Abstract
Background:
Pericardial effusion (PE) is a potential complication of transcatheter left atrial appendage occlusion (LAAO). The objective of this study was to investigate the incidence, associated characteristics, and outcomes of PE following LAAO.
Methods:
Patients in the NCDR LAAO Registry who underwent a Watchman procedure between January 1, 2016 and December 31, 2019 were included. The primary outcome was in-hospital PE requiring intervention (percutaneous drainage or surgery). Odds ratios [ORs] were calculated for adverse event rates associated with PE.
Results:
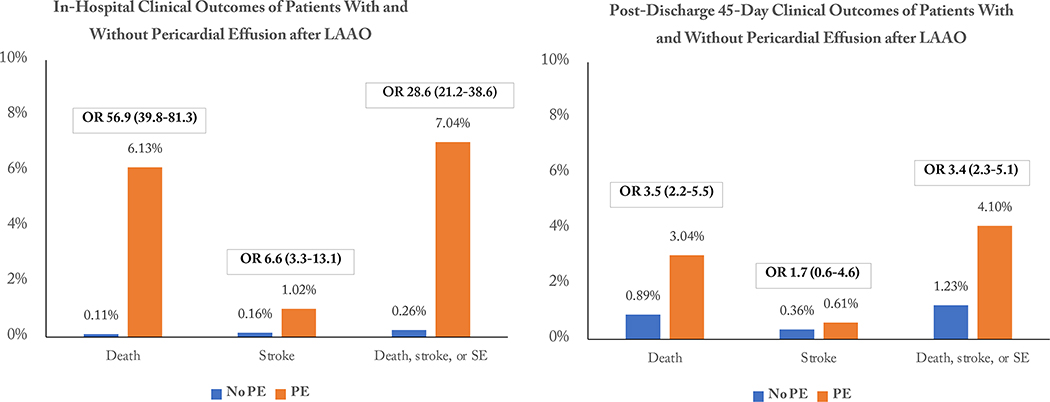
The study population consisted of 65,355 patients. The mean patient age was 76.2±8.1 years and the mean CHA2DS2-VASc score was 4.6±1.5. PE occurred in 881 patients (1.35%). Clinical variables independently associated with PE included older age, female sex, left ventricular function, paroxysmal atrial fibrillation, prior bleeding, lower serum albumin, and pre-procedural dual antiplatelet therapy; procedural variables included number of delivery sheaths used, sinus rhythm during the procedure, and moderate sedation rather than general anesthesia. PE was associated with increased risk of in-hospital stroke (OR, 6.58 [95% CI, 3.32 to 13.06], P<0.0001), death (OR, 56.88 [95% CI, 39.79 to 81.32], P<0.0001), and the composite of death, stroke, or systemic embolism (SE) (OR, 28.64 [95% CI, 21.24 to 38.61], P<0.0001). PE during the index hospitalization was associated with increased risk of death (OR 3.52 [95% CI, 2.23 to 5.54], P<0.0001) and the composite of death, stroke, or SE (OR 3.42 [95% CI, 2.31 to 5.07], P<0.0001) between discharge and 45-day follow-up.
Conclusions:
In-hospital PE during transcatheter LAAO is infrequent but associated with a substantially higher risk of adverse events, including in-hospital and early post-discharge mortality. Strategies to minimize PE are critical to improve the risk-benefit ratio for this therapy.
Keywords: Atrial fibrillation, left atrial appendage, bleeding, Watchman, pericardial effusion
Introduction
Transcatheter left atrial appendage occlusion (LAAO) is an alternative approach for stroke prevention in patients with non-valvular atrial fibrillation (AF) who are not suitable for long-term oral anticoagulation (OAC). Decision-making regarding the appropriate stroke prevention strategy in AF patients requires the integration of the procedural risk of LAAO, estimates of thromboembolic and bleeding risk with and without OAC therapy, and individual patient values and preferences. Pericardial effusion (PE) is a well-known complication of left atrial instrumentation. Transcatheter LAAO requires catheter and device manipulation within the thin-walled LAA, and the anchors and outward radial force of the device that interact with the thin LAA wall may cause acute or sub-acute trauma. The procedural safety of transcatheter LAAO was of major concern during the U.S. Food and Drug Administration (FDA) regulatory approval process, and the FDA Circulatory System Device Panel noted that although the PE rate had decreased with changes in training and procedural technique, post-approval studies would be important to further monitor this outcome.1 Through its Coverage with Evidence Development process, the Center for Medicare & Medicaid Services (CMS) requires U.S. hospitals to submit data for all Watchman procedures performed in Medicare beneficiaries into the National Cardiovascular Data Registry (NCDR) LAAO Registry to qualify for reimbursement and allow for the assessment of clinical outcomes. Given the gap in knowledge regarding the rates, risk factors, and sequelae of PE associated with LAAO in contemporary practice, we sought to perform a detailed study of PE using the prospective NCDR LAAO Registry.
Methods.
The authors declare that all supporting data are available within the article (and its online supplementary files).
NCDR LAAO Registry.
The American College of Cardiology NCDR LAAO Registry has been previously described.2 In brief, it is a prospective, national, audited registry that is currently the only registry approved by CMS to satisfy the coverage decision data submission requirements. A computer-based algorithm uses discrete combinations of registry data elements to adjudicate adverse events based on standard event definitions.2 The Human Investigation Committee of the Yale University School of Medicine approved the use of a limited data set from the NCDR for research and granted a waiver of informed consent.
Study population and endpoints.
Patients who underwent attempted or successful Watchman implantation between January 1, 2016, and December 31, 2019, were identified. Patients with cancelled procedures prior to vascular access (e.g., due to left atrial thrombus) were excluded. Patients undergoing procedures after December 31, 2019, were excluded to minimize any potential follow-up bias due to the COVID-19 pandemic. Patients undergoing concomitant cardiovascular procedures at the time of LAAO or who underwent another procedure prior to LAAO in the same hospital admission were excluded to isolate the risk of transcatheter LAAO. Patients receiving the next-generation Watchman FLX are not included in this analysis, as the device was introduced after the eligibility period had passed.
The primary outcome assessed was site-reported PE requiring intervention (percutaneous drainage, cardiac surgery, or both.) If a patient required both percutaneous drainage and cardiac surgery, the primary outcome was categorized as cardiac surgery alone. The individual endpoints of death, systemic embolism (SE) and stroke (ischemic, hemorrhagic, or undetermined) were centrally adjudicated as described above. Cases of death in which stroke was identified as the cause were also included in the stroke endpoint.
Statistical analysis.
Categorical variables are reported as counts (percentages), and continuous variables were reported as mean ± SD or median and interquartile range (IQR) where appropriate. Univariable and multivariable logistic regression analyses were performed to identify clinical and procedural variables associated with in-hospital PE requiring intervention. The following candidate variables were included: age, female sex, left ventricular dysfunction (left ventricular ejection fraction <40%), hypertension, diabetes mellitus, abnormal liver function, clinically relevant prior bleeding, AF classification (paroxysmal or not paroxysmal), body mass index, platelet count, serum albumin, serum creatinine, international normalized ratio, hemoglobin, sinus rhythm at start of procedure, number of devices used per case, largest device implant attempted, type of sedation used (moderate sedation or general anesthesia), and baseline anticoagulant/antiplatelet medication. Variables were retained in the multivariable model if the p value was less than 0.10 in the univariable analysis. Rates and unadjusted odds of in-hospital adverse events occurring for patients who did and did not experience in-hospital PE were calculated, then rates and unadjusted odds of adverse events occurring between discharge and the first follow-up visit at 45 days for those who did and did not experience in-hospital PE were calculated. Analyses were conducted using SAS, version 9.4 (Cary, N.C.).
Results
Baseline characteristics.
The study population consisted of 65,355 patients enrolled in the NCDR LAAO Registry who underwent LAAO between January 1, 2016, and December 31, 2019. Baseline clinical and procedural characteristics are shown in Table 1. The average patient age was 76.2±8.1, 41.3% were female, and 37.6% had diabetes. The mean CHA2DS2-VASc score was 4.6±1.5 and the mean HAS-BLED score was 3.0±1.1.
Table 1.
Baseline clinical and procedural characteristics of the study population.
| N=65355 | |
|---|---|
| Clinical characteristic | |
| Age (mean ± SD) | 76.2 ± 8.1 |
| Female sex (n, %) | 27008 (41.3%) |
| LV dysfunction (n, %) | 8274 (12.7%) |
| Hypertension (n, %) | 60182 (92.1%) |
| Diabetes mellitus (n, %) | 24554 (37.6%) |
| Renal dysfunction (n, %) | 9150 (14.0%) |
| Hepatic dysfunction (n, %) | 2077 (3.2%) |
| CHA2DS2VASc score (mean ± SD) | 4.6±1.5 |
| HAS-BLED score (mean ± SD) | 3.0 ± 1.1 |
| Clinically relevant prior bleeding | 44292 (67.9%) |
| AF classification, paroxysmal (n, %) | 35894 (54.9%) |
| Body mass index (mean ± SD) | 30.1 ± 10.4 |
| Platelet count (mean ± SD) | 208358 ± 71558 |
| Albumin (mean ± SD) | 3.84 ± 0.5 |
| Creatinine (mean ± SD) | 1.3 ± 1.1 |
| INR (mean ± SD) | 1.41 ± 1.0 |
| Hemoglobin (mean ± SD) | 12.7 ± 2.1 |
| Procedural characteristic | |
| Number of devices used per case (mean ± SD) | 1.2 ± 0.6 |
| Multiple delivery sheaths used | 2977 (4.6%) |
| Largest device size attempted (median) | 27mm |
| General anesthesia (N, %) | 63812 (97.6%) |
AF, atrial fibrillation; INR, international normalized ratio, SD standard deviation.
Pericardial Effusions.
In-hospital PE requiring intervention occurred in 881 patients (1.35%). Percutaneous drainage was performed in 771 cases (1.18%) and cardiac surgery in 177 (0.27%). Pericardial effusion not requiring intervention was reported in 669 patients (1.02%). The results of the multivariate model including baseline clinical and procedural characteristics that were statistically significant are shown in Table 2. Older age, female sex, normal left ventricular function, paroxysmal AF, lower serum albumin, prior clinically relevant bleeding and pre-procedural dual antiplatelet therapy were clinical variables that were independently associated with in-hospital PE requiring intervention; sinus rhythm during the procedure, no delivery sheath used, more than one delivery sheath used, and moderate sedation rather than general anesthesia were procedural characteristics independently associated PE requiring intervention. The number of devices used, and the largest device size attempted, were not associated with PE. Details of the full model are shown in Supplemental Tables S1 and S2.
Table 2.
Multivariable model of clinical and procedural characteristics independently associated with peri-procedural pericardial effusion requiring intervention.
| Characteristic | Odds Ratio | 95% Wald Confidence Limits | P value | |
|---|---|---|---|---|
| Age | 1.02 | 1.007 | 1.034 | 0.002 |
| Female Sex | 1.74 | 1.419 | 2.122 | <0.001 |
| Left ventricular function* | 1.58 | 1.087 | 2.288 | 0.017 |
| Clinically relevant prior bleeding | 0.77 | 0.631 | 0.947 | 0.013 |
| AF classification, paroxysmal | 1.48 | 1.183 | 1.854 | <0.001 |
| Serum albumin | 0.79 | 0.642 | 0.968 | 0.023 |
| Aspirin and P2Y12 regimen pre-procedure | 1.76 | 1.166 | 2.669 | 0.007 |
| No access system used | 7.90 | 4.606 | 13.541 | <0.001 |
| Multiple access systems used | 1.68 | 1.150 | 2.468 | 0.007 |
| Sinus rhythm during procedure | 1.26 | 1.016 | 1.556 | 0.036 |
| Moderate sedation intra-procedure | 2.10 | 1.391 | 3.155 | <0.001 |
C-statistic, 0.668
Ejection fraction >40%
AF, atrial fibrillation
In-hospital outcomes.
A total of 288 patients with PE (32.7%) required red-blood cell transfusion (median 2 units, interquartile range [IQR] 2 to 5), compared with 459 patients (0.71%) who did not have PE (median 2 units, IQR 1 to 3) (p<0.0001). PE requiring intervention was associated with a significantly longer hospital stay (median 4 days [IQR 3 to 6 days] vs 1 day [IQR, 1 to 1 day], p<0.0001), and patients with PE were less commonly discharged to home (72.1% vs 97.6%, P<0.0001).
In-hospital clinical outcomes are shown in Table 3. Patients experiencing a PE requiring intervention were at significantly higher risk for any stroke (OR 6.58 [95% CI, 3.32 to 13.05], P<0.0001), all-cause mortality (OR 56.88 [95% CI 39.79 to 81.32], P<0.0001), and the composite of death, stroke, or systemic embolism (OR 28.64 [95% CI, 21.24 to 38.61], P<0.001) compared to patients without PE. The in-hospital mortality rate of patients with PE who underwent catheter drainage alone was 4.7% (33 of 704 patients), and the mortality rate of patients with PE requiring cardiac surgery was 11.9% (21 of 177 patients). Details of in-hospital outcomes according to the need for percutaneous drainage or cardiac surgery are shown in Supplementary Table S3.
Table 3.
Clinical outcomes in patients with and without in-hospital pericardial effusion requiring intervention
| Event | Pericardial effusion (N=881) | No Pericardial effusion (N=64,4747) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| In-hospital | ||||
| Death | 54 (6.13%) | 74 (0.11%) | 56.88 (39.79–81.32) | <0.0001 |
| Stroke (any) | 9 (1.02%) | 101 (0.16%) | 6.58 (3.32–13.05) | <0.0001 |
| Systemic embolism | 0 | 3 (0.005%) | NA | |
| Death, stroke, or systemic embolism | 62 (7.04%) | 170 (0.26%) | 28.64 (21.24–38.61) | <0.0001 |
| 45-days follow-up * | (N=658) | (N=55338) | ||
| Death | 20 (3.04%) | 491 (0.89%) | 3.52 (2.23–5.54) | <0.0001 |
| Stroke (any) | 4 (0.61%) | 198 (0.36%) | 1.70 (0.63–4.60) | 0.29 |
| Systemic embolism | 3 (0.46%) | 24 (0.04%) | 10.56 (3.17–35.14) | 0.001 |
| Death, stroke, or systemic embolism | 27 (4.10%) | 683 (1.23%) | 3.42 (2.31–5.07) | <0.0001 |
Surviving patients with 45-day follow-up
At discharge, OAC alone or in combination with antiplatelet therapy were significantly less likely to be prescribed among the patients with PE than those without, while dual antiplatelet or single antiplatelet therapy were prescribed more frequently. Over 18% of patients who experienced periprocedural PE were discharged with no oral antithrombotic medications (Table 4).
Table 4.
Medications at discharge, stratified by the occurrence of in-hospital pericardial effusion requiring intervention.
| Discharge Medication | Total Cohort (N=65355) | Patients with PE (N=881) | Patients without PE (N=64474) | P value |
|---|---|---|---|---|
| Warfarin and aspirin | 19695 (30.1%) | 157 (17.8%) | 19538 (30.3%) | <0.0001 |
| Warfarin only | 5977 (9.2%) | 65 (7.4%) | 5912 (9.2%) | 0.0669 |
| DOAC and aspirin | 18702 (28.6%) | 154 (17.5%) | 18548 (28.8%) | <0.0001 |
| DOAC and P2Y12 | 1609 (2.5%) | 16 (1.8%) | 1593 (2.5%) | 0.213 |
| DOAC and aspirin and P2Y12 | 739 (1.1%) | 4 (0.5%) | 735 (1.1%) | 0.0558 |
| DOAC only | 8969 (13.7%) | 104 (11.8%) | 8865 (13.7%) | 0.0956 |
| P2Y12 and aspirin | 3896 (6.0%) | 78 (8.9%) | 3818 (5.9%) | 0.0003 |
| P2Y12 or aspirin alone | 2073 (3.2%) | 142 (16.1%) | 1931 (3.0%) | <0.0001 |
| None of the above | 1849 (5.65%) | 161 (18.3%) | 3534 (5.5%) | <0.0001 |
DOAC, direct oral anticoagulant; PE, pericardial effusion requiring intervention
Early post-discharge outcomes.
Follow-up at 45 days was available in a total of 55996 patients, representing 85.9% of patients surviving until discharge and 79.8% of the surviving patients with in-hospital PE (658 of 827 patients). Clinical outcomes between discharge and 45-day follow-up are shown in Table 3. The rate of post-discharge PE requiring intervention (i.e., recurrent PE) was 1.82% among the patients with in-hospital PE, compared with 0.11% in patients without in-hospital PE (OR 18.38 [95% CI, 9.80 to 34.45], P<0.001). Patients with in-hospital PE had a significantly higher risk of early post-discharge mortality (OR 3.52 [95% CI, 2.23 to 5.54], P<0.0001), as well as the composite of death, stroke, or systemic embolism (OR 3.42 [95% CI, 2.31 to 5.07], P<0.0001). Post-discharge outcomes according to the need for in-hospital percutaneous drainage or cardiac surgery are shown in Supplementary Table S4.
Discussion
Pericardial effusion is a potential complication of procedures involving the left atrium and was a key safety endpoint in the pivotal randomized clinical trials that assessed the safety and effectiveness of transcatheter LAAO with the Watchman device. In those trials, the rate of periprocedural PE declined over the clinical trial experience.3 However, the risk factors for and clinical sequelae of periprocedural PE could not be well-defined given the relatively small sample sizes enrolled in these trials. In this large, prospective, national cohort of patients undergoing commercial LAAO implantation with the first-generation Watchman device, we found that although the rates of PE requiring intervention were lower than in the randomized clinical trials that led to device approval, peri-procedural PE was associated with a substantial risk of morbidity and mortality both in-hospital and during the early post-discharge period. Furthermore, we identified several clinical and procedural characteristics independently associated with PE. Our findings highlight the importance of PE as a central safety endpoint in clinical trials of transcatheter LAAO and suggest strategies to identify high-risk patients and potentially mitigate this adverse outcome.
The absolute rate of PE requiring intervention in this “real-world” registry was relatively low at 1.35%. This is consistent with the continued reduction in PE events over the course of the Watchman clinical experience. In the PROTECT-AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial, the incidence of PE requiring percutaneous or surgical drainage was 4.8%,4 in the subsequent PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients with Atrial Fibrillation) trial, the incidence was 1.9%,3 and in the Watchman arm of the most recent AMULET IDE trial the incidence was 1.3%.5 The rate of PE in the current study is notably infrequent considering the higher risk profile of patients undergoing commercial transcatheter LAAO, as patients in the NCDR LAAO are generally older, more likely to be female, have more frequent prior bleeding and higher CHA2DS2-VASc scores than the clinical trial population of PROTECT-AF and PREVAIL.2 As the device used in this study is identical to that used in those two trials, the reduction in PE in current practice can likely be ascribed to evolution of procedural technique, manufacturer oversight and training, and communal operator experience.
The substantially increased risk of clinically important adverse outcomes associated with PE highlight the central importance of this complication compared with other safety events that can occur with transcatheter LAAO. For example, device-related thrombus occurs with a reported frequency between 1.7% and 4.5% over the first year post-implantation,5–7 and is associated with a 3.9-fold higher adjusted risk of ischemic stroke or systemic embolism.7 Yet device-related thrombus accounted for only 13% of thromboembolic events after LAAO and was not associated with cardiovascular or all-cause mortality.7 While device embolization is likely associated with significant morbidity, including the need for cardiac surgery, the incidence of this event in modern practice is extremely rare, occurring in 0.07% of commercial LAAO cases within the United States.2 Peri-device leak has also garnered attention as a primary “anatomic” or “mechanistic” endpoint in recent studies.5, 6 It occurs frequently, seen in as many as 30–55% of patients, and may or may not be associated with an increased risk of thromboembolic events; a large, randomized trial recently showed similar rates of ischemic stroke or systemic embolism between 2 devices despite large differences in peri-device leak rates.5 In comparison, peri-procedural PE is associated with very large increases in in-hospital mortality, stroke, and the composite of in-hospital death, stroke, or systemic embolism, and accounts for a large proportion of deaths that occur prior to discharge. These poor outcomes are seen with PE requiring both percutaneous and surgical intervention. Peri-procedural PE should therefore be a key, top-line endpoint reported in clinical trials of device safety and effectiveness.
New devices and new generations of approved devices, such as Watchman FLX, have been designed to reduce complications such as PE. Large post-market registries will be essential to confirm the apparently reduced rate of in-hospital PE observed in a recent study with that device.6 Further studies are also required to determine whether the higher rate of PE with the Amulet device5, 8 persists with increased operator experience, and to compare the rates of PE between the Amulet and the current-generation Watchman FLX. Even with improvements in device safety, PE will still occur to some extent with transcatheter LAAO and will remain one of the most important potential complications of the procedure.
Our findings provide insight into factors associated with peri-procedural PE, and may inform patient-centered decision making, patient management, and procedural execution. Female sex was associated with a significantly increased risk of PE, consistent with a prior NCDR report that identified female sex as a risk factor for major adverse events after transcatheter LAAO,9 and the known increased risk of cardiac tamponade in females undergoing AF ablation.10 Older age and lower albumin levels were also independently associated with PE. Although speculative, these may be markers of frailty and systemic illness which might influence the friability of the left atrium and/or LAA. Procedural factors independently associated with PE included sinus rhythm at the time of implantation. LAA contraction may exert force on the delivery sheath or on the device, particularly at the site of anchors, leading to PE. Univariate analysis also suggested a relationship between sinus rhythm and PE in the much smaller EWOLUTION (European Registry on Watchman Outcomes in Real-Life Utilization) study.11 The number of delivery sheaths used during the LAAO procedure was a strong and independent procedural characteristic associated with PE requiring intervention, with multiple sheaths or no sheath associated with an increased risk compared with a single sheath. The need for multiple sheaths might reflect more catheter and/or device manipulation within the LAA and/or left atrium, leading to a greater potential for LAA or left atrial laceration. The introduction of steerable delivery sheaths and/or device iterations which reduce the amount of catheter manipulation required for successful implantation might be beneficial in this regard. The procedural variable most strongly associated with PE was the use of no delivery sheath at all – suggesting that in these cases, PE occurred during or soon after transseptal puncture and before a delivery sheath was introduced into the body. This observation further suggests that a sizable fraction of PE events (7.3% in the current study) may not be addressable by device iteration alone but must focus on the transseptal puncture portion of the procedure. Moderate sedation, as opposed to general anesthesia, was also strongly associated with PE. The specific rationale for using moderate sedation within the study cohort is unknown. Several variables could potentially influence procedural safety in the setting of moderate sedation, including the inability to hold ventilation or prevent patient movement during the deep intubation of the delivery sheath that is required with the older generation Watchman device used in this study, the use of intracardiac echocardiographic guidance that might be favored in patients with moderate sedation, procedural differences, or unmeasured confounders.
We observed that patients experiencing in-hospital PE are significantly less likely to be discharged home on anticoagulants, and that approximately 24% were discharged on either single antiplatelet or no antithrombotic medications. Insufficient medical therapy following LAAO may potentially increase the risk of thromboembolism. In the current study, recurrent PE between discharge and 45-day follow-up occurred in 1.4%, and the composite of death, stroke, or systemic embolism occurred in 4.1% of patients, which were substantially higher than that observed in patients without in-hospital PE. While any analysis of the association between recurrent PE, thromboembolism and post-discharge therapy is subject to substantial confounding, it seems reasonable that in-hospital PE patients should be closely followed with serial imaging in the weeks and months after discharge.
Our study is consistent with a prior report derived from the publicly available, National Inpatient Sample administrative claims database.12 In that study, PE requiring intervention was also associated with a large increased risk of inpatient mortality, prolonged length of stay, and increased hospitalization costs. We expand upon that work with a larger, more comprehensive cohort (with 4 times as many PE events), prospectively collected data using case report forms which include detailed intraprocedural information, the use of endpoints subjected to a structured event adjudication process, analyses of discharge medications, and follow-up beyond discharge.
Limitations
Post-market registries have inherent limitations. Adverse event rates were captured through site reported data and as such under-reporting of adverse events is possible. However, the NCDR utilizes a rigorous Data Quality Reporting process to ensure that submissions are complete, valid, and accurate. The event rates at 45-day follow-up may be under-estimated due to loss of follow-up. We report unadjusted odd ratios for clinical outcomes, which may be subject to confounding. Although there was no independent association between pre-procedural OAC and the incidence of PE requiring intervention, the data collection forms did not allow us to identify whether OAC was continued or temporarily discontinued around the time of the procedure. LAA morphology was unavailable and therefore we could not explore the relationship between morphology and PE occurrence. Operator and/or institutional experience may influence PE rates, which was not addressed by this analysis as the data were unavailable. We did not directly assess whether guidance with intracardiac echocardiography was independently associated with pericardial effusion. Our study identified patient characteristics that were associated with increased risk of pericardial effusion, but we did not develop a formal prediction model and our study cannot demonstrate causality. Future efforts are ongoing to develop and validate a formal prediction model for major adverse events with LAAO procedures using the LAAO Registry. We captured a narrower cohort of patients with PE compared with the Munich Consensus definition of “clinically relevant” PE, which also includes cases that are not treated with percutaneous or surgical intervention but require blood transfusion or result in shock or death13. The presence of post-procedural PE that does not requiring intervention may also be clinically important. As such we may have underestimated the morbidity and mortality associated with the entire spectrum of in-hospital PE. Finally, due to the timeframe of the study, we included only patients with the older generation Watchman device, rather than the newer-generation Watchman FLX device that incorporates design modifications that may influence the rate of safety outcomes, including PE.6 However, our findings highlight the profound morbidity associated with transcatheter LAAO-associated PE.
Conclusions
Peri-procedural PE after transcatheter LAAO is infrequent but associated with substantially increased risk of adverse events, including stroke and both in-hospital and early post-discharge mortality. Strategies to minimize PE, potentially guided by an understanding of the associated patient and procedural risk factors, will be critical to improve the risk-benefit ratio for this therapy.
Supplementary Material
Outcomes of Pericardial Effusion Requiring Intervention Among 65,355 Patients Undergoing Transcatheter LAAO In the NCDR LAAO Registry
Clinical Perspective.
What is known:
Peri-procedural pericardial effusion is the main driver of in-hospital major adverse events after transcatheter left atrial appendage occlusion (LAAO)
The rates of pericardial effusions decreased over time during the LAAO randomized clinical trial experience
What this study adds:
Among patients undergoing commercial transcatheter LAAO in the United States with the first-generation Watchman device, in-hospital pericardial effusion requiring percutaneous drainage or surgery was infrequent but associated with substantially increased risk of adverse events, including stroke and in-hospital and early post-discharge mortality.
Given its associated morbidity and mortality, pericardial effusion should be a key safety endpoint reported in all transcatheter LAAO studies.
Strategies to minimize PE, potentially guided by an understanding of the associated patient and procedural risk factors, as well as through device iteration, will be critical to improve the risk-benefit ratio for this therapy.
Funding:
This study was funded by the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) and the National Heart, Lung and Blood Institute (NHLBI) grants R56HL142765 and R01HL142765.
Disclosures:
Dr. Price reports honoraria from Abbott Vascular, Boston Scientific, W.L. Gore, Baylis Medical, Biotronik, Medtronic, Biosense Webster and Shockwave, and has equity interest in Indian Wells.
Dr. Freeman has received salary support from the American College of Cardiology (ACC) NCDR and the NHLBI; and has received consulting fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, Biosense Webster and PaceMate, and has equity interest in PaceMate.
Dr. Kar reports honoraria from Abbott Vascular, Boston Scientific, and Medtronic.
Dr. Friedman has received research grants from the AHA, NCDR, Boston Scientific, Abbott, Medtronic, Merit Medical, and Biosense Webster and consulting fees from Abbott, AtriCure, and Sanofi.
Dr. Curtis has an institutional contract with the ACC for his role as Senior Scientific Advisor of the NCDR; has received salary support from the ACC and CMS; and has equity in Medtronic.
Dr. Masoudi has had an institutional contract with the ACC for his role as Chief Scientific Advisor of the NCDR. He serves on a steering committee for Bristol Meyers Squibb.
Dr. Valderrábano reports consulting honoraria, research support from Biosense Webster and Circa Scientific, consulting honoraria from Baylis Medical and NuVera, and speaker fees from Boston Scientific.
All other authors have no relationships relevant to the contents of this paper to disclose.
Non-standard Abbreviations and Acronyms
- AF
atrial fibrillation
- DOAC
direct oral anticoagulant
- FDA
Food and Drug Administration
- LAA
left atrial appendage
- LAAO
left atrial appendage occlusion
- NCDR
National Cardiovascular Data Registry
- OAC
oral anticoagulant
- PE
pericardial effusion
- PREVAIL
Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients with Atrial Fibrillation
- PROTECT-AF
Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation
Footnotes
References
- 1.Waksman R and Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am J Cardiol. 2015;115:378–84. [DOI] [PubMed] [Google Scholar]
- 2.Freeman JV, Varosy P, Price MJ, Slotwiner D, Kusumoto FM, Rammohan C, Kavinsky CJ, Turi ZG, Akar J, Koutras C, Curtis JP and Masoudi FA. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75:1503–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes DR Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K and Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM and Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42. [DOI] [PubMed] [Google Scholar]
- 5.Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, Gold MR, Hermiller J, Diener HC, Schmidt B, MacDonald L, Mansour M, Maini B, O’Brien L and Windecker S. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation. 2021;144:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, Stone J Jr., Shah M, Dukkipati SR, Adler S, Nair DG, Kim J, Wazni O, Price MJ, Asch FM, Holmes DR Jr., Shipley RD, Gordon NT, Allocco DJ, Reddy VY and Investigators PF. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation. 2021;143:1754–1762. [DOI] [PubMed] [Google Scholar]
- 7.Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, Maini B, Gordon NT, Main ML and Reddy VY. Device-Related Thrombus After Left Atrial Appendage Closure. Circulation. 2018;138:874–885. [DOI] [PubMed] [Google Scholar]
- 8.Galea R, De Marco F, Meneveau N, Aminian A, Anselme F, Grani C, Huber AT, Teiger E, Iriart X, Babongo Bosombo F, Heg D, Franzone A, Vranckx P, Fischer U, Pedrazzini G, Bedogni F, Raber L and Valgimigli M. Amulet or Watchman Device for Percutaneous Left Atrial Appendage Closure: Primary Results of the SWISS-APERO Randomized Clinical Trial. Circulation. 2022;145:724–738. [DOI] [PubMed] [Google Scholar]
- 9.Darden D, Duong T, Du C, Munir MB, Han FT, Reeves R, Saw J, Zeitler EP, Al-Khatib SM, Russo AM, Minges KE, Curtis JP, Freeman JV and Hsu JC. Sex Differences in Procedural Outcomes Among Patients Undergoing Left Atrial Appendage Occlusion: Insights From the NCDR LAAO Registry. JAMA Cardiol. 2021;6:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser DW, Fan J, Schmitt S, Than CT, Ullal AJ, Piccini JP, Heidenreich PA and Turakhia MP. Gender Differences in Clinical Outcomes after Catheter Ablation of Atrial Fibrillation. JACC Clin Electrophysiol. 2016;2:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt B, Betts TR, Sievert H, Bergmann MW, Kische S, Pokushalov E, Schmitz T, Meincke F, Mazzone P, Stein KM, Ince H and Boersma LVA. Incidence of pericardial effusion after left atrial appendage closure: The impact of underlying heart rhythm-Data from the EWOLUTION study. J Cardiovasc Electrophysiology. 2018;29:973–978. [DOI] [PubMed] [Google Scholar]
- 12.Munir MB, Khan MZ, Darden D, Pasupula DK, Balla S, Han FT, Reeves R and Hsu JC. Pericardial effusion requiring intervention in patients undergoing percutaneous left atrial appendage occlusion: Prevalence, predictors, and associated in-hospital adverse events from 17,700 procedures in the United States. Heart Rhythm. 2021;18:1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzikas A, Holmes DR Jr., Gafoor S, Ruiz CE, Blomstrom-Lundqvist C, Diener HC, Cappato R, Kar S, Lee RJ, Byrne RA, Ibrahim R, Lakkireddy D, Soliman OI, Nabauer M, Schneider S, Brachman J, Saver JL, Tiemann K, Sievert H, Camm AJ and Lewalter T. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints and data collection requirements for clinical studies. EuroIntervention. 2016;12:103–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.