in the mouse chromosome, as indicated in Figure 1a. sgRNA 1 targeted the 5’ IR region while sgRNA 2 targeted intron 6 of mTert locus. sgRNA oligos (Integrated DNA Technologies) were cloned into pSpCas9 (BB)-2A-GFP (pX458) plasmid as previously described (Ran et al., 2013). sgRNAs sequences were listed in the Table S1.
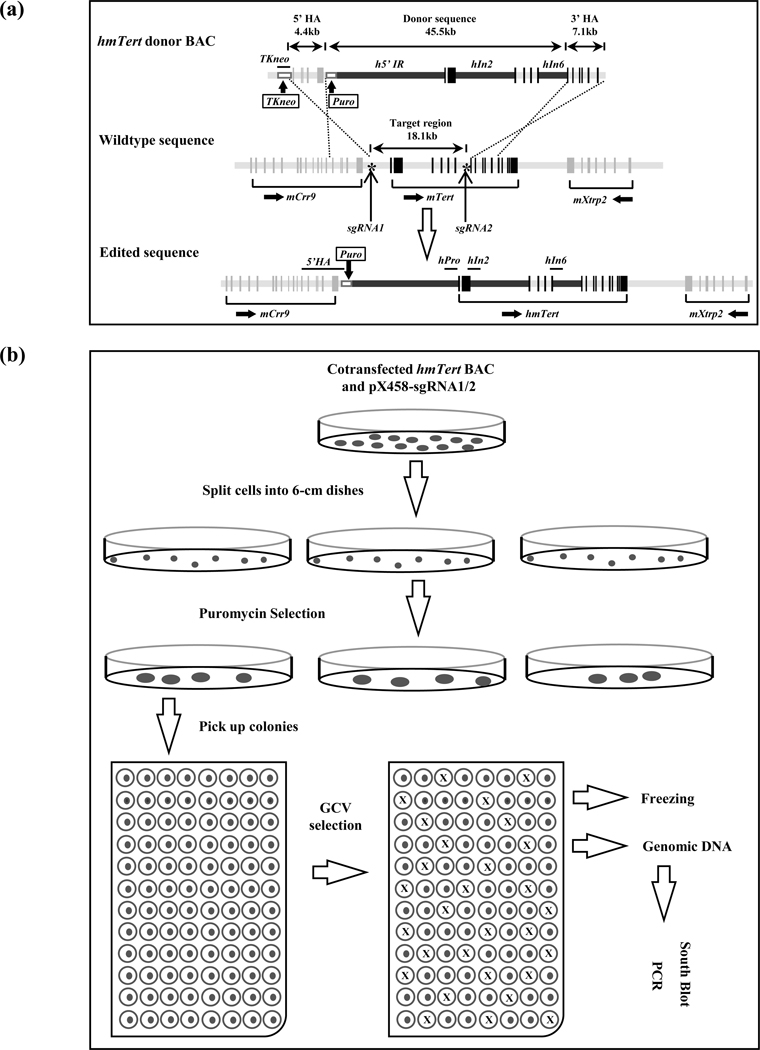
FIGURE 1.
Engineering of an hmTert allele via homologous recombination (HR). (a) Schematic illustration of the donor BAC construct and HR strategy. Sequences within the donor BAC contained the mTert gene components (light grey bars), in which its 5’IR, In2 and In6 were replaced by their counterparts from the hTERT gene (black bars). This 45.5-kb donor sequence was flanked by 5’ and 3’ homologous arms (HAs). The 5’ HA is a 4.4-kb sequence from the 3’ end of mCrr9 gene whereas the 3’ HA is a 7.1-kb region including exons 7–12 of the mTert gene. In addition, a TKneo and a puromycin-resistant cassette (white boxes) were inserted at upstream and downstream of 5’ HA, respectively. The target region is the 18.1-kb region from 5’IR to intron 6 of the mTert gene. Asterisk (*) represents Crispr/Cas9-sgRNAs targeting sites. Black vertical bars represent mTert exons. Horizontal lines above the edited sequence indicate PCR amplicons of TKneo, 5’HA, hPro, hIn2, and hIn6 used in assessing the integrities of hmTert genes in mESC clones. (b) Schematic description of transfection and colony screening strategies. mESCs were co-transfected with the donor BAC and Crispr/Cas9-sgRNA expressing plasmids (pX458-sgRNA1/2) and seeded into 6-cm dishes, followed by puromycin selection. Surviving colonies were individually picked and seeded into 96-well plates, followed by GCV negative selection. The resulting colonies, without cross, were expanded for further characterization by PCR and Southern analyses.
3. Cell culture and Analysis
TC1 and v6.5 mouse ESCs were cultured as previously described (Cheng, Wang, et al., 2017). Genomic DNA PCR and reverse transcription (RT)-PCR analyses were also performed as previously reported (Zhao et al., 2014). Primer sequences were listed in Table S1. Luciferase assays were performed using Dual Luciferase Assay system (Promega, Madison, WI).
4. Homologous recombination in mouse ESCs
mESCs were co-transfected with BAC construct with the hmTert donor BAC and two Crispr/Cas9-sgRNAs expressing vectors pX458-sgRNA1/2 using lipofectamine 2000 (Invitrogen, US) and selected with 1.5 μg/ml puromycin. One week later, puromycin resistant colonies were seeded individually in 96-well plates and treated with 50 μM Ganciclovir (GCV) for two days. The survived clones were expanded for further analysis. A diagram of this procedure is shown in Figure 1b.
5. Genomic DNA analyses
Genomic PCR analysis was used for the initial identification of homologous recombination at the 5’ end of chimeric locus and the integrated hmTert region. Primer sequences were listed in Table S1. Southern blot was performed as previously described (S. Wang et al., 2003) to validate PCR-positive clones. Briefly, 2 μg genomic DNA was digested with restriction enzymes, separated in 0.7% agarose gel, and transferred to nylon membranes. Membranes were incubated with 32P-labeled DNA probes and hybridization signals were detected with PhosphorImager system (GE healthcare). DNA probes were summarized in Table S2 and Figure S1.
6. Telomerase activity
Telomerase activities were determined using a modified telomeric repeat amplification protocol (TRAP) assay (Krupp et al., 1997; S. Wang et al., 2003). Primer sequences were listed in Table S1.
7. Generation of chimeric mice
hmTert/mTert mESCs were injected into C57BL/6J albino embryos at Stem Cell and Transgenic Core, Cornell University. Chimeric mice were identified by fur colors.
RESULTS
1. sgRNA guided large genomic deletion in mESCs
Our previous studies indicated that 5’IR and In2 & In6 of the hTERT gene likely contained cis regulatory elements for human-specific regulation of this gene (Cheng, Wang, et al., 2017; Cheng, Zhao, et al., 2017). To study these elements in mice, we set out to knock-in these human genomic regions into mouse ESCs to replace their counterparts in the mouse genome. A chimeric donor BAC, in which 5’IR, introns 2 and 6 of mTert gene were replaced by their human counterparts, was constructed and shown in Figure 1a. The donor sequence of chimeric hmTert exceeded 45-kb. Two flanking sequences, 4.4-kb and 7.1-kb, respectively, on each side of the chimeric hmTert region served as homologous arms. A puromycin-resistant cassette, flanked by two Lox511 sites, was inserted in front of hTERT 5’IR and acted as the positive selection for donor segment replacement. A negative selection marker, TKneo, was placed at upstream of 5’ homologous arm. This donor BAC contained the entire 45.5-kb replacement sequence, from 5’IR to intron 6 of the chimeric hmTert gene. In the first attempt to engineer the hmTert gene, the donor BAC was transfected into mESC line TC1. Transfected cells were subjected to consecutive puromycin and ganciclovir (GCV) selection and the surviving colonies were analyzed by genomic PCR and Southern analysis. Among 2016 colonies isolated and characterized, only one of them had anticipated recombination (Table 1). Therefore, this traditional method to induce homologous recombination over such a large fragment (over 45-kb) was extremely inefficient.
TABLE 1.
Efficiencies of unassisted homologous recombination (HR) at the mTert locus in mESCs
| Cell lines | # of cells | # of PuromycinR colonies | # of colonies isolated | GCVR colonies | Recombination at the 5’ end | TKneo+ by PCR | % of colonies via HR |
|---|---|---|---|---|---|---|---|
|
| |||||||
| TC1 | 8.5 × 106 | >8400 | 2016 | 1013 | 1/2016 (0.05%) | 949/1013 (94%) | 1/1013 (0.1%) |
To increase recombination efficiency, we used the Crispr/Cas9 system to introduce DNA DSBs within the targeted genomic region. Unlikely other reported genome editing methods using only one sgRNA cleavage, we utilized a pair of sgRNAs: sgRNA1 targeted the 5’IR region between mTert and mCrr9, and sgRNA2 targeted the end of mTert intron 6 (Figure 1a). The sequences at both targeted sites were unique in the mouse genome and not conserved in the human genome. Our goal was to induce efficient homologous recombination at both homology arms by cleaving these sites in mouse genome.
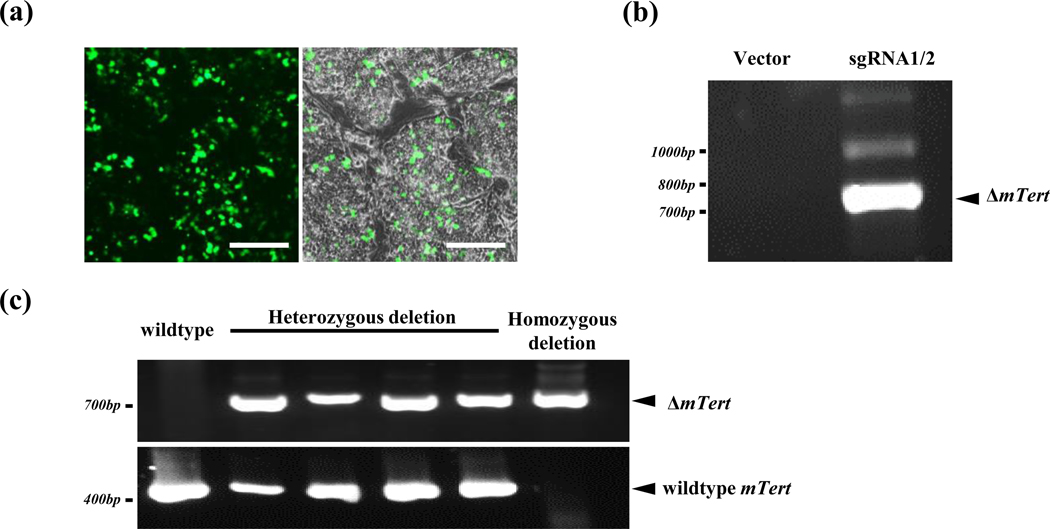
To test cutting efficiencies of these sgRNAs, pX458-sgRNA plasmids were co-transfected into TC1 mESCs, and GFP fluorescence imaging indicated that the transfection efficiencies ranged 40%~60% (Figure 2a). Genomic DNAs were harvested from ES cells 48 hours after transfection, and chromosomal deletion between two cleavage sites were detected by PCR with a paired-primer which located upstream and downstream of two cleaved sites, respectively. The mTert genomic sequence between two cleavage sites was over 18-kb and could not be amplified in untransfected cells in our PCR condition. An expected bright 730-bp band was detected from cells which transfected with sgRNA constructs, indicating that the sgRNAs guided Cas9 cleaved the intended chromosomal targets efficiently (Figure 2b). Furthermore, we isolated individual ESC colonies following transfection of pX458-sgRNA plasmids and examined the cleaved and deletion efficiency by PCR. Genomic deletion was achieved when a 730-bp band was detected in these colonies, whereas the wildtype mTert locus would be detected by the presence of a 478-bp PCR band using a pair of primers surrounding the downstream of sgRNA1 cleavage site (Figure 2c). Our data revealed that 38% of isolated clones contained at least one deleted mTert allele (Table S3), suggesting that the sgRNAs induced deletion at the targeted region in these cells.
FIGURE 2.
Characterization of sgRNAs in mESCs. (a) Transfection of mESCs with pX458-sgRNA plasmids. The green fluorescence was from the GFP marker in the pX458-sgRNA plasmids. Scale bar: 400 μm. (b-c) Chromosomal cleavage by sgRNA1/2 guided Crispr/Cas9. mESCs were transfected with pX458 vector or a mixture of pX458 plasmids containing sgRNA1&2 targeting upstream (5’IR) and downstream (In6) regions of the mTert gene, respectively. (b) Genomic DNAs were isolated two days after transfection and subjected to PCR analysis using primers located upstream of 5’ cleavage site and downstream of 3’ cleavage site. A 730-bp band was amplified when both cleaved their target sites and the chromosomal region between these sites was deleted. (c) Individual mESC colonies were isolated following transfection and analyzed by PCR. The undeleted mTert gene was identified by PCR using a pair of primers within the mTert locus.
2. Co-transfection of plasmids and BACs
To optimize transfection efficiencies of both plasmids and BAC constructs, pX458-sgRNA plasmids were transfected into mESCs together with BAC reporter H(wt), containing a 160-kb human genomic DNA with the whole hTERT locus and its neighboring genes, CRR9 and Xtrp2. In this BAC, a Renilla (Rluc) and a Firefly luciferase (Fluc) reporters were inserted downstream of hTERT and hCRR9 promoters, respectively (S. Wang et al., 2009b). Luciferase activities were measured two days after transfection. As shown in Table S4, increasing amounts of pX458-sgRNAs led to a drop of luciferase activities. On the other hand, increasing amounts of BAC DNA resulted in an increasing of luciferase activities (Table S5). Therefore, the ratio of 0.25 μg pX458-sgRNA plasmids to 1.0 μg BAC DNA was used for co-transfection in mESCs.
3. Long-range homologous recombination facilitated by Crispr/Cas9-mediated cleavages
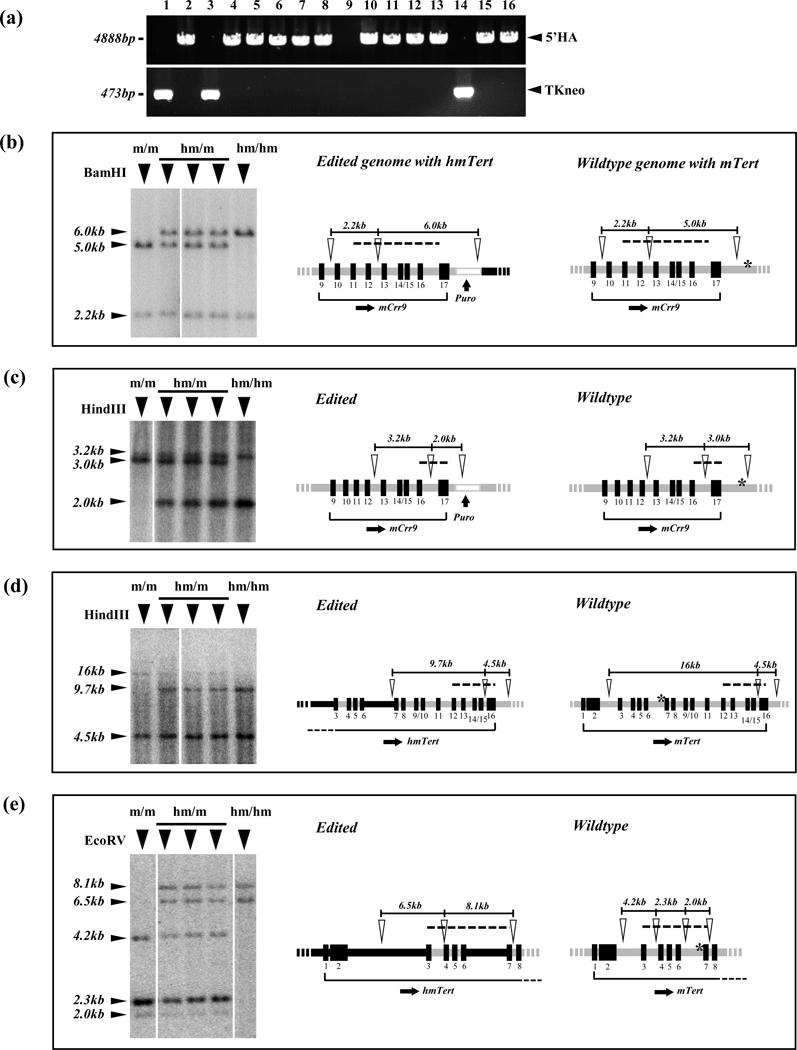
To generate the hmTert locus, the donor BAC was co-transfected into mESCs with two pX458-sgRNAs, sgRNA1 and sgRNA2. 800 colonies were picked from transfected TC1 cells following puromycin selection. Among them, 110 colonies survived after GCV selection (Table 2). To identify clones with successful recombination, genomic DNA PCR analysis was performed using primers just outside of the 5’ homology arm (5’HA). Correct recombination resulted in a 4.9-kb PCR fragment (Figure 3a). Furthermore, PCR detection of TKneo sequence would suggest random integration of the donor BAC. Among 110 puromycin and GCV resistant clones, 84 yielded 4.9-kb PCR fragment, indicating that these clones had undergone homologous recombination at the 5’ end (Table 2). Thus, the efficiency of homologous recombination was about 76% (84/110) in TC1 cells. A similar experiment was performed in v6.5 mESCs. Following co-transfection of pX458-sgRNAs, the rate of homologous recombination among puromycin and GCV resistant clones was 83% (130/156) (Table 2). However, without pX458-sgRNA1/2 assistance, the rate of homologous recombination was significantly lower: only one clone among 1031 puromycin and GCV resistant clones when the donor BAC was transfected alone (Table 1). Therefore, DSBs resulted from Crispr/Cas9-mediated cleavages dramatically increased homologous recombination efficiency in mESC lines.
TABLE 2.
Efficiencies of Crispr/Cas9 assisted homologous recombination at the mTert locus in mESCs
| Cell lines | # of cells | # of PuromycinR colonies | # of colonies isolated | GCVR clones | Recombination at the 5’ end | TKneo+ by PCR | % of colonies via HR |
|---|---|---|---|---|---|---|---|
|
| |||||||
| TC1 | 3.5 × 106 | 3400 | 800 | 110 | 84/800 (11%) | 16/110 (15%) | 84/110 (76%) |
| v6.5 | 2.2 × 106 | 1500 | 800 | 156 | 130/800 (16%) | 22/156 (14%) | 130/156 (83%) |
FIGURE 3.
Characterization of hmTert loci in mESC clones. (a) Identification of mESC clones with hmTert loci by PCR. The occurrence of 5’ HR was detected by the presence of a 4888-bp PCR band using a pair of primers just outside of 5’ HA. The presence of TKneo marker was indicated by a 473-bp PCR band using primers within the TKneo cassette. (b-e) Characterization of hmTert alleles by Southern analyses. Genomic DNAs (2 μg) from individual mESC clones were digested by BamHI (b), HindIII (c, d) and EcoRV (e), and hybridized with P32-labeled DNA probes, A (b), B (c), C (d), and D (e), respectively (Table S2). Left panels show Southern blot images and band sizes are indicated on the left. Genotypes: m/m, mTert/mTert; hm/m, hmTert/mTert; hm/hm, hmTert/hmTert. Diagrams on the right show schematic illustrations of edited and wildtype genomes with hmTert and mTert alleles, respectively. Open arrowheads indicate restriction enzyme sites and dash lines specify regions covered by DNA probes. Black rectangles with numbers below denote exons. Black and gray horizontal lines indicate human and mouse noncoding sequences, respectively. Asterisks (*) indicate sgRNAs cleavage sites.
Next, the chimeric hmTert region was examined by Southern blotting using probes spanning the entire edited region (Figure S1). As shown in Figure 3b–c, BamHI and HindIII digestion yielded the expected 6-kb and 2-kb restriction fragments, respectively, at 5’ end of the edited sequence. HindIII and EcoRV digestion resulted in correct 9.7-kb, and 6.5/8.1-kb bands, respectively, within the hmTert locus (Figure 3d–e). Restriction enzymes digestion in the intergenic region and the promoter region also yielded bands of expected sizes (Figure S2a–d). Together, these data indicated that the hmTert locus was correctly engineered in these ESC clones. Overall, about 30% of puromycin and GCV-resistant clones (34/110 in TC1 and 45/156 in v6.5) in two mESCs lines contained correct hmTert loci following Crispr/Cas9-mediated cleavages (Table S6). In comparison, the efficiency of homologous recombination in TC1 cells without Crispr/Cas9 cleavage was extremely low (Table 1).
From above mentioned experiments, we obtained a total of 27 clones with heterozygous hmTert/mTert and 3 clones with homozygous hmTert/hmTert alleles in TC1 and v6.5 mESCs (Table 3). Moreover, the number of clones with random insertion of the donor BAC also decreased, with only 15% clones in TC1 and 14% in v6.5 mESCs containing the TKneo cassette (Figure 3a and Table 2).
TABLE 3.
Southern blot analysis of the hmTert and mTert loci in mESC clones
| Cell lines | Genotype | # of colonies analyzed | # of clones with correct hmTert alleles | # of hm/m clones with an aberrant mTert allele |
|---|---|---|---|---|
|
| ||||
| TC1 | hm/m | 50 | 12 (24%) | 29 (58%) |
| hm/hm | 2 | 1 (50%) | N/A | |
| v6.5 | hm/m | 60 | 15 (30%) | 33 (55%) |
| hm/hm | 3 | 2 (67%) | N/A | |
4. Crispr/Cas9-induced aberrations at the unmodified mTert alleles
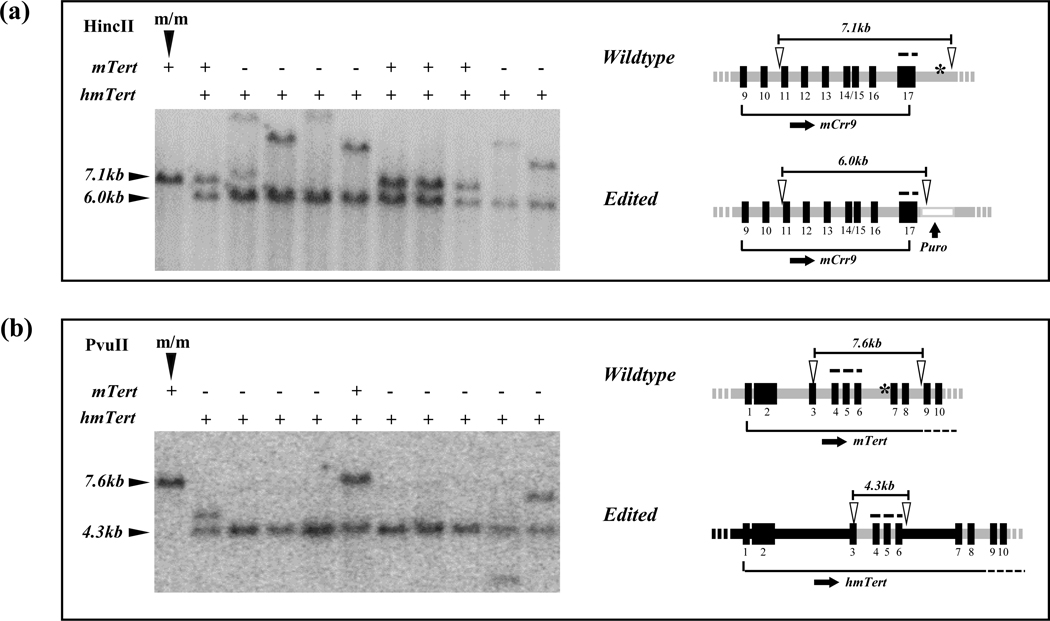
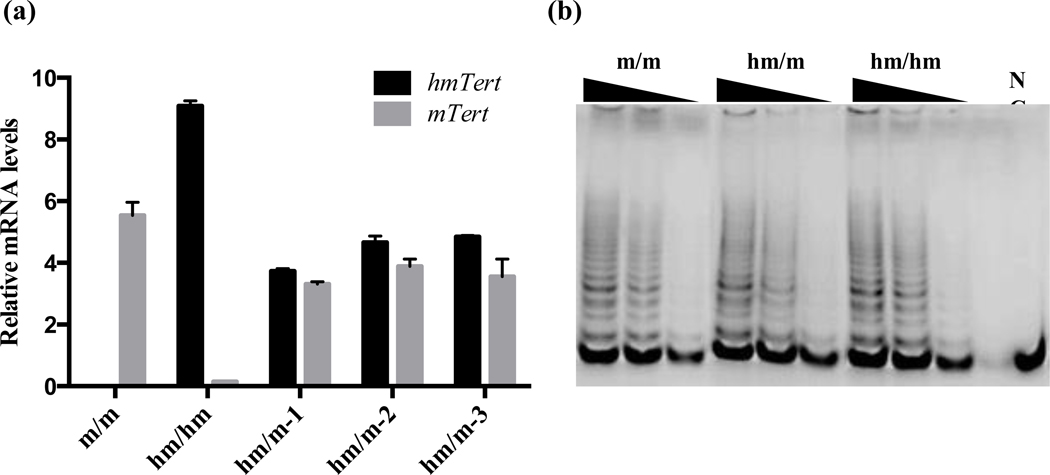
While generation of DSBs by Crispr/Cas9-sgRNAs considerably increased the efficiency of homologous recombination, DNA repairing following DSBs also introduced mutations and/or deletions at the cleavage sites. Southern analysis revealed that the mTert allele in over half of heterozygous mESC clones had a restriction fragment of aberrant sizes (Figure 4 and Table 3). To detect point mutations and small deletions, the chromosomal regions around the predicted cleavage sites in mTert allele were amplified by PCR and subjected to Sanger sequencing. Among six heterozygous clones with no apparent size changes of restriction fragments in Southern analysis, sequencing results showed that five clones contained deletions near the 5’ cleavage site, whereas four clones contained deletions at the 3’ cutting site (Table 4). Fortunately, most of these nucleotide changes did not affect mRNA expression of the mTert allele and telomerase activity (Figure 5a–b). cDNA sequencing results indicated that mTert mRNA splicing was unaffected (data not shown). Finally, mESC clones with both heterozygous hmTert/mTert alleles and homozygous hmTert alleles expressed similar levels of telomerase activity comparing with their parental mESCs (m/m), suggesting that edited hmTert alleles and unmodified mTert alleles were both functional (Figure 5b).
FIGURE 4.
Alterations induced by Crispr/Cas9 at unedited mTert alleles. Genomic DNAs were digested with HincII (a) and PvuII (b), followed by hybridization with probes A and E, respectively. Probes A & E (Table S2), marked by dash lines, recognized 5’ and 3’ ends of the recombination region, respectively. Southern blot images are shown on the left and diagrams of mTert and hmTert loci are on the right. Open arrowheads indicate restriction enzyme sites. The first lanes on the left are parental mESCs, and the rest are individual mESC clones. + and - indicate alleles that have correct and incorrect sizes of restriction fragments, respectively. Asterisks (*) indicate sgRNAs target sites.
TABLE 4.
Aberrations at the cleavage sites of the mTert allele in hm/m mESC clones
| mESC clones | Sequences at the 5’ cleavage site (5’−3’) | Sequences at the 3’ cleavage site (5’−3’) |
|---|---|---|
|
| ||
| v6.5 | GGAAAGGATGAGGTTGGGCCAATGGGCTA | GACTCTGCAATGGCGTGGTCCCACGGTGTTCTG |
| hm/m #1 | GGAAAGGATGAGGTT-----------------GGGCTA | GACTCTGCAATGGCGTGGTCCCACGGTGTTCTG |
| hm/m #2 | GGAAAGGATGAGGTTGGGC--AATGGGCTA | GACTCTGCAATGGCGTGG-----------------------TCTG |
| hm/m #3 | GGAAAGGATGAGGTTGGGCC--ATGGGCTA | GACTCTGCAATGGCGTGGTCCCACGGTGTTCTG |
| hm/m #4 | GGAAAGGATGAGGTTGGGC--AATGGGCTA | GACTCTGCAATGGCGTGGT----CACGGTGTTCTG |
| hm/m #5 | GGAAAGGATGAGGTTGGGCCAATGGGCTA | GACTCTGCAATGGCGTGGTCCCACGGTGTTCTG |
| hm/m #6 | GGAAAGGATGAGGTTGGGCC--ATGGGCTA | GACTCTGCAATGGCGTGG----CCACGGTGTTCTG |
Shaded sequences of V6.5 indicated the sgRNA targeted sequences.
FIGURE 5.
The expression of edited hmTert alleles in mESCs. (a) mRNA expression levels of hmTert and mTert genes in mESCs. mRNA expression was determined by RT-qPCR and the data was normalized to that of 18S ribosomal RNA. m/m, wildtype mESCs with homozygous mTert alleles; hm/hm, homozygous knock-in mESC clones with hmTert/hmTert alleles; hm/m, heterozygous knock-in mESC clones with hmTert/mTert alleles. −1, −2, & −3, three independent heterozygous clones. hmTert mRNA was detected by primers that distinguish hmTert mRNA from mTert mRNA by nine silent mutations near the 3’ end of exon 2 (Table S1). (b) Telomerase activities in m/m, hm/m, and hm/hm mESCs. Telomerase activities were determined by TRAP Assay. NC, Negative Control: RNase A treated m/m sample.
To determine whether the modified mESCs were able to undergo differentiation and embryonic development, chimeric mice were generated by injection of heterozygous TC1 cells (hmTert/mTert) into C57BL/6J albino embryos. Analysis of genomic DNAs from tissues of chimeric mice showed chimeric ratio of hmTert/mTert ES cells in most organs were between 20% and 40%, indicating the edited TC1 cells remained pluripotent (Figure 6).
FIGURE 6.
Contribution of edited mESCs to mouse embryonic development. (a) A chimeric mouse containing edited mESCs. Chimeric mice were generated by injection of heterozygous TC1 (hm/m) into mouse blastocysts (C57/BL6 albino). White fur color was from host embryo and gray fur was derived from injected mESCs. (b) Chimeric ratios of mESCs in tissues. Chimeric ratio was determined by ratio of hmTert and mTert copy number, as determined by genomic PCR analyses using primers specific for hmTert and mTert alleles, respectively. The ratio of mESCs (hm/m) was defined as 100%.
DISCUSSION
Genetic engineering or gene editing often requires the manipulation of large genomic regions. The procedures involving homologous recombination of large chromosomal regions are inherently inefficient due to the need to transfect large DNAs, such as bacterial artificial chromosomes, into cells and the need for recombination machineries to catalyze DNA recombination in a vast genomic region. In our approach to engineer a chimeric hmTert gene, we designed a recombination strategy and replaced an 18.1-kb chromosomal region within the mTert gene, from 5’ intergenic region to intron 6, with a 45.5-kb hTERT/mTert chimeric fragment. Our data showed that the efficiency of this recombination was dramatically increased from 0.05% to 11% by co-transfection of plasmids encoding the Cas9 enzyme and sgRNAs targeting mTert genomic sequences.
In side-by-side comparisons between Crispr/Cas9-mediated and -unmediated large fragment replacement, the efficiency of Crispr/Cas9-assisted homologous recombination was significantly higher than unassisted homologous recombination (11% vs 0.05%) in mESCs. At the same time, the ratio of random BAC integration to homologous recombination was much lower in the Crispr/Cas9-assisted experiments. The clones with 5’ recombination also underwent correct recombination at the 3’ end (Table S6). In addition, recombination involving homologous sequences in the middle of the donor BAC, resulting in the loss of human introns 2 and/or 6, also decreased upon Crispr/Cas9 cleavage (data not showed). Therefore, Crispr/Cas9-induced DSBs dramatically improved efficiency of homologous recombination over a large chromosomal region in mESCs.
Genome modifications are typically based on the HDR pathway and their efficiencies are increased by Crispr/Cas9 induced site-specific DNA cleavages. Recently, several reported approaches, such as PITCH (precise integration into target chromosome) and HMEJ (homology-mediated end-joining), have improved the precision and efficiency of chromosomal modifications in mammalian cells (Sakuma et al., 2016; Yao et al., 2017; Yao, Wang, et al., 2018). However, the efficiencies of these approaches are still limited by the sizes of targeted chromosomal regions and donor DNAs, and successful modifications involving chromosomal regions over 20-kb remain rare. Previously, Yoshimi et al. replaced a 58-kb rat chromosomal region using a 6.2-kb human CYP2D6 gene by combining Crispr-Cas9 with single-stranded oligodeoxynucleotides (ssODNs) and achieved an efficiency of one out of 130 in rat zygotes (Yoshimi et al., 2016). In the same study, they also inserted a BAC fragment of nearly 200-kb into a specific chromosomal site by co-injecting ssODNs and the BAC fragment into rat eggs. However, no homozygous modification was described. In our experiments, the replacement of an 18.1-kb mTert sequence by a 45.5-kb chimeric hmTert segment was mediated by homologous recombination within two homology arms (4.4-kb and 7.1-kb, respectively). Guided by a pair of sgRNAs, Crispr/Cas9 cleavages within the targeted mouse chromosomal region facilitated this recombination. In total, we obtained 27 clones with heterozygous hmTert/mTert alleles and 3 homozygous hmTert/hmTert clones out of 266 colonies (Table 3). These colonies contained accurate hmTert alleles encoding functional mTert proteins. Sequencing cDNAs from the hmTert alleles revealed no mutation in the protein-coding region (data not shown). The ESC clones with homozygous hmTert alleles are particularly useful for in vitro analysis of gene function in mESCs. To assess off-target risk of Crispr/Cas9 cleavages, we sequenced two potential off-target sites for each sgRNA, predicted by the software Cas-OFF find (Bae et al., 2014), and found no mutations in eight clones examined (Tables S7–8).
DNA repair occurred at the cutting sites, resulting in deletions, insertions, and/or mutations. Over half of the heterozygous hmTert colonies contained deletions/mutations at the sgRNA recognition sites in the unmodified mTert locus. Some of the clones even missed the entire region between two Crispr/Cas9 target sites (Figure 4a–b). Among 6 heterozygous clones that were subjected to sequencing analysis, most of them missed nucleotides at both sgRNA target sites in the unedited mTert allele (Table 4). Thus, sgRNAs should be designed to avoid targeting at critical sequences, such as exons.
In summary, we demonstrated a new Crispr/Cas9-assisted strategy to efficiently and precisely edit a large genomic region in mouse ESCs.
Supplementary Material
ACKNOWLEDGEMENT
We thank the core facilities at WSU Spokane campus. This work was supported by the National Institutes of Health [R01GM071725 to J.Z.], and the Spokane County Health Sciences and Service Authority.
Funding information
National Institutes of Health R01GM071725
Footnotes
CONFLICT OF INTEREST
None of the authors have professional or financial affiliations that could be perceived to bias the presentation of this manuscript.
REFERENCES
- Bae S, Park J, & Kim JS (2014). Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics, 30(10), 1473–1475. doi: 10.1093/bioinformatics/btu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Wang S, Jia W, Zhao Y, Zhang F, Kang J, & Zhu J. (2017). Regulation of human and mouse telomerase genes by genomic contexts and transcription factors during embryonic stem cell differentiation. Sci Rep, 7(1), 16444. doi: 10.1038/s41598-017-16764-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Zhao Y, Wang S, Zhang F, Russo M, McMahon SB, & Zhu J. (2017). Repression of telomerase gene promoter requires human-specific genomic context and is mediated by multiple HDAC1-containing corepressor complexes. FASEB J, 31(3), 1165–1178. doi: 10.1096/fj.201601111R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, & Kuhn R. (2015). Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol, 33(5), 543–548. doi: 10.1038/nbt.3198 [DOI] [PubMed] [Google Scholar]
- Chung ME, Yeh IH, Sung LY, Wu MY, Chao YP, Ng IS, & Hu YC (2017). Enhanced Integration of Large DNA Into E. coli Chromosome by CRISPR/Cas9. Biotechnology and Bioengineering, 114(1), 172–183. doi: 10.1002/bit.26056 [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, & Zhang F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339(6121), 819–823. doi: 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, & Zhang F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157(6), 1262–1278. doi: 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, & Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816–821. doi: 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatodia S, Bhatotia K, Passricha N, Khurana SM, & Tuteja N. (2016). The CRISPR/Cas Genome-Editing Tool: Application in Improvement of Crops. Front Plant Sci, 7, 506. doi: 10.3389/fpls.2016.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp G, Kuhne K, Tamm S, Klapper W, Heidorn K, Rott A, & Parwaresch R. (1997). Molecular basis of artifacts in the detection of telomerase activity and a modified primer for a more robust ‘TRAP’ assay. Nucleic Acids Res, 25(4), 919–921. doi: 10.1093/nar/25.4.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, & Copeland NG (2001). A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics, 73(1), 56–65. doi: 10.1006/geno.2000.6451 [DOI] [PubMed] [Google Scholar]
- Li W, & Ou G. (2016). The application of somatic CRISPR-Cas9 to conditional genome editing in Caenorhabditis elegans. Genesis, 54(4), 170–181. doi: 10.1002/dvg.22932 [DOI] [PubMed] [Google Scholar]
- Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng G, Sharp PA, & Zhang F. (2014). CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell, 159(2), 440–455. doi: 10.1016/j.cell.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, & Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc, 8(11), 2281–2308. doi: 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Nakade S, Sakane Y, Suzuki KT, & Yamamoto T. (2016). MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat Protoc, 11(1), 118–133. doi: 10.1038/nprot.2015.140 [DOI] [PubMed] [Google Scholar]
- Wang B, Li K, Wang A, Reiser M, Saunders T, Lockey RF, & Wang JW (2015). Highly efficient CRISPR/HDR-mediated knock-in for mouse embryonic stem cells and zygotes. BioTechniques, 59(4), 201–202, 204, 206–208. doi: 10.2144/000114339 [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao Y, Leiby M, & Zhu J. (2009a). A new positive/negative selection scheme for precise BAC recombineering. Mol Biotechnol, 42(1), 110–116. doi: 10.1007/s12033-009-9142-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhao Y, Leiby MA, & Zhu J. (2009b). Studying human telomerase gene transcription by a chromatinized reporter generated by recombinase-mediated targeting of a bacterial artificial chromosome. Nucleic Acids Res, 37(17), e111. doi: 10.1093/nar/gkp511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Zhu J. (2003). Evidence for a relief of repression mechanism for activation of the human telomerase reverse transcriptase promoter. J Biol Chem, 278(21), 18842–18850. doi: 10.1074/jbc.M209544200 [DOI] [PubMed] [Google Scholar]
- Yao X, Wang X, Hu X, Liu Z, Liu J, Zhou H, Shen X, Wei Y, Huang Z, Ying W, Wang Y, Nie YH, Zhang CC, Li S, Cheng L, Wang Q, Wu Y, Huang P, Sun Q, Shi L, & Yang H. (2017). Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res, 27(6), 801–814. doi: 10.1038/cr.2017.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Wang X, Liu J, Shi L, Huang P, & Yang H. (2018). CRISPR/Cas9-mediated Targeted Integration In Vivo Using a Homology-mediated End Joining-based Strategy. J Vis Exp(133). doi: 10.3791/56844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Zhang M, Wang X, Ying W, Hu X, Dai P, Meng F, Shi L, Sun Y, Yao N, Zhong W, Li Y, Wu K, Li W, Chen ZJ, & Yang H. (2018). Tild-CRISPR Allows for Efficient and Precise Gene Knockin in Mouse and Human Cells. Dev Cell, 45(4), 526–536 e525. doi: 10.1016/j.devcel.2018.04.021 [DOI] [PubMed] [Google Scholar]
- Yoshimi K, Kunihiro Y, Kaneko T, Nagahora H, Voigt B, & Mashimo T. (2016). ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat Commun, 7, 10431. doi: 10.1038/ncomms10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Li XL, Li GH, Chen W, Arakaki C, Botimer GD, Baylink D, Zhang L, Wen W, Fu YW, Xu J, Chun N, Yuan W, Cheng T, & Zhang XB (2017). Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol, 18(1), 35. doi: 10.1186/s13059-017-1164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cheng D, Wang S, & Zhu J. (2014). Dual roles of c-Myc in the regulation of hTERT gene. Nucleic Acids Res, 42(16), 10385–10398. doi: 10.1093/nar/gku721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang S, & Zhu J. (2011). A multi-step strategy for BAC recombineering of large DNA fragments. Int J Biochem Mol Biol, 2(3), 199–206. [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhao Y, & Wang S. (2010). Chromatin and epigenetic regulation of the telomerase reverse transcriptase gene. Protein Cell, 1(1), 22–32. doi: 10.1007/s13238-010-0014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.