ABSTRACT
Infectious diseases have been shown to disproportionately affect indigenous populations. Tuberculosis (TB) and malaria continue to impose a significant burden on humanity and are among the infectious diseases targeted within the 2030 Agenda for Sustainable Development. A systematic review and meta-analyses were undertaken to evaluate the prevalence of TB and malaria infections within minority indigenous populations of the South-East Asia and Western Pacific Regions. The review was undertaken in accordance with The Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines following a published protocol. A random effects meta-analysis was used to calculate the pooled prevalence of TB and malaria. A meta-regression analysis was applied to quantify associations with study covariates and a sub-group analysis undertaken where studies provided comparative data between minority indigenous and other population groups. From the 3,275 unique publications identified, 24 on TB, and 39 on malaria were included in the final analysis. The pooled prevalence of TB was 2.3% (95% CI: 1.7, 2.9) and the pooled prevalence of malaria was 19.9% (95% CI: 15.9, 24.2). There was significant (p = 0.000) heterogeneity (I2) between studies. Significant difference was not observed in TB and malaria prevalence between minority indigenous and other population groups, although the odds ratio of malaria infection in minority indigenous populations was 1.15 (95% CI 0.99, 1.34: p-value 0.06) compared to other population groups. The review identified a paucity of data on TB and malaria in minority indigenous populations despite the significant prevalence and burden of these diseases within these regions.
KEYWORDS: Tuberculosis, malaria, indigenous, minority, south-east asia, western pacific, systematic review
Introduction
In 2015, the 193 member states of the United Nations (UN) adopted the 2030 Agenda for Sustainable Development[1]. Amongst other diseases, Sustainable Development Goal (SDG) 3.3 aims to end the epidemics of tuberculosis (TB) and malaria by 2030[2]. With respect to morbidity and mortality, TB and malaria are among the three most important infectious diseases affecting humankind, the other being Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Virus (AIDS).
In 2019, an estimated 1.4 million people died as a result of TB and although the burden of disease is falling, the decline is not occurring at a rate sufficient to achieve the milestones within the World Health Organization (WHO) End TB Strategy and the SDG TB related target[3]. In 2018, approximately 10 million people fell ill with the disease and 87% of new cases occurred within 30 high TB burden countries[3]. Of the 30 high TB burden countries, 11 fall within the WHO South-East Asia (SEAR) and Western Pacific Region (WPR) [4] where 44% and 18% of 2018 new cases occurred respectively[3].
In 2018, there were an estimated 228 million cases and 405,000 deaths due to malaria, with the burden of disease in the SEAR second only to that occurring within the African Region[5]. Although the incidence of malaria is decreasing, the decline is not occurring at a rate sufficient to achieve the milestones of the Global Technology Strategy for Malaria 2016–20305 and the SDG target.
Mycobacterium tuberculosis, the bacterium responsible for TB, is globally ubiquitous[3]. The distribution of malaria caused by the protozoan parasite Plasmodium spp. is governed by seasonal temperature patterns and the distribution of the mosquito vector, Anopheles spp [6,7]. For both TB and malaria, research shows the prevalence of disease to be higher in populations living in poverty [8–10]. Indigenous people are disproportionately affected by poverty [11] and may be unduly impacted by TB and malaria in terms of both incidence and proximate determinants. [12–16] Access to health care provision for indigenous populations is inequitable due to social and cultural barriers, and the fact that they often live in remote locations[17]. These factors compound the health inequalities that are observed between indigenous and non-indigenous populations in both developing and industrialized nations[18]. The SEAR and WPR were chosen for this review to provide an opportunity to compare disease prevalence across countries with differing levels of socio-economic development whilst also capturing a significant proportion of the world’s minority indigenous people[19].
If health targets and the commitment of the 2030 Agenda for Sustainable Development that ‘no one will be left behind’ [20] are to be met, the prevalence of disease among vulnerable populations will need to be quantified so that effective interventions can be implemented. This systematic review analyzed available data to quantify the prevalence of TB and malaria in minority indigenous populations within the SEAR and WPR. The review also estimated the risk of infection in minority indigenous people relative to other populations groups from studies where direct comparative data were available.
Methods
Search strategy and selection criteria
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table 1: PRISMA Checklist)[21]. The full details of the search and selection criteria are available in a published protocol [22] (Open Science Framework registration: osf.io/m6sqc).
Table 1.
PRISMA Checklist[21].
| Section/topic | # | Checklist item | Reported on page # | |
|---|---|---|---|---|
| TITLE | ||||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 | |
| ABSTRACT | ||||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1–2 | |
| INTRODUCTION | ||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3–4 | |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 4 | |
| METHODS | ||||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number. | 5 | |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale. | 6–7 | |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5 | |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 36 | |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 5 | |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 7 | |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made. | 7 | |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 7 | |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means). | 7–8 | |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I[2]) for each meta-analysis. | 8 | |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies). | 8 | |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 8 | |
| RESULTS | ||||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 31 | |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations. | 24–26 | |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 37–39 | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 34–35 | |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 27, 29 | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 32, 33 | |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g. sensitivity or subgroup analyses, meta-regression [see Item 16]). | 28, 30 | |
| DISCUSSION | ||||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. healthcare providers, users, and policy makers). | 11–13 | |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias). | 13 | |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 14 | |
| FUNDING | ||||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review. | 15 | |
In summary, a systematic search for epidemiological studies was undertaken in Q4 2020 in four biomedical databases: Web of Science, Scopus, EMBASE (Ovid) and Medline (Ovid), without restriction on year of publication, using the search terms detailed in Appendix 1. In addition to the search results from the biomedical databases, reference lists from relevant studies were hand searched.
Screening
Articles identified from the search were uploaded into Endnote X9 (Clarivate Analytics) and duplicates were removed. Once the duplicates were removed, all remaining articles were uploaded into Rayyan Qatar Computing Research Institute (QCRI) software [23] and two authors (BG and KAA) independently screened the titles and the abstracts. The same authors independently screened the full text articles against the inclusion and exclusion criteria.
Any disagreements regarding the inclusion/exclusion of a study were resolved by discussion and when consensus could not be achieved, the third author (ACAC) was consulted. Where required, further clarification was sought from the corresponding author of relevant studies.
Inclusion criteria
To be included, studies were required to: relate to human infection, include minority indigenous populations within the SEAR or WPR and be representative surveys that reported sufficient data to enable the prevalence of disease to be calculated. Where studies reported on the impact of intervention regimes, only pre-intervention baseline data were recorded.
As detailed in the protocol[22], minority indigenous population groups where defined when each of the following criteria were met:
Descendants of the original or earliest known inhabitants of an area; people who have historical continuity with pre-invasion and pre-colonial societies, [24–26]
Distinct societies with languages, culture, customs, and social and political frameworks that vary significantly from those of the dominant population, [24–28]
Groups of people with strong cultural ties and dependence upon the environment and its resources for their survival, [24,26,28,29]
People self-identifying as indigenous, [26]
Exclusion criteria
Due to resource constraints, articles published in languages other than English were excluded. Studies were excluded if less than 90% of study participants in the study (or, for the comparative analyses, the minority indigenous category) were minority indigenous participants. Case studies and case series with less than 10 people, literature or systematic reviews, conference abstracts or posters and scientific correspondence e.g. letter to the editor, were excluded. Studies on latent TB were omitted from the analysis (i.e. those utilizing Mantoux testing as the sole diagnostic). Studies were excluded if only symptomatic participants were tested and details on the total population screened were not included.
Data extraction and quality assessment
Data were extracted into a Microsoft Excel 2014 spreadsheet (Microsoft, Redmond, Washington, USA) by one of the researchers (BG) and cross-checked by the second author (KAA).The data extraction spreadsheet was pilot tested and refined before subsequent extraction of the following data: first author; year of publication; year of data collection; country in which the study was undertaken; population group (whether minority indigenous or other population); infectious agent (for Plasmodium species); diagnostic methods; size of study population (n); age; sex; size of the disease positive population (n) and screening method (for TB studies). Where studies undertook a comparison between minority indigenous and other population groups, data were extracted for both groups to facilitate a comparison.
The quality of the included studies was assessed using a modified version of the Newcastle-Ottawa Quality Assessment Scale [32] the results of which are detailed in Appendix 2.
Data Analysis
For both TB and malaria, a random effects meta-analysis with 95% confidence intervals (CI) was used to estimate the prevalence of infection. For the prevalence of both diseases, a meta-regression model was used to quantify associations of population type and study characteristics with infection status. Where direct comparative data were available for minority indigenous and other population groups, sub-group analyses were undertaken to calculate the relative risk of infection between the two population groups.
Infection status (positive/negative) was derived using the case definitions used within each study.
Cochran’s Q test, utilized to measure heterogeneity between studies, was quantitatively assessed by the index of heterogeneity squared (I [2]) statistics with 95% CI[33]. As a result of the high heterogeneity (I [2] >75%) [33] identified, meta – regression was undertaken using the study characteristics as covariates. Where differentials in disease prevalence were identified across covariates, or between population groups, bivariate meta-regression was used to test significance (p < 0.05) when three or more studies were available for each comparison.
Potential publication bias was assessed utilizing funnel plots and asymmetry was evaluated with Egger’s method using a p < 0.05 to indicate significant bias[34].
Stata/MP version 16 (StataCorp, College Station, TX) was used to undertake the analyses.
Results
The search identified 3,275 unique publications and 233 articles remained after the title and abstract screening. After full text review, 63 were included in the final analysis. The PRISMA summary of the systematic review shortlisting process is detailed in Figure 1. Analysis of publication bias for the included studies is detailed in Figures 2 and 3. No publication bias was observed for the malaria studies (Figure 3), however asymmetry of the funnel plot (Figure 2) and a p = 0.003 for Egger’s regression test indicated publication bias for the included TB studies.
Figure 1.
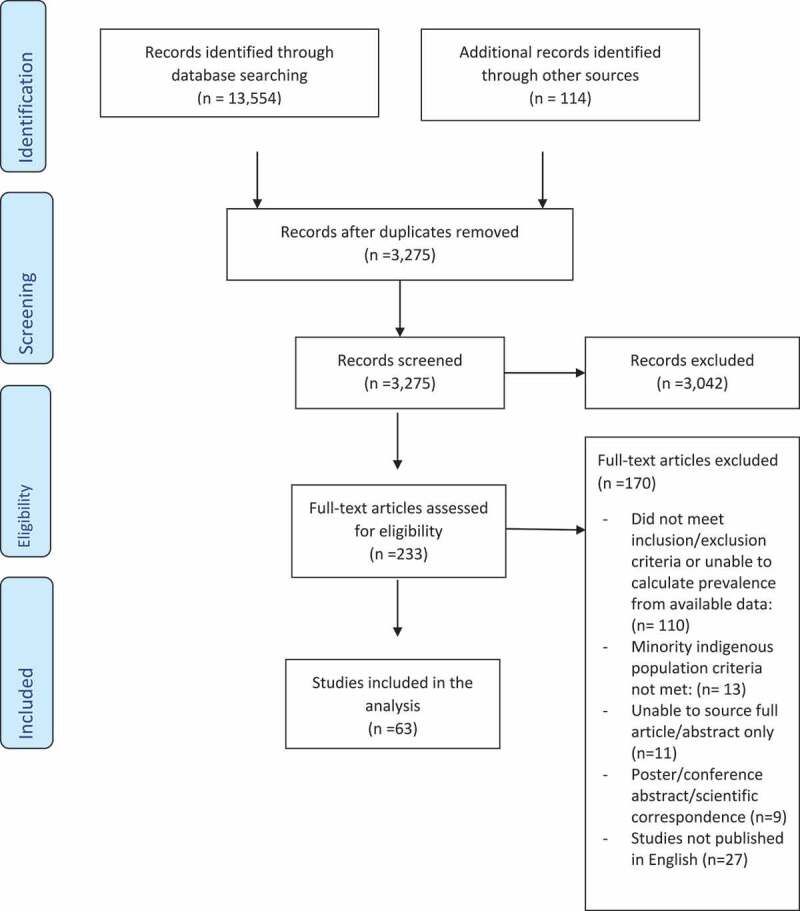
PRISMA summary of systematic review study selection process. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
Figure 2.
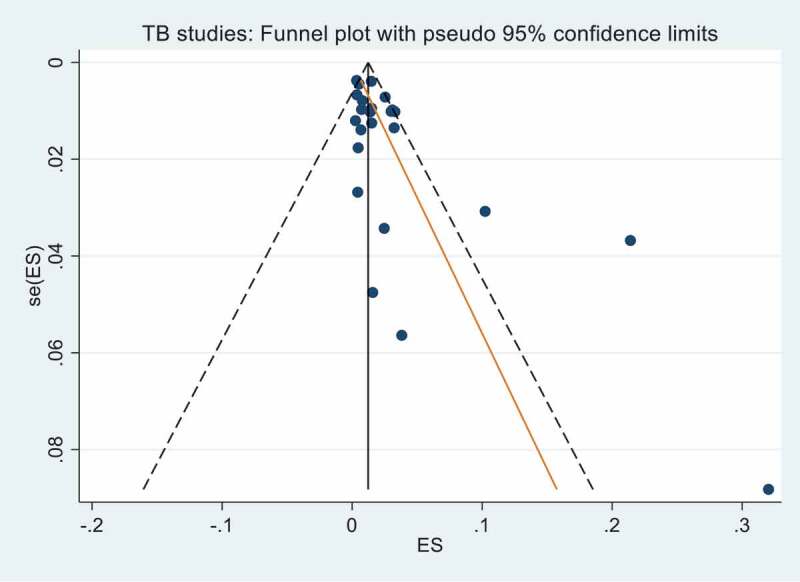
Funnel plot with pseudo 95% confidence limits for TB studies. Egger’s test for small study effects gave a bias coefficient of 1.78 (95% CI 0.66, 2.91) p-value 0.003 indicating significant publication bias.
Figure 3.
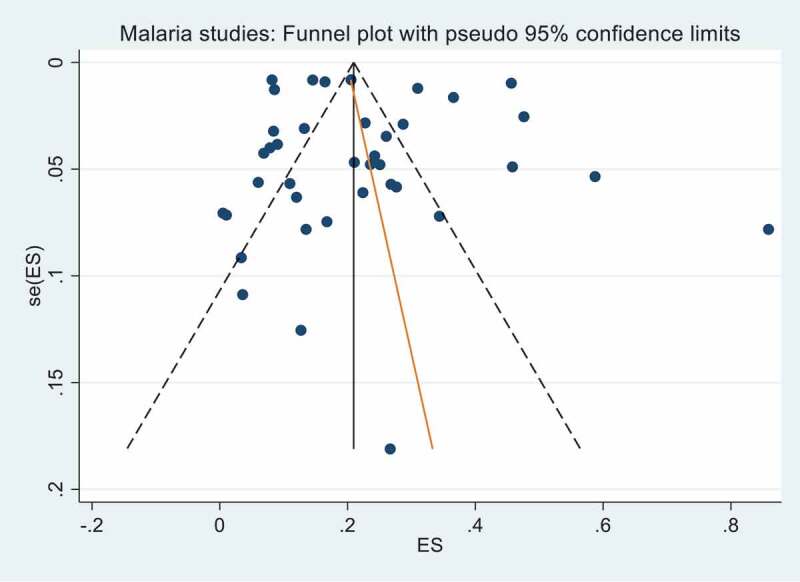
Funnel plot with pseudo 95% confidence limits for malaria studies. Egger’s test for small study effects gave a bias coefficient of 0.74 (95% CI −2.33, 3.81) and a p-value of 0.63 indicating no significant publication bias.
Characteristics of the included studies
The characteristics of the included studies are presented in Tables 2 and 3.
Table 2.
Summary of TB studies
| Study ID | First Author Year of Publication | Year of Data Collection^ |
WHO Region | WHO Mortality Strata |
Country | Diagnostic Method* | Population TB +ve Screening Screened Screened# (n) Method≠ Population (n) % Male |
Population | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bhat, 2009 | 2007–2008 | SEAR | D | India | Culture | 22,284 | 83 | Chest symptoms | 48.6 | |||||||
| 2 | Bhat, 2015 | 2012–2013 | SEAR | D | India | Culture | 19,409 | 494 | Chest symptoms | ||||||||
| 3 | Bhat, 2017 | 2013 | SEAR | D | India | Culture | 12,123 | 348 | Chest symptoms | ||||||||
| 4 | Bolton, 1975 | 1961–1971 | WPR | B | Malaysia | Smear | 71,748 | 249 | X-ray | ||||||||
| 5 | Chakma, 1996 | <1996 | SEAR | D | India | Culture | 11,097 | 142 | Chest symptoms | ||||||||
| 6 | Damon, 1974 | 1968 | WPR | B | Solomon Is | Clinical | 850 | 21 | No pre-screening | ||||||||
| 7 | Datta, 2001 | 1989 | SEAR | D | India | Culture | 16,017 | 126 | Chest symptoms +/or x-ray | 60.2 | |||||||
| 8 | Haddad, 2012 | <2012 | SEAR | D | India | Smear | 1,660 | 346 | Chest symptoms | ||||||||
| 9 | Hussain, 2020 | 2015–2017 | SEAR | D | India | Culture | 5,145 | 35 | Chest symptoms | ||||||||
| 10 | Kashyap, 2013 | <2013 | SEAR | D | India | Culture | 128 | 41 | No pre-screening | ||||||||
| 11 | Kerketta, 2009 | <2009 | SEAR | D | India | Clinical | 314 | 12 | No pre-screening | 43.0 | |||||||
| 12 | King, 1951 | 1950 | WPR | A | Australia | Clinical | 3,209 | 15 | Mantoux test | ||||||||
| 13 | Macken, 1952 | 1949–1951 | WPR | A | Australia | Clinical | 5,472 | 177 | Mantoux test | ||||||||
| 14 | Murhekar, 2004 | 2001–2002 | SEAR | D | India | Smear | 10,570 | 77 | Chest symptoms | ||||||||
| 15 | Purty, 2019 | 2015–2017 | SEAR | D | India | Smear | 6,898 | 18 | Chest symptoms | 47.8 | |||||||
| 16 | Rao, 2010A | 2008 | SEAR | D | India | Culture | 1,390 | 6 | Chest symptoms | ||||||||
| 17 | Rao, 2010B | 2007–2008 | SEAR | D | India | Culture | 11,116 | 166 | Chest symptoms | ||||||||
| 18 | Rao, 2011 | 2007–2008 | SEAR | D | India | Culture | 9,538 | 133 | Chest symptoms | 47.6 | |||||||
| 19 | Rao, 2015 | 2012–2013 | SEAR | D | India | Culture | 9,653 | 318 | Chest symptoms | 46.5 | |||||||
| 20 | Rao, 2019 | 2013 | SEAR | D | India | Culture | 9,756 | 293 | Chest symptoms | ||||||||
| 21 | Roy, 1969 | 1968 | WPR | B | Malaysia | Smear | 1,055 | 108 | X-ray | 55 | |||||||
| 22 | Sharma, 2010 | 2006–2007 | SEAR | D | India | Smear | 50,000 | 266 | Chest symptoms | ||||||||
| 23 | Vyas, 2019 | 2014–2015 | SEAR | D | India | Smear | 65,230 | 964 | Chest symptoms | ||||||||
| 24 | Yano, 1974 | 1972 | WPR | B | Malaysia | Clinical | 562 | 12 | No pre-screening | ||||||||
Notes:^ If the study has not detailed the year of data collection, it is assumed < year of publication
*Diagnostic method: Smear = smear or uncategorized sputum methodology. Clinical = current TB treatment, self-report, X-ray. If a paper uses multiple methods, it is classified according to the most sensitive method according to the following descending order: culture, smear and clinical (e.g. if smear + culture classified as culture, if x-ray and smear classified as smear)
# Population figures are inclusive of non-indigenous participants in the comparative studies
≠Where studies utilize a screening method to determine the population to be tested, this is detailed. Chest symptoms include-persistent cough, chest pain, fever, hemoptysis.
Table 3.
Summary of malaria studies
| Study ID | First Author Year of Publication | Year of Data Collection^ | WHO Region | WHO Mortality Strata |
Country | Diagnostic Method * | Population tested# | Malaria positive $ | Tested Population % Male | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abe, 2009 | 2006 | WPR | B | Vietnam | Microscopy | 552 | 38 | ||
| 2 | Chaturvedi, 2017 | 2013–2014 | SEAR | D | India | Microscopy | 6,761 | 2,094 | ||
| 3 | Choubisa, 1992 | <1992 | SEAR | D | India | Microscopy | 250 | 30 | 64 | |
| 4 | Chourasia, 2017a | 2013–2014 | SEAR | D | India | Microscopy | 293 | 81 | ||
| 5 | Chourasia, 2017b | 2016 | SEAR | D | India | PCR | 437 | 103 | 42.8 | |
| 6 | Damon, 1974 | 1966 + 1968 | WPR | B | Solomon Is | Microscopy + Enlarged Spleen | 1,542 | 734 | ||
| 7 | Das, 2000 | 1998 | SEAR | D | India | Microscopy | 435 | 109 | 53.8 | |
| 8 | Das, 2005 | 2001 | SEAR | D | India | Microscopy | 179 | 30 | 58.1 | |
| 9 | Das, 2017 | 2014–2016 | SEAR | D | India | RDT | 1,192 | 342 | ||
| 10 | Dev, 2006 | 1991–1993 | SEAR | D | India | Microscopy | 15,093 | 3,101 | ||
| 11 | Erhart, 2005 | 2003 | WPR | B | Vietnam | Microscopy | 3,932 | 1,385 | ||
| 12 | Ganguly, 2013 | 2012 | SEAR | D | India | PCR | 963 | 81 | ||
| 13 | Gordon, 1991 | <1991 | WPR | B | Malaysia | Microscopy | 268 | 60 | ||
| 14 | Haque, 2011 | 2009 | SEAR | D | Bangladesh | RDT | 1,400 | 161 | ||
| 15 | Jiram, 2016 | <2016 | WPR | B | Malaysia | PCR | 306 | 82 | 52.3 | |
| 16 | Kaur, 2009 | <2009 | WPR | B | Malaysia | Microscopy | 520 | 126 | 49.6 | |
| 17 | Luxemburger, 1996 | 1991–1992 | SEAR | B | Thailand | Microscopy + Enlarged Spleen | 677 | 61 | ||
| 18 | Mak, 1987 | 1984 | WPR | B | Malaysia | Microscopy | 191 | 17 | ||
| 19 | Marasabessy, 2019 | 2019 | SEAR | B | Indonesia | Microscopy | 84 | 3 | 60.7 | |
| 20 | Marchand, 2011 | 2010 | WPR | B | Vietnam | Microscopy | 624 | 49 | ||
| 21 | Nakabayashi, 1973 | 1970 | WPR | B | Philippines | Microscopy | 65 | 10 | ||
| 22 | Nithikathkul, 2003A | 2002 | SEAR | B | Thailand | Microscopy | 119 | 4 | 46.2 | |
| 23 | Nithikathkul, 2003B | <2003 | SEAR | B | Thailand | Microscopy | 195 | 2 | 42 | |
| 24 | Norhayati, 2001 | <2001 | WPR | B | Malaysia | Microscopy | 310 | 34 | ||
| 25 | Pichainarong, 2004 | 2001–2002 | SEAR | B | Thailand | Microscopy | 417 | 191 | 68.1 | |
| 26 | Rahmah, 1997 | 1996 | WPR | B | Malaysia | Microscopy | 200 | 1 | ||
| 27 | Rajagopalan, 1989 | 1986–1988 | SEAR | D | India | Microscopy + Enlarged Spleen | 29,932 | 3,501 | ||
| 28 | Roy, 2001 | 1997 | SEAR | D | India | Microscopy | 163 | 22 | ||
| 29 | Sahu, 2013 | 2009 | SEAR | D | India | Microscopy | 12,045 | 1,983 | 48.6 | |
| 30 | Sharma, 2004 | 2001 | SEAR | D | India | Microscopy | 6,136 | 525 | ||
| 31 | Sharma, 2006 | 2001–2003 | SEAR | D | India | Microscopy | 14,860 | 1,214 | ||
| 32 | Singh, 1989 | 1987–1988 | SEAR | D | India | Microscopy + Enlarged Spleen | 10,558 | 4,817 | ||
| 33 | Singh, 1998 | 1995–1996 | SEAR | D | India | Microscopy | 456 | 96 | 0 | |
| 34 | Singh, 2001 | 1999 | SEAR | D | India | Microscopy + Enlarged Spleen | 349 | 205 | ||
| 35 | Srivastava, 2000 | 1995 | SEAR | D | India | Microscopy | 833 | 217 | ||
| 36 | Stafford, 1980 | <1980 | SEAR | B | Indonesia | Microscopy | 316 | 19 | 52.8 | |
| 37 | Thomas, 1981 | <1981 | WPR | B | Malaysia | Microscopy + Enlarged Spleen + IFA∆ | 163 | 140 | ||
| 38 | Tipmontree, 2009 | <2009 | SEAR | B | Thailand | Self-report | 192 | 66 | ||
| 39 | Wharton, 1963 | 1960–1962 | WPR | B | Malaysia | Microscopy | 1,244 | 283 | ||
Notes: ^ If the study has not detailed the year of data collection, it is assumed < year of publication
* Where studies utilized multiple diagnostic methods, Rapid Diagnostic Test (RDT) + microscopy were classified as microscopy and RDT + microscopy + Polymerase Chain Reaction (PCR) were classified as PCR.
#Population figures are inclusive of non-indigenous participants in the comparative studies
$Where multiple diagnostic methods were used in the same study, the method which gave the greatest number of malarial cases was used to determine the number of cases.
∆Indirect Fluorescent Antibody (IFA)
A total of 24 studies on TB, representing 337,677 minority indigenous participants, met the review criteria and were included in the analysis. Within the 24 studies, four [35–38] undertook a comparison between minority indigenous and other population groups. These four studies represented 17,895 and 7,547 minority indigenous and non ‘minority indigenous’ participants, respectively.
Eighteen TB studies [35–37,39–52] where undertaken in the SEAR, all in India (WHO mortality stratum D)[53]. Six TB studies were identified in the WPR; two in Australia [54,55] (mortality stratum A)[53]; three studies where undertaken in Malaysia [38,56,57] (mortality stratum B) [53] and one study in the Solomon Islands [58] (mortality stratum B)[53]. Nineteen minority indigenous population groups were represented across the four countries – Table 4.
Table 4.
Minority indigenous population groups represented in the TB studies analyzed
| Country | # Minority Indigenous Study Participants | Minority Indigenous Population | Minority Indigenous Population % Representation |
|---|---|---|---|
| Australia | 8,681 | Aborigine | 100.0 |
| India | 254,901 | Saharia | 55.5 |
| Sahariya + Bhil | 19.6 | ||
| Tribal | 13.5 | ||
| Malayaali | 6.3 | ||
| Car Nicobarese | 4.1 | ||
| Bharia | 0.5 | ||
| Paniyas + other scheduled tribes | 0.3 | ||
| Langia Saora, Paudi Bhuiyan, Kutia Kondh + Dongria Kondh | 0.1 | ||
| Malaysia | 73,245 | Orang Asli | 98.0 |
| Murut | 1.4 | ||
| Iban | 0.6 | ||
| Solomon Islands | 850 | Nasioi, Kwaio, Lau + Baegu | 100.0 |
For malaria, a total of 39 studies representing 98,249 minority indigenous participants were included in the analysis. Within the 39 studies, four studies [59–62] undertook a comparison between minority indigenous and other populations, representing 4,841 and 747 participants, respectively.
Within the 39 studies, 26 were undertaken in the SEAR, and of these seven were within mortality stratum B53 (two in Indonesia [63,64] and five in Thailand [65–69]) and 19 within mortality stratum D53 (one in Bangladesh [60] and 18 in India [70–87]). Thirteen studies were undertaken in the WPR, all within mortality stratum B53 (eight in Malaysia [61,88–94], one in the Philippines [62], one in the Solomon Islands [58] and three in Vietnam [59,95,96]). Thirty-three minority indigenous population groups were represented across the eight countries – Table 5.
Table 5.
Minority indigenous population groups represented in malaria studies analyzed
| Country | # Minority Indigenous Study Participants | Minority Indigenous Population | Minority Indigenous Population % Representation |
|---|---|---|---|
| Bangladesh | 1,043 | Marma, Tripura, Tonchonga, Khiang + Chakma | 100.0 |
| India | 85,679 | Aboriginal tribes | 88.2 |
| Baiga | 7.9 | ||
| Munda,Oraon, Lohra, Bedia, Baraik + Kachhap | 1.4 | ||
| Gond | 1.3 | ||
| Gond, Halba + Muria | 0.5 | ||
| Santhals + Adivasis | 0.5 | ||
| Jarawas | 0.2 | ||
| Indonesia | 400 | Nuaulu | 21.0 |
| Torajans | 79.0 | ||
| Malaysia | 3,074 | Orang Asli | 100.0 |
| Philippines | 30 | Palawano | 100.0 |
| Solomon Islands | 1,542 | Nasioi, Kwaio, Lau + Baegu | 100.0 |
| Thailand | 1,600 | Karen | 61.9 |
| Hill Tribe | 26.1 | ||
| Karen + Mon | 12.0 | ||
| Vietnam | 4,881 | Rag Lays | 75.9 |
| Raglai | 12.8 | ||
| Steing | 11.3 |
Prevalence of TB
Within minority indigenous populations, the pooled prevalence of TB was 2.3% (95% CI 1.7, 2.9); ranging from 0.3% (95% CI 0.2, 0.4) [46] to 32.0% (95% CI 24.6, 40.5)[43]. These data are represented in a Forest Plot – Figure 4, which shows the significant heterogeneity between studies. The pooled prevalence of TB in minority indigenous people between study populations and across study covariates is detailed in Table 6 and associations with covariates are detailed in Table 7.
Figure 4.
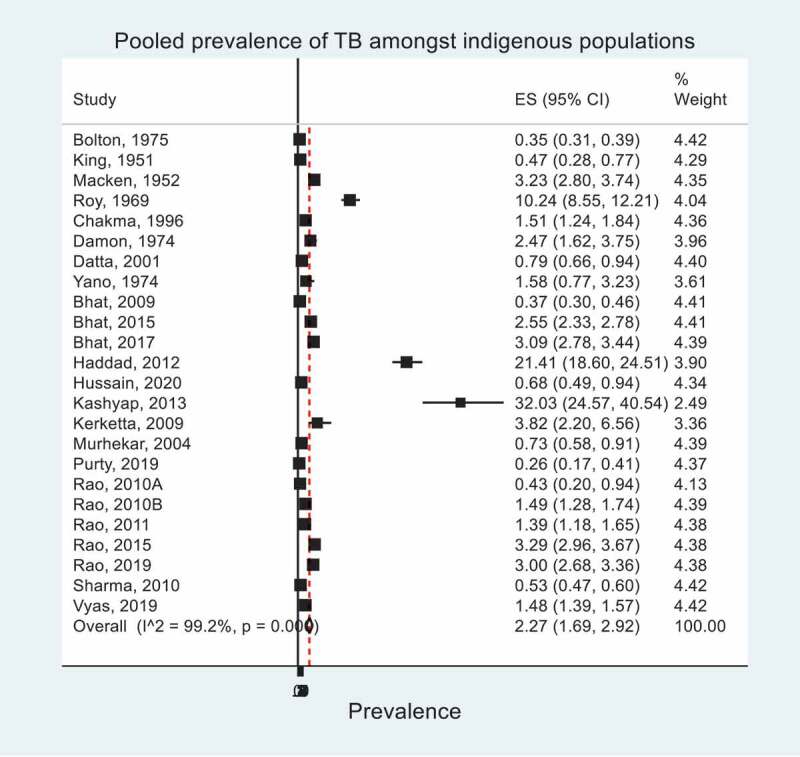
Pooled prevalence of TB within minority indigenous study populations. The forest plot shows overall effect sizes (ES) and their 95% confidence intervals (CI). I^2 statistic describes the percentage of variation due to heterogeneity.
Table 6.
Pooled prevalence of TB within population groups and across study covariates within minority indigenous populations
| Studies (n) | Pooledα Prevalence TB (95% CI) | |
|---|---|---|
| Study Population | ||
| Minority indigenous populations | 24 | 2.27 (1.69, 2.92) |
| Comparative Studies | ||
| Non ‘minority indigenous’ populations | 4 | 4.96 (0.32, 14.23) |
| Minority indigenous populations | 4 | 5.04 (1.72, 9.93) |
| Analysis on indigenous populations only | ||
| WHO regions | ||
| SEAR | 18 | 2.23 (1.61, 2.95) |
| WPR | 6 | 2.31 (0.65, 4.91) |
| WHO Mortality Strata | ||
| A | 2 | 1.93 (1.65, 2.23) |
| B | 4 | 2.77 (0.08, 8.78) |
| D | 18 | 2.23 (1.61, 2.95) |
| Countries | ||
| Australia | 2 | 1.93 (1.65, 2.23) |
| India | 18 | 2.23 (1.61, 2.95) |
| Malaysia | 3 | 2.87 (0.00, 11.99) |
| Solomon Islands | 1 | 2.47 (1.62, 3.75) |
| Year of data collection | ||
| 1945–1970 | 4 | 2.46 (0.46, 5.95) |
| 1971–1995 | 4 | 1.44 (0.81, 2.24) |
| 1996–2020 | 16 | 2.44 (1.72, 3.28) |
|
Age <15 years ≥15 years |
1 15 |
13.64 (7.34, 23.93) 2.40 (1.55, 3.41) |
|
Sex Female Male |
9 | 1.01 (0.53, 1.63) 2.89 (1.56, 4.59) |
| Diagnostic methods | ||
| Clinical | 7 | 2.05 (1.34, 2.89) |
| Culture | 12 | 2.08 (1.28, 3.08) |
| Smear | 8 | 2.18 (1.34, 3.22) |
| Screening Method | ||
| Chest symptoms | 16 | 1.76 (1.2, 2.41) |
| Mantoux skin test | 2 | 1.93 (1.65, 2.23) |
| No pre-screening | 4 | 6.86 (1.37, 15.91) |
| X-ray | 2 | 0.37 (0.33, 0.42) |
Note: α Prevalence pooled when >1 data set, otherwise result is presented from a single study
Table 7.
Bivariate regression between TB study covariates
| Pooled prevalence of TB infection |
||
|---|---|---|
| 95% CI | p-value | |
| Comparative Studies | ||
| Non ‘minority indigenous’ populations | 1.00 | |
| Minority indigenous populations | 1.00032 (0.85, 1.18) | 0.996 |
| WHO regions | ||
| SEAR | 1.00 | |
| WPR | 0.99 (0.95, 1.04) | 0.783 |
| WHO Mortality Strata | ||
| B | 1.00 | |
| D | 1.00 (0.95, 1.06) | 0.985 |
| Countries | ||
| India | 1.00 | |
| Malaysia | 1.00 (0.94, 1.07) | 0.895 |
| Year of data collection | ||
| 1945–1970 | 1.00 | |
| 1971–1995 | 0.98 (0.94, 1.03) | 0.380 |
| 1996–2020 | 1.00 (0.95, 1.06) | 0.863 |
|
Sex Female Male |
1.00 1.02 (0.99, 1.05) |
0.218 |
| Diagnostic methods | ||
| Clinical | 1.00 | |
| Culture | 1.01 (0.98, 1.04) | 0.581 |
| Smear | 1.02 (0.97, 1.07) | 0.468 |
| Screening Method | ||
| Chest symptoms | 1.00 | |
| No pre-screening | 1.06 (0.94, 1.19) | 0.354 |
Note: Bivariate meta-regression analysis was only undertaken where there were 3 or more data sets
In the four studies that undertook a comparison between population groups [35–38], no difference in TB prevalence was observed between minority indigenous (5.0% 95% CI 1.7, 9.9) and non ‘minority indigenous’ participants (5.0% 95% CI 0.3, 14.2).
Within minority indigenous populations only, there were no significant differences in TB prevalence between the regions (SEAR and WPR), WHO mortality strata, countries of study, year of data collection, sex of study participants, diagnostic method, or method of population screening. Insufficient studies were available to examine age as a covariate.
Prevalence of malaria
The prevalence of malaria across the study covariates is detailed in Table 8 and the analysis of associations between malaria and covariates is detailed in Table 9.
Table 8.
Pooled prevalence of malaria within population groups and across study covariates within minority indigenous populations
| Categories | Pooled α prevalence of malaria* |
|
|---|---|---|
| Studies (n) | Pooled Prevalence (95% CI) | |
| Population group | ||
| Minority indigenous populations | 39 | 19.87 (15.89, 24.16) |
| Comparative Studies | ||
| Non ‘minority indigenous’ populations | 4 | 8.20 (4.89, 12.22) |
| Minority indigenous populations | 4 | 21.50 (7.81, 39.42) |
| Analysis on indigenous populations only | ||
| WHO regions | ||
| SEAR | 26 | 18.37 (13.93, 23.27) |
| WPR | 13 | 23.11 (14.27, 33.32) |
| WHO Mortality Strata | ||
| B | 20 | 18.71 (11.72, 26.86) |
| D | 19 | 21.03 (15.67, 26.94) |
| Countries | ||
| Bangladesh | 1 | 13.23 (11.31, 15.42) |
| India | 18 | 21.51 (15.93, 27.67) |
| Indonesia | 2 | 5.36 (3.29, 7.85) |
| Malaysia | 8 | 23.21 (11.41, 37.61) |
| Philippines | 1 | 26.67 (14.18, 44.45) |
| Solomon Islands | 1 | 47.60 (45.12, 50.10) |
| Thailand | 5 | 14.84 (2.29, 35.27) |
| Vietnam | 3 | 15.20 (1.23, 40.35) |
| Infectious agent$ | ||
| P.falciparum | 22 | 12.90 (9.37, 16.90) |
| P.falciparum + P.malariae | 2 | 0.00 (0.00, 0.003) |
| P.falciparum +/or P.vivax | 13 | 5.04 (2.81, 7.84) |
| P.falciparum +/or P.vivax +/or P.malariae | 3 | 0.91 (0.42, 1.58) |
| P.knowlesi | 1 | 7.52 (5.06, 11.03) |
| P.malariae | 8 | 0.61 (0.23, 1.14) |
| P.vivax | 22 | 4.75 (3.16, 6.63) |
| P.vivax + P.malariae | 1 | 1.12 (0.38, 3.24) |
| Plasmodium spp | 14 | 27.47 (17.21, 39.10) |
| Year of data collection | ||
| 1960–1980 | 5 | 36.44 (15.98, 59.82) |
| 1981–2000 | 14 | 19.21 (12.58, 26.85) |
| 2001–2020 | 20 | 16.89 (12.28, 22.07) |
| Diagnostic methods^ | ||
| Enlarged spleen | 6 | 40.17 (23.90, 57.68) |
| IFA | 1 | 85.89 (79.72, 90.41) |
| Microscopy | 33 | 17.19 (13.19, 21.59) |
| PCR | 3 | 18.70 (7.52, 33.41) |
| RDT | 2 | 20.93 (19.27, 22.65) |
| Self-report | 1 | 34.38 (28.02, 41.34) |
Notes: α Prevalence pooled when >1 data set, otherwise result from single study
*All species consolidated to give malaria prevalence, where a study uses different diagnostic methods on the same study population, the result from the method which gives the highest number of positives is taken as the number of malaria cases.
$P.falciparum + P.vivax and P.falciparum or P.vivax classified together as P.falciparum +/or P.vivax.
^ RDT + microscopy classified as microscopy; RDT + microscopy + PCR classified as PCR
Table 9.
Bivariate regression between malaria study covariates
| Categories | Pooled prevalence of malaria |
|
|---|---|---|
| 95% CI | p value | |
| Comparative Studies | ||
| Non ‘minority indigenous’ populations | 1.00 | |
| Minority indigenous populations | 1.15 (0.99, 1.34) | 0.063 |
| Analysis on indigenous populations only | ||
| WHO regions | ||
| SEAR | 1.00 | |
| WPR | 1.05 (0.92, 1.21) | 0.433 |
| WHO Mortality Strata | ||
| B | 1.00 | |
| D | 1.003 (0.90, 1.12) | 0.963 |
| Countries | ||
| Thailand | 1.00 | |
| Vietnam | 0.98 (0.76, 1.28) | 0.896 |
| Malaysia | 1.07 (0.82, 1.40) | 0.601 |
| India | 1.04 (0.85, 1.26) | 0.704 |
| Year of data collection | ||
| 1960–1980 | 1.00 | |
| 1981–2000 | 0.844 (0.64,1.11) | 0.223 |
| 2001–2020 | 0.82 (0.63, 1.08) | 0.158 |
| Diagnostic methods | ||
| Microscopy | 1.00 | |
| Enlarged spleen | 1.25 (1.02, 1.53) | 0.035 |
| PCR | 1.00 (0.90, 1.12) | 0.954 |
Note: Bivariate meta-regression analysis was only undertaken where there were 3 or more data sets
The pooled prevalence of malaria across minority indigenous participants was 19.9% (95% CI 15.9, 24.2), ranging from 0.5% (95% CI 0.1, 2.8) [92] to 85.9% (95% CI 79.7, 90.4)[93]. These data are represented in a Forest Plot (Figure 5). Where the species of plasmodium was identified by the study, the most prevalent was Plasmodium falciparum (12.9%, 95% CI 9.4, 16.9) followed by Plasmodium knowlesi (7.5%, 95% CI 5.1, 11.0) and Plasmodium vivax (4.8%, 95% CI 3.2, 6.6).
Figure 5.
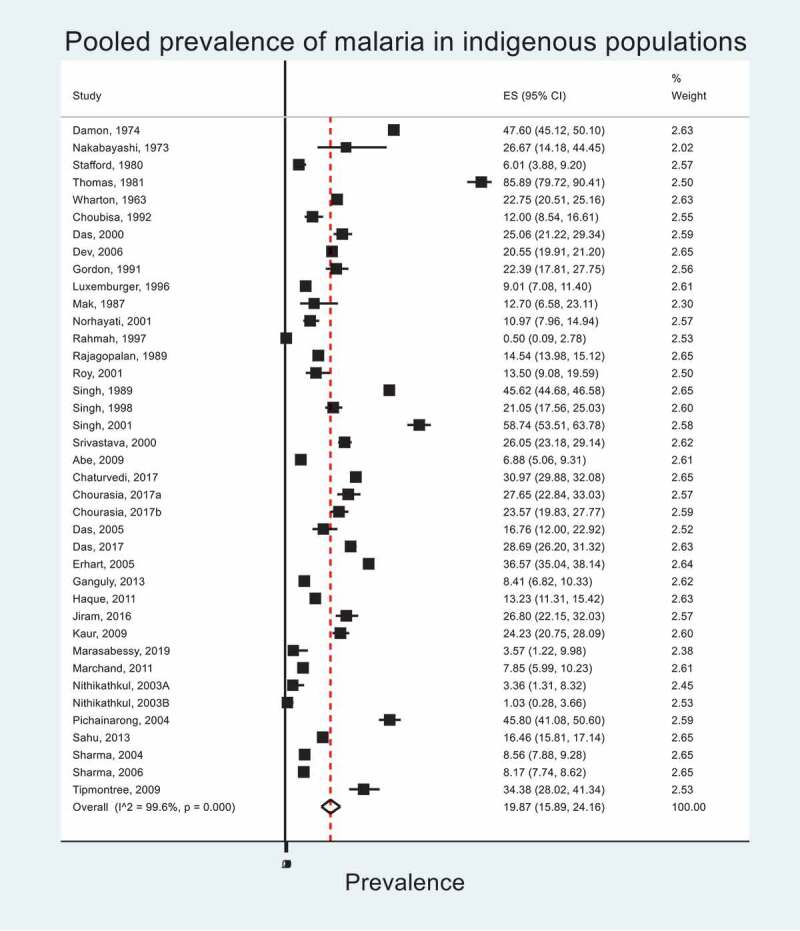
Pooled prevalence of malaria within minority indigenous study populations. The forest plot shows overall effect sizes (ES) and their 95% confidence intervals (CI). I^2 statistic describes the percentage of variation due to heterogeneity.
Across the four studies [59–62] that undertook a comparison between population groups, the prevalence of malaria was 21.5% (95% CI 7.8, 39.4) in minority indigenous people and 8.2% (95% CI 4.9, 12.2) in the non ‘minority indigenous’ population. The difference was not significant at the 5% level, but only marginally not so (p = 0.06), with an odds ratio of 1.15 (95% CI 0.99, 1.34).
Prevalence of malaria in minority indigenous populations was found not to be significantly different for the regions (WPR and SEAR), nor for the mortality strata, country of study, or year of data collection.
The difference in malaria prevalence between studies using microscopy 17.2% (95% CI 13.2, 21.6) and spleen palpitation (40.2% (95% CI 23.9, 57.7)) was found to be significant (p = 0.035).
Discussion
This systematic review highlights the paucity of TB data for minority indigenous populations within the high TB burden countries of the SEAR and WPR as defined by the WHO. From these high TB burden countries, data were only available for India. From the studies that are available, no improvement in disease prevalence was observed over time. The disease is a global problem that continues to prevail across all mortality strata.
The review only found four studies for each disease that undertook a direct comparison of disease prevalence between minority indigenous and other population groups. Based on the data from these four studies, there was no difference in TB prevalence between the population groups. The literature is conflicting regarding the impact of indigenous status on TB prevalence [13] highlighting the need for further research. It has been suggested that the isolation of some tribal communities from cultural contact has provided a safeguard from TB disease [58,97]. Where disease prevalence is comparable between population groups, research has shown indigenous populations to be at an increased risk of TB as they transition to a more modern lifestyle[39]. The risk factors associated with lifestyle transition include increased exposure to both the disease and its proximate determinants [14,39,98].
The review identified a high prevalence of malaria among minority indigenous peoples and comparative studies showed these populations to be at greater risk of disease relative to other groups (although marginally not statistically significant). The environments that minority indigenous people inhabit put them at increased risk of infection with malaria [59] and due to their geographic isolation, these populations can present one of the last barriers to disease elimination[99]. The human population interface with alternate hosts of zoonotic Plasmodium spp., may also impact the prevalence of disease. Notably P.knowlesi, a zoonotic malaria parasite, was the second most prevalent amongst study participants, ahead of P.vivax. The review includes a study published in 2016 showing a high prevalence of malaria in minority indigenous peoples of Malaysia, a country which was classified as malaria free in 2017[100]. This finding maybe due to the exclusion of zoonotic species from the definition of ‘malaria free’[101] and although the definition is complex[102], data on all Plasmodium spp., infections will be required to effectively combat the disease.
Although light microscopy is the recommended gold standard for malarial parasite detection[103], its ability to detect asymptomatic infections is low in comparison to molecular techniques[104]. Data from the systematic review showed a wide range in malaria prevalence across the diagnostic methods. Although splenomegaly has many potential causes and low sensitivity for a definitive malaria diagnosis, the results of the review recommend further diagnostics be used when an enlarged spleen is identified in malaria endemic areas.
The review demonstrated high heterogeneity in the prevalence of TB and malaria between studies and within and across co-variates. This variation in disease prevalence highlights the need for targeted and relevant data to inform effective control strategies. The review identified a paucity of data for minority indigenous populations in countries that report a high prevalence of infection across their total population. Where studies were available, the data were often historic making current conclusions difficult to draw.
Although progress has been made in reducing the prevalence of these diseases over recent decades, achievements may be derailed by the Coronavirus Disease 2019 (COVID-19) pandemic as control and treatment programmes are disrupted and resources are re-allocated. [105–108] Modeling suggests that over a five-year period in high TB and malaria settings, the COVID-19 pandemic could result in a 20% and 36% increase in TB and malaria deaths respectively[109]. To date empirical evidence regarding the impact of the COVID-19 pandemic on TB and malaria is limited [106,110].The interrelationship between the diseases is geospatially and temporally complex but the pandemic is likely to further exacerbate the TB and malaria epidemics in vulnerable population groups [106,110,111].
There were several limitations to the current study. Publication bias and reliance on the use of secondary data are limitations of the systematic review process. Due to resource constraints, the review restricted studies to those published in English. Studies on small sample populations may decrease the accuracy of estimating disease prevalence. The implementation of treatment and intervention programs have not been taken into consideration, which may impact disease prevalence over time. There is no universal definition of minority indigenous peoples, and each country has its own definition.
The review shows the prevalence of malaria to be higher in minority indigenous than comparative populations, but for there to be no difference for TB. The reason for this finding may be the limited number of comparative studies and the relatively small size of the study population groups[13]. The different findings for TB and malaria, may also be partly attributable to the very different ecologies of the two diseases, and how these ecologies have interfaced with indigenous lifestyles over time. The year of data collection for the comparative TB studies may have impacted the findings of the systematic review. Recent results from countries that disaggregate data by ethnicity, show indigenous populations to carry a significant and disproportionate burden of TB[112]. Time may be an important factor as increased exposure of indigenous people to the social and proximate determinants of the disease occurs as they move away from their traditional lifestyles[14].
The results show however, that further research and current data are required, if the burden of TB and malaria are to be accurately quantified in vulnerable populations and appropriate and effective interventions are to be developed.
Conclusions
The review shows there to be a paucity of recent data on TB and malaria prevalence within minority indigenous populations of the SEAR and WPR, despite the significant burden of these diseases within these regions. If SDG 3.3 is to be achieved, accurate and current data on the prevalence of TB and malaria within vulnerable population groups is required.
Acknowledgment(s)
Not applicable
Appendix 1: Summary of systematic review search terms
| Descriptor | Search Terms |
|---|---|
| TB terms | Tuberculosis OR TB OR ‘Mycobacterium tuberculosis’ OR |
| Malaria terms | malaria* OR plasmodi* AND |
| ∆ Countries of SEAR and WPR | Indonesia OR ‘Sri Lanka’ OR Ceylon OR Thailand OR Timor* OR Bangladesh OR Bhutan OR ‘Democratic People’s Republic of Korea’ OR India OR Maldives OR Myanmar OR Burma OR Nepal OR Australia OR Brunei OR Japan OR ‘New Zealand’ OR Cambodia OR China OR ‘Cook Islands’ OR Fiji OR Kiribati OR Lao* OR Malaysia OR ‘Marshall Islands’ OR Micronesia OR Mongolia OR Nauru OR Niue OR Palau OR ‘Papua New Guinea’ OR Philippines OR ‘Republic of Korea’ OR Samoa OR ‘Solomon Islands’ OR Tonga OR Tuvalu OR Vanuatu OR Vietnam AND |
| α Indigenous terms | Indigenous OR aborigin* OR native OR ‘first nation*’ OR ‘ethnic group’ OR tribal OR tribe OR autochthonous |
∆ Countries within the SEAR and WPR were defined according to the WHO Global Burden of Disease (GBD) regional classification system [53]. Singapore was excluded from the search as it does not have any minority indigenous populations according to the definition used in this review.
α In addition to these indigenous terms, those relevant to each country as derived from the World Directory Listing of Minorities and Indigenous People [113]; Native Planet – Indigenous Mapping [114] and International Working Group on Indigenous Affairs [24], were included. Studies were included if populations were not on the search criteria list, but the author identified them as minority indigenous groups.
Appendix 2: Quality Assessment (QA) based on modified Newcastle-Ottawa QA Scale
| # | References | Study Population 1 = The study population is clearly defined 0 = The study population is not clearly defined | Representativeness of the sample2 = Study sample is representative of the study population (all subjects or random sampling)1 = Study sample comprises a select group of the study population (nonrandom sampling)0 = No description of the sampling strategy. | Ascertainment of specimen collection methods1 = The study clearly defines specimen collection methodologies 0 = The study does not detail specimen collection methodologies | Sample size1 = Justified and satisfactory (sample size and power calculation included) 0 = Not justified | Non-respondents1 = Comparability between respondents and non-respondents characteristics are established. 0 = No description of the response rate or the characteristics of the responders and the non-responders. | Impact of Bias (selection bias, measurement bias, participant reporting, confounders)1 = Where relevant, the study acknowledges and mitigates for potential bias. When comparisons are made between different study populations results are adjusted for confounders0 = Where appropriate, the study does not acknowledge or mitigate for potential bias. When comparisons are made between different study populations results are not adjusted for confounders | Assessment of the outcome (TB or Malaria infection) 1 = Objective diagnostic methodology with units of measurement and /or definitions 0 = No definitive diagnosis or self report | Statistical analysis1 = The statistical method used is clearly described and appropriate for the analysis undertaken. Where comparisons are made between population groups, the measurement of the association is presented, including confidence intervals and the probability level (p value) 0 = The statistical test is inappropriate/not described/incomplete | Total Score |
| MALARIA STUDIES | ||||||||||
| 1 | Abe, 2009 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 2 | Chaturvedi, 2017 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 3 | Choubisa, 1992 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 4 | Chourasia, 2017a | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 5 | Chourasia, 2017b | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 6 | Damon, 1974 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 7 | Das, 2000 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| 8 | Das, 2005 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| 9 | Das, 2017 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 10 | Dev, 2006 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 11 | Erhart, 2005 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| 12 | Ganguly, 2013 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 13 | Gordon, 1991 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 14 | Haque, 2011 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 15 | Jiram, 2016 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 16 | Kaur, 2009 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 17 | Luxemburger, 1996 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 18 | Mak, 1987 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 19 | Marasabessy, 2019 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 20 | Marchand, 2011 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 21 | Nakabayashi, 1973 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 22 | Nithikathkul, 2003A | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 23 | Nithikathkul, 2003B | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| 24 | Norhayati, 2001 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 25 | Pichainarong, 2004 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| 26 | Rahmah, 1997 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 27 | Rajagopalan, 1989 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 28 | Roy, 2001 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 29 | Sahu, 2013 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| 30 | Sharma, 2004 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 31 | Sharma, 2006 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 32 | Singh, 1989 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 33 | Singh, 1998 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 34 | Singh, 2001 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 35 | Srivastava, 2000 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 36 | Stafford, 1980 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 37 | Thomas, 1981 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 38 | Tipmontree, 2009 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| 39 | Wharton, 1963 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| TB STUDIES | ||||||||||
| 1 | Bhat, 2009 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| 2 | Bhat, 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| 3 | Bhat, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 4 | Bolton, 1975 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 5 | Chakma, 1996 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| 6 | Damon, 1974 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 7 | Datta, 2001 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 8 | Haddad, 2012 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 9 | Hussain, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| 10 | Kashyap, 2013 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| 11 | Kerketta, 2009 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 12 | King, 1951 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 13 | Macken, 1952 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 14 | Murhekar, 2004 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 15 | Purty, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| 16 | Rao, 2010A | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| 17 | Rao, 2010B | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| 18 | Rao, 2011 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 19 | Rao, 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 20 | Rao, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 21 | Roy, 1969 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| 22 | Sharma, 2010 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 23 | Vyas, 2019 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| 24 | Yano, 1974 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
The average QA total score across the malaria studies was 5.5 and 6.0 across the TB studies out of a total possible score of 9
List of Abbreviations
AIDS: Acquired Immunodeficiency Syndrome; CI: Confidence Interval; COVID-19: Coronavirus Disease 2019; ES: Effect Size; GBD: Global Burden of Disease; HIV: Human Immunodeficiency Virus; IFS: Indirect Fluorescent Antibody; PCR: Polymerase Chain Reaction; PRISMA: Preferred Reporting Items for Systematic Review and Meta-Analyses; QA: Quality Assessment; QCRI: Qatar Computing Research Institute; RDT: Rapid Diagnostic Test; SDG: Sustainable Development Goal; SEAR: South-East Asia Region; TB.. Tuberculosis; UN: United nations; WHO: World Health Organization; WPR: Western Pacific Region.
Declarations
Ethical approval and consent to participate
Ethics approval and participant consent was not required for this study as it was based upon a review of published work.
Consent for publication
Not applicable
Availability of Data and Materials
All required information is available in the manuscript and supporting documentation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].United Nations . Sustainable Development Goals Officially Adopted by 193 Countries [Internet]. 2015. [cited 2020 Oct 10]. Available from: http://www.un.org.cn/info/6/620.html
- [2].World Health Organization . Sustainable Development Goals The goals within a goal: health targets for SDG 3 [Internet]. n.d.. [cited 2020 Oct 10]. Available from: https://www.who.int/sdg/targets/en/
- [3].World Health Organization . Tuberculosis Key Facts [Internet]. 2020. cited 2020 Oct 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- [4].World Health Organization . Use of high burden country lists for TB by WHO in the post-2015 era. Geneva: World Health Organization; 2015. [Google Scholar]
- [5].World Health Organization . World Malaria Report 2019; 2019. Report No.: Licence: CC BY-NC-SA 3.0 IGO.
- [6].Sinka ME. Global distribution of the dominant vector species of malaria. In: Anopheles mosquitoes-New insights into malaria vectors. Chapter 4, 109-143. Sylvie Manguin: IntechOpen; 2013. [Google Scholar]
- [7].Sachs J, Malaney P.. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. [DOI] [PubMed] [Google Scholar]
- [8].Centres for Disease Control and Prevention. Malaria’s Impact Worldwide [Internet]. n.d.. [cited 2020 Nov 10]. Available from: https://www.cdc.gov/malaria/malaria_worldwide/impact.html
- [9].Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? A review of the literature. Trop Med Int Health. 2005;10(10):1047–1059. [DOI] [PubMed] [Google Scholar]
- [10].The Lancet . Tackling poverty in tuberculosis control Elsevier. ;The lancet. 2005 Vol366(9503), p2063. [DOI] [PubMed] [Google Scholar]
- [11].The World Bank . Partnering with Indigenous Peoples and Ethnic Minorities Through Community Driven Development [Internet]. 2016. [cited 2019 Jul 1].Available from: https://www.worldbank.org/en/news/feature/2016/05/11/partnering-with-indigenous-peoples-and-ethnic-minorities-through-community-driven-development
- [12].Assembly of First Nations . A Strategic Framework for Action on Tuberculosis (TB) Control in Indigenous Communities. Global Indigenous STOP-TB Experts Meeting 12-14 Nov 2008, Toronto, ON. E/CN.19/2009/CRP.5 21. Inuit Tapiriit Kanatami; 2009. [Google Scholar]
- [13].Tollefson D, Bloss E, Fanning A, et al. Burden of tuberculosis in indigenous peoples globally: a systematic review [Review article]. Int J Tuberc Lung Dis. 2013;17(9):1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cormier M, Schwartzman K, N’Diaye DS, et al. dos Santos AM, Gaspar J, et al. Proximate Determinants of Tuberculosis in Indigenous Peoples Worldwide: A Systematic Review. The Lancet Global Health 2019;7(1):e68–e80. [DOI] [PubMed] [Google Scholar]
- [15].Hotez PJ. Aboriginal populations and their neglected tropical diseases. PLoS Negl Trop Dis. 2014;8(1):e2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mendes AM, MdS L, Maciel AGP, et al. Malaria among indigenous peoples on the Brazil-French Guiana border, 2007-2016: a descriptive study. Epidemiologia E servicos de saude: Revista Do Sistema Unico de Saude Do Brasil. 2020;29(2):e2019056. [DOI] [PubMed] [Google Scholar]
- [17].Davy C, Harfield S, McArthur A, et al. Access to primary health care services for Indigenous peoples: a framework synthesis. Int J Equity Health. 2016;15(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].The Lancet Editorial . Indigenous Health: a Worldwide Focus. The Lancet. 2016;388(10040):104. DOI: 10.1016/S0140-6736(16)31020-0. [DOI] [PubMed] [Google Scholar]
- [19].Amnesty International . Indigenous Peoples [Internet]. n.d.. [cited 2020 Oct 10]. Available from: https://www.amnesty.org/en/what-we-do/indigenous-peoples/
- [20].United Nations General Assembly . Transforming our World: the 2030 Agenda for Sustainable Development. 2015. United Nations. sustainabledevelopment.un.org [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gilmour B, Alene KA, Clarke NE, et al. The Prevalence of Tuberculosis, Malaria and Soil Transmitted Helminth Infection in Minority Indigenous People of South East Asia and the Western Pacific: protocol for a Systematic Review and Meta-Analysis. Systematic Reviews. 2021;10(1):1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].International Work Group for Indigenous Affairs . Who we are: indigenous Peoples in Asia; 2009. [cited 2019 July 10]. Available from: https://www.iwgia.org/en/resources/publications/306-briefings/3127-who-we-are-indigenous-peoples-in-asia-
- [25].World Health Organization . Indigenous Populations [Internet]. n.d.. [cited 2019 July 07]. Available from: https://www.who.int/topics/health_services_indigenous/en/
- [26].United Nations . Factsheet: who are indigenous peoples? [Internet]. n.d.. [cited 2019 July 07].Available from: https://www.un.org/esa/socdev/unpfii/documents/5session_factsheet1.pdf
- [27].The World Bank . Partnering with Indigenous Peoples and Ethnic Minorities Through Community-Driven Development [Internet]. 2016. [cited 2019 July 01].Available from: https://www.worldbank.org/en/news/feature/2016/05/11/partnering-with-indigenous-peoples-and-ethnic-minorities-through-community-driven-development
- [28].The World Bank . Indigenous Peoples [Internet]. n.d.. [cited 2020 Feb 24].Available from: https://www.worldbank.org/en/topic/indigenouspeoples
- [29].Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374(9683):65–75. cited 2019/07/28]. [DOI] [PubMed] [Google Scholar]
- [30].The Science of Man in the World Crisis. New York (US): Columbia University Press; 1945. Linton, Ralph Ed. [Google Scholar]
- [31].Schratz A, Pineda MF, Reforma LG, et al. Neglected diseases and ethnic minorities in the Western Pacific Region exploring the links. Adv Parasitol. 2010;72:79–107. [DOI] [PubMed] [Google Scholar]
- [32].Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute oxford. Asp; 2011. [Google Scholar]
- [33].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- [34].Ioannidis JP, Stanley TD, Doucouliagos H. The power of bias in economics research. UK: Oxford University Press Oxford; 2017. [Google Scholar]
- [35].Bhat J, Rao V, Sharma R, et al. Investigation of the risk factors for pulmonary tuberculosis: a case–control study among Saharia tribe in Gwalior district, Madhya Pradesh, India. Indian J Med Res. 2017;146(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chakma T, Vinay Rao P, Pall S, et al. Survey of pulmonary tuberculosis in a primitive tribe of Madhya Pradesh. Indian Journal of Tuberculosis. 1996;43:85–90. [Google Scholar]
- [37].Haddad S, Mohindra KS, Siekmans K, et al. “Health divide” between indigenous and non-indigenous populations in Kerala, India: population based study. BMC Public Health. 2012;12(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yano K. Pulmonary tuberculosis in a rural area of Sarawak, Malaysia. Southeast Asian J Trop Med Public Health. 1974;5(3):417–423. [PubMed] [Google Scholar]
- [39].Bhat J, Rao VG, Gopi PG, et al. Prevalence of pulmonary tuberculosis amongst the tribal population of Madhya Pradesh, central India. Int J Epidemiol. 2009;38(4):1026–1032. [DOI] [PubMed] [Google Scholar]
- [40].Bhat J, Rao V, Yadav R, et al. Situation of drug resistant tuberculosis in Saharia tribe of central India. Indian J Med Res. 2015;141(5):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Datta M, Radhamani M, Sadacharam K, et al. Survey for tuberculosis in a tribal population in North Arcot District. Int J Tuberc Lung Dis. 2001;5(3):240–249. [PubMed] [Google Scholar]
- [42].Hussain T, Tripathy SS, Das S, et al. Prevalence, risk factors and health seeking behaviour of pulmonary tuberculosis in four tribal dominated districts of Odisha: comparison with studies in other regions of India. PloS One. 2020;15(4):e0227083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kashyap RS, Nayak AR, Gaherwar HM, et al. Laboratory investigations on the diagnosis of tuberculosis in the malnourished tribal population of melghat, India. PLoS One. 2013;8(9):e74652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kerketta AS, Bulliyya G, Babu BV, et al. Health status of the elderly population among four primitive tribes of Orissa, India: a clinico-epidemiological study. Zeitschrift für Gerontologie und Geriatrie. 2009;42(1):53–59. [DOI] [PubMed] [Google Scholar]
- [45].Murhekar MV, Kolappan C, Gopi P, et al. Tuberculosis situation among tribal population of Car Nicobar, India, 15 years after intensive tuberculosis control project and implementation of a national tuberculosis programme. Bull World Health Organ. 2004;82(11):836–843. [PMC free article] [PubMed] [Google Scholar]
- [46].Purty AJ, Mishra AK, Chauhan RC, et al. Burden of pulmonary tuberculosis among tribal population: a cross-sectional study in tribal areas of Maharashtra, India. Indian J Commun Med. 2019;44(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rao V, Bhat J, Yadav R, et al. Prevalence of pulmonary tuberculosis among the Bharia, a primitive tribe of Madhya Pradesh, central India. Int J Tuberc Lung Dis. 2010A;14(3):368–370. [PubMed] [Google Scholar]
- [48].Rao VG, Gopi PG, Bhat J, et al. Pulmonary tuberculosis: a public health problem amongst the Saharia, a primitive tribe of Madhya Pradesh, Central India. Inter J Infect Dis. 2010;14(8):e713–e716. [DOI] [PubMed] [Google Scholar]
- [49].Rao VG, Gopi P, Bhat J, et al. Selected risk factors associated with pulmonary tuberculosis among Saharia tribe of Madhya Pradesh, central India. Eur J Public Health. 2012;22(2):271–273. [DOI] [PubMed] [Google Scholar]
- [50].Rao V, Bhat J, Yadav R, et al. Pulmonary tuberculosis - a health problem amongst Saharia tribe in Madhya Pradesh. Indian J Med Res. 2015;141(5):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rao V, Bhat J, Yadav R, et al. Declining tuberculosis prevalence in Saharia, a particularly vulnerable tribal community in Central India: evidences for action. BMC Infect Dis. 2019;19(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vyas A, Creswell J, Codlin A, et al. Community-based active case-finding to reach the most vulnerable: tuberculosis in tribal areas of India. Int J Tuberc Lung Dis. 2019;23(6):750–755. [DOI] [PubMed] [Google Scholar]
- [53].World Health Organization . Global Burden of Disease Regions used for WHO-CHOICE Analyses [Internet]. n.d.. [cited 2017 Jun 07]. Available from: https://www.who.int/choice/demography/regions/en/
- [54].King A, Edwards G, Gibson P. A survey of Australian aborigines for pulmonary tuberculosis. Med J Aust. 1951;1(26):934–935. [DOI] [PubMed] [Google Scholar]
- [55].Macken FM. Initial Comments on Tuberculosis Case-Finding Survey among Australian Aboriginals (State of Queensland). Tubercle. 1952;33(12):376–381. [Google Scholar]
- [56].Bolton J, Snelling M. Review of tuberculosis among the Orang AsIi (aborigines) in West Malaysia from 1951-1970. Medical Journal of Malaysia. 1975;30(1):10–29. [PubMed] [Google Scholar]
- [57].Roy R. TUBERCULOSIS IN MURUTS OF PENSIANGAN IN SABAH. Med J Aust. 1969;1(17):842–848. [DOI] [PubMed] [Google Scholar]
- [58].Damon A. Human ecology in the Solomon Islands: biomedical observations among four tribal societies. Hum Ecol. 1974;2(3):191–215. [Google Scholar]
- [59].Erhart A, Thang ND, Van Ky P, et al. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Haque U, Sunahara T, Hashizume M, et al. Malaria prevalence, risk factors and spatial distribution in a hilly forest area of Bangladesh. PLoS One. 2011;6(4):e18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mak J, Lim P, Tan M, et al. Parasitological and serological surveys for malaria among the inhabitants of an aborigine village and an adjacent Malay village. Acta Trop. 1987;44(1):83–89. [PubMed] [Google Scholar]
- [62].Nakabayashi T, Tsukamoto M, Motomura I, et al. Epidemiologic survey on malaria in some rural areas, especially in Palawan Island, of the Philippines. 熱帯医学 Tropical Medicine. 1973;15(3):154–168. [Google Scholar]
- [63].Marasabessy NB, Soedirham O, Dachlan YP. Association of Hunting Behavior and Malaria incidence: a cross Sectional Study on Nuaulu Tribe community in Mesoendemic Area of Malaria PAGE 285. Indian J Public Health Res Dev. 2019;10(9):792–796. [Google Scholar]
- [64].Stafford E, Dennis D, Masri S, et al. Intestinal and blood parasites in the Torro Valley, Central Sulawesi, Indonesia. Southeast Asian J Trop Med Public Health. 1980;11(4):468–472. [PubMed] [Google Scholar]
- [65].Luxemburger C, Thwai KL, White N, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90(2):105–111. [DOI] [PubMed] [Google Scholar]
- [66].Nithikathkul C, Changsap B, Wannapinyosheep S, et al. Parasitic infections among Karen in Kanchanaburi province. Western Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2003A;34:86–89. [PubMed] [Google Scholar]
- [67].Nithikathkul C, Polseela P, Poodendan W, et al. Malaria and enterobiasis among Karen long-neck tribe in Mae Hong Son Province. Southeast Asian J Trop Med Public Health. 2003B;34:25–28. [PubMed] [Google Scholar]
- [68].Pichainarong N, Chaveepojnkamjorn W. Malaria infection and life-style factors among hilltribes along the Thai-Myanmar border area, northern Thailand. Southeast Asian journal of tropical medicine and public health, 2004;35(4):834-839. [PubMed] [Google Scholar]
- [69].Tipmontree R, Fungladda W, Kaewkungwal J, et al. Migrants and malaria risk factors: a study of the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2009;40(6):1148. [PubMed] [Google Scholar]
- [70].Chaturvedi N, Krishna S, Bharti PK, et al. Prevalence of afebrile parasitaemia due to Plasmodium falciparum & P. vivax in district Balaghat (Madhya Pradesh): implication for malaria control. Indian J Med Res. 2017;146(2):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Choubisa S, Choubisa L. Prevalence of intestinal and malaria parasitic infections in tribal students of Dungarpur (Rajasthan). Indian Journal of Parasitology. 1992;16(2):101–103. [Google Scholar]
- [72].Chourasia M, Raghavendra K, Bhatt R, et al. Burden of asymptomatic malaria among a tribal population in a forested village of central India: a hidden challenge for malaria control in India. Public Health. 2017A;147:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chourasia MK, Raghavendra K, Bhatt RM, et al. Additional burden of asymptomatic and sub-patent malaria infections during low transmission season in forested tribal villages in Chhattisgarh, India. Malar J. 2017;16(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Das N, Bhuyan M, Das S. Entomological and epidemiological studies on malaria in Rajmahal range, Bihar. Indian Journal of Malariology. 2000;37(3–4):88–96. [PubMed] [Google Scholar]
- [75].Das M, Joshi H, Verma A, et al. Malaria among the Jarawas, a primitive and isolated tribe on the Andaman Islands, India. Ann Trop Med Parasitol. 2005;99(6):545–552. [DOI] [PubMed] [Google Scholar]
- [76].Das MK, Prajapati BK, Tiendrebeogo RW, et al. Malaria epidemiology in an area of stable transmission in tribal population of Jharkhand, India. Malar J. 2017;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dev V, Phookan S, Sharma V, et al. Malaria parasite burden and treatment seeking behavior in ethnic communities of Assam, Northeastern India. J Infect. 2006;52(2):131–139. [DOI] [PubMed] [Google Scholar]
- [78].Ganguly S, Saha P, Guha SK, et al. High prevalence of asymptomatic malaria in a tribal population in eastern India. J Clin Microbiol. 2013;51(5):1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rajagopalan P, Pani S, Das P, et al. Malaria in Koraput district of Orissa. Indian J Pediatr. 1989;56(3):355–364. [DOI] [PubMed] [Google Scholar]
- [80].Roy A, Tyagi P, Sharma SK. Serological appraisal of malaria status in tribal area of Orissa, India. Indian Journal of Malariology. 2001;38(3–4):84–90. [PubMed] [Google Scholar]
- [81].Sahu SS, Gunasekaran K, Vanamail P, et al. Persistent foci of falciparum malaria among tribes over two decades in Koraput district of Odisha State, India. Malar J. 2013;12(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sharma S, Tyagi P, Padhan K, et al. Malarial morbidity in tribal communities living in the forest and plain ecotypes of Orissa, India. Ann Trop Med Parasitol. 2004;98(5):459–468. [DOI] [PubMed] [Google Scholar]
- [83].Sharma SK, Tyagi PK, Padhan K, et al. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100(10):917–925. [DOI] [PubMed] [Google Scholar]
- [84].Singh N, Sharma V, Mishra A, et al. Bio-environmental control of malaria in a tribal area of Mandla district, Madhya Pradesh, India. Indian Journal of Malariology. 1989;26(2):103–120. [PubMed] [Google Scholar]
- [85].Singh N, Saxena A, Chand S, et al. Studies on malaria during pregnancy in a tribal area of central India (Madhya Pradesh). Southeast Asian J Trop Med Public Health. 1998;29(1):10–17. [PubMed] [Google Scholar]
- [86].Singh N, Shukla M. An assessment of the usefulness of a rapid immuno-chromatographic test, ”Determine™ malaria pf” in evaluation of intervention measures in forest villages of central India. BMC Infect Dis. 2001;1(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Srivastava H, Yadav R. Malaria outbreak in a tribal area of Gujarat state, India. Southeast Asian J Trop Med Public Health. 2000;31(2):219–224. [PubMed] [Google Scholar]
- [88].Gordon DM, Davis DR, Lee M, et al. Significance of circumsporozoite-specific antibody in the natural transmission of Plasmodium falciparum, Plasmodium vivax, and Plasmodium malariae in an aboriginal (Orang Asli) population of central peninsular Malaysia. Am J Trop Med Hyg. 1991;45(1):49–56. [DOI] [PubMed] [Google Scholar]
- [89].Jiram AI, Hisam S, Reuben H, et al. Submicroscopic evidence of the simian malaria parasite, Plasmodium knowlesi, in an orang asli community. SE Asian J Trop Med Public Health. 2016;47:591–599. [Google Scholar]
- [90].Kaur G. Prevalence of clinical malaria among an Orang Asli community in Malaysia. Southeast Asian J Trop Med Public Health. 2009;40(4):665. [PubMed] [Google Scholar]
- [91].Noryahati M, Rohani A, Hayati MN, et al. Clinical features of malaria in Orang Asli population in Pos Piah, Malaysia. Medical Journal of Malaysia. 2001;56(3):271–274. [PubMed] [Google Scholar]
- [92].Rahmah N, Ariff R, Abdullah B, et al. Parasitic infections among aborigine children at Post Brooke, Kelantan, Malaysia. Med J Malaysia. 1997;52(4):412–415. [PubMed] [Google Scholar]
- [93].Thomas V, Hock SK, Leng YP. Seroepidemiology of malaria: age-specific pattern of Plasmodium falciparum antibody, parasite and spleen rates among children in an endemic area in peninsular Malaysia. Trop Doct. 1981;11(4):149–154. [DOI] [PubMed] [Google Scholar]
- [94].Wharton R, Laing A, Cheong W. Studies on the distribution and transmission of malaria and filariasis among aborigines in Malaya. Ann Trop Med Parasitol. 1963;57(2):235–254. [DOI] [PubMed] [Google Scholar]
- [95].Abe T, Honda S, Nakazawa S, et al. Risk factors for malaria infection among ethnic minorities in Binh Phuoc, Vietnam. Southeast Asian J Trop Med Public Health. 2009;40(1):18. [PubMed] [Google Scholar]
- [96].Marchand RP, Culleton R, Maeno Y, et al. Co-infections of Plasmodium knowlesi, P. Falciparum, and P. Vivax among Humans and Anopheles Dirus Mosquitoes, Southern Vietnam. Emerging Infectious Diseases 2011;17(7):1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Levy S. The Evolution of Tuberculosis: genetic analysis offers new insight on the spread of an ancient disease. BioScience. 2012;62(7):625–629. [Google Scholar]
- [98].Gracy M, King M. Indigenous health Part 1: determinants and disease patterns. In: The Lancet. 2009;374(9683): p. 65–75. [DOI] [PubMed] [Google Scholar]
- [99].Leandro-Reguillo P, Thomson-Luque R, Monteiro WM, et al. Urban and architectural risk factors for malaria in indigenous Amazonian settlements in Brazil: a typological analysis. Malar J. 2015;14(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].World Health Organization . Malaria Country Profiles [Internet]. 2018. [cited 2020 Nov 13]. Available from: https://www.who.int/malaria/publications/country-profiles/en/
- [101].Organization WH . A framework for malaria elimination. Geneva. World Health Org. 2017. Licence: CC BY-NC-SA 3.0 IGO [Google Scholar]
- [102].Schapira A. Malaria elimination- definitions. Criteria and Possible Variants. 2013. [cited 2020 Dec]. who.int/malaria/mpac/malaria_elimination_definitions_criteria_presentation.pdf [Google Scholar]
- [103].Mathison BA, Pritt BS. Update on malaria diagnostics and test utilization. J Clin Microbiol. 2017;55(7):2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhao Y, Zhao Y, Lv Y, et al. Comparison of methods for detecting asymptomatic malaria infections in the China–Myanmar border area. Malar J. 2017;16(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Weiss DJ, Bertozzi-Villa A, Rumisha SF, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. 2020;21(1):59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rogerson SJ, Beeson JG, Laman M, et al. Identifying and combating the impacts of COVID-19 on malaria. BMC Med. 2020;18(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cilloni L, Fu H, Vesga JF, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28:100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].The Global Fund . Results Report 2020; 2020.
- [109].Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].McQuaid CF, Vassall A, Cohen T, et al. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25(6):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Alene KA, Wangdi K, Clements AC. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020;5(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bright A, Denholm J, Coulter C, et al. Tuberculosis notifications in Australia, 2015-2018. Communicable Diseases Intelligence (2018). 2020;44. [DOI] [PubMed] [Google Scholar]
- [113].Minority Rights Group International . World Directory of Minorities and Indigenous Peoples [Internet]. n.d.. [cited 2019 May15].Available from: https://minorityrights.org/directory/
- [114].Native Planet . Indigenous Mapping: ethnic Communities from Asia [Internet]. n.d.. [cited 2019 May 19]. Available from: https://www.nativeplanet.org/indigenous/ethnicdiversity/indigenous_data_asia.shtml
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All required information is available in the manuscript and supporting documentation.


