Abstract
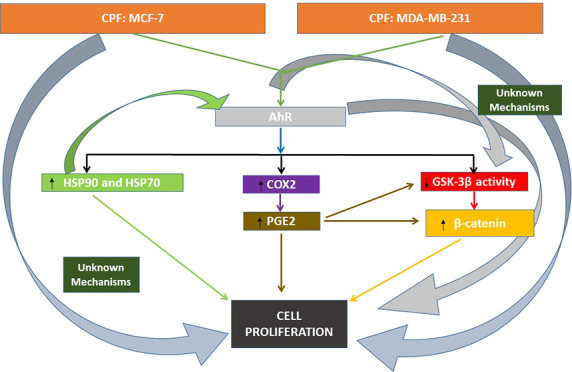
The biocide chlorpyrifos (CPF) was described to increase breast cancer risk in humans, to produce breast cancer in animals, and to induce cell proliferation in MCF-7 and MDA-MB-231 cells after 1 and 14 days of treatment. The entire mechanisms related to these CPF actions remain unknown. CPF induced cell proliferation in MCF-7 and MDA-MB-231 cells after 1 and 14 days of treatment by AhR activation through the PGE2/Wnt/β-catenin pathway and HSP90 and HSP70 overexpression. Our results reveal new information on CPF toxic mechanisms induced in human breast cancer cell lines, which could assist in elucidating its involvement in breast cancer.
Chlorpyrifos (CPF), an extensively employed biocide, was reported to generate breast cancer after repeated exposure at low doses in rats1 and to increase the risk of breast cancer development in women.2 In addition, it was reported to produce cell proliferation in human breast cancer cell lines expressing (MCF-7) or not (MDA-MB-231) estrogen receptor after unique and long-term treatment.3,4 However, to date the complete mechanisms through which CPF could induce this effect remain to be discovered.
CPF was reported to induce cell proliferation in MCF-7 and MDA-MB-231 cells, in part, through Wnt/β-catenin signaling disruption, aromatic hydrocarbon receptor (AhR) activation, arylacetamide deacetylase-like 1 (AADACL1, also known as KIAA1363) and acetylcholinesterase R (AChE-R) variant overexpression, reactive oxygen species (ROS) generation, and increase of ACh levels after 24 h and 14 days of treatment and only through estrogen receptor alpha (ERα) activation in MCF-7 cells after 24 h of treatment, but additional mechanisms seem to be implicated.3,4 Heat shock proteins (HSPs) were reported to protect against ROS, toxic misfolded or aberrant proteins, and cell death.5 HSP overexpression was associated with the induction of cell proliferation, migration, and invasion in different cancer types, like breast cancer.6,7 HSP90 and HSP70 overexpression was reported to induce cell proliferation in human breast cancer tissues8,9 and in MCF-7 and MDA-MB-231 cells.10−12 CPF was reported to induce HSP90 and HSP70 overexpression in different species after single and repeated exposure.13−15 Thus, CPF could also contribute to cell proliferation through the overexpression of these HSPs. In addition, HSP90 was shown to be essential in the regulation of the AhR activity, and its inhibition, or downregulation, inhibits AhR activity.16 However, AhR activation was shown to regulate the HSP90 and HSP70 expression among other HSPs in different species,17,18 so the AhR activation induced by CPF could mediate the HSP overexpression intensifying the AhR action on cell proliferation.
Prostaglandin E2 (PGE2), which is synthesized first by the rate-limiting enzyme cyclo-oxygenase-2 (COX2) and finally by the Prostaglandin E synthase, was also related to the induction of cell proliferation, migration, and invasiveness in MCF-7 and MDA-MB-231 breast cancer cells.19 In addition, PGE2 was reported to induce this effect through Wnt/β-catenin signaling activation by GSK-3β deactivation and β-catenin induction.19 CPF was reported to increase PGE2 in mouse brain samples after prenatal exposure and in rat hippocampus samples after a single treatment.20,21 Therefore, CPF could also induce cell proliferation through Wnt/β-catenin signaling pathway dysfunction mediated by PGE2. Otherwise, AhR was reported to regulate the synthesis of PGE2 and Wnt/β-catenin signaling pathway.22,23 Accordingly, we hypothesized that CPF could activate AhR, producing the upregulation of HSP90 and HSP70 proteins and the induction of Wnt/β-catenin signaling pathway, mediated by the increment of PGE2 levels, leading to cell proliferation in MCF-7 and MDA-MB-231 cells. To evaluate our hypothesis, wild type or HSP90 and HSP70 silenced MCF-7 and MDA-MB-231 cells were exposed to several CPF concentrations either alone or in combination with CH-223191 (AhR antagonist, 20 nM) and/or MF-63 (prostaglandin E synthase inhibitor, 1 μM) for 24 h or repeatedly for 14 days.
CPF toxic effects in tissues were reported to be developed by the combination of CPF and its main locally formed metabolite, chlorpyrifos oxon (CPFO).3,4 MCF-7 and MDA-MB-231 cells express different cytochrome P450 isoforms that metabolize CPF to CPFO.3,4 We used CPF for this study because we previously did not observe different actions between CPF and CPFO on cell proliferation,3,4 because CPF is the compound to which human population and animals are naturally exposed and because the toxic effect of CPF is produced, by CPF and CPFO, after its local metabolism in the tissues.
MCF-7 and MDA-MB-231 cell lines, used as a model of estrogen-dependent and estrogen-independent breast cancer cells, were cultured according to Moyano et al.3,4 Cells (passages 7–15) were seeded with complete medium (Dulbecco’s modified Eagle’s medium/F12 at 1:1 with 10% fetal bovine serum (FBS), penicillin/streptomycin, 2 mM l-glutamine, and 6 ng/mL insulin for MCF-7 or without insulin for MDA-MB-231) and left to attach for 24 h. Following attachment, phenol red-free medium with 2.5% charcoal-treated FBS was used for 24 h as experimental medium; afterward, the experimental medium was renewed, and the experimental compounds were added for either 1 day or daily with new medium for 14 consecutive days. The described conditions and times were followed for all different cotreatments.
We choose 0.01 μM to 100 μM CPF concentrations because according to the literature and previous studies they are relevant to study cell proliferation in breast cancer.3,4 In addition, we chose 1 μM CPF concentration, because it was the maximum concentration to induce cell proliferation.3 Finally, we chose CH-223191 (CH22) and MF-63 concentrations because they were the minimum concentrations that completely blocked AhR activation and PGE2 synthesis, respectively.
BrdU ELISA Cell Proliferation Assay Kit (colorimetric) (ab126556, Abcam, Cambridge, U.K.) was used, following the manufacturer’s guidelines, to elucidate the mechanisms involved on MCF-7 and MDA-MB-231 cell proliferation induced by CPF after single and repeated treatment, which were confirmed by MTT and crystal violet staining test according to Moyano et al.3 The GSK-3β enzymatic activity was determined by a GSK-3β Activity Assay Kit (CS0990; Sigma, Madrid, Spain) following the manufacturer’s guidelines. GSK-3β enzymatic activity values are expressed as percentages of the untreated control. PGE2 concentration in culture media was analyzed employing a commercial ELISA kit (ab133021; Abcam, Cambridge, U.K.) following the manufacturer’s protocols.
Gene expression analysis was developed employing validated primers (SA Biosciences) for mRNAs encoding β-catenin (PPH00643F), beta-actin (ACTB; PPH00073G), HSP90 (PPH00643F), HSP70 (PPH01188C), cytochrome P450 isoenzyme 1A1 (CYP1A1; PPH01271F), and COX2 (PPH01271F) according to Moyano et al.3 QPCR data were analyzed following the Ct (cycle threshold) method.24 COX2, β-catenin, HSP90, and HSP70 protein expression were determined using commercial ELISA kits (MBS264304, MBS724736, MBS2702622, and MBS012990, respectively; MyBioSource, CA, United States), following the manufacturer’s protocol. Finally, cells were transfected using siRNA (Qiagen; Barcelona, Spain) homologous to mouse HSP90 (GS3320), HSP70 (GS3303), and β-catenin (GS1499) target genes, and the transfection efficiency was measured, performing gene’s expression analysis of silenced genes, showing statistically significant reduction on the expression of these targets (Supplementary Figure 1). Our results are representative of (at least) three experiments performed for each research study in triplicate (n = 9). Results are presented as means ± standard error of the mean (SEM). One-way (concentration–response analysis) and two-way (gene manipulation vs treatment) ANOVA analyses followed by Tukey posthoc test were performed to identify statistically significant differences between treatments (p ≤ 0.05), using GraphPad Software Inc.’s (San Diego, CA, United States).
Our results show that HSP90 and HSP70 protein expression were upregulated in a concentration-dependent way, respectively, after 1 and 14 days of CPF treatment (starting at 0.1 μM) in MCF-7 and MDA-MB-231 cells (Figure 1A–D), which was correlated with the gene expression results (data not shown). CPF was reported to induce HSP90 and HSP70 overexpression in different species after single and repeated exposure,13−15 supporting our results. In addition, CH22 treatment reversed, in part, the overexpression of HSP90 and HSP70, noticing the mediation of AhR in the CPF upregulation of these targets (Figure 1A–D). AhR was reported to regulate the expression of different HSPs like HSP90 and HSP70,17,18 corroborating our data. However, other mechanisms seem to be involved. CPF was reported to activate estrogen receptor and induce NRF2 pathway,3,4 which were also reported to regulate HSP expression,25 so these mechanisms could also participate in this effect. In addition, CPF treatment of HSP90 silenced cells reversed completely the CYP1A1 overexpression induced by AhR activation (data not shown). HSP90 was shown to be essential for the AhR activity,16 and its inhibition blocks its activity, supporting our results.
Figure 1.
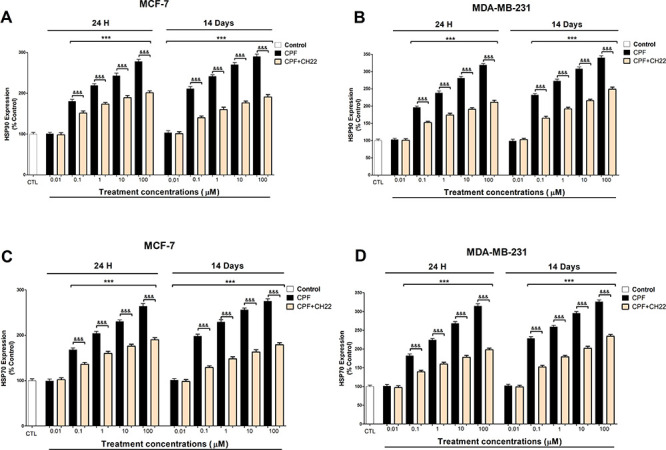
HSP90 (A and B) and HSP70 (C and D) expression analysis results. Data represent the mean ± SEM of three separate experiments from cells of different cultures, each performed in triplicate. ***p ≤ 0.001, significantly different from controls; &&&p ≤ 0.001, compared to CPF treatment.
In addition, COX2 protein expression (Figure 2A,B), which was correlated with the gene expression results (data not shown), and PGE2 levels (Figure 2C,D) were upregulated in a concentration-dependent way after 1 and 14 days of CPF treatment (starting at 0.1 μM), and these effects were completely reversed after CH22 cotreatment with CPF. These data show that AhR mediates this effect. AhR was reported to regulate COX2 expression and PGE2 levels.22 CPF was reported to induce COX2 expression in MCF-7 cells through AhR26 and to increase PGE2 levels after single treatment,21 which supports our data.
Figure 2.
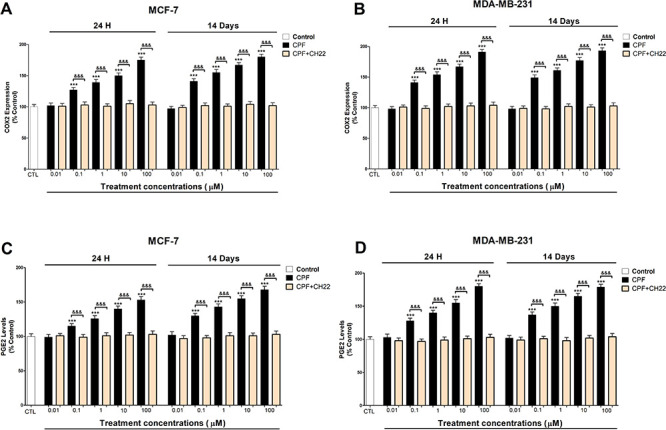
COX2 (A and B) expression and PGE2 (C and D) concentration analysis results. Data represent the mean ± SEM of three separate experiments from cells of different cultures, each performed in triplicate. ***p ≤ 0.001, significantly different from controls; &&&p ≤ 0.001 compared to CPF treatment.
CPF also decreased GSK-3β activity and increased β-catenin expression after single and repeated treatment from 0.1 μM to 10 μM concentration, and after CH22 or MF-63 cotreatment with CPF, they were partially reversed (Figure 3A–D). However, these effects were the opposite after 100 μM (1 day) and 10 μM (14 days) concentrations, and only the CH22 cotreatment with CPF was able to partially revert these effects (Figure 3A–D). As we indicated previously,4 β-catenin accumulation induces cell proliferation, but its downregulation produces cell viability reduction, which may explain these opposite effects on cell proliferation/viability reduction observed after CPF treatment.3 In addition, our data indicate that CPF induces Wnt/β-catenin signaling pathway, in part, through the action of AhR and PGE2, which is in turn induced by AhR.22 PGE2 and AhR were reported to induce Wnt/β-catenin signaling activation by GSK-3β deactivation and β-catenin induction,19,23 supporting our results. AhR was also reported to downregulate Wnt/β-catenin pathway activity,23 which could explain the opposite effect on this pathway observed from 100 μM (1 day) and 10 μM (14 days) concentrations. AhR signaling pathway presents a very complex regulation, and AhR has different variants that could mediate different effects.3 Thus, the differences observed could result from the action of different pathway modulators or the expression of different AhR variants, depending on the concentration. Otherwise, additional mechanisms seem to be involved in the observed effects. We previously described that CPF also mediated this effect through ROS generation,4 and we hypothesized that other targets, which were reported to be affected by CPF-like protein kinase C and histone deacetylase 1 that regulate GSK-3β,27,28 could also contribute to this effect.4
Figure 3.
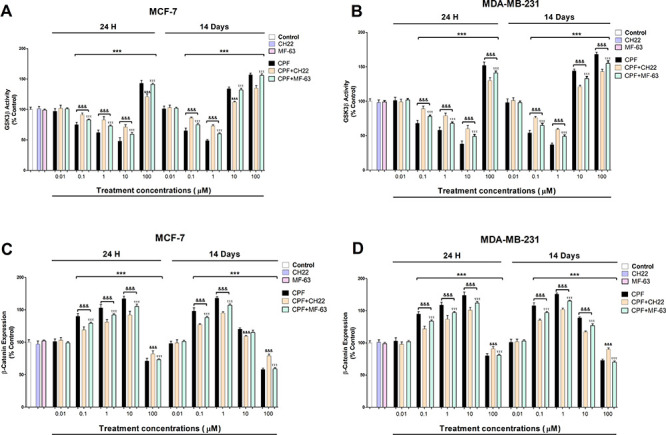
GSK3β (A and B) activity and β-catenin (C and D) expression analysis results. Data represent the mean ± SEM of three separate experiments from cells of different cultures, each performed in triplicate. ***p ≤ 0.001, significantly different from controls; &&&p ≤ 0.001 compared to CPF treatment.
Lastly, BrdU results show that CPF produced proliferation of MCF-7 and MDA-MB-231 cells after 1 and 14 days of treatment (Figure 4A,B), which was previously described.3,4 These results were partially reverted after CPF treatment of simultaneously HSP90 and HSP70 silenced cells, of β-catenin silenced cells, or after cotreatment with CH22 or MF-63, which shows that HSP90, HSP70, AhR, PGE2, and β-catenin mediated the cell proliferation observed after CPF treatment alone. These data were corroborated by an MTT test (data not shown). We previously showed that CPF mediated this effect through AhR and Wnt/β-catenin signaling pathway activation.4 In addition, PGE2 was reported to induce cell proliferation in MCF-7 and MDA-MB-231 breast cancer cells.19 HSP90 and HSP70 overexpression was reported to induce cell proliferation in MCF-7 and MDA-MB-231 cells.10−12 Therefore, these data support our results. In addition, the CH22 and MF-63 concomitant treatment of simultaneously HSP90, HSP70, and β-catenin silenced cells induced a greater reversion, but it was still incomplete, suggesting that other mechanisms may be implicated. In this regard, we previously described that CPF mediates this effect through the AChE-R variant and KIAA1363 overexpression, increase of acetylcholine levels, activation of estrogen receptor, and ROS generation;3,4 therefore, all these mechanisms together could be contributing to this effect, but we cannot discard other mechanisms. Paraoxonase overexpression was reported to induce tumor growth.29 CPF exposure was shown to upregulate paraoxonases expression;30 therefore, this mechanism could also contribute to the effect observed.
Figure 4.
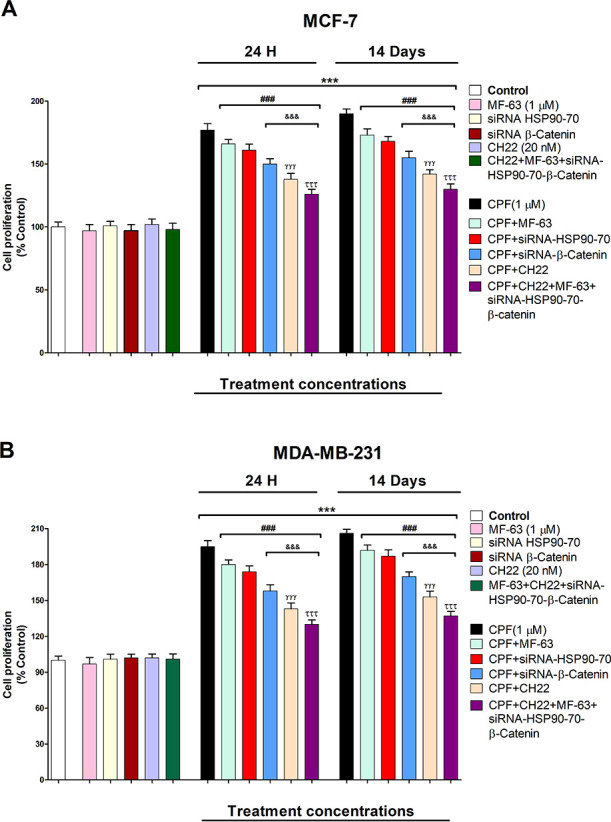
CPF (1 μM) effect on the proliferation of wild-type or single or simultaneous HSP90-70 and β-catenin-silenced MCF-7 (A) and MBA-MB-231 cells (B) cotreated with or without CH22 (20 nM) and with or without MF-63 (1 μM). Data represent the mean ± SEM of three separate experiments from cells of different cultures, each performed in triplicate. ***p ≤ 0.001 compared to the control; ###p ≤ 0.001 compared to CPF treatment; &&&p ≤ 0.001 compared to CPF treatment of HSP90–70 silenced cells; γγγp ≤ 0.001 compared to CPF treatment of β-catenin silenced cells; τττp ≤ 0.001 compared to CPF cotreatment with CH22.
These data show that CPF induces cell proliferation in MCF-7 and MDA-MB-231 cells after 1 and 14 days of treatment by AhR activation through PGE2/Wnt/β-catenin pathway and HSP90 and HSP70 overexpression. These data may help explain the CPF action in the proliferation of breast cancer cells. Further studies should be performed to determine if all mechanisms that we described mediate cell proliferation or whether additional mechanisms are involved and to confirm that these mechanisms mediate cell proliferation in vivo. Our results are of interest because they supply novel insights on the mechanisms that mediate cell proliferation induced following CPF exposure in human breast cancer cell lines.
Acknowledgments
The authors thank Professor Miguel Capo for his counseling during the preparation of the present work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.1c00258.
Supplementary Figure 1: Transfection efficiency for single or simultaneous siRNA targeting HSP90, HSP70, and β-catenin (PDF)
This work was supported by research grants 172C126PMA from Alborada Foundation/Catedra extradordinaria de Patologia y Medioambiente, UCM.
The authors declare no competing financial interest.
Supplementary Material
References
- Ventura C.; Nieto M. R.; Bourguignon N.; Lux-Lantos V.; Rodriguez H.; Cao G.; Randi A.; Cocca C.; Núñez M. Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J. Steroid Biochem. Mol. Biol. 2016, 156, 1–9. 10.1016/j.jsbmb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Engel L. S.; Werder E.; Satagopan J.; Blair A.; Hoppin J. A.; Koutros S.; Lerro C. C.; Sandler D. P.; Alavanja M. C.; Beane Freeman L. E. Insecticide Use and Breast Cancer Risk among Farmers’ Wives in the Agricultural Health Study. Environ. Health Perspect. 2017, 125 (9), 097002. 10.1289/EHP1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano P.; García J.; García J. M.; Pelayo A.; Muñoz-Calero P.; Frejo M. T.; Anadon M. J.; Lobo M.; Del Pino J. Chlorpyrifos-induced cell proliferation in human breast cancer cell lines differentially mediated by estrogen and aryl hydrocarbon receptors and KIAA1363 enzyme after 24 h and 14 days exposure. Chemosphere 2020, 251, 126426. 10.1016/j.chemosphere.2020.126426. [DOI] [PubMed] [Google Scholar]
- Moyano P.; García J. M.; García J.; Pelayo A.; Muñoz-Calero P.; Frejo M. T.; Anadon M. J.; Naval M. V.; Flores A.; Mirat V. A.; Del Pino J. Chlorpyrifos induces cell proliferation in MCF-7 and MDA-MB-231 cells, through cholinergic and Wnt/β-catenin signaling disruption, AChE-R upregulation and oxidative stress generation after single and repeated treatment. Food Chem. Toxicol. 2021, 152, 112241. 10.1016/j.fct.2021.112241. [DOI] [PubMed] [Google Scholar]
- Moyano P.; García J. M.; García J.; Anadon M. J.; Naval M. V.; Frejo M. T.; Sola E.; Pelayo A.; Pino J. D. Manganese increases Aβ and Tau protein levels through proteasome 20S and heat shock proteins 90 and 70 alteration, leading to SN56 cholinergic cell death following single and repeated treatment. Ecotoxicol. Environ. Saf. 2020, 203, 110975. 10.1016/j.ecoenv.2020.110975. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig L. M.; Fanelli M. A.; López L. A.; Gago F. E.; Tello O.; Aznar J. C.; Ciocca D. R. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer. Detect. Prev. 1997, 21 (5), 441–51. [PubMed] [Google Scholar]
- Kim L. S.; Kim J. H. Heat shock protein as molecular targets for breast cancer therapeutics. J. Breast. Cancer. 2011, 14 (3), 167–174. 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M.; Naito Z.; Tanaka S.; Asano G. Expression and roles of heat shock proteins in human breast cancer. Jpn. J. Cancer Res. 1996, 87 (9), 908–915. 10.1111/j.1349-7006.1996.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimas D. T.; Perlepe C. D.; Sergentanis T. N.; Misitzis I.; Kontzoglou K.; Patsouris E.; Kouraklis G.; Psaltopoulou T.; Nonni A. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2018, 38 (3), 1551–1562. 10.21873/anticanres.12384. [DOI] [PubMed] [Google Scholar]
- Dong H.; Zou M.; Bhatia A.; Jayaprakash P.; Hofman F.; Ying Q.; Chen M.; Woodley D. T.; Li W. Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90α) to Survive a Hostile Hypoxic Environment. Sci. Rep. 2016, 6, 20605. 10.1038/srep20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z. W.; Zeng Q.; Pei H. J.; Ren L. D.; Bai H. Z.; Na R. N. HSP90 expression and its association with wighteone metabolite response in HER2-positive breast cancer cells. Oncol. Lett. 2016, 11 (6), 3719–3722. 10.3892/ol.2016.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish N.; Parashar D.; Gupta N.; Agarwal S.; Suri V.; Kumar R.; Suri V.; Sadasukhi T. C.; Gupta A.; Ansari A. S.; Lohiya N. K.; Suri A. Heat shock protein 70–2 (HSP70–2) is a novel therapeutic target for colorectal cancer and is associated with tumor growth. BMC Cancer 2016, 16, 561. 10.1186/s12885-016-2592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H.; Liu T.; Zhang Z.; Wang X.; Xu S. Acute and subchronic toxic effects of atrazine and chlorpyrifos on common carp (Cyprinus carpio L.): Immunotoxicity assessments. Fish Shellfish Immunol. 2015, 45 (2), 327–333. 10.1016/j.fsi.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Bagchi D.; Bhattacharya G.; Stohs S. J. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology 1996, 112 (1), 57–68. 10.1016/0300-483X(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Benedetto A.; Brizio P.; Squadrone S.; Scanzio T.; Righetti M.; Gasco L.; Prearo M.; Abete M. C. Oxidative stress related to chlorpyrifos exposure in rainbow trout: Acute and medium term effects on genetic biomarkers. Pestic. Biochem. Physiol. 2016, 129, 63–69. 10.1016/j.pestbp.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Hughes D.; Guttenplan J. B.; Marcus C. B.; Subbaramaiah K.; Dannenberg A. J. Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev. Res. 2008, 1 (6), 485–493. 10.1158/1940-6207.CAPR-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aluru N.; Jenny M. J.; Hahn M. E. Knockdown of a zebrafish aryl hydrocarbon receptor repressor (AHRRa) affects expression of genes related to photoreceptor development and hematopoiesis. Toxicol. Sci. 2014, 139 (2), 381–395. 10.1093/toxsci/kfu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S.; Park S. Y.; Yoo K. Y.; Lee S. K.; Jung W. W. Induction of Heat Shock Proteins and Antioxidant Enzymes in 2,3,7,8-TCDD-Induced Hepatotoxicity in Rats. Korean J. Physiol. Pharmacol. 2012, 16 (6), 469–476. 10.4196/kjpp.2012.16.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Chen Y.; Liu H.; Yang J.; Song X.; Zhao J.; He N.; Zhou C. J.; Wang Y.; Huang C.; Dong Q. Celecoxib targets breast cancer stem cells by inhibiting the synthesis of prostaglandin E2 and down-regulating the Wnt pathway activity. Oncotarget. 2017, 8 (70), 115254–115269. 10.18632/oncotarget.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A.; Greco A.; Calamandrei G.; Minghetti L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J. Neuroinflammation 2016, 13 (1), 149. 10.1186/s12974-016-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Granero C.; Canadas F.; Cardona D.; Yu Y.; Gimenez E.; Lozano R.; Avila D. S.; Aschner M.; Sanchez-Santed F. Chlorpyrifos-, diisopropylphosphorofluoridate-, and parathion-induced behavioral and oxidative stress effects: are they mediated by analogous mechanisms of action?. Toxicol. Sci. 2013, 131, 206–216. 10.1093/toxsci/kfs280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P.; Zhou K.; Lu S.; Bai Y.; Qi R.; Zhang S. Modulation of aryl hydrocarbon receptor inhibits esophageal squamous cell carcinoma progression by repressing COX2/PGE2/STAT3 axis. J. Cell. Commun. Signal. 2020, 14 (2), 175–192. 10.1007/s12079-019-00535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A. J.; Branam A. M.; Peterson R. E. Intersection of AHR and Wnt signaling in development, health, and disease. Int. J. Mol. Sci. 2014, 15 (10), 17852–17885. 10.3390/ijms151017852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Bozaykut P.; Ozer N. K.; Karademir B. Nrf2 silencing to inhibit proteolytic defense induced by hyperthermia in HT22 cells. Redox Biol. 2016, 8, 323–332. 10.1016/j.redox.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate L. V.; Pontillo C. A.; Español A.; Miret N. V.; Chiappini F.; Cocca C.; Álvarez L.; de Pisarev D. K.; Sales M. E.; Randi A. S. Angiogenesis signaling in breast cancer models is induced by hexachlorobenzene and chlorpyrifos, pesticide ligands of the aryl hydrocarbon receptor. Toxicol. Appl. Pharmacol. 2020, 401, 115093. 10.1016/j.taap.2020.115093. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A.; Seidler F. J. Protein Kinase C Is a Target for Diverse Developmental Neurotoxicants: Transcriptional Responses to Chlorpyrifos, Diazinon, Dieldrin and Divalent Nickel in PC12 Cells. Brain Res. 2009, 1263, 23–32. 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C.; Zappia C. D.; Lasagna M.; Pavicic W.; Richard S.; Bolzan A. D.; Monczor F.; Núñez M.; Cocca C. Effects of the pesticide chlorpyrifos on breast cancer disease. Implication of epigenetic mechanisms. J. Steroid Biochem. Mol. Biol. 2019, 186, 96–104. 10.1016/j.jsbmb.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Bacchetti T.; Ferretti G.; Sahebkar A. The role of paraoxonase in cancer. Semin. Cancer Biol. 2019, 56, 72–86. 10.1016/j.semcancer.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Jasna J. M.; Anandbabu K.; Bharathi S. R.; Angayarkanni N. Paraoxonase enzyme protects retinal pigment epithelium from chlorpyrifos insult. PLoS One 2014, 9 (6), e101380 10.1371/journal.pone.0101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


