Abstract
Background
Epidemiologic evidence of the effect of dietary selenium intake on stroke risk remains controversial. This study aimed to examine the cross-sectional correlation between dietary selenium intake and the risk of stroke in adults.
Materials and methods
We retrospectively analysed 39,438 participants from the National Health and Nutrition Examination Survey 2003–2018, aged 20–85 years. Participants were divided into quartiles depending on daily dietary selenium intake: quartile 1 (0–77 μg), quartile 2 (77–108 μg), quartile 3 (108–148 μg), and quartile 4 (148–400 μg). The dose-response relationship was assessed using the restricted cubic spline function.
Results
The adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of stroke were 0.70 (0.55, 0.88) for participants in quartile 2, 0.71 (0.53, 0.93) for quartile 3, and 0.61 (0.43, 0.85) for quartile 4 compared with that in quartile 1. p-Value for trend through quartiles was .007. A non-linear negative correlation between dietary selenium intake and stroke was observed in the threshold effect analysis and restricted cubic spline function (p-value for non-linearity < .001). An initial decrease in odds of stroke lower than 105 μg/day selenium intake (0.61 [0.44, 0.85], p = .004) was followed by a platform beyond 105 μg/day (0.97 [0.81, 1.16], p = .723). In the subgroup analysis, adjusted ORs (95% CIs) of stroke were 0.51 (0.36, 0.70) for female participants, 0.63 (0.40, 0.99) for participants with age <60 years, 0.63 (0.47, 0.85) for participants with poverty-income ratio < 2.14, 0.66 (0.50, 0.87) for participants with overweight and obesity, 0.66 (0.52, 0.84) for participants with hypertension, 0.72 (0.53, 0.97) for participants without diabetes, and 0.72 (0.56, 0.92) for non-anaemic participants.
Conclusions
Dietary selenium had a negative and non-linear correlation with the risk of stroke in adults. The correlation varied across different population subgroups.
KEY MESSAGES
Dietary selenium had a negative and non-linear correlation with the risk of stroke in adults.
Non-linear negative correlation trends were observed in subpopulations of females, age <60 years, poverty-income ratio <2.14, overweight and obesity, hypertension, non-diabetes, and non-anaemia.
Dietary selenium intake of approximately 105 μg per day has an optimum effect on stroke.
Keywords: Dietary selenium intake, stroke, negative correlation, non-linear model, adults, National Health And Nutrition Examination Survey
Introduction
Stroke is a significant cause of mortality and disability worldwide [1,2]. Millions of Americans experience a new or recurrent stroke every year, which can lead to long-term disability [1,2]. The global lifetime risk of stroke has increased from 22% to 24% over the past three decades [3]. With the ageing population, the burden of stroke continues to increase, especially in developing countries [3]. There are limited treatment options for patients that have undergone a stroke. Hence, there remains an urgent need to identify novel strategies for stroke prevention. Selenium, as an essential trace element, has recently attracted significant attention because of its beneficial effects on stroke risk [4,5].
Selenium, as selenocysteine, is a crucial component of selenoproteins, a class of proteins primarily involved in anti-oxidation and redox regulation [6,7]. Oxidative stress has recently been considered a critical pathophysiological mechanism in stroke [8]. Previous observational studies have reported the beneficial effects of selenium on cardiovascular diseases, including stroke [9,10]. However, secondary analyses in the Nutritional Prevention of Cancer (NPC) trial did not reveal any benefit of selenium supplementation on the risk of stroke [9,10].
Notably, epidemiological studies have mainly focussed on the benefits of circulating selenium levels but not on selenium intake. In the NPC trial, we believe selenium supplementation (200 μg/day) could be slightly high for stroke prevention [11]. Following the hypothesis that selenium supplementation has a threshold effect or “U” shape effect on the risk of stroke, we examined the dose-response correlation between dietary selenium intake and the risk of stroke in the National Health and Nutrition Examination Survey (NHANES) 2003–2018.
Materials and methods
Study population
The NHANES is a cross-sectional investigation that provides comprehensive information about the nutrition and health of the national population in the United States [12]. Data from eight consecutive NHANES with 2-year cycles (2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, 2017–2018) were collected. All participants provided informed consent, and the ethics approval was obtained from the research ethics review board at the National Centre for Health Statistics [13].
Exposure and outcomes
Dietary selenium intake from foods was calculated using the US Department of Agriculture's Food and Nutrient Database for Dietary Studies. Two non-consecutive days of intake data were available for each participant during 2003–2018. The first day’s data was collected at a mobile examination centre, and the second day’s data was collected over telephone 3–10 days later. Selenium intake from supplements reflects average daily selenium intake from non-prescription and prescription dietary supplements during the 30-day period before the survey date. In this analysis, the average selenium intake from foods and supplements was added together as dietary selenium intake. The tolerable upper level of selenium intake for adults is 400 μg [14]. Therefore, the upper limit of daily selenium intake was set at 400 μg in this study.
A questionnaire was used to record whether the patients had pre-existing medical conditions. Stroke was defined using self-reported history by asking the following question: "Has a doctor ever told you that you had a stroke?" The exclusion criteria were as follows: (1) individuals who refused to answer this question; (2) individuals who did not know if they ever had a stroke.
Covariates
Variables of interest obtained by questionnaires included basic information of participants on age (years), sex (male, %), race, education level, marital status (married, %), hypertension (self-reported), diabetes (self-reported), family poverty-income ratio (PIR), body mass index (BMI), smoking (smoked at least 100 cigarettes during their lifetime or not), alcohol use (consumed at least 12 alcoholic drinks per year or not), and physical activity (never, moderate, and vigorous). According to the analysis of previous related studies [15,16], the following covariates were included: the levels of total cholesterol, high-density lipoprotein-cholesterol (HDL-cholesterol), triglycerides, glycohemoglobin, and the daily intake of total energy and cholesterol from the diet. Haemoglobin and uric acid levels were included because of anaemia and hyperuricaemia association with stroke [17,18].
The PIR was calculated as family income divided by the poverty threshold specific to family size and the appropriate year and state. The patients were divided into two groups with a median of 2.14 for subgroup analysis. BMI was calculated as body weight divided by height squared, and the participants were categorised as normal weight (<25.0 kg/m2), overweight (25.0–29.9 kg/m2) and obesity (≥30.0 kg/m2) [19]. Vigorous physical activity was defined as activity that significantly increased breathing or heart rate, whereas moderate physical activity was defined as activity that slightly increased breathing rate. Haemoglobin levels were categorised into non-anaemia (≥12 g/dL) and anaemia (<12 g/dL).
Statistical analysis
The recommended sample weights were used in all analyses. The recommended 2-year sample weight for the 2003–2018 period was used to calculate the new 16-year sample weights for all participants.
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as numbers (percentage). Odds ratios (ORs) and 95% confidence intervals (95% CIs) of stroke were estimated with EmpowerStats software using multivariate logistic regression and a piece-wise linear regression model. Three adjusted models were developed: Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for education level, marital status, PIR, BMI, smoking, alcohol use, hypertension, diabetes, and physical activity based on Model 1. Model 3 was adjusted for levels of haemoglobin, uric acid, total cholesterol, HDL-cholesterol, triglyceride, glycohemoglobin, daily intake of total energy, and cholesterol from the diet based on Model 2. The dose-response relationship between dietary selenium intake and stroke was assessed using Stata version 15.0, using a restricted cubic spline function with four knots located at the 25th, 50th, 75th, and 99th percentiles, and p-value for non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0 [20]. Statistical significance was set at p < .05.
Results
Clinical characteristics of included participants
A total of 44,790 participants (aged 20–85 years) responded to the question, “Has a doctor ever told you that you had a stroke?” After excluding 68 participants who refused to answer this question or did not know whether they had a stroke, 941 participants who were pregnant, 4200 participants without information on dietary selenium intake, and 143 participants whose dietary selenium intake >400 μg/day, 39,438 participants were finally included in this study. Among the 39,438 participants, there are 38,695 participants providing information on selenium intake from foods and 8635 participants providing information on selenium intake from supplements. The characteristics of the included participants are shown as quartiles in Table 1.
Table 1.
Description of participants included in the present study.
| Quartiles of dietary selenium intake (100 μg/day) | Quartile1 (0.00–0.77) |
Quartile2 (0.77–1.08) |
Quartile3 (1.08–1.48) |
Quartile4 (1.48–4.00) |
p-Value |
|---|---|---|---|---|---|
| Case number | 9858 | 9861 | 9855 | 9864 | |
| Incidence of stroke | 584 (5.92%) | 411 (4.17%) | 339 (3.44%) | 256 (2.60%) | <.001 |
| Age, years | 53.07 ± 18.63 | 50.93 ± 18.14 | 49.28 ± 17.63 | 47.63 ± 16.95 | <.001 |
| Male, n (%) | 3014 (30.57%) | 4086 (41.44%) | 5281 (53.59%) | 7078 (71.76%) | <.001 |
| Race, n (%) | <.001 | ||||
| Mexican American | 1583 (16.06%) | 1624 (16.47%) | 1583 (16.06%) | 1472 (14.92%) | |
| Other Hispanic | 921 (9.34%) | 860 (8.72%) | 875 (8.88%) | 817 (8.28%) | |
| Non-Hispanic White | 4045 (41.03%) | 4367 (44.29%) | 4420 (44.85%) | 4482 (45.44%) | |
| Non-Hispanic Black | 2455 (24.90%) | 2106 (21.36%) | 1951 (19.80%) | 1929 (19.56%) | |
| Other Race | 854 (8.66%) | 904 (9.17%) | 1026 (10.41%) | 1164 (11.80%) | |
| Education level, n (%) | <.001 | ||||
| Less than 9th grade | 1549 (15.74%) | 1155 (11.72%) | 931 (9.46%) | 649 (6.58%) | |
| 9–11th grade | 1603 (16.28%) | 1411 (14.32%) | 1342 (13.63%) | 1227 (12.45%) | |
| High school graduate | 2414 (24.52%) | 2313 (23.48%) | 2210 (22.45%) | 2248 (22.81%) | |
| Some college or AA degree | 2661 (27.03%) | 2882 (29.25%) | 2970 (30.17%) | 3017 (30.61%) | |
| College graduate or above | 1617 (16.43%) | 2091 (21.22%) | 2392 (24.30%) | 2716 (27.55%) | |
| Married, n (%) | 4608 (46.78%) | 4971 (50.43%) | 5335 (54.17%) | 5402 (54.79%) | <.001 |
| Alcohol use, n (%) | 2359 (23.93%) | 2344 (23.77%) | 2093 (21.24%) | 1954 (19.81%) | <.001 |
| Smoking, n (%) | 4355 (44.18%) | 4372 (44.34%) | 4496 (45.62%) | 4704 (47.69%) | <.001 |
| Diabetes, n (%) | 1465 (14.86%) | 1321 (13.40%) | 1227 (12.45%) | 1101 (11.16%) | <.001 |
| Hypertension, n (%) | 3990 (40.47%) | 3662 (37.14%) | 3427 (34.77%) | 3330 (33.76%) | <.001 |
| Physical activity, n (%) | <.001 | ||||
| Never | 4215 (43.34%) | 3608 (36.87%) | 3153 (32.15%) | 2646 (26.88%) | |
| Moderate | 3018 (31.03%) | 3141 (32.10%) | 3088 (31.49%) | 2817 (28.62%) | |
| Vigorous | 2493 (25.63%) | 3037 (31.03%) | 3566 (36.36%) | 4379 (44.49%) | |
| Poverty-income ratio | 2.24 ± 1.54 | 2.49 ± 1.60 | 2.62 ± 1.63 | 2.78 ± 1.65 | <.001 |
| Body mass index, kg/m2 | 29.15 ± 7.02 | 29.17 ± 6.84 | 29.27 ± 6.97 | 28.90 ± 6.74 | .002 |
| Haemoglobin, g/dL | 13.71 ± 1.52 | 13.96 ± 1.52 | 14.21 ± 1.52 | 14.55 ± 1.46 | <.001 |
| Uric acid, mg/dL | 5.32 ± 1.48 | 5.38 ± 1.43 | 5.51 ± 1.42 | 5.67 ± 1.39 | <.001 |
| Total cholesterol, mg/dL | 195.01 ± 42.43 | 194.44 ± 42.28 | 193.74 ± 41.46 | 192.16 ± 41.98 | <.001 |
| HDL-cholesterol, mg/dL | 54.31 ± 16.59 | 53.73 ± 16.15 | 52.54 ± 15.90 | 51.49 ± 15.60 | <.001 |
| Triglyceride, mg/dL | 144.29 ± 108.55 | 148.01 ± 122.15 | 157.49 ± 141.16 | 160.48 ± 155.04 | <.001 |
| Glycohemoglobin, % | 5.80 ± 1.10 | 5.76 ± 1.07 | 5.76 ± 1.09 | 5.72 ± 1.04 | <.001 |
| Blood selenium (ng/mL) | 177.59 ± 34.78 | 180.62 ± 32.39 | 185.60 ± 30.91 | 192.28 ± 33.48 | <.001 |
| Dietary intake per day | |||||
| Total energy (kcal) | 1337.85 ± 469.55 | 1791.64 ± 501.70 | 2176.21 ± 622.98 | 2771.57 ± 934.23 | <.001 |
| Cholesterol (mg) | 148.84 ± 91.73 | 240.99 ± 124.00 | 316.41 ± 160.27 | 439.91 ± 238.74 | <.001 |
| Selenium (100 μg) | 0.55 ± 0.16 | 0.92 ± 0.09 | 1.26 ± 0.12 | 2.01 ± 0.49 | <.001 |
Continuous variables were described using mean ± standard deviation (SD) and were analysed by the t-test. Categorical variables were expressed as numbers (percentage) and were analysed by the chi-square test.
Correlation between dietary selenium intake and risk of stroke
As shown in Table 2, when dietary selenium intake was assessed as quartiles after multivariate adjustment for all the above-mentioned covariates in Model 3, the OR (95% CI) of stroke was 0.70 (0.55, 0.88) for participants in quartile 2 (77–108 μg/day), 0.71 (0.53, 0.93) for quartile 3 (108–148 μg/day), and 0.61 (0.43, 0.85) for quartile 4 (148–400 μg/day) compared with that in quartile 1 (0–77 μg/day). The p-value for trend through quartiles was .007.
Table 2.
Adjusted odds ratios of stroke correlated with dietary selenium intake.
| Dietary selenium intake (100 μg/day, n = 39,438) |
Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Quartile 1 (0.00–0.78) | Ref. | Ref. | Ref. |
| Quartile 2 (0.78–1.08) | 0.65 (0.54, 0.78) | 0.70 (0.56, 0.86) | 0.70 (0.55, 0.88) |
| p-Value | <.001 | .001 | .004 |
| Quartile 3 (1.08–1.48) | 0.62 (0.49, 0.77) | 0.72 (0.56, 0.91) | 0.71 (0.53, 0.93) |
| p-Value | <.001 | .009 | .017 |
| Quartile 4 (1.48–4.00) | 0.48 (0.39, 0.60) | 0.62 (0.48, 0.80) | 0.61 (0.43, 0.85) |
| p-Value | <.001 | <.001 | .005 |
| p-Value for trend | <.001 | <.001 | .007 |
Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for education level, marital status, poverty-income ratio, body mass index, smoking, alcohol use, hypertension, diabetes, physical activity based on Model 1. Model 3 was adjusted for levels of haemoglobin, uric acid, total cholesterol, HDL-cholesterol, triglyceride, glycohemoglobin, daily intake of total energy and cholesterol from the diet based on Model 2.
Threshold effect of dietary selenium intake on risk of stroke
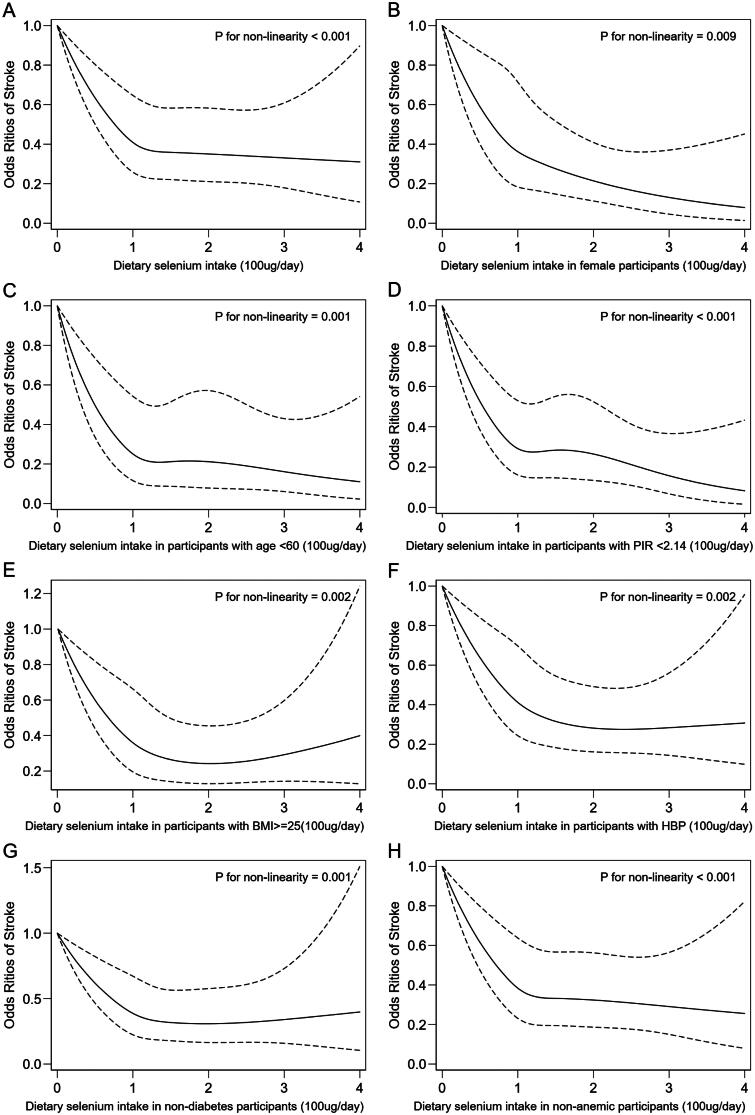
After multivariate adjustment for all covariates mentioned above in Model 3, a non-linear negative correlation was observed between dietary selenium intake and the risk of stroke, with an obvious breakpoint at 105 μg/day (p-value for non-linearity = .025). There was an initial decrease (<105 μg/day) (0.61 [0.44, 0.85], p = .004) in odds followed by a platform beyond 105 μg/day (0.97 [0.81, 1.16], p = .723) (Table 3). A non-linear negative correlation between dietary selenium intake and risk of stroke was observed by the spline smoothing plot in Figure 1(A) (p-value for non-linearity <.001).
Table 3.
Threshold effect of dietary selenium intake on risk of stroke.
| Dietary selenium intake (100 μg/day, n = 39,438) |
Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Linear model | |||
| OR (95% CI) p-value |
0.71 (0.64, 0.78) <.001 | 0.81 (0.72, 0.90) <.001 | 0.86 (0.74, 1.00) . 051 |
| Non-linear model | |||
| Breakpoint (K) | 2.00 | 1.20 | 1.05 |
| OR1(95% CI), <K p-value |
0.63 (0.55, 0.71) <.001 | 0.62 (0.49, 0.77) <.001 | 0.61 (0.44, 0.85) . 004 |
| OR2(95% CI), >K p-value |
1.22 (0.88, 1.71) .237 | 0.99 (0.83, 1.18) .889 | 0.97 (0.81, 1.16) .723 |
| p-Value for non-linearity | .002 | .007 | .025 |
The piece-wise linear regression model was applied to show the threshold effect of dietary selenium intake on the risk of stroke. Linear model: model that presumes the correlation between dietary selenium intake and the risk of stroke is linear. Non-linear model: model that presumes the correlation between dietary selenium intake and the risk of stroke is non-linear and has a breakpoint. p-Value for non-linearity <.05 means that the non-linear model may better describe the correlation. Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for education level, marital status, poverty-income ratio, body mass index, smoking, alcohol use, hypertension, diabetes, physical activity based on Model 1. Model 3 was adjusted for levels of haemoglobin, uric acid, total cholesterol, HDL-cholesterol, triglyceride, glycohemoglobin, daily intake of total energy and cholesterol from the diet based on Model 2. OR (95% CI): odds ratio and 95% confidence interval.
Figure 1.
The weighted odds ratio of stroke correlated with dietary selenium intake. (A) All participants. (B) Female participants. (C) Participants with age <60 years. (D) Participants with PIR <2.14. (E) Participants with overweight and obesity. (F) Participants with hypertension. (G) Participants without diabetes. (H) Participants without anaemia. The solid and long dash lines represent the estimated odds ratio and 95% confidence interval. Odds ratios were adjusted for age, sex, race, education level, marital status, poverty-income ratio, body mass index, smoking, alcohol use, hypertension, diabetes, physical activity, levels of haemoglobin, uric acid, total cholesterol, HDL-cholesterol, triglyceride, glycohemoglobin, daily intake of total energy and cholesterol from the diet. PIR: family poverty-income ratio.
Stratified correlations between dietary selenium intake and stroke
Non-linear negative correlation trends were observed in subgroups of female, age <60, PIR <2.14, overweight and obesity, hypertension, non-diabetes, and non-anaemia (Figure 1(B–H)). Adjusted ORs (95% Cls) of stroke were 0.51 (0.36, 0.70) for female participants, 0.63 (0.40, 0.99) for participants with age <60 years, 0.63 (0.47, 0.85) for participants with PIR <2.14, 0.66 (0.50, 0.87) for participants with overweight and obesity, 0.66 (0.52, 0.84) for participants with hypertension, 0.72 (0.53, 0.97) for participants without diabetes, and 0.72 (0.56, 0.92) for non-anaemic participants (Table 4). All p-values for non-linearity in these subgroups <.01.
Table 4.
Subgroups analysis for the correlation between dietary selenium intake and the risk of stroke.
| Subgroups | Incidence of stroke | Levels of blood selenium (ng/mL) | Dietary selenium intake (100 μg/day) | ORs (95%CIs) of stroke | p-Value |
|---|---|---|---|---|---|
| Male | 2.62% | 186.16 ± 33.21 | 1.37 ± 0.65 | 1.00 (0.74, 1.35) | .986 |
| Female | 3.23% | 182.11 ± 33.43 | 1.01 ± 0.49 | 0.51 (0.36, 0.70) | <.001 |
| Age <60 years | 1.28% | 187.13 ± 30.06 | 1.23 ± 0.61 | 0.63 (0.40, 0.99) | .046 |
| Age > =60 years | 7.64% | 179.22 ± 37.63 | 1.10 ± 0.56 | 0.82 (0.64, 1.06) | .127 |
| PIR <2.14 | 4.36% | 183.31 ± 33.35 | 1.13 ± 0.59 | 0.63 (0.47, 0.85) | .002 |
| PIR > =2.14 | 2.11% | 184.53 ± 33.89 | 1.25 ± 0.61 | 0.86 (0.62, 1.19) | .349 |
| Normal weight | 2.29% | 183.68 ± 33.52 | 1.20 ± 0.61 | 1.03 (0.68, 1.55) | .902 |
| Overweight/obesity | 3.05% | 184.54 ± 33.22 | 1.19 ± 0.59 | 0.66 (0.50, 0.87) | .004 |
| Non-smoking | 2.27% | 186.17 ± 32.32 | 1.17 ± 0.60 | 0.79 (0.57, 1.10) | .168 |
| Smoking | 3.73% | 181.58 ± 34.45 | 1.20 ± 0.61 | 0.72 (0.51, 1.01) | .055 |
| Non-hypertension | 1.11% | 185.43 ± 32.32 | 1.21 ± 0.61 | 0.97 (0.64, 1.47) | .875 |
| Hypertension | 6.81% | 182.12 ± 34.84 | 1.15 ± 0.59 | 0.66 (0.52, 0.84) | .001 |
| Non-diabetes | 2.19% | 183.92 ± 33.00 | 1.20 ± 0.60 | 0.72 (0.53, 0.97) | .034 |
| Diabetes | 9.55% | 184.63 ± 34.56 | 1.13 ± 0.58 | 0.77 (0.53, 1.13) | .188 |
| Non-anaemia | 2.70% | 185.09 ± 33.48 | 1.21 ± 0.60 | 0.72 (0.56, 0.92) | .010 |
| Anaemia | 7.14% | 172.59 ± 29.96 | 1.00 ± 0.49 | 1.02 (0.52, 2.01) | .947 |
Continuous variables were described by using mean ± standard deviation (SD). ORs were adjusted for age, sex, race, education level, marital status, poverty-income ratio, body mass index, smoking, alcohol use, hypertension, diabetes, physical activity, levels of haemoglobin, uric acid, total cholesterol, HDL-cholesterol, triglyceride, glycohemoglobin, daily intake of total energy and cholesterol from the diet. ORs (95% CIs): odds ratios and 95% confidence intervals; PIR: poverty-income ratio.
Discussion
In this study, we observed a non-linear negative correlation between dietary selenium intake and the risk of stroke in adults. A significant decrease in the risk of stroke was observed until 105 μg dietary selenium was consumed per day. Previous studies have shown a correlation between selenium deficiency and cardiovascular diseases [21,22]. Therefore, selenium supplementation to improve health is becoming increasingly popular. However, selenium is toxic at high levels [23]. The recommended daily adequate selenium intake is 70 μg for adults with a tolerable upper intake level of 400 μg/day [14,24]. Our research shows that a selenium intake of approximately 105 μg per day has an optimum beneficial effect on the risk of stroke. Increasing selenium intake has no further benefit. This finding validates our previous hypothesis and may explain why selenium supplementation (200 μg/day) had no effect on stroke risk in the NPC trial.
The correlation between selenium levels and stroke may vary across different population subgroups. The decline in the risk of stroke was significant in women and participants with lower income, overweight and obesity, or hypertension, probably because of the low serum selenium concentration and low habitual selenium intake [25–27]. The incidence of stroke was high among these participants. This could be improved by appropriate selenium supplementation, suggesting that selenium deficiency may play a role in the incidence of stroke in these populations.
We observed a negative correlation between dietary selenium intake and the risk of stroke in non-anaemic individuals. In contrast, no correlation was found between dietary selenium intake and the risk of stroke in anaemic individuals despite low blood selenium levels and low selenium intake, which suggested that anaemia might attenuate the beneficial effects of selenium intake on stroke. This may be related to the critical role of erythrocytes in selenium transport [28,29]. The significantly reduced erythrocytes in anaemic patients are insufficient for the transport and utilisation of selenium, which affects the physiological role of selenium.
There is conflicting evidence regarding the association between selenium intake and diabetes. Early case-control studies have shown that diabetes influences serum selenium levels as decreased selenium levels were observed in patients with diabetes [30]. In contrast, observational studies found a positive correlation between serum selenium levels and the incidence of diabetes [30,31]. Secondary analyses in a randomised clinical trial reported that selenium supplementation (200 μg/day) increases the incidence of diabetes [31]. However, other trials did not find an overall significant effect of selenium supplementation (200 μg/day) on incident diabetes [32,33]. Similar contradictions have been reported in relevant systematic reviews and meta-analyses [34,35]. We observed a beneficial effect of dietary selenium intake on the risk of stroke in non-diabetic individuals but not in those with diabetes. It should be noted that the odds ratios of stroke in the two groups were very close [0.72 (0.53, 0.97) for non-diabetes vs. 0.77 (0.53, 1.13) for diabetes]. Therefore, the impact of selenium intake on stroke in diabetes is not yet clear. Further prospective studies are needed.
Limitation
As a cross-sectional study, stroke history was self-reported, and bias cannot be avoided for some of the results. However, this is likely limited as a previous study confirmed the validity of using self-reported illness to measure objective health [36]. Furthermore, although many covariates were adjusted in the regression models, unmeasured confounders correlated with a stroke cannot be excluded. We can only show correlation, not causality. The impact of selenium on stroke and its specific manifestations in the general population requires further prospective studies.
Conclusions
In conclusion, Dietary selenium had a negative and non-linear correlation with the risk of stroke in adults. Non-linear negative correlation trends were observed in subpopulations of females, age <60 years, PIR <2.14, overweight and obesity, hypertension, non-diabetes, and non-anaemia. Dietary selenium intake of approximately 105 μg/day has an optimum beneficial effect on stroke.
Acknowledgments
The authors thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for data sharing.
Funding Statement
This work was supported by grants from the Weifang Health Committee to Jianxin Dou [grant number: WFWSJK-2020-025] and Liang Su [grant number: WFWSJK-2020-006].
Author contributions
Wenrui Shi and Liang Su analysed the data and drafted the manuscript. The corresponding author ensured that the descriptions were accurate and agreed upon by all the authors. All authors made substantial contributions to the interpretation of the data and provided critical revisions to the manuscript.
Disclosure statement
All authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Data availability statement
The data used in this study are openly available from the Centres for Disease Control and Prevention at https://www.cdc.gov/nchs/nhanes/index.htm
References
- 1.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1395–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Y, Yuan Y, Liu Y, et al. Circulating multiple metals and incident stroke in Chinese adults. Stroke. 2019;50(7):1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu XF, Stranges S, Chan L.. Circulating selenium concentration is inversely associated with the prevalence of stroke: results from the Canadian Health Measures Survey and the National Health and Nutrition Examination Survey. J Am Heart Assoc. 2019;8(10):e012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariharan S, Dharmaraj S.. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology. 2020;28(3):667–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeberle SC, Gollowitzer A, Laoukili J, et al. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms. Redox Biol. 2020;28:101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orellana-Urzúa S, Rojas I, Líbano L, et al. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des. 2020;26(34):4246–4260. [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Yuan Y, Xiao Y, et al. Associations of plasma metal concentrations with the risks of all-cause and cardiovascular disease mortality in Chinese adults. Environ Int. 2021;157:106808. [DOI] [PubMed] [Google Scholar]
- 10.Ding J, Zhang Y.. Relationship between the circulating selenium level and stroke: a Meta-analysis of observational studies. J Am Coll Nutr. 2021. Mar 30;1–9. doi: 10.1080/07315724.2021.1902880. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Stranges S, Marshall JR, Trevisan M, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163(8):694–699. [DOI] [PubMed] [Google Scholar]
- 12.Chen TC, et al. National Health and Nutrition Examination Survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2020;2(184):1–35. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Research Ethics Review Board (ERB) Approval 2003-2018. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm.
- 14.Micronutrients I. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. 2001. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- 15.Lin J, Shen T.. Association of dietary and serum selenium concentrations with glucose level and risk of diabetes mellitus: a cross sectional study of national health and nutrition examination survey, 1999–2006. J Trace Elem Med Biol. 2021;63:126660. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, et al. Comparison of prevalence, awareness, treatment, and control of cardiovascular risk factors in China and the United States. J Am Heart Assoc. 2018;7(3):e007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Hou W, Zhang X, et al. Hyperuricemia and risk of stroke: a systematic review and Meta-analysis of prospective studies. Atherosclerosis. 2014;232(2):265–270. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura Y, Wakabayashi H, Shiraishi A, et al. Hemoglobin improvement is positively associated with functional outcomes in stroke patients with anemia. J Stroke Cerebrovasc Dis. 2021;30(1):105453. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25_suppl_2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desquilbet L, Mariotti F.. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. [DOI] [PubMed] [Google Scholar]
- 21.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. [DOI] [PubMed] [Google Scholar]
- 22.Rees K, Hartley L, Day C, et al. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;2013(1):CD009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadrup N, Ravn-Haren G.. Acute human toxicity and mortality after selenium ingestion: a review. J Trace Elem Med Biol. 2020;58:126435. [DOI] [PubMed] [Google Scholar]
- 24.Gać P, Czerwińska K, Macek P, et al. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ Toxicol Pharmacol. 2021;82:103553. [DOI] [PubMed] [Google Scholar]
- 25.Hu XF, Chan HM.. Factors associated with the blood and urinary selenium concentrations in the Canadian population: Results of the Canadian Health Measures Survey (2007–2011). Int J Hyg Environ Health. 2018;221(7):1023–1031. [DOI] [PubMed] [Google Scholar]
- 26.Combs GF, Watts JC, Jackson MI, et al. Determinants of selenium status in healthy adults. Nutr J. 2011;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baudry J, Kopp JF, Boeing H, et al. Changes of trace element status during aging: results of the EPIC-Potsdam cohort study. Eur J Nutr. 2020;59(7):3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai T, Mihara H, Kurihara T, et al. Selenocysteine is selectively taken up by red blood cells. Biosci Biotechnol Biochem. 2009;73(12):2746–2748. [DOI] [PubMed] [Google Scholar]
- 29.Haratake M, Fujimoto K, Hirakawa R, et al. Hemoglobin-mediated selenium export from red blood cells. J Biol Inorg Chem. 2008;13(3):471–479. [DOI] [PubMed] [Google Scholar]
- 30.Kljai K, Runje R.. Selenium and glycogen levels in diabetic patients. BTER. 2001;83(3):223–229. [DOI] [PubMed] [Google Scholar]
- 31.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(4):217–223. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PA, et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016;108(12):djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohler L, Foote J, Kelley C, et al. Selenium and type 2 diabetes: systematic review. Nutrients. 2018;10(12):1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinceti M, Filippini T, Rothman KJ.. Selenium exposure and the risk of type 2 diabetes: a systematic review and Meta-analysis. Eur J Epidemiol. 2018;33(9):789–810. [DOI] [PubMed] [Google Scholar]
- 36.Bourne PA. The validity of using self-reported illness to measure objective health. N Am J Med Sci. 2009;1(5):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are openly available from the Centres for Disease Control and Prevention at https://www.cdc.gov/nchs/nhanes/index.htm