Abstract
American foulbrood is a disease of larval honeybees (Apis mellifera) caused by the bacterium Paenibacillus larvae. Over the years attempts have been made to develop a selective medium for the detection of P. larvae spores from honey samples. The most successful of these is a semiselective medium containing nalidixic acid and pipermedic acid. Although this medium allows the growth of P. larvae and prevents the growth of most other bacterial species, the false-positive colonies that grow on it prevent the rapid confirmation of the presence of P. larvae. Here we describe a PCR detection method which can be used on the colonies that grow on this semiselective medium and thereby allows the rapid confirmation of the presence of P. larvae. The PCR primers were designed on the basis of the 16S rRNA gene of P. larvae and selectively amplify a 973-bp amplicon. The PCR amplicon was confirmed as originating from P. larvae by sequencing in both directions. Detection was specific for P. larvae, and the primers did not hybridize with DNA from closely related bacterial species.
American foulbrood, caused by the spore-forming bacterium Paenibacillus larvae (White), is the most serious disease of honeybee broods around the world and causes considerable economic losses to beekeepers. The only known host of this bacterium is the honeybee Apis mellifera. In the field the disease is detected by inspection, and a positive diagnosis is based on clinical symptoms. In an infected colony, spores from P. larvae can be isolated from honey, wax, pollen, and hive walls (5). It has been reported that the P. larvae spores can remain infective for at least 35 years (10). The disease spreads when spores are transported on drifting bees, hive parts, clothing, and contaminated pollen or honey (4). The examination of honey for spores may therefore be of value in tracing disease outbreaks, and there have been a number of studies using honey for this purpose (1, 6, 7, 13, 14). The detection of these inapparent or latent infections would identify sources of pathogens which may cause fully developed disease in these hives or spread of infection to other hives.
P. larvae was previously grouped in the genus Bacillus. However, comparative 16S rRNA gene sequence analysis has demonstrated that the genus Bacillus consists of at least five phyletic lines (2). P. larvae and some other close relatives have subsequently been assigned to the new genus Paenibacillus (2). The other bacteria in this genus include P. polymyxa and P. alvei, which have also been found in honeybees, although they have not been demonstrated to be pathogenic.
There have been a number of reports describing media for the growth of P. larvae spores from honey samples. Hansen and colleagues (6–9) developed a technique to detect P. larvae spores by direct inoculation onto J agar of undiluted honey samples that had been heated to 80°C for 5 min. The J agar consisted of 0.5% tryptone (Oxoid), 0.3% K2HPO4, 1.5% yeast extract (Difco, Detroit, Mich.), 2% agar, and 0.2% glucose (autoclaved separately); the pH was 7.3 to 7.5. Shimanuki and Knox (13) employed an alternative method which involved the dilution of honey samples, dialysis, centrifugation, resuspension, and heat treatment of honey before its inoculation onto brain heart infusion agar. These techniques, while allowing for the growth of P. larvae, did not suppress the growth of the many other Bacillus species contaminating honey samples. First attempts to produce a medium selective for P. larvae were carried out by Hornitzky and Clark (11). Their method involved centrifugation of the diluted honey samples, heat treatment of the sediment, and culturing onto sheep blood agar plates containing nalidixic acid to prevent the development of motile colonies of P. alvei. The incorporation of nalidixic acid in the culture medium inhibited the growth of P. alvei but other Bacillus species overgrew the plates, making it difficult to detect the presence of P. larvae (1). Alippi (1) described a semiselective medium that incorporated nalidixic acid and pipermidic acid in J agar. This medium successfully isolated P. larvae while at the same time preventing the development of most other Bacillus and Paenibacillus species which normally develop on plates before P. larvae spores can germinate.
Prior to performing a nationwide survey of South African honeybee diseases we have investigated the most suitable methods for bee pathogen detection. Although the semiselective medium reported by Alippi (1) seems to be the most suitable for the screening of bulk honey samples for the presence of P. larvae, it has not been found to be completely selective. In the present study other Bacillus species occasionally grew on J agar plates containing nalidixic acid and pipermidic acid, regardless of the concentration of these antibiotics used, during the screening of bulk honey samples (data not shown). While these colonies were very few compared to the total number of colonies growing on parallel J agar plates without nalidixic acid or pipermidic acid, these false-positive colonies made it impossible to categorically confirm the presence of P. larvae by colony growth alone.
Here we describe a PCR detection technique that can be used on colonies that are able to grow on the semiselective medium to quickly and unambiguously determine the presence of P. larvae. The complete procedure takes less than 4 h. PCR primers were designed on the basis of the 16S rRNA gene of P. larvae (GenBank accession no. X60619). Since the 16S rRNA gene has remained largely unchanged throughout the evolution of bacteria, very small differences in sequences from one bacterial species to another can be used to classify and identify organisms correctly (3). The PCR primers used here were based on a region of the P. larvae 16S rRNA gene that was not homologous to other closely related bacterial 16S rRNA genes deposited in the available databases. The primers amplify a 973-bp PCR amplicon unique to P. larvae. Primer 1 (5′ AAGTCGAGCGGACCTTGTGTTTC 3′) was compared to all known DNA sequences in the available databases and showed homology only to P. larvae (100%), on which its design was based. When primer 1 was visually compared to the same genome region of the other closely related Paenibacillus species, it was found to have 7 nucleotide differences compared to P. alvei and 10 nucleotide differences compared to P. polymyxa (Table 1). Primer 2 (5′ GGAGACTGGCCAAAACTCTATCT 3′) had 100% homology to P. larvae, on which its design was based. When compared to other closely related Paenibacillus species, it was found to have three nucleotide differences compared to P. polymyxa and nine nucleotide differences compared to P. alvei. Table 1 shows alignment of primer 1 and primer 2 to nucleotide sequences of two closely related Paenibacillus species and of two Bacillus species, including Bacillus subtilis, the Bacillus type species. All of the bacterial species shown in Table 1 have been previously found in honeybees.
TABLE 1.
Comparison of P. larvae PCR primer sequences to nucleotide sequences of other speciesa
| P. larvae primer and species | Sequence | % Identity to P. larvae |
|---|---|---|
| Primer 1 | AAGTCGAGCGGACCTTGTGTTTC | 100 |
| P. alvei | AAGTCGAGCGGACTTGATGGAGT | 70 |
| P. polymyxa | AAGTCGAGCGGGGTTAATTAGAA | 57 |
| B. pumilus | AAGTCGAGCGGACAGAAGGGAGC | 66 |
| B. subtilis | AAGTCGAGCGGACAGATGGGAGC | 66 |
| Primer 2 | GGAGACTGGCCAAAACTCTATCT | 100 |
| P. alvei | ACTTACTGGCGGGATCTCTATCC | 61 |
| P. polymyxa | GGAGACTGGCCAGATCTCTATCC | 88 |
| B. pumilus | GGAGACTGTTGGGATCTCTATCC | 70 |
| B. subtilis | GGAGACTGTTAGGATCTCTATCC | 70 |
The nucleotide sequences of P. larvae PCR primers and nucleotide sequences of two closely related Paenibacillus species and two Bacillus species were compared. The bacteria used in this comparison have all been previously isolated from bee honey samples. Nucleotide sequence identity with P. larvae is indicated in boldface type.
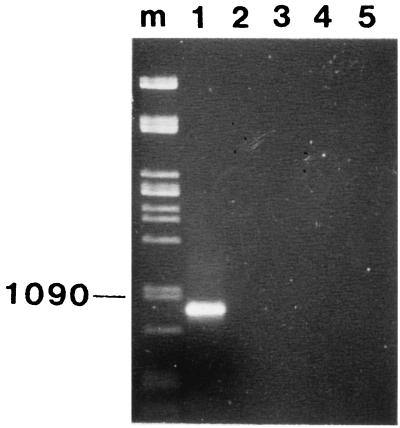
The PCR primers were tested against five bacteria: P. larvae, P. alvei, P. polymyxa, Bacillus pumilus, and B. subtilis. All of these bacteria have been previously reported for honeybees (1), and all except P. larvae were found and isolated in the present study. The P. larvae type strain was obtained from the Belgium culture collection (LMG 9820). A colony of each bacterial strain was suspended in 50 μl of distilled water and heated to 95°C for 15 min. Following centrifugation at 5,000 × g for 5 min, 1 μl of the supernatant was amplified in a 50-μl PCR mixture in a Hybaid OMN-E thermocycler in 700-μl PCR tubes. The PCR was optimized by using the following: 2 mM MgCl2, 50 pmol of each primer per μl, a 25 mM concentration of each deoxynucleoside triphosphate, and 1 U of Taq polymerase per μl. The PCR conditions consisted of a 95°C (1-min) step; 30 cycles of 93°C (1 min), 55°C (30 s), and 72°C (1 min); and a final cycle of 72°C (5 min). The molecular weights of the PCR products were determined by electrophoresis in a 0.8% agarose gel and staining with ethidium bromide. Under these PCR conditions, only the P. larvae type strain produced a PCR product. As expected, this product banded just below the 1,090-bp lambda Pst marker; none of the other Bacillus or Paenibacillus species produced a PCR product under these PCR conditions (Fig. 1). The sensitivity of this method had a detection limit of 50 CFU/ml (data not shown).
FIG. 1.
Agarose gel (0.8%) showing PCR products from different lysed bacterial species (pure cultures). Lanes: m, Pst lambda DNA marker (the 1,090-bp band is indicated); 1, P. larvae; 2, P. alvei; 3, P. polymyxa; 4, B. pumilus; 5, B. subtilis.
To confirm that the PCR product was from P. larvae, the PCR product was cloned into pCR-Script Amp SK(+) cloning vector and transformed using the pCR-Script Amp SK(+) cloning kit (Stratagene). Plasmids were purified with the Nucleobond kit (Maherey-Nagel) for plasmid isolation. The PCR product was sequenced in both directions by standard methods (12). A sequence similarity search was done by using the Blast server at the National Center for Biotechnology Information. The PCR product was found to be 973 bp long, which was the same size as the region on the P. larvae 16S rRNA gene between the two primers. An alignment of the PCR product and of the P. larvae 16S rRNA gene sequence showed the two sequences to be identical. Figure 2 shows the sequence of this 973-bp PCR amplicon.
FIG. 2.
Nucleotide sequence of the 973-bp P. larvae PCR amplicon. The PCR binding regions are indicated in boldface type.
Since the only reported semiselective medium for the isolation of P. larvae does not eliminate all spore-forming Bacillus species found in beehives, it is necessary to have additional techniques to confirm the isolation of P. larvae on this medium. Although several tests are available to do this, the PCR technique described here is particularly useful because of the short time required to carry it out and the certainty of the results obtained. Since primer 1 used here had no significant homology to any other Bacillus or Paenibacillus species, it was not possible for the PCRs carried out here to detect any Bacillus or Paenibacillus species other than P. larvae. This was confirmed here against four nonpathogenic Bacillus and Paenibacillus species that have previously been isolated from honeybees. It would be interesting to carry out this PCR technique directly on field-infected bees. This was not possible in the present survey because there have been no confirmed reports of American foulbrood in southern Africa and strict laws in South Africa prohibit the importation of P. larvae-infected bees, even for research purposes.
Acknowledgments
This work was partly funded by the Foundation for Research Development, Pretoria, South Africa.
REFERENCES
- 1.Allipi A M. Detection of Bacillus larvae spores in Argentinian honeys by using a semi-selective medium. Microbiol Semin. 1995;11:343–350. [PubMed] [Google Scholar]
- 2.Ash C, Priest F G, Collins M D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks, and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Leeuwenhoek. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- 3.Avaniss-Aghjani K, Jones K, Chapman D, Brunk C. A molecular technique for identification of bacteria using small subunit rRNA sequences. BioTechniques. 1994;17:144–146. [PubMed] [Google Scholar]
- 4.Delaplane K S. American foulbrood. Am Bee J. 1991;131:700–702. [Google Scholar]
- 5.Gochnauer T A. The distribution of Bacillus larvae spores in the environs of colonies infected with American foulbrood disease. Am Bee J. 1981;121:332–335. [Google Scholar]
- 6.Hansen H. Methods for determining the presence of the foulbrood bacterium Bacillus larvae in honey. Dan J Plant Soil Sci. 1984;88:325–328. [Google Scholar]
- 7.Hansen H. The incidence of the foulbrood bacterium Bacillus larvae in honeys retailed in Denmark. Dan J Plant Soil Sci. 1984;88:329–336. [Google Scholar]
- 8.Hansen H, Rasmussen B. The investigation of honey from bee colonies for Bacillus larvae. Dan J Plant Soil Sci. 1986;90:81–86. [Google Scholar]
- 9.Hansen H, Rasmussen B, Christensen F. A preliminary experiment involving induced infection from Bacillus larvae. Dan J Plant Soil Sci. 1988;92:11–15. [Google Scholar]
- 10.Haseman L. How long can spores of American foulbrood live? Am Bee J. 1961;101:298–299. [Google Scholar]
- 11.Hornitzky M A Z, Clark S. Culture of Bacillus larvae from bulk honey samples for the detection of American foulbrood. J Apic Res. 1991;30:13–16. [Google Scholar]
- 12.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimanuki H, Knox D A. Improved method for the detection of Bacillus larvae spores in honey. Am Bee J. 1988;128:353–354. [Google Scholar]
- 14.Steinkraus K H, Morse R A. American foulbrood incidence in some US and Canadian honeys. Apidologie. 1992;23:497–500. [Google Scholar]