Abstract
BACKGROUND
In most of the Americas, the recommended treatment to prevent relapse of Plasmodium vivax malaria is primaquine at a total dose of 3.5 mg per kilogram of body weight, despite evidence of only moderate efficacy.
METHODS
In this trial conducted in Brazil, we evaluated three primaquine regimens to prevent relapse of P. vivax malaria in children at least 5 years of age and in adults with microscopy-confirmed P. vivax monoinfection. All the patients received directly observed chloroquine for 3 days (total dose, 25 mg per kilogram). Group 1 received a total primaquine dose of 3.5 mg per kilogram (0.5 mg per kilogram per day) over 7 days with unobserved administration; group 2 received the same regimen as group 1 but with observed administration; and group 3 received a total primaquine dose of 7.0 mg per kilogram over 14 days (also 0.5 mg per kilogram per day) with observed administration. We monitored the patients for 168 days.
RESULTS
We enrolled 63 patients in group 1, 96 in group 2, and 95 in group 3. The median age of the patients was 22.4 years (range, 5.4 to 79.8). By day 28, three P. vivax recurrences were observed: 2 in group 1 and 1 in group 2. By day 168, a total of 70 recurrences had occurred: 24 in group 1, 34 in group 2, and 12 in group 3. No serious adverse events were noted. On day 168, the percentage of patients without recurrence was 58% (95% confidence interval [CI], 44 to 70) in group 1, 59% (95% CI, 47 to 69) in group 2, and 86% (95% CI, 76 to 92) in group 3. Survival analysis showed a difference in the day 168 recurrence-free percentage of 27 percentage points (97.5% CI, 10 to 44; P<0.001) between group 1 and group 3 and a difference of 27 percentage points (97.5% CI, 12 to 42; P<0.001) between group 2 and group 3.
CONCLUSIONS
The administration of primaquine at a total dose of 7.0 mg per kilogram had higher efficacy in preventing relapse of P. vivax malaria than a total dose of 3.5 mg per kilogram through day 168. (Supported by the U.S. Agency for International Development; ClinicalTrials.gov number, NCT03610399.)
Malaria remains a public health concern in the Americas. In 2020, a total of 144,887 cases were reported in the Brazilian Amazon; of these cases, 83.6% were caused by Plasmodium vivax and 16.4% by P. falciparum or mixed infections.1 Treatment for P. vivax malaria presents additional challenges for the control and elimination of malaria because the infection involves both blood-stage parasites and hypnozoites, dormant parasite forms in the liver that can cause relapse weeks after an acute infection. P. vivax blood-stage parasites are sensitive to chloroquine in most of the world, except in Southeast Asia and Oceania, but hypnozoite eradication requires the use of primaquine or tafenoquine.2-4
The efficacy of primaquine therapy depends on the total dose given during a round of therapy (either 3.5 mg or 7.0 mg per kilogram of body weight), because of variable drug susceptibility worldwide.5,6 In Brazil, and in most of the Americas, the recommended P. vivax treatment consists of a combination of chloroquine (total dose, 25 mg per kilogram) and primaquine at a total dose of 3.5 mg per kilogram, despite the only moderate efficacy of this regimen (60 to 70%) in preventing recurrence within 6 months.7,8
We evaluated the therapeutic efficacy of chlooquine and primaquine to treat and prevent relapse of P. vivax malaria in the Acre state of Brazil. In this trial, we compared a lower total dose of primaquine (3.5 mg per kilogram) with a higher total dose (7.0 mg per kilogram).
METHODS
TRIAL DESIGN AND RANDOMIZATION
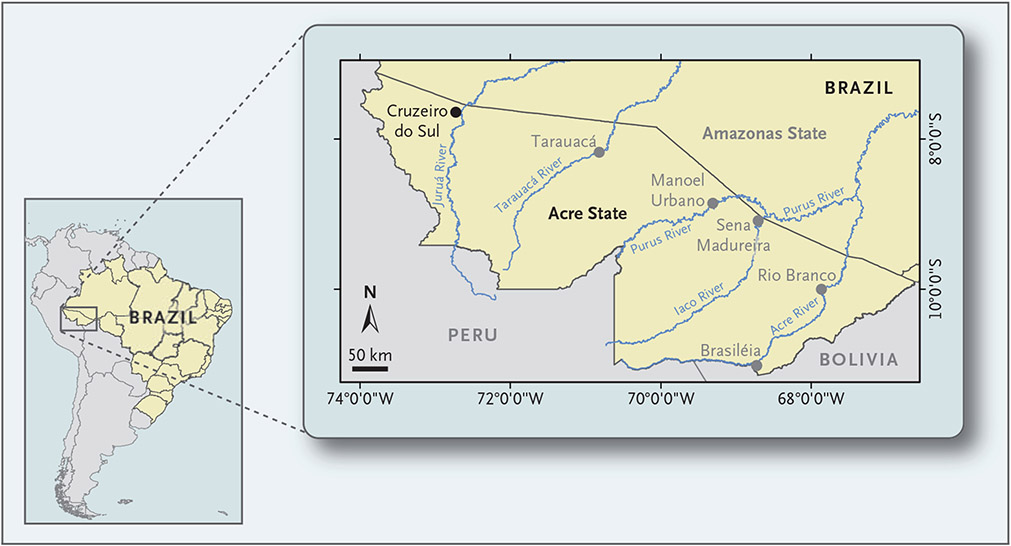
Our trial was conducted during the malaria transmission season at eight malaria diagnostic posts in Cruzeiro do Sul, a city in the western Brazilian Amazon with 85,000 residents and an annual parasite incidence of 147.5 malaria cases per 1000 residents (Fig. 1).
Figure 1. Location of Cruzeiro do Sul, Acre State, Brazil.
The trial was conducted at eight malaria diagnostic posts in Cruzeiro do Sul, a city with 85,000 residents and an annual parasite incidence of 147.5 malaria cases per 1000 residents.
All the patients received directly observed chloroquine for 3 days (total dose, 25 mg per kilogram of body weight) and then were randomly assigned to one of three groups depending on the primaquine regimen. Those in group 1 were instructed to take primaquine as unobserved therapy for a total dose of 3.5 mg per kilogram over 7 days (0.5 mg per kilogram per day), and those in group 2 received the same regimen but under directly observed therapy. Patients in group 3 received directly observed primaquine therapy for a total dose of 7.0 mg per kilogram for 14 days (also 0.5 mg per kilogram per day). We adjusted the primaquine dose according to body weight in patients who weighed more than 70 kg.
OVERSIGHT
The protocol (available with the full text of this article at NEJM.org) was reviewed and approved by the institutional review boards at the Centers for Disease Control and Prevention (CDC) and at the Brazilian Instituto Evandro Chagas. All the medications that were used in the trial were procured by the Brazilian Ministry of Health under quality-assurance programs.
The U.S. Agency for International Development, which funded the trial, had no role in the design or in the collection, analysis, or interpretation of the data. All the authors vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol.
Written informed consent was obtained from adult patients and from children’s parents or guardians; children between the ages of 7 and 17 years also provided verbal assent. Patients who declined to participate in the trial were evaluated by malaria diagnostic staff members and were treated according to the standard of care at each malaria post.
PATIENTS AND PROCEDURES
In the trial, we included both children who were at least 5 years of age and adults with a body weight of less than 120 kg, documented fever (axillary temperature, ≥37.5°C) or a history of fever during the previous 48 hours, P. vivax monoinfection, and a parasite density between 100 and 200,000 asexual parasites per cubic millimeter.8 Patients with suspicion of severe malaria or abnormal glucose-6-phosphate dehydrogenase (G6PD) activity were excluded,10 since G6PD deficiency can result in severe hemolysis during primaquine therapy.
After informed consent had been provided, we randomly assigned the patients to one of the three trial groups. We collected demographic and clinical information at enrollment and drew venous blood for malaria confirmation using thick and thin smears and for measurement of hemoglobin and G6PD activity; women of childbearing age underwent pregnancy testing. We also prepared dried blood spots for microsatellite genotyping and analysis of the gene encoding cytochrome P-450 2D6 (CYP2D6) as a measure of drug metabolism and activation.
We initiated oral treatment with chloroquine (Institute of Drug Technology [Farmanguinhos]) at the time of enrollment (day 0). Patients returned to the trial site on days 1 and 2 to complete chloroquine treatment, clinical evaluation, and microscopy testing; all daily chloroquine doses were directly observed. After the results of G6PD testing became available (usually within 2 weeks), oral primaquine (Medopharm) was initiated in patients who were found to have a normal G6PD level (≥7.0 IU per gram of hemoglobin). Primaquine treatment lasted for 7 or 14 days, according to the trial group.
In addition to days 1 and 2, patients were evaluated on days 3, 7, 14, 21, and 28 and then every 4 weeks until day 168. During those visits, staff assessed patients’ clinical status, measured axillary temperature, collected blood smears, and prepared dried blood spots in case of a positive microscopy result on or after day 4. We instructed patients to contact the trial team for evaluation if malaria symptoms developed. Data for patients who missed their scheduled visit were censored on that day, with tolerance of ±1 day given for visits on or after day 14.
MEASUREMENT OF BLOOD SMEARS AND CHLOROQUINE LEVELS
Parasite density was calculated by counting the number of asexual parasites and gametocytes against white cells on the basis of a white-cell count of 6000 cells per cubic millimeter. Two microscopists (or three in case of discrepancies in determinations of asexual parasite density) who were unaware of one another’s results examined blood smears. We used the geometric mean of the two closest readings to estimate asexual parasite densities and the arithmetic mean for gametocyte densities.
In cases of treatment failure on or before day 28, plasma levels of chloroquine and desethylchloroquine were measured by high-performance liquid chromatography on day 7 and on the day of recurrence.11
MICROSATELLITE GENOTYPING
Seven neutral microsatellites (MS2, MS6, Pv3.502, Pv11.162, Pv12.35, Pvms038, and Pv10.29) were used to genotype parasites on day 0 and on the day of recurrence.8,12 We analyzed microsatellite fluorescent-labeled polymerase-chain-reaction (PCR) products with the 3130 Genetic Analyzer 16-Capillary Array (Applied Biosystems) and determined allele sizes using genotype analysis software GeneMarker, version 1.95. Alleles with a difference of at least two nucleotides in length were considered to be different.
We determined multiplicity of infection and haplotype frequencies using day 0 samples obtained from patients with recurrence and additional randomly selected day 0 samples to reach 128 samples.13 Samples that were obtained on day 0 and on the day of recurrence were processed in parallel; paired samples with at least one microsatellite difference were considered to be heterologous haplotypes, whereas all other paired samples were considered to be homologous haplotypes.8,14
CYP2D6 GENOTYPING AND PHENOTYPING OF METABOLIC ACTIVITY
We analyzed CYP2D6 polymorphisms and correlated them with metabolic activity phenotypes.15,16 Day 0 samples were genotyped for seven single-nucleotide polymorphisms — C-1584G (rs1080985), C100T (rs1065852), C1023T (rs28371706), G1846A (rs3892097), G31A (rs769258), G3183A (rs59421388), and G4180C (rs1135840) — and one deletion, 2615_2617delAAG (rs5030656), with the use of the TaqMan SNP Genotyping Assay (Thermo Fisher Scientific). We assigned an enzyme metabolic activity score for each haplotype: poor activity (0), intermediate activity (0.25 to 1.00), normal activity (1.25 to 2.25), and ultrarapid activity (>2.25).15,17
OUTCOME MEASURES
Treatment failure by day 28 was defined as clinical deterioration leading to hospitalization with parasitemia on day 28 or earlier, parasitemia and fever from days 3 through 28, or parasitemia from days 7 through 28.10 Parasitemia from days 28 through 168, with or without fever, was classified as extended failure during follow-up. If patients did not meet the criteria for treatment failure by day 28, they were described as having had an adequate clinical and parasitologic response. We also determined the percentage of patients who were free from recurrence by day 168. Patients with treatment failure were withdrawn and referred for rescue treatment.7
STATISTICAL ANALYSIS
We determined that the enrollment of 74 patients in group 2 and in group 3 would provide a power of 90% to compare an expected recurrence-free percentage of 70% in group 2 and 90% in group 3 at day 168, assuming a significance level of 5%.8,9 Similarly, we determined that in order to compare a recurrence-free percentage of 60% in group 1 and 90% in group 3, we would need to enroll 50 patients in each group, since the difference between the recurrence-free estimates was larger for the comparison between group 1 and group 3 than it was for the comparison between group 2 and group 3.10 We added 30% more patients in each trial group to account for loss to follow-up. Thus, the final required sample size was 257 patients: 65 in group 1 and 96 each in group 2 and group 3.
We entered data into an Epi Info database (version 7.2) and used SAS software, version 9.4 (SAS Institute), for data cleaning and analysis. We used Kaplan–Meier survival analysis in the intention-to-treat population to estimate the primary efficacy outcomes of an adequate clinical and parasitologic response by day 28 and freedom from P. vivax recurrence by day 168. We compared the day 28 response and day 168 recurrence-free percentage between group 1 and group 3 and between group 2 and group 3 using the Wald test. We used a Bonferroni correction to account for multiple testing when conducting these pairwise comparisons in which P values of less than 0.025 were considered to indicate statistical significance and 97.5% confidence intervals were reported. An unadjusted Cox proportional-hazards regression model was used to estimate hazard ratios. We also performed an analysis in which we calculated freedom from recurrence by day 168 that considered only homologous recurrences.
In addition, we conducted per-protocol analyses of the day 28 response and freedom from recurrence by day 168 and fit a Cox proportional-hazards regression model for association with recurrence by day 168 after adjustment for selected variables. (Results of the per-protocol and Cox regression analyses are provided in the Supplementary Appendix, available at NEJM.org.)
RESULTS
PATIENTS
Patients were enrolled from April through October 2018; follow-up ended in March 2019. Infection with P. vivax was confirmed in 1526 patients during the enrollment period at the trial malaria posts. Participation was offered to 342 patients who presented during regular clinic hours; 291 patients (85.1%) provided consent and were conditionally enrolled. Of these patients, 37 were later excluded, which resulted in a total enrollment of 254 patients (Fig. 2).
Figure 2. Enrollment and Outcomes.
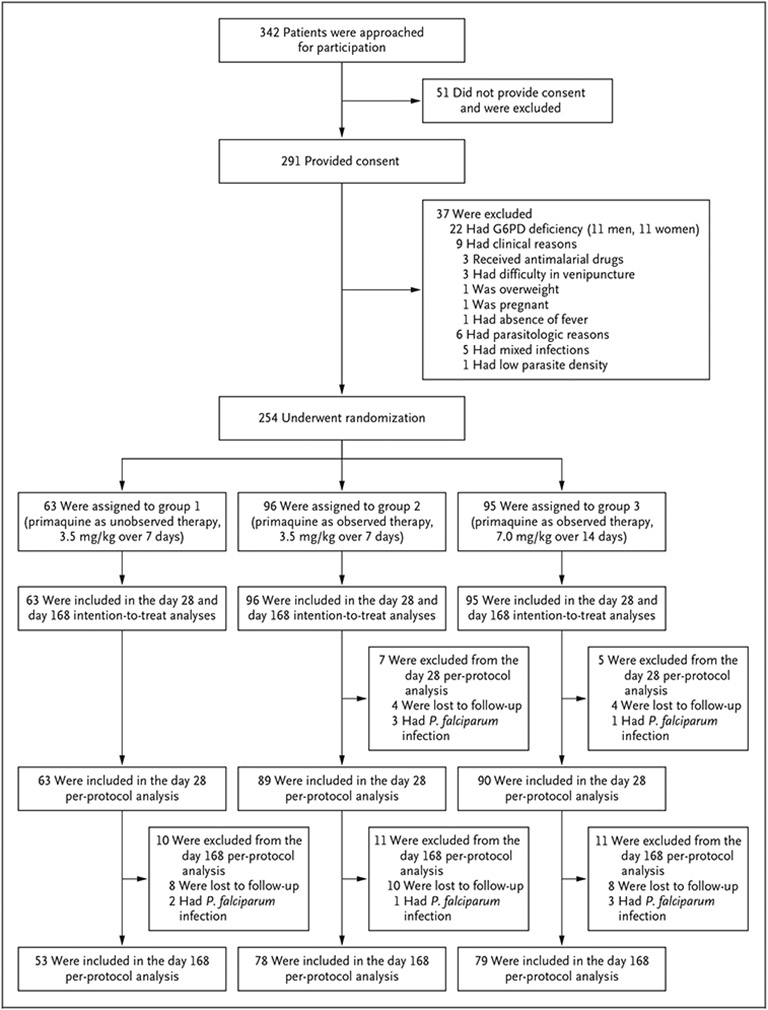
All the patients received directly observed chloroquine for 3 days after being randomly assigned to one of three groups. The groups varied according to the primaquine regimen and whether receipt of the drug was observed or unobserved. G6PD denotes glucose-6-phosphate dehydrogenase.
The median age of the patients was 22.4 years (Table 1). The median duration of fever was 2 days, and 54 patients (21%) had documented fever at enrollment. Commonly reported symptoms were headache, chills, and calf pain. The geometric mean of asexual parasite density at enrollment was 2300 parasites per cubic millimeter. Parasitemia remained in 92 patients (36%) on day 2 and in 22 (9%) on day 3; infection was cleared in all the patients by day 7 (Fig. S1 in the Supplementary Appendix).
Table 1.
Demographic and Clinical Characteristics of the Patients at Enrollment and during Follow-up.*
| Characteristic | Group 1 (N = 63) | Group 2 (N = 96) | Group 3 (N = 95) | All Patients (N = 254) |
|---|---|---|---|---|
| Demographic | ||||
| Median age (range) — yr | 26.5 (8.1–79.8) | 20.3 (5.6–73.9) | 23.5 (5.4–64.8) | 22.4 (5.4–79.8) |
| Male sex — no. (%) | 35 (56) | 52 (54) | 52 (55) | 139 (55) |
| Place of residence — no. (%) | ||||
| Rural | 16 (25) | 24 (25) | 34 (36) | 74 (29) |
| Urban | 47 (75) | 72 (75) | 61 (64) | 180 (71) |
| Clinical | ||||
| Median weight (range) — kg | 61.5 (21.0–110.0) | 58.5 (15.6–118.6) | 64.0 (19.3–96.0) | 61.0 (15.6–118.6) |
| Fever | ||||
| At enrollment — no. (%) | 13 (21) | 21 (22) | 20 (21) | 54 (21) |
| Median duration (range) — days | 2 (1–5) | 2 (1–7) | 2 (1–10) | 2 (1–10) |
| Median hemoglobin (range) — g/dl | ||||
| Day 0 | 13.8 (10.7–18.6) | 13.6 (10.8–17.9) | 13.5 (9.3–17.0) | 13.6 (9.3–18.6) |
| Day 14 | 13.6 (10.2–17.0) | 13.3 (9.3–18.4) | 13.4 (11.1–17.0) | 13.4 (9.3–18.4) |
| Day 28 | 14.0 (9.7–16.0) | 13.4 (10.4–16.4) | 13.9 (10.6–17.8) | 13.8 (9.7–17.8) |
| Geometric mean of asexual parasite density (range) — no./mm3 | 2080 (130–14,828) | 2286 (107–20,467) | 2475 (125–14,193) | 2300 (107–20,467) |
| Mean gametocyte density (range) — no./mm3 | 39 (0–232) | 52 (0–308) | 60 (0–531) | 52 (0–531) |
| Positive gametocytemia — no. (%) | ||||
| Day 0 | 29 (46) | 48 (50) | 59 (62) | 136 (54) |
| Day 2 | 3 (5) | 2 (2) | 4 (4) | 9 (4) |
| Day 3 | 0 | 0 | 1 (1) | 1 (<1) |
| Median G6PD enzyme activity (range) — IU/g of hemoglobin | 10.4 (7.1–13.9) | 10.1 (7.0–14.7) | 10.6 (7.1–15.8) | 10.4 (7.0–15.8) |
| CYP2D6 enzyme phenotype — no./total no. (%) | ||||
| Poor metabolic activity | 0 | 4/96 (4) | 5/94 (5) | 9/252 (4) |
| Intermediate metabolic activity | 21/62 (34) | 24/96 (25) | 26/94 (28) | 71/252 (28) |
| Normal metabolic activity | 41/62 (66) | 68/96 (71) | 63/94 (67) | 172/252 (68) |
All data were obtained at the time of enrollment unless otherwise indicated. CYP2D6 denotes cytochrome P-450 2D6, and G6PD glucose-6-phosphate dehydrogenase.
The median total dose of chloroquine was 23.4 mg per kilogram (range, 12.6 to 39.1) among the 254 patients. The median total dose of primaquine was 3.3 mg per kilogram (range, 2.5 to 4.2) in group 1, 3.4 mg per kilogram (range, 2.7 to 4.5) in group 2, and 6.8 mg per kilogram (range, 6.1 to 8.4) in group 3. The median day of primaquine initiation was day 17 (interquartile range, day 15 to day 21) after enrollment. Although adverse events were not systematically assessed, no serious adverse events related to chloroquine or primaquine were reported. Median hemoglobin levels were similar within and between trial groups on days 0, 14, and 28.
RECURRENCE OF P. VIVAX INFECTION
By day 28, among the 254 patients, 8 (3%) had been lost to follow-up, and P. falciparum had been diagnosed in 4 patients (2%) (Fig. 2). Recurrence of P. vivax had occurred in 3 patients (1%): 2 in group 1 and 1 in group 2. On the day of recurrence in these patients, plasma levels of at least 15 ng per milliliter of chloroquine plus desethylchloroquine were reported, a finding that suggested the presence of chloroquine resistance (Table S3).10 A total of 97% or more of the patients in each trial group had an adequate clinical and parasitologic response by day 28 (Table 2).
Table 2.
Adequate Clinical and Parasitologic Response (ACPR) by Day 28 and Freedom from P. vivax Recurrence by Day 168 (Intention-to-Treat Population).*
| Group | No. of Patients | ACPR by Day 28 | Recurrence-free by Day 168† | |
|---|---|---|---|---|
| Any Recurrence | Homologous Recurrence | |||
| percent (95% CI) | percent (95% CI) | |||
| Group 1 | 63 | 97 (91–100) | 58 (44–70)‡ | 78 (64–87)§ |
| Group 2 | 96 | 99 (96–100) | 59 (47–69)‡ | 78 (66–86)¶ |
| Group 3 | 95 | 100 (NA) | 86 (76–92) | 95 (87–98) |
All listed data were calculated by means of Kaplan–Meier analysis. NA denotes not applicable.
Samples that were obtained on day 0 and on the day of recurrence were processed in parallel; paired samples with at least one microsatellite difference were considered to be heterologous recurrences, whereas all other paired samples were considered to be homologous recurrences.
P<0.001 for the comparison with group 3 as reference.
P = 0.006 for the comparison with group 3 as reference.
P = 0.001 for the comparison with group 3 as reference.
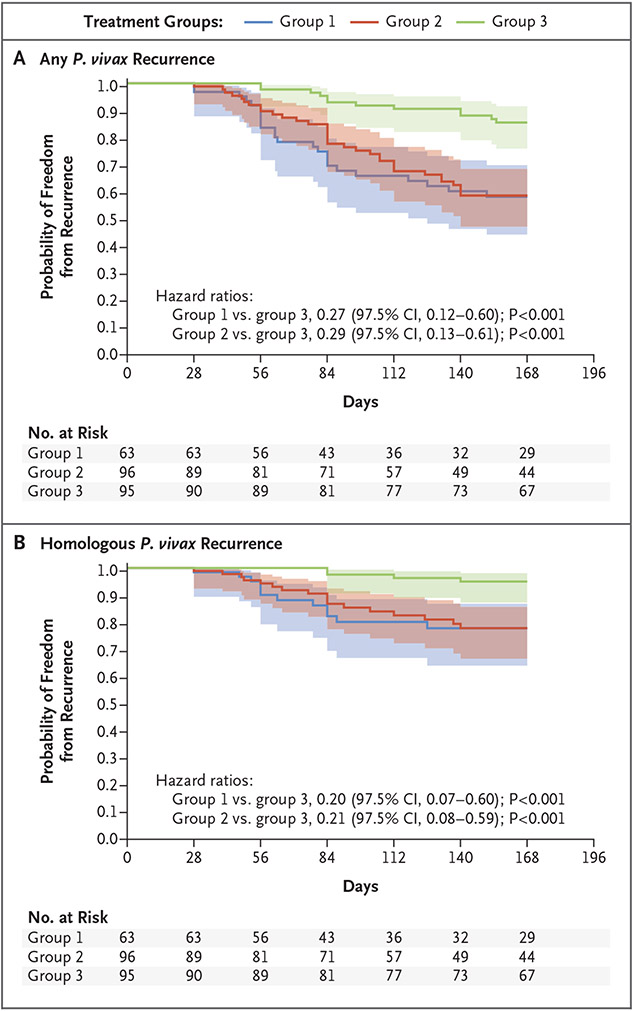
By day 168, among 254 patients, 34 (13%) had been lost to follow-up, P. falciparum had been diagnosed in 10 (4%), and P. vivax recurrence had been reported in 70 patients (28%); 12 of these cases were diagnosed at malaria posts (Table S4). P. vivax recurrence was reported from day 28 to day 155 regardless of the timing of primaquine initiation. Of the 70 patients with recurrence, 42 (60%) reported having had fever. By day 168, the percentage of patients who were recurrence-free was 58% (95% confidence interval [CI], 44 to 70) in group 1, 59% (95% CI, 47 to 69) in group 2, and 86% (95% CI, 76 to 92) in group 3. The between-group difference among patients who were recurrence-free by day 168 was 27 percentage points (97.5% CI, 10 to 44; P<0.001) between group 1 and group 3 and 27 percentage points (97.5% CI, 12 to 42; P<0.001) between group 2 and group 3 (Table S6). Figure 3 shows day 168 recurrence-free survival curves. Results of the per-protocol analysis are presented in Table S7.
Figure 3. Freedom from Plasmodium vivax Recurrence at Day 168.
Panel A shows Kaplan–Meier curves indicating freedom from any recurrence of P. vivax infection among the 254 patients according to trial group. The shaded areas indicate 95% confidence intervals. In the calculation of the hazard ratios for between-group comparisons, a 97.5% confidence interval was used to account for multiple comparisons. Panel B shows freedom from homologous P. vivax recurrence (with homologous recurrence defined as no microsatellite difference between the baseline sample and the recurrence sample).
Of the 128 samples that were obtained on day 0, polyclonal infection was identified in 8 samples (frequency of multiplicity of infection, 1%); the median number of alleles per locus was 7 (range, 4 to 9). Among the 70 patients with recurrence of P. vivax, microsatellite results were available for 58 (83%): 33 (57%) with homologous haplotypes and 25 (43%) with heterologous haplotypes (Table S8). By day 168, among the patients who had a homologous recurrence, the percentage who were recurrence-free was 78% (95% CI, 64 to 87) in group 1, 78% (95% CI, 66 to 86) in group 2, and 95% (95% CI, 87 to 98) in group 3.
Among the 252 patients with CYP2D6 data on day 0, 172 (68%) had normal activity (Table 1). Because of the low frequency of patients with poor drug-metabolizing activity, poor and intermediate phenotypes were combined in subsequent analyses. In group 2, 14 of 22 patients (63%) with a poor or intermediate drug-metabolizing phenotype in the per-protocol population remained recurrence-free by day 168, as compared with 30 of 56 patients (54%) among those with a normal phenotype; in group 3, the corresponding percentages were 79% (22 of 28 patients) and 88% (45 of 51 patients). The trial-group assignment remained associated with freedom from recurrence by day 168 in the Cox analysis in the per-protocol population (Table S9).
DISCUSSION
Among the patients in our trial who received directly observed therapy, the recurrence-free percentage by day 168 was higher among those who had received a higher total dose of primaquine (86%) than among those who had received a lower total dose (59%). Because all the patients received the same dose of chloroquine and no significant between-group difference was observed for the day 28 response to treatment, our findings suggest that a higher total dose of primaquine prevented P. vivax recurrence, which generally occurs more than 28 days after the initiation of treatment.
There are limitations in the use of genotyping to categorize P. vivax recurrence, since relapse can be caused by both homologous and heterologous hypnozoites.18,19 However, homologous recurrence more directly reflects recrudescence and relapse, so such recurrence is more indicative of lower treatment efficacy.14 We found that the percentage of patients receiving higher-dose primaquine who were recurrence-free by day 168 was greater when we limited the analysis to those with a homologous recurrence (95%), an approach that has also been used in other studies.8,14,20
Options for the treatment of P. vivax infection expanded with the approval of tafenoquine, which is administered as a single dose, unlike the 7-day or 14-day primaquine regimens.2,21 A recent study involving patients who had acquired infections in South America showed similar 6-month recurrence-free efficacy among those who had received tafenoquine (63%) and those who had received lower-dose primaquine (61%).9 Although no side-by-side comparison of tafenoquine and higher-dose primaquine has been performed in the Americas so far, our results show a better response in the prevention of P. vivax recurrence within 168 days with higher-dose primaquine than with tafenoquine, as reported in the previous study. Although we recognize the additional effort that a 14-day primaquine regimen requires to guarantee patient adherence (e.g., directly observed therapy and telephone reminders), our findings suggest that this regimen should be considered for the prevention of P. vivax recurrence.
The effects of such host factors as the CYP2D6 phenotype in the response to primaquine and tafenoquine have been discussed previously.22,23 Higher doses of primaquine seem to improve antirelapse efficacy in patients with impaired CYP2D6 metabolism.24 However, the effect of CYP2D6 polymorphisms in the response to tafenoquine therapy is unclear, since there is no consensus on whether tafenoquine needs to be metabolized into an active form by CYP2D6-complex enzymes as primaquine does.25,26 We did not find differences in recurrence prevention among patients with different CYP2D6 phenotypes, which may be due to a weaker role of CYP2D6 phenotypes in the response to antirelapse treatment with primaquine in the Americas or to an insufficient sample size in our trial.
No serious adverse events were observed, but we enrolled only patients with normal G6PD activity. Clinicians in the Americas often administer lower-dose primaquine without previous G6PD testing on the basis of the historically low incidence of adverse events.7 However, since such events have been reported during malaria treatment, efforts should be made to implement regular G6PD testing before primaquine use, especially if higher doses are considered.27,28 A recent study evaluated daily primaquine doses of 1 mg per kilogram administered during a 7-day period as compared with the same total dose (7 mg per kilogram) given over a 14-day period. Although the two regimens had similar efficacy, three cases of severely reduced hemoglobin levels occurred in the 7-day group as compared with one case in the 14-day group, mostly among female G6PD heterozygotes.29 In addition, tafenoquine is contraindicated in patients with any level of G6PD deficiency.30 Therefore, overcoming barriers to broad implementation of robust G6PD testing programs is needed for clinicians in countries where the intensification of antirelapse treatment is being considered for P. vivax malaria.
Effective relapse prevention is essential for proper case management of P. vivax infection.3,22 Deciding on the appropriate antirelapse treatment in the Americas and expanding G6PD testing as a means of avoiding adverse events are likely to become the top priorities in preventing relapse.31 Alternatives to current treatment include higher total doses of primaquine over 14 days or single-dose tafenoquine with a clear understanding of the benefits (e.g., potentially higher efficacy of higher-dose primaquine) and limitations of each option. A side-by-side comparison of these options would allow for a better evaluation of the response to antirelapse treatment and would help to clarify uncertainties concerning P. vivax treatment.
Supplementary Material
Footnotes
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development (USAID) or the Centers for Disease Control and Prevention (CDC).
Supported by the USAID through the Latin America and Caribbean Regional Malaria Program.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their caregivers who participated in this trial; the staff members at Ministério da Saúde do Brasil and Secretaria Estadual de Saúde do Acre for their support; the members of the trial team, especially Francisca Ingrid de Souza Conceição, Mônica Barboza, Jéssica Coelho, Isabela da Costa César, Mateus Araújo Castro e Souza, and Naialy Fernandes Araújo Reis, for their assistance with implementation and sample processing; José Maria de Souza Nascimento, José Mário Veloso Peres, Michel Platini Caldas de Souza, Jéssica Cristina da Silva Marvão, Danielle Regina Lima Barbosa, Izis Mônica Carvalho Sucupira, and Marinete Póvoa of Instituto Evandro Chagas; John Williamson, Michael Green, Venkatachalam Udhayakumar, and Naomi Lucchi of the CDC; and staff members in the Brazilian and Washington, D.C., offices of the Pan-American Health Organization for their technical, logistic, and administrative support.
Contributor Information
Nathália N. Chamma-Siqueira, Instituto Evandro Chagas, Ministério da Saúde do Brasil, Ananindeua, Brazil; Programa de Pós-Graduação em Biologia de Agentes Infecciosos e Parasitários, Brazil
Suiane C. Negreiros, Universidade Federal do Pará, Belém, Secretaria de Saúde do Estado do Acre, Cruzeiro do Sul, Brazil
Sarah-Blythe Ballard, Epidemic Intelligence Service, Center for Surveillance, Epidemiology, and Laboratory Services, Atlanta; Malaria Branch, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta
Sâmela Farias, Universidade Federal do Pará, Belém, Secretaria de Saúde do Estado do Acre, Cruzeiro do Sul, Brazil
Sandro P. Silva, Instituto Evandro Chagas, Ministério da Saúde do Brasil, Ananindeua, Brazil
Stella M. Chenet, Instituto de Investigaciones en Ciencias Biomedicas, Universidad Ricardo Palma, Lima, and Instituto de Enfermedades Tropicales, Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas, Chachapoyas, Peru
Eduardo J.M. Santos, Programa de Pós-Graduação em Biologia de Agentes Infecciosos e Parasitários, Brazil; Laboratório de Genética de Doenças Complexas, Brazil
Luann W. Pereira de Sena, Instituto de Ciências Biológicas, and Laboratório de Farmacocinética de Drogas Antimaláricas, Instituto de Ciências da Saúde, Brazil
Flávia Póvoa da Costa, Programa de Pós-Graduação em Biologia de Agentes Infecciosos e Parasitários, Brazil; Laboratório de Genética de Doenças Complexas, Brazil
Amanda G.N. Cardoso-Mello, Instituto de Ciências Biológicas, and Laboratório de Farmacocinética de Drogas Antimaláricas, Instituto de Ciências da Saúde, Brazil
Paola B. Marchesini, Grupo Técnico da Malária, Coordenação-Geral de Vigilância de Zoonoses e Doenças de Transmissão Vetorial, Departamento de Imunização e Doenças Transmissíveis, Secretaria de Vigilância em Saúde, Ministério da Saúde, Brazil
Cássio R.L. Peterka, Diretoria de Vigilância Epidemiológica, Subsecretaria de Vigilância em Saúde, Secretaria Estadual de Saúde do Distrito Federal, Brasília, Brazil
Giselle M.R. Viana, Instituto Evandro Chagas, Ministério da Saúde do Brasil, Ananindeua, Brazil; Programa de Pós-Graduação em Biologia de Agentes Infecciosos e Parasitários, Brazil
Alexandre Macedo de Oliveira, Malaria Branch, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta
REFERENCES
- 1.Ministério da Saúde. Dados para o cidadão: Malária — Brasil, Sivep-malária e Sinan. (https://public.tableau.com/app/profile/mal.ria.brasil/viz/Dadosparacidado_201925_03_2020/Incio). (In Portuguese.)
- 2.Centers for Disease Control and Prevention. Malaria treatment (United States). November 2, 2020. (https://www.cdc.gov/Malaria/Diagnosis_Treatment/Treatment.Html).
- 3.World Health Organization. Guidelines for the treatment of malaria, third edition. 2015. (https://www.afro.who.int/publications/guidelines-treatment-malaria-third-edition). [PubMed]
- 4.Llanos-Cuentas A, Lacerda MVG, Hien TT, et al. Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019;380:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006;75:402–15. [PubMed] [Google Scholar]
- 6.Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg 1996;55:243–9. [DOI] [PubMed] [Google Scholar]
- 7.Guia de tratamento da malária no Brasil. Brasília, Brazil: Ministério da Saúde, 2020. (https://bvsms.saude.gov.br/bvs/publicacoes/guia_tratamento_malaria_brasil.pdf). (In Portuguese.) [Google Scholar]
- 8.Negreiros S, Farias S, Viana GMR, et al. Efficacy of chloroquine and primaquine for the treatment of uncomplicated Plasmodium vivax malaria in Cruzeiro do Sul, Brazil. Am J Trop Med Hyg 2016;95:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacerda MVG, Llanos-Cuentas A, Krudsood S, et al. Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019;380:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Methods for surveillance of antimalarial drug efficacy. 2009. (https://www.who.int/docs/default-source/documents/publications/gmp/methods-for-surveillance-of-antimalarial-drug-efficacy.pdf?sfvrsn=29076702_2).
- 11.Miranda TA, Silva PHR, Pianetti GA, César IC. Simultaneous quantitation of chloroquine and primaquine by UPLC-DAD and comparison with a HPLC-DAD method. Malar J 2015;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imwong M, Sudimack D, Pukrittayakamee S, et al. Microsatellite variation, repeat array length, and population history of Plasmodium vivax. Mol Biol Evol 2006;23:1016–8. [DOI] [PubMed] [Google Scholar]
- 13.Anderson TJ, Haubold B, Williams JT, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol 2000;17:1467–82. [DOI] [PubMed] [Google Scholar]
- 14.Beck H-P, Wampfler R, Carter N, et al. Estimation of the antirelapse efficacy of tafenoquine, using Plasmodium vivax genotyping. J Infect Dis 2016;213:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci 2020;13:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008;83:234–42. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich DC, Genro JP, Sortica VA, et al. Distribution of CYP2D6 alleles and phenotypes in the Brazilian population. PLoS One 2014;9(10):e110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imwong M, Snounou G, Pukrittayakamee S, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 2007;195:927–33. [DOI] [PubMed] [Google Scholar]
- 19.Imwong M, Boel ME, Pagornrat W, et al. The first Plasmodium vivax relapses of life are usually genetically homologous. J Infect Dis 2012;205:680–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durand S, Cabezas C, Lescano AG, et al. Efficacy of three different regimens of primaquine for the prevention of relapses of Plasmodium vivax malaria in the Amazon Basin of Peru. Am J Trop Med Hyg 2014;91:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebstie YA, Abay SM, Tadesse WT, Ejigu DA. Tafenoquine and its potential in the treatment and relapse prevention of Plasmodium vivax malaria: the evidence to date. Drug Des Devel Ther 2016;10:2387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JK, Battle KE, Howes RE. Primaquine ineligibility in anti-relapse therapy of Plasmodium vivax malaria: the problem of G6PD deficiency and cytochrome P-450 2D6 polymorphisms. Malar J 2018;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daher A, Aljayyoussi G, Pereira D, et al. Pharmacokinetics/pharmacodynamics of chloroquine and artemisinin-based combination therapy with primaquine. Malar J 2019;18:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird JK, Louisa M, Noviyanti R, et al. Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Netw Open 2018;1(4):e181449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett JW, Pybus BS, Yadava A, et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 2013;369:1381–2. [DOI] [PubMed] [Google Scholar]
- 26.St Jean PL, Xue Z, Carter N, et al. Tafenoquine treatment of Plasmodium vivax malaria: suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the phase 2b DETECTIVE trial. Malar J 2016;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avalos S, Mejia RE, Banegas E, et al. G6PD deficiency, primaquine treatment, and risk of haemolysis in malaria-infected patients. Malar J 2018;17:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro WM, Moura-Neto JP, Recht J, Bassat Q, Lacerda MVG. Fatal primaquine-induced hemolysis in a patient with Plasmodium vivax malaria and G6PD A(−) variant in the Brazilian Amazon. Clin Infect Dis 2016;62:1188. [DOI] [PubMed] [Google Scholar]
- 29.Taylor WRJ, Thriemer K, von Seidlein L, et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet 2019;394:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rueangweerayut R, Bancone G, Harrell EJ, et al. Hemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PD Mahidol variant) versus G6PD-normal volunteers. Am J Trop Med Hyg 2017;97:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brito-Sousa JD, Santos TC, Avalos S, et al. Clinical spectrum of primaquine-induced hemolysis in glucose-6-phosphate dehydrogenase deficiency: a 9-year hospitalization-based study from the Brazilian Amazon. Clin Infect Dis 2019;69:1440–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.