Abstract
Atopic dermatitis (AD) is a prevalent protracted inflammatory skin condition that affects approximately 12% of children globally. Topical remedies, such as pharmacologic and nonpharmacologic management, and off-label systemic medicines, have traditionally been used to treat pediatric AD patients. To minimize comorbidities, sleep disturbances, pruritus, and signs of inflammation and improve the patient’s quality of life, it is vital to optimize severe AD management in pediatric patients. Treatment resistance can be caused by a variety of circumstances, including deficient obedience or inappropriate medicine usage, a shortage of adequate pharmaceuticals, hypersensitivity reciprocation to local application of therapeutics, cutaneous infections, and other infuriating ecological provoking factors. If these elements are eliminated, a skin biopsy is required to exclude other AD-like cutaneous disorders. New regimens that target peculiar avenues with improved proficiency and promise minimal adverse events have resulted from recent developments and understanding of the etiology of AD. Although the condition of most patients improves quickly with this treatment, some do not respond well. In this review, the author discusses the management of treatment-resistant atopic dermatitis, with an emphasis on the pediatric population.
Keywords: atopic dermatitis, pediatrics, skin disorder, systemic medicines, treatment resistance
What is known about this subject in regard to women and their families?
Atopic dermatitis is the most frequently encountered skin disease in pediatric dermatology.
About half of all children with atopic dermatitis have a negative influence on their quality of life, which ultimately have detrimental effect on women and their families.
Very few studies have addressed the problems confronted by treatment-resistant atopic dermatitis in pediatric population.
What is new from this article as messages for women and their families?
Treatment resistance in pediatric atopic dermatitis patients can be caused by deficient obedience or inappropriate medicine usage, a shortage of pharmaceuticals, hypersensitivity reciprocation to local application of therapeutics, cutaneous infections, and other infuriating ecological provoking factors.
If treatment-resistant root-causes are ruled out, skin biopsy is necessary to exclude other cutaneous disorders.
A systemic flowchart can be employed for integrated management of treatment-resistant or refractory cases of atopic dermatitis in pediatric population, which is ultimately summary of guidelines from major global dermatological societies. This compendious review also provides the insights into contemporary status of modern medicines into the treatment aspect of pediatric atopic dermatitis.
Introduction
Atopic dermatitis (AD) is an itchy, incendiary cutaneous condition commonly observed in children. An impaired cutaneous barrier and dysregulated inflammation are hallmarks of AD. Inflammation in acute AD is attributed to T-helper (Th)-2 cells and increased predisposition to skin infections. About half of all children with AD have a negative influence on their quality of life.1 A variety of mechanisms contribute to the pathogenesis of AD, including changes in the skin microbiome, immune retort deficiencies, dysregulation of innate immune responses, and skin barrier deficiencies. Dominant predicaments that induce AD encompass the categorical ancestry of atopy and retrogressive shift in the filaggrin gene.2,3
Many cohort studies on filaggrin mutations in AD have found that filaggrin transfigurations can propagate AD in 25–50% of patients.4 A study by Wadonda-Kabondo et al.5 reported that there was a link between childhood AD and parental eczema with an odds ratio 2.72 (95% CI, 2.09 to 3.53) for eczema in both parents.
According to the current guidelines for AD treatment, most cases of AD can be successfully controlled by eliminating aggravating factors, practicing proper skincare, and using topical medicines.6 Because pediatric AD is chronic and recurring, it necessitates both maintenance and active therapy to maintain the integrity of the cutaneous roadblock and to avoid imminent fierce episodes. Systemic remedies are approved for patients who have exiguous disorder restraint, notwithstanding pertinent therapies with local medications and/or phototherapy.7
A child with moderate-to-severe AD can have as much as a 50% risk of developing asthma and a 75% risk of developing hay fever.8 Severe AD covers larger areas of the skin and is extremely itchy and associated with rash. Systemic regimens are frequently necessary for severe AD, even though they have fluctuating intensities of efficiency and divergent adverse event (AE) vignettes that demand regular monitoring and counseling. Although phototherapy is helpful in the treatment of challenging astringent patients with AD, various factors, such as cost and accessibility, can limit its efficacy and utility. As a result, new treatments for AD are being developed that target specific pathways.3,9 Dupilumab was the first biological medicine introduced for moderate-to-severe AD. Although this medicine helps in rapidly improving the condition of most patients, some patients do not respond well. Even after these systemic therapies have been prescribed, a small number of patients continue to have widespread severe pruritus and skin lesions, causing physical and emotional distress. Skin infections, hypersensitivity reactions to topical therapies, a lack of access to appropriate medications, inappropriate medication use or poor compliance, and other exacerbating environmental triggers must all be evaluated in cases of treatment resistance. If these factors are ruled out, skin biopsy is necessary to exclude other cutaneous disorders.3 In this comprehensive review, the author discusses the management of treatment-resistant refractory AD in the pediatric population.
Epidemiology
AD is the most frequently encountered skin disease in pediatric dermatology. AD in children is a chronic, pruritic, inflammatory disorder of the skin. It is estimated that 10–20% of children in developed countries are affected. Recently, Silverberg et al.10 conducted an international web-based survey to estimate the prevalence of AD among pediatric populations in 18 different countries. Based on the survey, the authors reported that the prevalence of AD among pediatric populations ranged between 2.7% and 20.1% across countries.
Management of AD
A long-term strategy that includes treatment, trigger avoidance, excellent skin care, and education is vital.11,12 Whether used as a condensed blueprint to contain a flare or as a durable sustainment plan, topical or systemic medication can help; the therapeutic agent used is critical. Moreover, factors related to the patient, disease, and treatment must be considered. As novel molecular and biological treatments focusing on disease pathways have been discovered, the treatment landscape for pediatric AD, just like any other disease, is constantly changing.
Standard of care
Therapeutic protocols have been endorsed by distinct dermatologic federations. The collation of treatment standards in the United States and European countries portraying distinct sectors was the topic of the guideline comparison.13 Usually, topical corticosteroids (TCS) should be used initially, followed by topical calcineurin inhibitors as the second-line therapy.14 More aggressive therapy is advised in cases with severe disease symptoms, although they often have a poor safety profile for patients.
Nonpharmacologic treatments
Moisturizers and emollients
Emollients are essential in preventing, managing, and maintaining pediatric AD as skin barrier disruption is critical to disease etiology, which leads to transepidermal water loss and marked xerosis. Emollients are moisturizing factors that impede the depletion of water and provide conservative layering. Unscented emollients are advised for all pediatric patients with AD because they assist in restoring the integrity of the skin barrier. Using an ointment help alleviate preservative induced stinging.15
Pertinent utilization of moisturizers curtails the requirement of aggressive therapy and diminishes the chances of flare-ups. Globally, dermatologists recommend liberal use of moisturizers 2–3 times per day with average utilization of 250 g of moisturizers per week. Therapeutic moisturizers which have peer-reviewed clinical efficacy data for cutaneous barrier improvement should be recommended with consideration of cost.16 Dermatologists recommend that after bath, pediatric AD patients should be dried gently, and subtle water should be left on skin which feels damp. Next, thick layer of eczema medication (depending on disease stage) cream should be applied within 3 minutes of bathing followed by slathering of moisturizer.17
Bathing
Patients should bathe in warm water and use moisturizers shortly after.6 While most patients with AD prefer to take a shower, soaking in a bathtub filled with dilute sodium hypochlorite (“bleach baths”) can help reduce the severity of disease; nevertheless, studies are mixed, and bleach baths may not be any more effective than conventional water baths.18,19
Research in pediatric AD regarding bathing frequency is limited. However, an interesting randomized, cross-over study comparing frequent (“wet method”) versus infrequent (“dry method”) soak and seal baths demonstrated that wet method, twice-daily soak, and seal bath, reduce the SCORing AD (SCORAD) by 21.2. The same study also depicted more than 30% improvement for wet method. Even AD severity (AD Quickscore) also demonstrated significant improvement. Wet method, that is, twice-daily soak and seal bath is recommended as an acute treatment intervention in moderate-to-severe cases of pediatric AD.20 Bathing with body wash containing lipids can help in curtailing the requirement of corticosteroid and improves the healthy skin microorganisms in comparison with a mild synthetic bar soap.21
Skincare products
Patients should be counseled to use high-quality laundry and skincare products in general that are hypoallergenic and fragrance-free.
Alternative Therapies
Most alternative therapies for treating pediatric AD have limited evidence to support them. Even though the research is growing, many studies lack sufficient evidence to demonstrate effectiveness.6
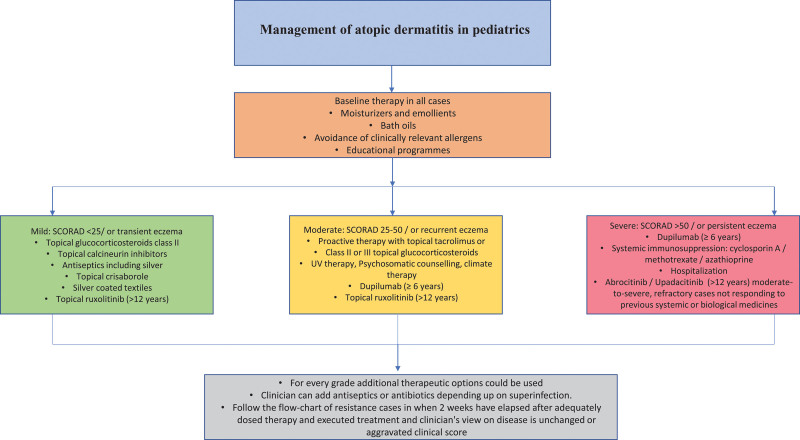
A brief outline of AD management in pediatric patients at various severity levels is shown in Figure 1.
Fig. 1.
Management of pediatric atopic dermatitis.
Pharmacologic treatments
Local application of corticosteroids
TCSs are the linchpins of local therapeutics for pediatric AD. They act by binding to the host’s DNA’s glucocorticoid response sites. This limits the release of proinflammatory cytokines by interfering with specific immune cells’ antigen processing.15 Topical corticosteroids have been approved by the FDA as the first line treatment in AD patients of any age. Apply medium-potency to high-potency topical corticosteroids twice-daily to pediatric with AD. Even once daily application of medium-to-high-potency TCSs for pediatric patients with AD is helpful. Cutaneous atrophy and striae are common side effects associated with local application of corticosteroids.22
A rare-but-significant side effect is the suppression of the hypothalamic-pituitary-adrenal axis by virtue of systemic penetration of corticosteroids on the grounds that the pediatric population has an elevated body surface area to volume ratio.23 TCS systemic absorption sufficient to have harmful effects is uncommon, although it has been linked to impaired linear development in children, decreased bone density and hypothalamic-pituitary-adrenal axis suppression in adults. Because of their large body surface area to weight ratio, babies and children are especially vulnerable to systemic absorption.24 Low potency TCSs are appropriate for minor disease forms and sensitive places, including the axilla, groin, and face.25 During a flare, wet wrap therapy with a medium-potency TCS or an ointment moisturizer can be used.26
Pustular eruptions, hypertrichosis, hypopigmentation, telangiectasia, skin atrophy, infection, and striae are all possible local side effects. Thinner skin (flexures, younger age, and face), high-potency TCS, prolonged and continuous use, and occlusion further increase these risks. Because local absorption of TCS can cause posterior subcapsular cataracts and glaucoma, continuous periopthalmic use is particularly problematic. Local application of corticosteroids can be used in amalgamation with calcineurin inhibitors, keratolytic, tar, and topical vitamin D analogs to improve efficacy and prevent AEs.15
Steroid phobia continues to be present in parents of AD patients. Socioeconomic status, cultural tendencies, education, type of healthcare system and time allotted to patients affect this phobia. However, video-assisted elucidation, written pamphlets, practical demonstrations by nurses, and establishing doctor–patient trust can alleviate steroid phobia.27
Topical phosphodiesterase 4 inhibitors
Phosphodiesterase 4 (PDE4) inhibitors are a novel class of nonsteroidal, anti-inflammatory drugs that are currently being studied for the treatment of AD. To inhibit the release of inflammatory cytokines and enhance the levels of intracellular cyclic adenosine monophosphate, PDE is a therapeutic target for AD.28 Crisaborole ointment is an inaugural PDE4 inhibitor used to treat AD in patients aged two years and above.29 Skin burning is the most prevalent AE associated with therapy.30
Topical calcineurin inhibitors
Topical calcineurin inhibitors (TCIs) are immunosuppressive drugs that limit early T-cell activation and cytokine production by inhibiting calcineurin in the skin. They help decrease inflammation associated with AD. Because this does not produce atrophy, striae, or telangiectasia, they are appealing alternatives to TCS. They are useful for areas such as the face and flexures where the skin is thin.24 The perpetual maintenance usage of TCIs is preferable for long-term usage of TCS; data confirming the efficacy and safety of TCI in children aged <12 years were found to be solid in an analysis on the use of localized treatment in this pediatric population.31 TCI can be used in any region of the body, including delicate areas, for lengthy periods; nonetheless, a poignant sensation can follow if applied to inflamed skin.32 Despite the FDA’s boxed warning about the potential of developing cancer with TCI use, current scientific evidence does not support this, and patients should be carefully counseled.33,34 TCIs have been used in children for over 15 years, with no evidence of increased malignancy.24 Skin “stinging” or “burning” is the most common AE of TCI, which may drive some patients to quit taking TCIs too soon. However, after a week of use, this sensation usually alleviates.
Methotrexate
Methotrexate is an immunosuppressive drug that inhibits folic acid production. Recently, a retrospective study conducted by Anderson et al.35 on 55 pediatric patients showed improvement in AD with methotrexate treatment. Approximately 50% of patients experience nausea and gastrointestinal disorders. Another retrospective study by Deo et al.36 conducted on 31 pediatric patients reported that methotrexate was ineffective in 25% of the patients. A small randomized controlled trial (RCT) comparing cyclosporine and methotrexate found no difference in symptom improvement or tolerance between the two drugs, while individuals on methotrexate had more prolonged remissions.37 In this trial, cyclosporine had a faster onset of effect (2–3 weeks) than methotrexate (3–5 weeks), while methotrexate was correlated with a protracted term to recidivate than cyclosporine.6 Gastrointestinal symptoms, nausea, and stomatitis are common AEs. The most severe AEs were pulmonary fibrosis, hepatotoxicity, and bone marrow suppression. Methotrexate is favored as a third-line treatment for children (8 years) with moderate-to-severe AD. Patients who are not cyclosporine candidates may benefit most from this medication.
Azathioprine
Many inflammatory conditions, including refractory AD, are treated with azathioprine, a corticosteroid-free agent. A study by Caufield and Tom38 assessed the efficacy of azathioprine in pediatric AD patients. Except for one individual, azathioprine medication was associated with clinical improvement; nonetheless, patients had modest gastrointestinal disturbances for a few weeks. Another study by Fuggle et al.39 evaluated the AEs of oral azathioprine in pediatric AD patients. In this cohort, oral azathioprine was linked with minimal noticeable side effects, given the period of usage and dosage. Because sparse thiopurine methyltransferase (TPMT) action is connected to exalted myelotoxicity in pediatric patients, investigations screening TPMT activity should be performed immediately.7 Prolonged wields could lead to non-melanoma skin cancer, lymphopenia, elevated liver enzymes for a short time, and progressive anemia.7,40
Cyclosporine
Among the short-term remedies of severe pediatric AD, cyclosporin is highly effective and well-tolerated; however, long-term data is currently limited. Systemic cyclosporine medication is efficacious and safe in pediatric patients.41 The efficacy of cyclosporine A was evaluated by Saricaoglu et al.42 in 43 children with AD. Approximately 32.6% of patients did not respond to cyclosporine A treatment, and a small number of individuals experienced several side effects. Recently, a retrospective study by Patro et al.43 evaluated the efficacy of cyclosporine in 30 pediatric AD patients. According to this study, few children developed side effects, which were reversible primarily with dose adjustments. The most prevalent side effects of cyclosporine are nephrotoxicity and hypertension. Other AEs include low serum magnesium, gingival hyperplasia, diarrhea, nausea, headaches, and hypertrichosis.40
Phototherapy
Phototherapy is a treatment option for children with severe AD who do not respond to conventional treatments, although evidence supporting its effectiveness remains limited. Moreover, once phototherapy is discontinued, recurrence is prevalent. Phototherapy involves the application of ultraviolet light to the skin, which is thought to have immunosuppressive properties. The National Institute for Health and Clinical Excellence guidelines for atopic eczema management in children state that phototherapy is recommended when other treatments have failed. Because of its availability, efficacy, and safety, narrowband (NB)-UVB is considered the first phototherapy option in pediatric populations.7 In a study by Dayal et al.,44 both unbiased austerity grades and proclaimed scores were reduced in pediatric patients with AD. However, several individuals were reported to have experienced side effects, such as reactivation of herpes labialis, chickenpox, and Grade II erythema in this trial. All patients required a brief break from treatment and were reintroduced on reduced doses of NB-UVB. NB-UVB is shown to be effective in children as young as three years old; nevertheless, universal NB-UVB therapy is suggested for older age groups.45
Patients typically tolerate phototherapy smoothly. Skin burning, pruritus, erythema, and xerosis are all possible AEs.46 In the United States, NB-UVB phototherapy is the most widely used method for treating AD.47 Phototherapy, specifically NB-UVB and medium-dose UVA1, is proposed as a second-line treatment option for both short-and long-term control of moderate-to-severe AD in children, based on the impregnability and competency of eminent RCTs. To minimize flare-ups, phototherapy can be used with TCSs and emollients. Phototherapy is usually not recommended for infants or young children until they can remain motionless in the system and wear suitable eye guards.
Dupilumab
Dupilumab is a fully humanized monoclonal antibody that thwarts the effects of classic Th2 cytokines identified in the cutaneous layers of AD patients.48 Dupilumab is the only systemic biological treatment approved for AD in children aged ≥6 years. It offers a particular mode of operation, barricading the common receptor subunit for IL-4 and IL-13, with meticulous clinical trial outcomes in pediatric AD to date. Subcutaneously given injectable dupilumab significantly improves the AD symptoms, signs, and quality of life of moderate-to-severe AD adolescents’ patients and severe cases in children.49,50 Pediatric moderate-to-severe AD patients for whom topical treatment has failed show good effectiveness to this treatment.12 Studies also support use of dupilumab as a continuous long-term treatment for pediatric AD cases aged ≥6 years with severe disease.49,51 Dupilumab has an acceptable safety profile and is generally well-tolerated.50 In general, dupilumab is a safe and effective treatment FDA-approved option for children (≥6 years) and adolescents with moderate-to-severe AD whose disease is not adequately controlled with topical therapeutics or when these therapies are not advisable. Dupilumab can be used with or without topical corticosteroids.52
Several AEs in pediatric patients have been documented in the literature. Treister and Lio reported AEs in their case series, and nasopharyngitis was the most reported AE in the phase 2a pediatric study phase. In Europe’s open-label phase 2a research, injection site responses occurred in approximately 5% of children aged 6–11 years, with conjunctivitis occurring in 11%.53
A poorly defined facial eruption of unknown origin has recently been reported as a possible AE of dupilumab. Based on a putative drug-related increase in sensitivity towards type 1 helper T-cell-biased haptens, allergic contact dermatitis has been postulated in some of these instances. Developing antidrug antibodies, a growing consequence of the more well-established biological medicines, is also a concern.54 When TCSs were added to dupilumab treatment, the improvement in signs and symptoms of AD was more significant than when dupilumab was used alone. Dry eye, noninfectious conjunctivitis, and blepharitis were the most common AEs. There is no need for laboratory testing, tuberculosis, or hepatitis B/C screening with dupilumab.55
Recent therapies for pediatric AD
Ruxolitinib
A locally applied JAK1/JAK2 restrictor, ruxolitinib, was the first medicine tested in pediatric patients aged 12–17 years.56 In addition, studies are recruiting patients aged 12 years and above (NCT03745651 and NCT03745638). Only topical application ruxolitinib which has got the FDA approval for short-term treatment of mild-to-moderate cases of AD in patients aged 12 years and above whose disease is not properly controlled with other local applications or when those local medications are not advisable.57
Abrocitinib
Abrocitinib is an oral JAK1 inhibitor that has been evaluated in 12- to 17-year-old adolescents. Abrocitinib was found to be efficacious and safe in adults and adolescents in moderate-to-severe patients of AD when used alone as once daily dose.58 It has few AEs, the most common of which are URTI and AD aggravation.59 Abrocitinib has been evaluated in various phase III studies in patients aged 12 years and above (NCT03349060, NCT03627767, NCT03575871 NCT03422822).
Delgocitinib
A topical JAK/TYK2 inhibitor, delgocitinib, is being studied in pediatric AD patients aged 12–17 years and in adult with AD. Nasopharyngitis, erysipelas, and lymphopenia are the most common AEs.60 A phase II study including children aged 2–15 years has been completed; however, no findings have been published (JapicCTI-173553). Additional phase II/III studies (NCT03725722) in children are ongoing.
Upadacitinib
An oral JAK1 inhibitor of upadacitinib is being considered in children with AD aged 2–17 years. Upadacitinib is FDA approved for moderate to severe and refractory adults as well as adolescent patients of AD who are not responding to other previous treatments or biologics.61 The most common AE is upper respiratory tract infection (URTI).62 Upadacitinib is now being tested in adolescents and adults in phase III trials (NCT03661138, NCT03568318, NCT03569293, and NCT03607422). In extension, a phase I trial (NCT03646604) for children aged 2–12 years with severe AD has begun. Because of the promising outcomes, the FDA designated upadacitinib as a breakthrough treatment in January 2018.
Omalizumab
Omalizumab is an anti-IgE monoclonal antibody that inhibits the activation of mast cells and basophils by preventing IgE from attaching to its receptor, FcεRI. FcεRI receptors are downregulated when serum IgE is depleted, stabilizing mast cells and basophils.63 Omalizumab was found to be no better than placebo for the SCORAD score and clinical evaluation in two RCTs involving children and adults aged ≥4 years.64 Participants were recruited for a phase IV trial (NCT02300701 for children aged 4–18 years).
Impact of AD in pediatrics and families of patients
AD is a serious disease that has a series of impacts on the quality of life of pediatric patients. Because of the chronicity of the disease and sleep deprivation caused by pruritus, it affects mental and physical functioning.65,66 A study of 380 AD patients found that anxiety symptoms were more common than depressive symptoms considering the psychiatric impact of the disease. In addition, half of the participants were diagnosed with AD for more than 27 years, and 40% were diagnosed in adulthood.66 In a study of AD patients aged 6–12 years, a higher frequency of ADHD symptoms was observed in pediatric AD patients as compared to controls.67 Nocturnal scratching, psychological distress, and sleep disturbances are all linked to pruritus, which results in daytime exhaustion and impairment of everyday tasks.68–70
Impact on the quality of life of affected children’s families is also immense. As care of pediatric AD patients consumes a lot of time, weaken interpersonnel relationship, reduced psychosocial functioning, and sleep deprivation are end results in family members of affected AD patients.28 Dermatitis Family Impact scoring system was devised to identify quality of life impairment in family members of people with AD. This is a 10-item questionnaire and can be used in families of children added 6 months to 10 years. This score ranged between 4.8 and 9.4 in these affected children’s family members.71 In a study of psychosocial impact of AD in families, Dermatitis Family Impact was significantly higher in families where pediatric AD cases were severe (9.71, SD 7.78 vs 0.57, SD 1.62; P < .001).72
AD leads to significant straight cost that affects patients, families, and payers no matter what the socioeconomic status of family is. Indirect costs from absenteeism at school or workplace and hospital visit costs are considerable. Overall costs occurring from learning difficulties have after-effects on socioeconomic status of families as well as entire economy.73 Generally, total cost escalates along with disease severity. However, mild AD can also inflict considerable cost.74 Economic burden of pediatric AD on families is well-known. In a recent retrospective study, data analysis showed vast prevalence of financial insecurity among patients with AD. Problems in paying medical bills, medical care delayed due to cost, overtime while paying medical bills and nonaffordance of medical care were observed.75
Though families of patients are affected at multiple levels, parents’ education remains the cornerstone of entire management of pediatric AD. The Berlin education program for parents of children with AD is one such model. This consist of collaborative efforts of pediatrician/dermatologist, psychologist, dietician discussing basic information on AD, skin care, triggering factors, and therapeutics of symptoms with parents. Next, recommendations for general nutrition are provided along with information on nutritional allergies and different forms of diet. Furthermore, sessions on stress management, dealing with itching, coping of the child and family are given.76
Strategies to manage treatment-resistant pediatric AD cases
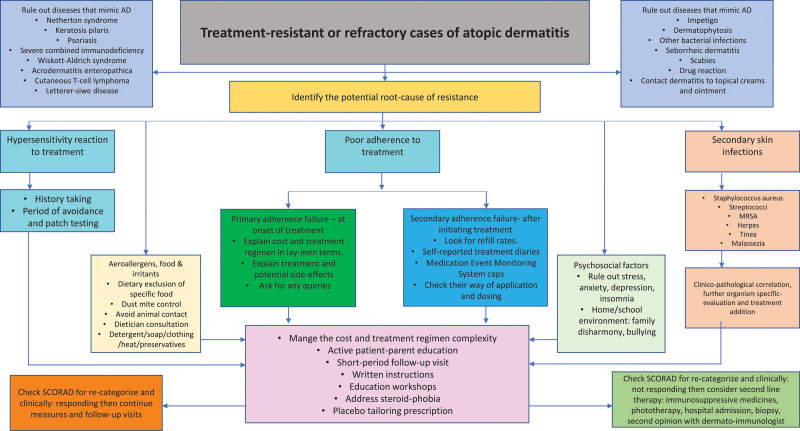
Considering all treatment options and scenarios mentioned above, a review of the literature has identified the following guidelines and strategies to successfully manage treatment-resistant or refractory cases of AD in pediatric patients.6,9,77,78 (Fig. 2).
Fig. 2.
Management of treatment-resistant or refractory cases of atopic dermatitis in pediatrics.
Conclusion
Many novel therapeutic options are being developed with a greater understanding of the pathophysiology of AD; thus, patients can look forward to a better future. For example, the FDA has approved dupilumab for use in pediatric patients (≥6 years), and in clinical practice, it has demonstrated promising results with a low rate of AEs. In addition, distinct novel therapies in phase III clinical studies for the treatment of mild-to-moderate and moderate-to-severe AD showed significant indications of success. These novel therapies will provide more therapeutic alternatives for treatment-resistant or refractory diseases and pave way for a more customized approach. Concrete decisive outcomes can motivate patients and parents to cohere to the therapeutic plans; therefore, the greater efficacy of novel medications can improve treatment adherence.
Author contributions
PPN: Conception and design, drafting of the article, revision, final approval, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest
None.
Funding
None.
Study approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author. No patient consent was required.
Footnotes
Published online 25 May 2022
References
- 1.Nguyen M, Pona A, Kolli S, Feldman S, Strowd L. Recent insights in atopic dermatitis pathogenesis, treatment, and disease impact. J Dermatology Dermatologic Surg. 2019;23:66. [Google Scholar]
- 2.Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol 2021;32:23–33. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol 2019;12:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osawa R, Akiyama M, Shimizu H. Filaggrin gene defects and the risk of developing allergic disorders. Allergol Int 2011;60:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Wadonda-Kabondo N, Sterne JA, Golding J, Kennedy CTC, Archer CB, Dunnill MGS; ALSPAC Study Team. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Arch Dis Child 2004;89:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollenberg A, Barbarot S, Bieber T, et al. Consensus based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatology Venereol. 2018;32:657–682. [DOI] [PubMed] [Google Scholar]
- 7.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014;71:327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catherine Mack Correa M, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract 2012;2012:836931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollenberg A, Christen-Zäch S, Taieb A, et al. ; European Task Force on Atopic Dermatitis/EADV Eczema Task Force. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020;34:2717–2744. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population. Ann Allergy Asthma Immunol. 2021;126:417–428.e2. [DOI] [PubMed] [Google Scholar]
- 11.Jordaan H, Visser W. The diagnosis and management of atopic dermatitis. South African Fam Pract. 2009;51:368–374. [Google Scholar]
- 12.Lansang P, Lam JM, Marcoux D, Prajapati VH, Spring S, Lara-Corrales I. Approach to the assessment and management of pediatric patients with atopic dermatitis: a consensus document. Section III: treatment options for pediatric atopic dermatitis. J Cutan Med Surg. 2019;23(suppl 5):19S–31S. [DOI] [PubMed] [Google Scholar]
- 13.Kowalska-Olędzka E, Czarnecka M, Baran A. Comparison of treatment standards in Atopic Dermatitis management across selected geographies prior to emerging targeted therapies onset. J Drug Assess 2019;8:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs 2013;15:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicol NH, Rippke F, Weber TM, Hebert AA. Daily moisturization for atopic dermatitis: importance, recommendations, and moisturizer choices. J Nurse Pract. 2021;17:920–925. [Google Scholar]
- 17.Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis—A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology Guidelines. Alergol Pol Polish J Allergol. 2017;4:158–168. [DOI] [PubMed] [Google Scholar]
- 18.Chopra R, Vakharia PP, Sacotte R, Silverberg JI. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2017;119:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hon KL, Tsang YC, Lee VW, et al. Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: a randomized, placebo-controlled cross-over trial. J Dermatolog Treat 2016;27:156–162. [DOI] [PubMed] [Google Scholar]
- 20.Cardona ID, Kempe EE, Lary C, Ginder JH, Jain N. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract 2020;8:1014–1021. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Liu X, Niu Y, et al. Skin benefits of moisturising body wash formulas for children with atopic dermatitis: a randomised controlled clinical study in China. Australas J Dermatol 2020;61:e54–e59. [DOI] [PubMed] [Google Scholar]
- 22.Frantz T, Wright EG, Balogh EA, Cline A, Adler-Neal AL, Feldman SR. Topical and oral therapies for childhood atopic dermatitis and plaque psoriasis. Children (Basel) 2019;6:E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica 1976;152(suppl 1):173–180. [DOI] [PubMed] [Google Scholar]
- 24.Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol 2016;61:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol 2018;14(suppl 2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdman-Grinshpoun Y, Ben-Amitai D, Zvulunov A. Barrier-restoring therapies in atopic dermatitis: current approaches and future perspectives. Dermatol Res Pract 2012;2012:923134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koster ES, Philbert D, Zheng X, Moradi N, de Vries TW, Bouvy ML. Reducing corticosteroid phobia in pharmacy staff and parents of children with atopic dermatitis. Int J Clin Pharm 2021;43:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Wang J, Zhang X, et al. Application of topical phosphodiesterase 4 inhibitors in mild to moderate atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol 2019;155:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016;75:494–503.e6. [DOI] [PubMed] [Google Scholar]
- 30.Papier A, Strowd LC. Atopic dermatitis: a review of topical nonsteroid therapy. Drugs Context 2018;7:212521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 2016;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynde CW, Bergman J, Fiorillo L, et al. Clinical insights about topical treatment of mild-to-moderate pediatric and adult atopic dermatitis. J Cutan Med Surg 2019;23(suppl 3):3S–13S. [DOI] [PubMed] [Google Scholar]
- 33.Castellsague J, Kuiper JG, Pottegård A, et al. A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study). Clin Epidemiol 2018;10:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis DJ, Abuabara K, Hoffstad OJ, Wan J, Raimondo D, Bilker WB. Association between malignancy and topical use of pimecrolimus. JAMA Dermatol 2015;151:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson K, Putterman E, Rogers RS, Patel D, Treat JR, Castelo-Soccio L. Treatment of severe pediatric atopic dermatitis with methotrexate: a retrospective review. Pediatr Dermatol 2019;36:298–302. [DOI] [PubMed] [Google Scholar]
- 36.Deo M, Yung A, Hill S, Rademaker M. Methotrexate for treatment of atopic dermatitis in children and adolescents. Int J Dermatol 2014;53:1037–1041. [DOI] [PubMed] [Google Scholar]
- 37.El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr 2013;172:351–356. [DOI] [PubMed] [Google Scholar]
- 38.Caufield M, Tom WL. Oral azathioprine for recalcitrant pediatric atopic dermatitis: clinical response and thiopurine monitoring. J Am Acad Dermatol 2013;68:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuggle NR, Bragoli W, Mahto A, Glover M, Martinez AE, Kinsler VA. The adverse effect profile of oral azathioprine in pediatric atopic dermatitis, and recommendations for monitoring. J Am Acad Dermatol 2015;72:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014;133:429–438. [DOI] [PubMed] [Google Scholar]
- 41.Slater NA, Morrell DS. Systemic therapy of childhood atopic dermatitis. Clin Dermatol 2015;33:289–299. [DOI] [PubMed] [Google Scholar]
- 42.Saricaoglu H, Yazici S, ZorluORLU Ö, Başkan EB. Cyclosporine-A for severe childhood atopic dermatitis: clinical experience on efficacy and safety profile. Turkish J Med Sci. 2018;48:933–938. [DOI] [PubMed] [Google Scholar]
- 43.Patro N, Panda M, Dash M. Cyclosporine a in recalcitrant pediatric dermatoses-A retrospective analysis of thirty children. Indian J Paediatr Dermatol. 2020;21:98. [Google Scholar]
- 44.Dayal S, Pathak K, Sahu P, Jain VK. Narrowband UV-B phototherapy in childhood atopic dermatitis: efficacy and safety. An Bras Dermatol 2017;92:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dogra S, Mahajan R; Indian Association of Dermatologists, Venereologists and Leprologists. Phototherapy for atopic dermatitis. Indian J Dermatol Venereol Leprol 2015;81:10–15. [DOI] [PubMed] [Google Scholar]
- 46.Garritsen FM, Brouwer MW, Limpens J, et al. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol 2014;170:501–513. [DOI] [PubMed] [Google Scholar]
- 47.Patrizi A, Raone B, Ravaioli GM. Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol 2015;8:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson EL, Bieber T, Guttman-Yassky E, et al. ; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–2348. [DOI] [PubMed] [Google Scholar]
- 49.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb) 2021;11:1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol 2021;184:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghazal S, Ridha Z, D’Aguanno K, et al. Treatment guidelines for atopic dermatitis since the approval of dupilumab: a systematic review and quality appraisal using AGREE-II. Front Med (Lausanne) 2022;9:821871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cork MJ, Thaci D, DiCioccio T, Davis JD, Zhang Q, Ardeleanu M. Pharmacokinetics, safety, and efficacy of dupilumab in a pediatric population with moderate-to-severe atopic dermatitis: results from an open-label phase 2a trial. In: American Academy of Dermatology 75th Annual Meeting. 2017:3–7. [Google Scholar]
- 54.Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol 2019;36:172–176. [DOI] [PubMed] [Google Scholar]
- 55.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389:2287–2303. [DOI] [PubMed] [Google Scholar]
- 56.Raedler LA. Jakafi (Ruxolitinib): first FDA-Approved medication for the treatment of patients with polycythemia vera. Am Health Drug Benefits 2015;8(Spec Feature):75–79. [PMC free article] [PubMed] [Google Scholar]
- 57.Witkoff B, Logas CM, Glick BP, Del Rosso JQ. JAK inhibitors in the treatment of atopic dermatitis. Dermatological Rev. 2022;3:20–28. [Google Scholar]
- 58.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020;156:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peeva E, Hodge MR, Kieras E, et al. Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: a phase 1, randomized, placebo-controlled, dose-escalation study. Br J Clin Pharmacol 2018;84:1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa H, Nemoto O, Yamada H, Nagata T, Ninomiya N. Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. J Dermatol 2018;45:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol 2022;158:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R. Efficacy and safety of upadacitinib treatment over 32 weeks for patients with atopic dermatitis from a phase 2b, randomized, placebo-controlled trial. In: 27th European Academy of Dermatology and Venerology (EADV) Congress. Vol 12; 2018. [Google Scholar]
- 63.Kawakami T, Blank U. From IgE to Omalizumab. J Immunol 2016;197:4187–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol 2013;162:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris VR, Cooper AJ. Atopic dermatitis: the new frontier. Med J Aust 2017;207:351–356. [DOI] [PubMed] [Google Scholar]
- 66.Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016;74:491–498. [DOI] [PubMed] [Google Scholar]
- 67.Schmitt J, Buske-Kirschbaum A, Tesch F, et al. Increased attention-deficit/hyperactivity symptoms in atopic dermatitis are associated with history of antihistamine use. Allergy 2018;73:615–626. [DOI] [PubMed] [Google Scholar]
- 68.Yu SH, Attarian H, Zee P, Silverberg JI. Burden of sleep and Fatigue in US adults with atopic dermatitis. Dermatitis 2016;27:50–58. [DOI] [PubMed] [Google Scholar]
- 69.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol 2015;135:56–66. [DOI] [PubMed] [Google Scholar]
- 70.Hajar T, Gontijo JRV, Hanifin JM. New and developing therapies for atopic dermatitis. An Bras Dermatol 2018;93:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang J, Choo YJ, Smith HE, Apfelbacher C. Quality of life in atopic dermatitis in Asian countries: a systematic review [e-pub ahead of print]. Arch Dermatol Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedersen CJ, Uddin MJ, Saha SK, Darmstadt GL. Prevalence and psychosocial impact of atopic dermatitis in Bangladeshi children and families. PLoS One 2021;16:e0249824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol 2019;122:360–366. [DOI] [PubMed] [Google Scholar]
- 74.Hebert AA, Stingl G, Ho LK, et al. Patient impact and economic burden of mild-to-moderate atopic dermatitis. Curr Med Res Opin 2018;34:2177–2185. [DOI] [PubMed] [Google Scholar]
- 75.Zheng DX, Cwalina TB, Jella TK, et al. Financial insecurity among children with atopic dermatitis in the United States [e-pub ahead of print]. J Am Acad Dermatol. doi: [DOI] [PubMed] [Google Scholar]
- 76.Wenninger K, Kehrt R, von Rüden U, et al. Structured parent education in the management of childhood atopic dermatitis: the Berlin model. Patient Educ Couns 2000;40:253–261. [DOI] [PubMed] [Google Scholar]
- 77.Kelly KA, Ewulu A, Emmerich VK, Heron CE, Feldman SR. Refractory pediatric psoriasis and atopic dermatitis: the importance of therapeutical adherence and biological management. Biomedicines 2021;9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A; Atopic Dermatitis Working Group of the Allergic Skin Diseases Committee of the AAAAI. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract 2013;1:142–151. [DOI] [PubMed] [Google Scholar]