Abstract
Purpose
We report the first case of neuroretinitis after administration of a second dose of a messenger RNA vaccine for coronavirus disease-2019 (COVID-19).
Observations
An 83-year-old healthy woman presented with subacute, painless, and progressive visual loss in the right eye that started 2 days after the second injection of the COVID-19 vaccine (Comirnaty®) from Pfizer (New York, NY, USA) and BioNTech (Mainz, Germany). Visual acuities were hand motion perception in the right eye and 20/30 in the left eye. There was optic nerve head swelling in the right eye and subretinal fluid and disruption of the photoreceptor layers in both eyes. Magnetic resonance imaging revealed an enhancement of the right optic nerve, consistent with optic neuritis. She was treated with intravenous corticosteroids, and the optic nerve swelling in the right eye resolved promptly. However, the amount of subretinal fluid worsened for 1 month and did not improve until 6 months from onset. Her visual acuity was slightly improved to finger count perception in the right eye and 20/20 in the left eye during an examination 6 months from onset.
Conclusions and Importance
Considering the temporal relation between the second dose of vaccination and the symptom onset in our patient, the ophthalmic symptoms here reported might be considered a rare adverse effect of the Comirnaty® COVID-19 vaccine. Although a causal relationship is not established, to our knowledge, this is the first report of neuroretinitis after vaccination with Comirnaty®, and any further similar cases should be examined in detail.
Keywords: Neuroretinitis, SARS-CoV-2-vaccine
1. Introduction
The coronavirus disease-2019 (COVID-19) vaccine (Comirnaty®) from Pfizer (New York, NY, USA) and BioNTech (Mainz, Germany) was approved for emergency use by the United States Food and Drug Administration (FDA) on December 11, 2020,1 after clinical trials indicated it had an excellent safety and efficacy profile.2, 3, 4 Following the implementation of the vaccination, reports of immediate side effects, such as anaphylaxis, emerged5,6; however, potential complications of the vaccine that are non-immediate or rare also need to be identified.
Neuroretinitis is an inflammatory disorder of the eye presenting with optic disc swelling with marked peripapillary and macular exudates.7,8 Only a single case report describing a temporal association between chick embryo cell anti-rabies vaccination and neuroretinitis has been published,9 and no other reports of neuroretinitis following COVID-19 vaccination have been released. Herein, we report a case of neuroretinitis after administration of a second dose of the Comirnaty® COVID-19 vaccine.
2. Case presentation
An 83-year-old healthy woman was referred to our clinic with subacute, painless, and progressive visual loss in the right eye for about 1 month. She denied any experience of visual discomfort before this recent onset of unilateral vision loss. The patient had hypertension and hyperlipidemia, which were well-controlled with anti-hypertensive medication and a lipid-lowering agent, and she was not on any other medication. There was no significant surgical history or social history, and she denied any trauma history. She reported that 1 month prior and 2 days before her visual symptoms has started, she had received a second dose of the Comirnaty® COVID-19 vaccine, from Pfizer (New York, NY, USA) and BioNTech (Mainz, Germany), then immediately developed malaise and limb pain. Two days later, the pain in her upper and lower extremities had decreased, but blurriness developed in the inferior visual field of the right eye. Over the next month, the blurriness in her right eye had worsened, and her central vision had deteriorated.
Upon the review of systems, no symptoms other than progressive visual loss in the right eye were noted. During the ocular examination, a visual acuity assessment registered hand motion perception in the right eye and 20/30 in the left eye. Her color vision was decreased to 0/10 in the right eye and 9/10 in the left eye on the Hardy, Rand, and Rittler pseudoisochromatic plates. The pupil reacted normally to direct light in both eyes, though a relative afferent pupillary defect was apparent in the right eye. Findings from ocular motility and anterior segment examinations were normal. Fundus photography and optical coherence tomography revealed optic nerve head swelling in the right eye and subretinal fluid and disruption of the photoreceptor layers in both eyes (Fig. 1). Fluorescein angiography showed multiple focal leakages from the retinal vessels in both eyes (Fig. 1). The Humphrey field analyzer (Carl Zeiss, Oberkochen, Germany) showed a generalized field defect in the right eye and superior arcuate scotoma in the left eye (Fig. 1). Magnetic resonance imaging (MRI) showed a subtle enhancement of the right optic nerve in the retrobulbar area, while MRI findings in the area from the optic nerve head to the chiasm in the left eye were normal (Fig. 2). No periventricular white matter lesion or brain parenchymal lesion was found. Laboratory tests including complete blood cell count with differential, chemistry panel, electrolytes, lipid profile, C-reactive protein level, erythrocyte sedimentation rate, vitamin B12, folate, serum angiotensin-converting enzyme, lupus anticoagulants, antinuclear antibody, anticardiolipin antibodies, neuromyelitis optica immunoglobulin G, serologic tests (for syphilis and human immunodeficiency virus 1 and 2), and paraneoplastic antibody testing with a commercial panel (EUROIMMUN AG, Lübeck, Germany) were all normal. She was given 1 g of intravenous methylprednisolone daily for 3 days, followed by oral prednisolone with a tapering dosage, and the optic nerve swelling in the right eye resolved promptly after this treatment. However, the amount of subretinal fluid slightly increased, and her visual acuity had not improved 1 month after steroid treatment. She was on close follow-up with a retina specialist and 6 months after the first event, visual acuity registered finger count perception in the right eye and 20/20 in the left eye. Follow-up fundus photography showed mild pallor without swelling in the right optic nerve head (Fig. 3). There was no significant change in subretinal fluid and disruption of the photoreceptor layers on optical coherence tomography and visual field defect on Humphrey field analyzer in both eyes (Fig. 3).
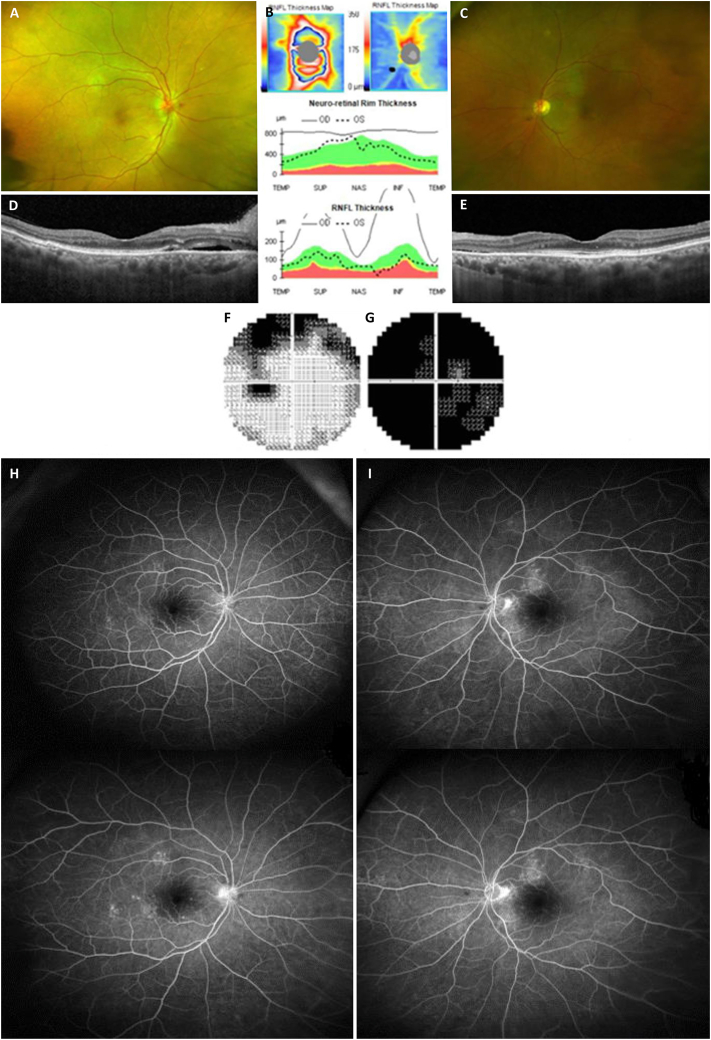
Fig. 1.
Fundus photography (A: right eye, C: left eye) and optical coherence tomography (B: retinal nerve fiver layer thickness and significance map, D: macular cross-sectional image in the right eye, E: macular cross-sectional image in the left eye) revealed optic nerve head swelling in the right eye and subretinal fluid and disruption of the photoreceptor layers in both eyes. The Humphrey field analyzer showed a generalized field defect in the right eye (G) and superior arcuate scotoma in the left eye (F). Fluorescein angiography (H: right eye, I: left eye) showed multiple focal leakages from the retinal vessels without neovascularization at both early (upper images) and late (lower images) phases in both eyes.
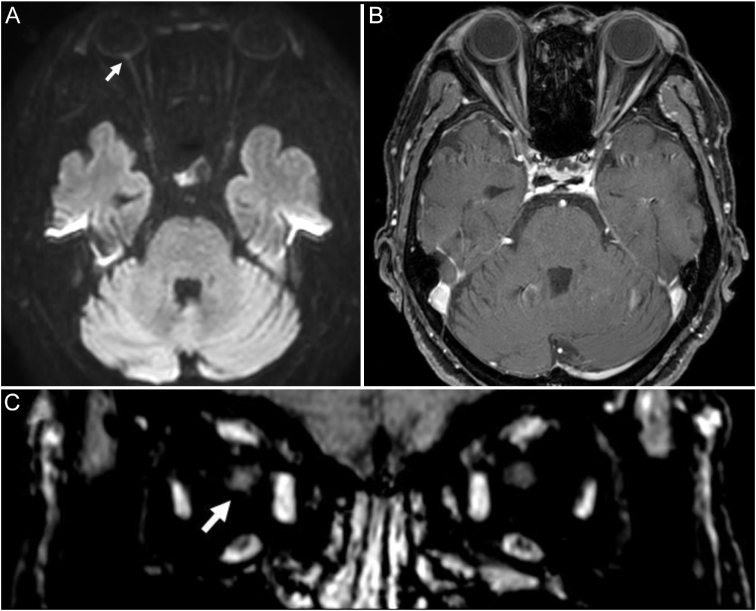
Fig. 2.
Magnetic resonance imaging (MRI) revealed discoid elevation and restricted diffusion in the right optic nerve head on the diffusion-weighted image (A). There was no significant signal change in the axial fat-suppressed T1-weighted image (B); however, the coronal multiplanar reconstruction image showed a subtle enhancement in the right optic nerve (C).
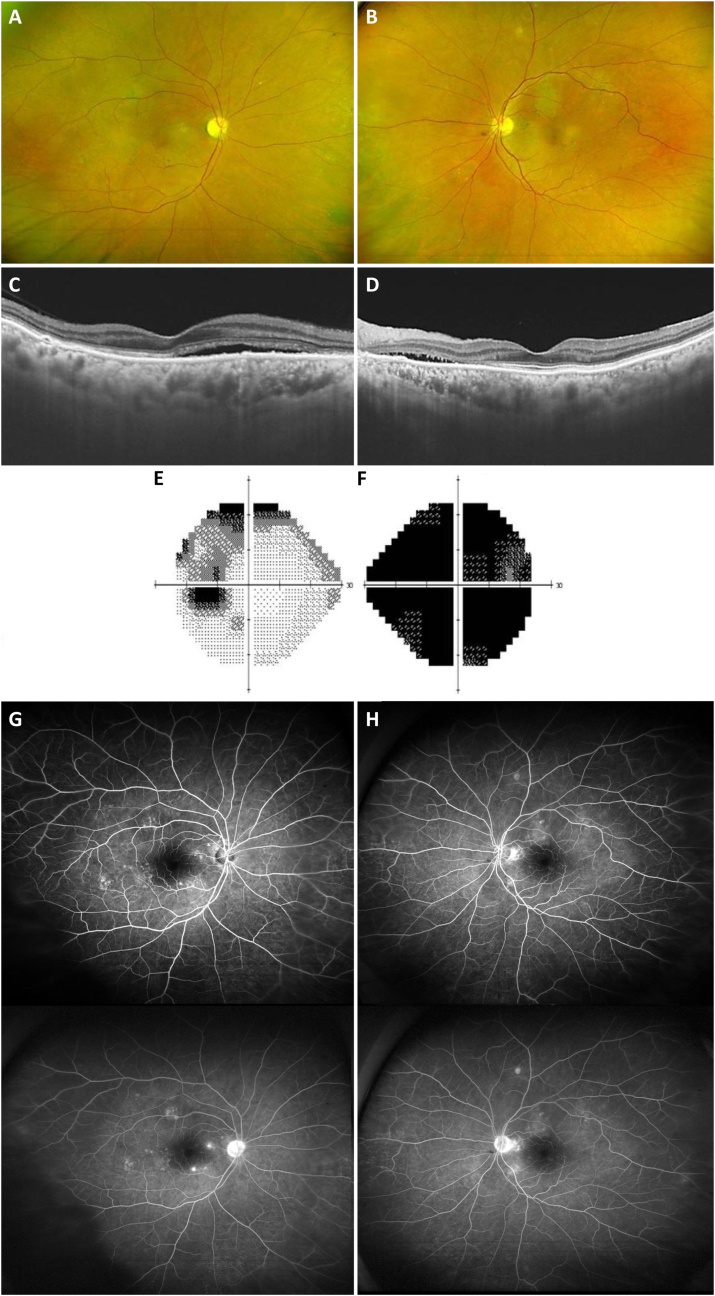
Fig. 3.
Six months after the onset, fundus photography (A: right eye, B: left eye) showed mild pallor without swelling in the right optic nerve head. And subretinal fluid and disruption of the photoreceptor layers on optical coherence tomography (C: right eye, D: left eye) persisted stationary in both eyes. The Humphrey field analyzer showed a superior arcuate scotoma in the left eye (E) and generalized field defect in the right eye (F). Fluorescein angiography (G: right eye, H: left eye) showed a mild increase in focal leakages from the retinal vessels in both eyes.
3. Discussion and conclusions
The Comirnaty® COVID-19 vaccine, a nucleoside-modified messenger RNA vaccine that encodes the full length of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, was the first COVID-19 vaccine approved by the United States Food and Drug Administration.1 Its most common side effects are pain and redness at the site of injection, fever, chills, and headache, although diarrhea, nausea, vomiting, and dermatitis have also been reported.2 Most documented symptoms occur after the second injection, typically starting the first or second day after the injection and lasting for days.2
Several ocular conditions have been reported following the administration of various vaccines.10, 11, 12, 13 Uveitis, a form of eye inflammation that is characterized by blurred and decreased vision, floaters, and sensitivity to light, is one of the serious adverse reactions found to be associated with vaccination.10 Interestingly, many cases of vaccine-induced uveitis are related to the hepatitis B vaccine, which contains the HepB surface antigen, inserted into yeast cells using recombinant DNA technology.10 Flu vaccination has also been reported to be associated with numerous ophthalmic adverse effects; in addition to uveitis,10 multiple evanescent white dot syndrome14 and a variety of types of inflammation involving the optic nerve, including optic nerve head inflammation,15 retrobulbar optic nerve inflammation,16 and acute macular neuroretinopathy17 have been documented. Optic neuritis has also been reported to be associated with administration of the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap)-inactivated polio vaccine,18 hepatitis A virus (HAV) vaccine and typhoid fever vaccine,19 measles vaccine,20 anti-rabies vaccine,21,22 Tdap vaccine,23 and varicella-zoster virus vaccine.24 Although the exact mechanism is unknown, vaccine-induced optic neuritis is believed to occur because the T-cells activated by vaccines cross the blood-brain barrier, resulting in type IV hypersensitivity.25,26 Neuroretinitis, an inflammatory disorder of the optic nerve head associated with exudates or edema around the macula,7,8 was associated with anti-rabies vaccination in the case report9; of a patient who developed acute, painless visual loss 1 day after the third injection and 5 days after the second injection of a chick embryo cell anti-rabies vaccine (i.e., injection days 0, 3, and 7 after a dog bite).9 The patient showed optic disc swelling and macular exudates in both eyes.9 Our case did not show typical macular star or subretinal fluid affecting the foveal center, and MRI revealed inflammation extending to the retrobulbar area; however, certain findings, including predominant optic nerve head inflammation and exudates and swelling from the disc distributed toward the macular area were considered to be compatible with neuroretinitis.
The etiology of neuroretinitis is generally divided into infectious and non-infectious causes, and non-infectious cases can be idiopathic or associated with systemic inflammatory disorders.13 In the previously reported case with neuroretinitis and our case, the visual symptoms occurred 5 days (1 day after the third injection)9 and 2 days, respectively, after the second vaccination. Cheng and Margo suggested that adverse events occurring within days to 1 month after vaccination suggest that the occurrences may not be coincidental.13 Optic neuropathies occurring within hours of a booster vaccination might be attributed to an immediate hypersensitivity reaction, while those presenting 2–3 weeks after exposure are consistent with a B- or T-cell–mediated reaction.12,13,27,28
The optimum treatment strategy for noninfectious neuroretinitis is unclear, and there is no class 1 evidence to support a specific treatment strategy.29 In our case, the optic nerve swelling promptly responded to steroid treatment. However, the subretinal fluid slightly increased 1 month after treatment and remained for a long time. The visual acuity showed improvement in both eyes but was not fully recovered. The cause of the partial improvement in vision in our case might be attributed to the effects of both photoreceptor disruption and continued subretinal fluid. We presume that a complex mechanism involving not only neuroretinitis was responsible for the clinical course after the acute phase. The initial clinical features, such as optic nerve edema, optic nerve enhancement on MRI, and accompanying subretinal fluid and photoreceptor disruption, are presumed to be mainly due to neuroretinitis. Subretinal fluid, which was increased after 1 month of steroid treatment in this patient and continued thereafter, is presumed to have a similar mechanism to central serous retinopathy. A high dose of steroid treatment in the setting of neuroretinitis could have induced decompensation of the retinal pigment epithelium, central serous retinopathy-like feature. To confirm the baseline pathomechanism of this case and seek an optimal treatment strategy, a longer-term follow-up and analysis of other similar cases are necessary.
Considering the temporal relationship between the second COVID-19 vaccine and symptom onset in our patient, the ophthalmic symptoms here reported might be a rare adverse effect of the Comirnaty® COVID-19 vaccine. Although a causal relationship is not established, to our knowledge, this is the first report of neuroretinitis after vaccination with Comirnaty®, and any further similar cases should be examined in detail.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Samsung Medical Center. We adhered to the ethical standards laid out in the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this case report. The funder had no role in the study concept, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2021R1A2C1007718) to Kyung-Ah Park.
Study funding
The authors report no targeted funding.
Authors’ contributions
C. L. and K. A. P. analyzed the data and finished drafting the manuscript. G. I. L. provided technical support. K. A. P., D. I. H., and S. Y. O. provided critical revision of the manuscript. K. A. P. was responsible for supervision. All authors have read and approved the final manuscript.
Disclosures
The authors report no disclosures relevant to this manuscript.
Consent for publication
Written consent for publication was obtained from the patient for publication of this case report and any accompanying images.
Availability of data and materials
All data supporting the findings are contained within the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Contributor Information
Kyung-Ah Park, Email: kparkoph@skku.edu.
Don-Il Ham, Email: oculus@naver.com.
References
- 1.FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers): Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Revised January 2021. Available at http://lps3.www.fda.gov.libproxy.samsunghospital.com/media/144413/download. Accessed January 17, 2021.
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenck R.W., Jr., Klein N.P., Kitchin N., et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis After receipt of the first dose of pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Team C.C.-R., Food Drug A. Allergic reactions including anaphylaxis After receipt of the first dose of pfizer-BioNTech COVID-19 vaccine - United States, december 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelhakim A., Rasool N. Neuroretinitis: a review. Curr Opin Ophthalmol. 2018;29(6):514–519. doi: 10.1097/ICU.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 8.Maitland C.G., Miller N.R. Neuroretinitis Arch Ophthalmol. 1984;102(8):1146–1150. doi: 10.1001/archopht.1984.01040030924014. [DOI] [PubMed] [Google Scholar]
- 9.Saxena R., Sethi H.S., Rai H.K., Menon V. Bilateral neuro-retinitis following chick embryo cell anti-rabies vaccination--a case report. BMC Ophthalmol. 2005;5:20. doi: 10.1186/1471-2415-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benage M., Fraunfelder F.W. Vaccine-associated uveitis. Mo Med. 2016;113(1):48–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Santovito L.S., Pinna G. Acute reduction of visual acuity and visual field after Pfizer-BioNTech COVID-19 vaccine 2nd dose: a case report. Inflamm Res. 2021 doi: 10.1007/s00011-021-01476-9.1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stubgen J.P. A literature review on optic neuritis following vaccination against virus infections. Autoimmun Rev. 2013;12(10):990–997. doi: 10.1016/j.autrev.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J.Y., Margo C.E. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2021 doi: 10.1016/j.survophthal.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Samra A., Tarabishy A.B. Multiple evanescent white dot syndrome following intradermal influenza vaccination. Ocul Immunol Inflamm. 2019;27(4):528–530. doi: 10.1080/09273948.2017.1423334. [DOI] [PubMed] [Google Scholar]
- 15.Jun B., Fraunfelder F.W. Atypical optic neuritis after inactivated influenza vaccination. Neuro Ophthalmol. 2018;42(2):105–108. doi: 10.1080/01658107.2017.1335333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford C.M., Grazko M.B., Raymond WRt, Rivers B.A., Munson P.D. Retrobulbar optic neuritis and live attenuated influenza vaccine. J Pediatr Ophthalmol Strabismus. 2013;50(1):61. doi: 10.3928/01913913-20121108-01. [DOI] [PubMed] [Google Scholar]
- 17.Shah P., Zaveri J.S., Haddock L.J. Acute macular neuroretinopathy following the administration of an influenza vaccination. Ophthalmic Surg Lasers Imaging Retina. 2018;49(10):e165–e168. doi: 10.3928/23258160-20181002-23. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien P., Wong R.W. Optic neuritis following diphtheria, tetanus, pertussis, and inactivated poliovirus combined vaccination: a case report. J Med Case Rep. 2018;12(1):356. doi: 10.1186/s13256-018-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Dowd S., Bafiq R., Ryan A., Cullinane A., Costello D. Severe bilateral optic neuritis post hepatitis A virus (HAV) and typhoid fever vaccination. J Neurol Sci. 2015;357(1-2):300–301. doi: 10.1016/j.jns.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 20.Joshi J., Seth A., Aneja S., Singh A.K., Aggarwal M.K., Gupta N. Rapid onset optic neuritis following measles vaccine in India: case report. Vaccine Reports. 2016;6:86–88. [Google Scholar]
- 21.Agarwal A., Garg D., Goyal V., Pandit A.K., Srivastava A.K., Srivastava M.P. Optic neuritis following anti-rabies vaccine. Trop Doct. 2020;50(1):85–86. doi: 10.1177/0049475519872370. [DOI] [PubMed] [Google Scholar]
- 22.Dadeya S., Guliani B.P., Gupta V.S., Malik K.P., Jain D.C. Retrobulbar neuritis following rabies vaccination. Trop Doct. 2004;34(3):174–175. doi: 10.1177/004947550403400319. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera-Maqueda J.M., Hernandez-Clares R., Baidez-Guerrero A.E., Pio-Rendon J.I.B., Fernandez J.J.M. Optic neuritis in pregnancy after Tdap vaccination: report of two cases. Clin Neurol Neurosurg. 2017;160:116–118. doi: 10.1016/j.clineuro.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Han S.B., Hwang J.M., Kim J.S., Yang H.K. Optic neuritis following Varicella zoster vaccination: report of two cases. Vaccine. 2014;32(39):4881–4884. doi: 10.1016/j.vaccine.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Michael N.D.B., Jaffar T.N.T., Hussein A., Hitam W.-H.W. Simultaneous bilateral optic neuritis following human papillomavirus vaccination in a young child. Cureus. 2018;10 doi: 10.7759/cureus.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shams P., Plant G. Optic neuritis: a review. Int MS J. 2009;16:82–89. [PubMed] [Google Scholar]
- 27.Moradian S., Ahmadieh H. Early onset optic neuritis following measles-rubella vaccination. J Ophthalmic Vis Res. 2008;3(2):118–122. [PMC free article] [PubMed] [Google Scholar]
- 28.Arshi S., Sadeghi-Bazargani H., Ojaghi H., et al. The first rapid onset optic neuritis after measles-rubella vaccination: case report. Vaccine. 2004;22(25-26):3240–3242. doi: 10.1016/j.vaccine.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 29.Fairbanks A.M., Starr M.R., Chen J.J., Bhatti M.T. Treatment strategies for neuroretinitis: current options and emerging therapies. Curr Treat Options Neurol. 2019;21(8):36. doi: 10.1007/s11940-019-0579-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings are contained within the manuscript.