Abstract
INTRODUCTION:
Two antitumor necrosis factor therapies (infliximab [IFX] and adalimumab [ADA]) have been approved for the treatment of pediatric Crohn's disease (CD) but have not been compared in head-to-head trials. The aim of this study was to compare the efficacy and safety of ADA and IFX by propensity score matching in a prospective cohort of pediatric patients with luminal CD and at least a 24-month follow-up.
METHODS:
Among 100 patients, 75 met the inclusion criteria, and 62 were matched by propensity score. We evaluated time to treatment escalation as the primary outcome and primary nonresponse, predictors of treatment escalation and relapse, serious adverse events, pharmacokinetics, and effect of concomitant immunomodulators as secondary outcomes.
RESULTS:
There was no difference between ADA and IFX in time to treatment escalation (HR = 0.63 [95% CI 0.31–1.28] P = 0.20), primary nonresponse (P = 0.95), or serious adverse events. The median (interquartile range) trough levels at the primary outcome were 14.05 (10.88–15.40) and 6.15 (2.08–6.58) µg/mL in the ADA and IFX groups, respectively. On a multivariate analysis, the combination of anti-Saccharomyces cerevisiae antibody negativity and antineutrophil cytoplasmic antibody positivity was a strong independent predictor of treatment escalation (HR 5.19, [95% CI 2.41–11.18], P < 0.0001). The simple endoscopic score for CD, L3 disease phenotype, and use of concomitant immunomodulators for at least the first 6 months revealed a trend toward significance on a univariate analysis.
DISCUSSION:
Propensity score matching did not reveal substantial differences in efficacy or safety between ADA and IFX. The anti-S. cerevisiae antibody negativity and antineutrophil cytoplasmic antibody positivity combination is a strong predictor of treatment escalation.
INTRODUCTION
To date, 2 anti-tumor necrosis factor (TNF) agents have been approved for the treatment of pediatric Crohn's disease (CD): infliximab (IFX) and adalimumab (ADA). Both agents have been proven to be effective and safe in randomized controlled trials (RCTs) (1,2). However, these RCTs differed in some aspects of methodology. In the REACH trial, only patients who responded to induction IFX therapy were randomized, and in the IMAgINE trial, patients who previously failed on anti-TNF therapy were enrolled. Moreover, cessation of immunomodulator (IMM) therapy was permitted from week 26. Age at enrollment and disease activity based on the Pediatric Crohn's Disease Activity Index (PCDAI) were similar in both studies. However, no direct head-to-head comparison of both anti-TNF agents has been performed in pediatric or adult patients. Several indirect comparisons, including network meta-analyses, have been published, but these rarely consider pediatric populations (3–9). Owing to the low number of pediatric patients per center, it is difficult to perform RCTs that can demonstrate differences between these drugs. In particular, a noninferiority design would require a high number of patients. Therefore, we aimed to perform a propensity score analysis of our cohorts of prospectively followed up patients.
Study aims
The primary aim of this study was to compare the time to treatment escalation between patients treated with ADA and those treated with IFX. Secondary aims were to evaluate primary nonresponse to anti-TNF, predictors of treatment escalation and relapse, safety, pharmacokinetics (PK), and effect of concomitant IMM treatment.
METHODS
Study design and ethical considerations
This prospective observational cohort study was performed using propensity score matching. The study was approved by the local ethics committee, and all participants and/or parents signed written informed consent.
Study subjects and dosage of anti-TNF
Patients naive to biologic therapy, newly started on anti-TNF treatment between 2013 and 2017 (Motol PIBD cohort), were recruited into the study and prospectively followed up according to the standard protocol reflecting usual clinical practice (see Supplementary Figure 1, http://links.lww.com/CTG/A798). Patients were initiated on an anti-TNF agent based on a detailed discussion between the family and the treating physician. The minimal follow-up period required for evaluation of study outcomes was 24 months. Inclusion and exclusion criteria are listed in Supplementary Digital Content (see Supplementary Table 1, http://links.lww.com/CTG/A799). Patients were initiated on a standard dose of anti-TNF: ADA (Humira) 160-80-40 mg s.c. every other week, followed by 40 mg s.c. every other week, and IFX (Remicade) 5 mg/kg i.v. at weeks 0, 2, and 6 and every 8 weeks. No biosimilars were used in this study. In patients weighing less than 40 kg, the dose of ADA was calculated according to the body surface area. When applicable, a decision on therapy intensification (ADA up to 40 mg weekly and IFX up to 10 mg/kg every 4 weeks) was made by the treating physician, based primarily on clinical and laboratory data, and secondarily on trough levels and anti-drug antibodies (ATI) to the respective anti-TNF (reactive therapeutic drug monitoring [TDM]). No proactive TDM was applied during the study period. All patients, except for 3, received IMM (97% azathioprine [AZA], 3% methotrexate [MTX]) from diagnosis until the start of anti-TNF treatment (Table 1).
Table 1.
Characteristics of both study groups before propensity score matching
| ADA (N = 31) | IFX (N = 44) | P value | |
| Basic characteristics | |||
| Age | 14.18 (11.64–16.34), NA = 0 | 14.46 (13.24–16.27), NA = 0 | 0.36 |
| Sex (male) | 21 (0.68), NA = 0 | 24 (0.55), NA = 0 | 0.25 |
| Smoking | 3 (0.1), NA = 0 | 1 (0.02), NA = 0 | 0.16 |
| Ethnicity (White) | 29 (0.94), NA = 0 | 43 (0.98), NA = 0 | 0.37 |
| Family history of IBD | 1 (0.03), NA = 0 | 8 (0.08), NA = 0 | 0.03 |
| Concomitant immunopathology | 3 (0.1), NA = 0 | 3 (0.07), NA = 0 | 0.66 |
| Body height (cm) | 162 (136.95–170.75), NA = 0 | 156.55 (148.88–171.52), NA = 0 | 0.41 |
| Body height (z score) | −1.49 (−5.56–0.53), NA = 0 | −2.12 (−3.94–0.26), NA = 0 | 0.41 |
| Body weight (kg) | 44.3 (28.65–58.5), NA = 0 | 47.25 (37.12–56.08), NA = 0 | 0.43 |
| BMI (z score) | −1.77 (−2.58–0.53), NA = 0 | −1.34 (−1.97–0.4), NA = 0 | 0.27 |
| Paris classification | |||
| L1 | 11 (0.35), NA = 0 | 10 (0.23), NA = 0 | 0.23 |
| L2 | 1 (0.03), NA = 0 | 4 (0.09), NA = 0 | 0.3 |
| L3 | 19 (0.61), NA = 0 | 30 (0.68), NA = 0 | 0.54 |
| L4a or L4b | 22 (0.71), NA = 0 | 31 (0.7), NA = 0 | 0.96 |
| B1 | 23 (0.74), NA = 0 | 36 (0.82), NA = 0 | 0.43 |
| B2 | 4 (0.13), NA = 0 | 5 (0.11), NA = 0 | 0.84 |
| B3 | 4 (0.13), NA = 0 | 2 (0.05), NA = 0 | 0.19 |
| B2+B3 | 0 (0), NA = 0 | 1 (0.02), NA = 0 | 0.3 |
| Perianal disease | 7 (0.23), NA = 0 | 12 (0.27), NA = 0 | 0.64 |
| Growth impairment | 10 (0.33), NA = 1 | 10 (0.23), NA = 0 | 0.32 |
| Disease activity and labs | |||
| wPCDAI (points) | 22.5 (16.88–40.62), NA = 7 | 32.5 (16.88–40), NA = 4 | 0.64 |
| CRP (mg/L) | 13.6 (8.35–26.25), NA = 4 | 17 (4.85–29.85), NA = 1 | 0.55 |
| F-CPT (μg/g) | 1,800 (1,080–2,883), NA = 20 | 1,000 (801–1,720), NA = 11 | 0.09 |
| Albumin (g/L) | 42.8 (40.2–43.8), NA = 6 | 41.1 (39.4–43.4), NA = 3 | 0.32 |
| ESR (mm/hr) | 28.5 (20–41.25), NA = 3 | 30 (18–46.5), NA = 1 | 0.59 |
| ASCA positivity | 23 (0.88), NA = 5 | 34 (0.81), NA = 2 | 0.4 |
| pANCA positivity | 5 (0.19), NA = 5 | 6 (0.14), NA = 1 | 0.57 |
| SES-CD (points) | 20 (13–27), NA = 2 | 18 (11.75–21.5), NA = 4 | 0.2 |
| Treatment | |||
| Time since dg. to anti-TNF start (yr) | 1.04 (0.51–1.61), NA = 0 | 0.6 (0.17–1.23), NA = 0 | 0.14 |
| EEN during dg. | 21 (0.68), NA = 0 | 37 (0.84), NA = 0 | 0.1 |
| CS during dg. | 6 (0.19), NA = 0 | 6 (0.14), NA = 0 | 0.51 |
| IMM during dg. | 29 (0.94), NA = 0 | 43 (0.98), NA = 0 | 0.37 |
| EEN during anti-TNF start | 5 (0.16), NA = 0 | 7 (0.16), NA = 0 | 0.98 |
| CS during anti-TNF start | 1 (0.03), NA = 0 | 2 (0.05), NA = 0 | 0.77 |
| IMM during anti-TNF start | 29 (0.94), NA = 0 | 38 (0.86), NA = 0 | 0.31 |
Values are listed as median and interquartile range or median and fraction (%); NA = number of missing values.
ADA, adalimumab; ASCA, anti-Saccharomyces cerevisiae antibodies; BMI, body mass index; CRP, C-reactive protein; CS, corticosteroids; dg., diagnosis; EEN, exclusive enteral nutrition; ESR, erythrocyte sedimentation rate; F-CPT, fecal calprotectin; IBDinflammatory bowel disease; IFX, infliximab; IMM, immunomodulators; pANCA, antineutrophilic antibodies; SES-CD, simple endoscopic score for Crohn's disease; TNF, tumor necrosis factor; wPCDAI, weighted pediatric Crohn's disease activity index.
Primary outcome
The primary outcome of the study was the time to treatment escalation on anti-TNF therapy evaluated by survival analysis after propensity score matching.
Secondary outcomes
The following secondary outcomes were considered: (i) Proportion of patients with a primary nonresponse to ADA or IFX, (ii) identification of predictors of treatment escalation and relapse, (iii) rate of serious adverse events (SAEs) occurring on-treatment, (iv) PK of both drugs, and (v) effect of concomitant IMM treatment.
Definition of treatment escalation, relapse and primary nonresponse
Treatment escalation was defined as dose escalation or interval shortening due to a lack of drug efficacy (not due to adjustment for body weight) or bowel surgery due to disease activity, development of abscess, perianal or intra-abdominal fistula, change of anti-TNF therapy (due to side effects or ineffectivity), need for reinduction (corticosteroids, exclusive enteral nutrition, or antibiotics), or change of IMM treatment (AZA to MTX or vice versa), not dose adjustment for body weight (see Supplementary Figure 1, http://links.lww.com/CTG/A798). For the purpose of secondary subanalysis, dose escalation or interval shortening due to a lack of drug efficacy (not due to adjustment for body weight) was omitted from the abovementioned definition. This situation was marked as relapse.
Primary nonresponse was defined as the need for treatment change (switch to another anti-TNF therapy, treatment interruption, bowel surgery, or persisting need for induction therapy corticosteroids, exclusive enteral nutrition, antibiotics) until week 14 (12–16) due to clinical symptoms (weighted pediatric Crohn's disease activity index [wPCDAI], fistula, and stricture), laboratory signs of disease activity (erythrocyte sedimentation rate, C-reactive protein [CRP], fecal calprotectin [F-CPT]), endoscopic disease activity, need for bowel surgery, drug intolerance (side effects), or noncompliance.
Clinical and laboratory data
At the onset of anti-TNF therapy, we recorded general patient characteristics, factors that may influence the outcome or allocation of patients to the respective treatment group (ADA and IFX), and factors considered as potential predictors of treatment efficacy (Table 1). The data underlying this article will be shared on reasonable request to the corresponding author.
During follow-up, we prospectively recorded the following every 3 months: body height; weight; wPCDAI; CRP; F-CPT; perianal fistulas; extraintestinal manifestations; SAEs; dose and interval of anti-TNF; need for treatment escalation, cessation, or switch, including the reason; concomitant medication; trough levels and ATI to anti-TNF if applicable; and occurrence of primary and secondary outcomes.
Regarding clinical indication, the following checks were performed: bowel ultrasound, magnetic resonance enterography, or endoscopy. Endoscopy, including biopsies and evaluation of simple endoscopic score for CD (SES-CD), was performed before the decision on anti-TNF treatment and before any major therapeutic decision (e.g., switch to another anti-TNF therapy, bowel surgery, and nonresponse).
Patient allocation and statistical analysis
All data were analyzed using R statistical software (version 3.6.0; www.r-project.org). Continuous variables were described as medians and interquartile ranges (IQRs). Categorical variables were described as absolute frequencies and percentages. Missing data were not imputed. The difference between patients treated with ADA and IFX was assessed using the likelihood ratio test on the odds ratio or 2-sample t test, as appropriate. Propensity score matching was performed using the R package MatchIt (version 3.0.2). The model for propensity matching consisted of the SES-CD, stricturing behavior, penetrating behavior, perianal disease, z score of body mass index, and age at the time of anti-TNF onset. Variables were selected based on the clinical decision, according to factors that could influence the outcome or choice of therapy. Matching was performed using nearest neighbor matching with a ratio of 1:1. The covariate balance in the matched sample was checked by visual inspection of plots showing the mean of each covariate against the estimated propensity score, separately by treatment status. The effect of concomitant IMM therapy was evaluated as the percentage of time on concomitant IMM out of the complete follow-up time, as a continuous variable, and as a categorical variable if the patient received IMM for at least 6 months.
The primary outcome of the study was evaluated using a Cox proportional hazards model, subsequently adjusted for the proportion of time on IMM therapy. The preselected predictors were tested using unadjusted Cox regression. To assess the importance of particular variables, we further tested the association of time to relapse with the variables using multivariable Cox proportional hazards models.
We used a generalized linear mixed model to assess the association between SAE and the type of anti-TNF therapy. All mixed models were adjusted for follow-up time and IMM use. When values were missing, the time point was omitted from the current analysis.
P < 0.05 was considered significant. A 95% confidence interval (CI) was used. Figures were constructed using R package ggplot2. According to powerSurfEpi R-package, our study with 31 experimental subjects and 31 control subjects was able to detect hazard ratio (HR) of < 0.34 or > 2.90 with probability (power) 0.80.
RESULTS
Of 100 patients screened for inclusion in the study (50 ADA and 50 IFX), 25 met the exclusion criteria. The basic characteristics of patients in each study group before propensity score matching (31 patients in the ADA group and 44 patients in the IFX group) are presented in Table 1. No significant differences were found, except for family history of inflammatory bowel disease (IBD), which was more frequent in the IFX group (P = 0.03). Finally, propensity score matching allowed us to directly compare 31 pairs of patients (Figure 1).
Figure 1.
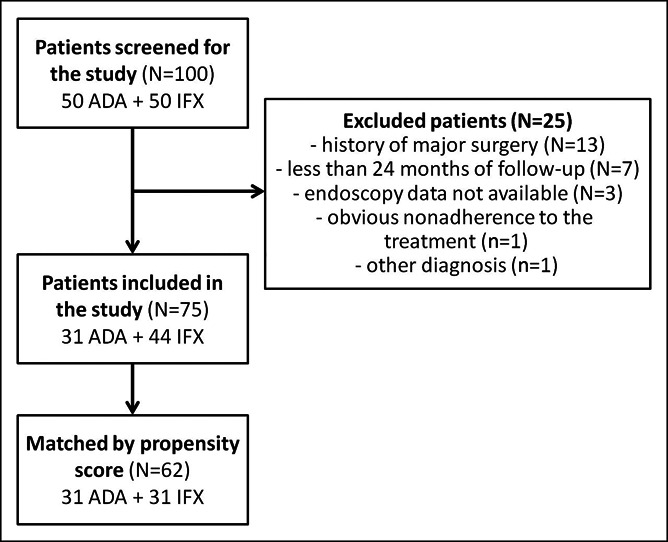
Flowchart of patient recruitment into the study and propensity score matching.
Primary outcome—time to treatment escalation
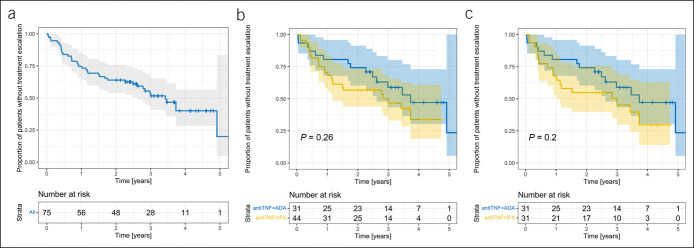
The overall time to treatment escalation in the whole study group (N = 75) is presented in Figure 2a, showing an approximate rate of 50% during the 3-year follow-up. Neither subanalysis of the whole study group (N = 75, HR = 0.68 [95% CI 0.35.1.33], P = 0.26, Figure 2b) nor of patients matched by propensity score (N = 62, HR = 0.63 [95% CI 0.31–1.28], P = 0.20, Figure 2c) revealed any significant difference in time to treatment escalation between ADA and IFX. The results were not affected by adjusting this model to concomitant IMM (HR = 0.63 [95% CI 0.31–1.28], P = 0.20).
Figure 2.
(a) Survival curve of time to treatment escalation in the whole study group (pooled data, N = 75). (b) Time to treatment escalation according to the type of anti-TNF therapy in the whole study group (N = 75). (c) Time to treatment escalation according to the type of anti-TNF therapy after propensity score matching (N = 62). ADA, adalimumab; IFX, infliximab; TNF, tumor necrosis factor.
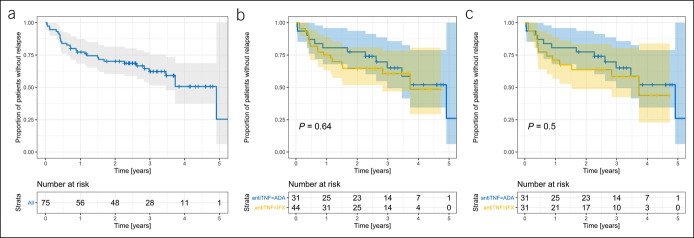
When the need for treatment intensification was omitted (situation classified as relapse) (see Figure 3a for pooled data on ADA + IFX), there was no significant difference in relapse rate between the ADA and IFX groups, in the whole study group (N = 75, HR = 0.83 [95% CI 0.40–1.76], P = 0.64, Figure 3b), or after propensity score matching (N = 62, HR = 0.76 [95% CI 0.35–1.68], P = 0.50, Figure 3c). Adjusting this model to concomitant IMM did not affect the results (HR = 0.76 [95% CI 0.34–1.67], P = 0.49).
Figure 3.(.
a) Survival curve of time to relapse (when dose and interval adjustment were omitted as a reason) in the whole study group (pooled data, N = 75). (b) Time to relapse (when dose and interval adjustment were omitted as a reason) according to the type of anti-TNF therapy in the whole study group (N = 75). (c) Time to relapse (when dose and interval adjustment were omitted as a reason) according to the type of anti-TNF therapy after propensity score matching (N = 62). ADA, adalimumab; IFX, infliximab; TNF, tumor necrosis factor.
Supplementary Digital Content (see Supplementary Table 2, http://links.lww.com/CTG/A800) presents various reasons for treatment escalation during the follow-up period in both groups after propensity score matching (N = 62). There was no significant difference between the 2 groups in any of the reasons listed.
Secondary outcomes
Primary nonresponse
There was no statistically significant difference in the primary nonresponse rate before propensity score matching (2/31 [6%] in the ADA group and 3/44 [7%] in the IFX group; P = 0.95) nor after matching (2/31 [6%] in the ADA group and 3/31 [10%] in the IFX group; P = 0.64). There was no significant difference in inflammatory markers (CRP, erythrocyte sedimentation rate, and F-CPT) or wPCDAI between ADA and IFX at the end of the induction period (week 12–16).
Predictors of treatment escalation and relapse
On a univariate analysis of the whole study group (N = 75, pooled data), antineutrophilic antibody (pANCA) positivity and anti-Saccharomyces cerevisiae antibody (ASCA) negativity were identified as potentially strong predictors of treatment escalation. The SES-CD, L3 disease phenotype, and use of concomitant IMM for at least the first 6 months demonstrated a trend toward significance (Table 2). In a subsequent multivariate analysis, the combination of ASCA negativity and pANCA positivity was identified as the only and very strong independent predictor of treatment escalation (HR 5.19, 95% CI 2.41.11.18, P < 0.0001, Figure 4). There was no effect of disease phenotype, concomitant IMM, or type of anti-TNF when added to the model (Table 3).
Table 2.
Risk factors of treatment escalation identified by univariate analysis in the whole study group (N = 75)
| HR (95% CI) | P value | |
| pANCA positivity | 3.221 (1.521–6.820) | 0.002 |
| ASCA negativity | 3.093 (1.469–6.514) | 0.003 |
| SES-CD | 0.960 (0.923–0.999) | 0.045 |
| L3 phenotype | 0.571 (0.300–1.087) | 0.088 |
| Concomitant IMM (at least 6 mo) | 0.472 (0.196–1.134) | 0.093 |
| Concomitant immunopathology | 0.197 (0.027–1.469) | 0.113 |
| B2 disease phenotype | 2.011 (0.837–4.833) | 0.118 |
| Family history of IBD | 1.892 (0.827–4.327) | 0.131 |
| L1 disease phenotype | 1.580 (0.807–3.094) | 0.182 |
| Time to anti-TNF onset | 1.213 (0.876–1.680) | 0.244 |
| F-CPT | 1.000 (1.000–1.001) | 0.249 |
| Perianal disease | 1.489 (0.749–2.962) | 0.257 |
| L2 disease phenotype | 1.808 (0.553–5.907) | 0.327 |
| B1 disease phenotype | 0.704 (0.331–1.497) | 0.363 |
| B3 disease phenotype | 0.526 (0.122–2.260) | 0.388 |
| BMI z score | 1.047 (0.901–1.218) | 0.547 |
| CRP | 0.996 (0.982–1.010) | 0.559 |
| ESR | 0.996 (0.982–1.010) | 0.560 |
| Growth impairment | 1.213 (0.610–2.410) | 0.582 |
| Concomitant IMM (as continuous) | 0.774 (0.286–2.092) | 0.614 |
| wPCDAI | 0.995 (0.976–1.015) | 0.616 |
| Age | 0.979 (0.875–1.096) | 0.716 |
| Sex (male) | 0.907 (0.476–1.727) | 0.766 |
| Height z score | 0.989 (0.919–1.065) | 0.775 |
| Albumin | 1.005 (0.959–1.054) | 0.826 |
| Year of anti-TNF administration (era) | 1.024 (0.721–1.453) | 0.896 |
| Smoking | 0.945 (0.214–4.178) | 0.941 |
Predictive factors were evaluated during anti-TNF onset. Values are listed as hazard ratio (HR) with 95% confidence interval (CI) and sorted by the raising P-value. In the multivariate model, factors in bold were tested, and composite predictive factor (pANCA+ and ASCA-) was used (Table 3).
ASCA, anti-Saccharomyces cerevisiae antibodies; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; F-CPT, fecal calprotectin; IBD, inflammatory bowel disease; IMM, immunomodulators; pANCA, antineutrophilic antibodies; SES-CD, simple endoscopic score for Crohn's disease; TNF, tumor necrosis factor; wPCDAI, weighted pediatric Crohn's disease activity index.
Figure 4.
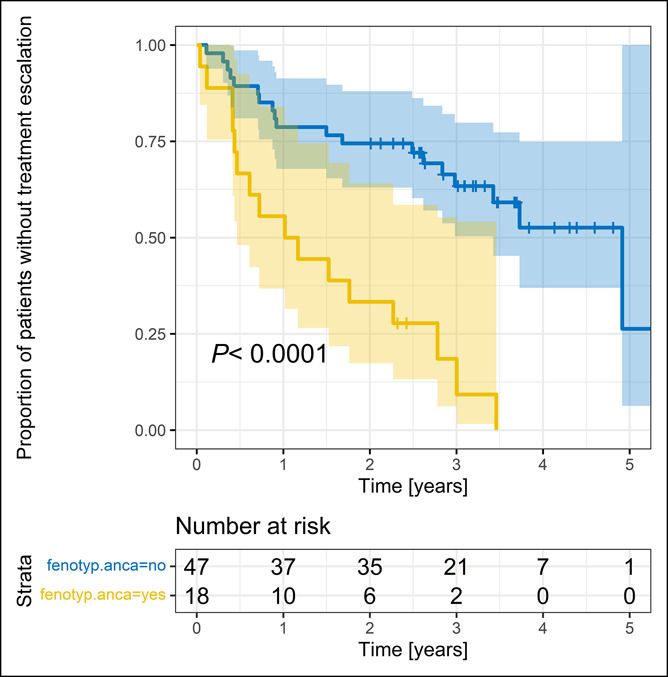
Time to dose and interval adjustment in the whole study group (pooled data, N = 75) stratified by composite predictor (combination of ASCA negativity and pANCA positivity [fenotyp ANCA = yes] vs combination of ASCA positivity and pANCA negativity [fenotyp ANCA = no]). ASCA, anti-Saccharomyces cerevisiae antibodies; pANCA, antineutrophilic antibodies.
Table 3.
Risk factors of treatment escalation tested by multivariate analysis in the whole study group (N = 75)
| HR (95% CI) | P value | Significance | |
| pANCA+ and ASCA− | 5.19 (2.41–11.18) | 0.00003 | *** |
| L3 phenotype | 0.49 (0.23–1.07) | 0.073 | NS |
| SES-CD | 0.98 (0.94–1.03) | 0.523 | NS |
| Concomitant IMM (at least 6 mo) | 0.78 (0.27–2.28) | 0.650 | NS |
| Type of anti-TNF | 0.95 (0.45–2.03) | 0.901 | NS |
Predictive factors were evaluated during anti-TNF onset (Table 2). Values are listed as hazard ratio (HR) with 95% confidence interval (CI) and sorted by the raising P-value. In the multivariate model, factors in bold were tested, and composite predictive factor (pANCA+ and ASCA−) was used (Table 3).
ASCA, anti-Saccharomyces cerevisiae antibodies; CI, confidence interval; HR, hazard ratio; IMM, immunomodulators; pANCA, antineutrophilic antibodies; SES-CD, simple endoscopic score for Crohn's disease; TNF, tumor necrosis factor.
***p<0.001; NS = not significant.
Predictors of relapse (as defined earlier) were similar to those of treatment escalation (L3, SES-CD, pANCA positivity, and B2 being statistically significant (P < 0.05) and L1, family history of IBD, and ASCA negativity being of borderline significance). A combination of pANCA and ASCA remained a strong predictor (P = 0.0091). There was no effect of concomitant IMM or type of anti-TNF when added to the model.
SAEs
A comparison of SAE occurrence in the treatment groups before and after propensity score matching is summarized in Tables 4 and 5. No significant difference was identified between the ADA and IFX groups, except for pneumonia after propensity score matching (3 cases in the IFX group and no cases in the ADA group; P = 0.04). A subsequently performed mixed model reflecting the occurrence of SAEs during each patient visit and adjusted to concomitant IMM treatment and length of follow-up did not reveal any differences between the study groups (Table 6). In 1 patient receiving ADA, serious dermatological side effects led to cessation of ADA. In 1 patient receiving IFX, infusion allergic reaction led to the cessation of IFX.
Table 4.
SAE according to the treatment group before propensity score matching (N = 75)
| ADA (N = 31) | IFX (N = 44) | P value | |
| Pneumonia | 0 (0), NA = 0 | 3 (0.07), NA = 0 | 0.07 |
| Meningitis | 0 (0), NA = 0 | 2 (0.05), NA = 0 | 0.14 |
| Pancreatitis | 0 (0), NA = 0 | 2 (0.05), NA = 0 | 0.14 |
| Leukopenia | 1 (0.03), NA = 0 | 2 (0.05), NA = 0 | 0.77 |
| Anemia | 2 (0.06), NA = 0 | 4 (0.09), NA = 0 | 0.67 |
| HSV | 3 (0.1), NA = 0 | 6 (0.14), NA = 0 | 0.6 |
| VZV | 2 (0.06), NA = 0 | 1 (0.02), NA = 0 | 0.37 |
| Other | 12 (0.39), NA = 0 | 15 (0.34), NA = 0 | 0.68 |
| Hospitalization | 14 (0.45), NA = 0 | 15 (0.34), NA = 0 | 0.33 |
| Any SAE | 20 (0.65), NA = 0 | 29 (0.66), NA = 0 | 0.9 |
ADA, adalimumab; HSV, herpes simplex virus; IFX, infliximab; SAE, serious adverse event; VZV, varicella zoster virus.
Table 5.
SAE according to the treatment group after propensity score matching (N = 62)
| ADA (N = 31) | IFX (N = 31) | P value | |
| Pneumonia | 0 (0), NA = 0 | 3 (0.1), NA = 0 | 0.04 |
| Meningitis | 0 (0), NA = 0 | 1 (0.03), NA = 0 | 0.24 |
| Pancreatitis | 0 (0), NA = 0 | 2 (0.06), NA = 0 | 0.09 |
| Leukopenia | 1 (0.03), NA = 0 | 0 (0), NA = 0 | 0.24 |
| Anemia | 2 (0.06), NA = 0 | 4 (0.13), NA = 0 | 0.39 |
| HSV | 3 (0.1), NA = 0 | 4 (0.13), NA = 0 | 0.69 |
| VZV | 2 (0.06), NA = 0 | 1 (0.03), NA = 0 | 0.55 |
| Other | 12 (0.39), NA = 0 | 11 (0.35), NA = 0 | 0.79 |
| Hospitalization | 14 (0.45), NA = 0 | 10 (0.32), NA = 0 | 0.3 |
| Any SAE | 20 (0.65), NA = 0 | 22 (0.71), NA = 0 | 0.59 |
ADA, adalimumab; HSV, herpes simplex virus; IFX, infliximab; SAE, serious adverse event; VZV, varicella zoster virus.
Table 6.
Mix model presenting occurrence of any SAE—adjusted to type of anti-TNF, concomitant IMM, and length of follow-up
| OR (95% CI) | P value | |
| Type of anti-TNF | 1.072 (0.566–2.031) | 0.831 |
| Concomitant IMM | 1.613 (0.627–4.148) | 0.322 |
| Length of follow-up | 1.167 (0.934–1.459) | 0.175 |
Numbers in Tables 4 and 5 are listed as No. (and fraction, %) of cases that presented with respective SAE at least once during the follow-up. There were no cases of hepatopathy, thrombosis, malignities, or deaths identified in any of the groups. The subsequently performed mix model (Table 6) did not find any difference in the occurrence of SAE between both groups.
CI, confidence interval; IMM, immunomodulator; SAE, serious adverse event; TNF, tumor necrosis factor.
PK
Regarding reactive TDM performed during the study period, TDM data were only available from selected visits (12% of all anti-TNF visits; 4% in the ADA group, and 21% in the IFX group). The median (IQR) trough levels at the time of the primary outcome were 14.05 (10.88–15.40) µg/mL in the ADA group and 6.15 (2.08–6.58) µg/mL in the IFX group (1 patient in the IFX group had undetectable trough levels). Positive ATI were only detected in the IFX group (5 observations in 3 patients during the follow-up period).
Because we did not intend to compare the PK of both anti-TNF agents, the PK subanalysis on propensity score–matched subgroups was not performed.
Concomitant IMM
After the onset of anti-TNF for at least 6 months, 29/31 (94%) patients in the ADA group and 38/44 (86%) in the IFX group received concomitant IMM therapy (97% AZA and 3% MTX). Adjusting the Cox model of time to treatment escalation (primary outcome) to concomitant IMM treatment did not affect the results (see the section on primary outcome). In the pooled data (N = 75), concomitant IMM (as a continuous or categorical variable) was not identified as a strong independent predictor of treatment escalation on either univariate or multivariate analysis (Table 2). Because only a minority of patients received anti-TNF monotherapy, and limited PK data were available, a subanalysis investigating the effects of IMM on drug PK was not performed.
DISCUSSION
In accordance with guidelines on the management of pediatric CD, the selection of anti-TNF therapy (ADA vs IFX) in anti-TNF naive patients is based on patient and family preference, drug availability, administration route, and cost (10,11). This approach is based on early adult (mainly retrospective) studies that did not demonstrate any difference in efficacy between ADA and IFX (12–22), and subsequent large adult prospective studies (23,24) and retrospective studies with the longest follow-up to date (up to 5 years) (25,26). Beyond clinical efficacy, no difference was found in mucosal and histological healing (27). Two recent large propensity score–matched comparison studies in adult patients revealed no significant difference in clinical benefit between the 2 therapies. In addition, large nationwide population-based studies revealed no differences in real-world settings (28,29). Even studies showing some differences do not consistently demonstrate an effect in one direction (30–34). Thus, to date, there is no firm evidence that initiating either ADA or IFX in anti-TNF naive adult patients would make a difference, even for long-term prognosis (a median follow-up of 64 months) after switching to second-line anti-TNF (35). Neither a Markov model (3-month cycle) developed to simulate the therapeutic sequences of initiating biological treatment with ADA or IFX revealed any significant differences in persistence after 3 years in patients with active luminal CD (36).
Along with anti-TNF agents, anti-integrins and anti-IL 12/23 biologics have been proven by RCTs to be effective in adults with active CD (37). Because head-to-head trials would require a high number of patients for a noninferiority design and are unlikely to be performed unless funded by academic (nonindustry) institutes, indirect comparisons based on systematic reviews and network meta-analyses may help clinicians to guide first-line biological treatment. In the study by Singh et al. (7), both ADA and IFX were ranked highest among all biologicals as first-line therapy for the induction of remission in adult patients with moderate to severe CD confirming the results of a previous network meta-analysis evaluating various biologics in both the induction and maintenance phases and including IMM as a comparator (3). By contrast, in an older network meta-analysis, despite both ADA and IFX being effective, IFX was found to have the highest probability of being ranked as the most efficacious agent for induction (86%) and ADA for the maintenance of remission (48%) (8).
In pediatric clinical practice, adult data are relied upon because evidence in children is very scarce. In previous RCTs, both ADA and IFX were shown to be effective and safe in pediatric populations (1,2). Early retrospective observational studies revealed no difference between both therapies for up to 3 years of follow-up after the induction phase (6). A recent systematic review and meta-analysis identified 4 prospective cohort studies comparing ADA and IFX in pediatric populations (4). Three of these were abstracts; the only study that was published as a full text evaluated mucosal healing with anti-TNF therapy in 37 patients (12 ADA and 25 IFX) with biologically naive CD (5). No significant difference was found between the 2 therapies in achieving complete mucosal healing over 1 year of follow-up (P = 0.74). High rates of clinical benefit (remission + response 65%–93%) within 2 years of follow-up with no significant difference between ADA and IFX were recently reported among 87 children with CD from a prospective cohort of the Sicilian IBD Network (9).
Thus, to date, there is no evidence that ADA is superior to IFX or vice versa in adult or pediatric patients with CD (38). This is also supported by our data based on propensity score matching. However, our power calculations showed that using 31 experimental and 31 control subjects, we were not able to detect HR of approximately 0.34–2.90. Thus, we can only conclude that there does not seem to be a substantial difference in the efficacy of both drugs.
Nevertheless, in real-life clinical practice, ADA is often considered as a second-line anti-TNF therapy in pediatric patients (39,40). This may be based on historically stronger experience with IFX, which, for many years, has been the only anti-TNF therapy approved for pediatric patients with CD. IFX may also be perceived as being more potent and adjustable than ADA by some clinicians due to the intravenous route of administration and weight-based dosing schedule, which allows a more precise dosage, especially in smaller children (41). In perianal disease, IFX may be preferred in clinical practice; however, available data probably do not allow first-line anti-TNF therapy to be determined based on disease phenotype (24,38,42,43).
The overall time to treatment escalation in our study was approximately 50% within 3 years of follow-up. However, our definition of treatment escalation also included the need for dose and interval adjustment, which increases the rates compared with a recently published systematic review of pediatric cohort studies reporting the probability of continuing IFX therapy 83%–97% after 1 year and 67%–91% and 61%–85% after 2 and 3 years, respectively. No conclusions can be made for ADA in this review due to the limited number of time-to-event studies (44). When we omitted the need for dose and interval adjustment from the definition, our relapse rate was similar (40% during 3 years) to those published. In the study focused on loss of response in primary responders, the reported random effects pooled incidence of dose intensification was 38% (95% CI 28–50) for IFX and 36% (95% CI 30–43) for ADA, with substantial heterogeneity in both cases. In pediatric patients, the mean percentage loss of response was 25.5%, with no possibility to compare anti-TNFs because of the lack of data (45).
Primary nonresponse to anti-TNF therapy is a substantial obstacle in IBD treatment, especially in adults, and is associated with an inferior response to second-line biologics (7). Primary nonresponse rates in our study (6% with the ADA group and 7% with the IFX group) were lower than those reported for both adults and children (1,2,37). However, these rates do not seem to be underestimated because the inflammatory markers and wPCDAI significantly decreased in both groups up to week 12–16. It is unlikely that patients would continue anti-TNF therapy due to the physicians' decision despite any signs of improvement. Moreover, nonresponse rates may be higher in RCTs, which follow a strict protocol, and different definitions of nonresponse are used in various studies, preventing direct comparison of results. Furthermore, a recently proposed tight TDM strategy during the induction phase (11), which could identify early nonresponders by PK, was not performed in our Center during the study. Several predictors of primary nonresponse are described in the literature (38,46); however, we did not perform these analyses because the rates of primary nonresponse were very low in our patient population.
Concerning predictors of long-term anti-TNF response, traditional factors that appear in the literature, and are derived mainly from adult data are younger age (younger than 40 years), being naive to anti-TNF, and concomitant use of IMM (38). In our study, only the latter was considered relevant and is discussed further. In pediatric patients, these data are generally very scarce. A recently published study within the pediatric inflammatory bowel disease (PIBD) Ahead project identified several risk factors (especially phenotypic, serological, and genetic) for unfavorable disease course; however, predictors of anti-TNF response/relapse were not specifically addressed (47). In the largest pediatric prospective inception cohort study (RISK study) in 913 CD patients, several risk factors of B2 and B3 disease behavior were identified but with no specific conclusions regarding the prediction of anti-TNF efficacy (48). Recent studies identified various serological or genetic predictors of anti-TNF response (49–54); however, these factors were not measured in our patients and thus cannot be discussed. We identified a combination of ASCA negativity and pANCA positivity as the strongest independent predictors of treatment escalation in the multivariate model. To the best of our knowledge, this serological combination has not previously been described in the literature and should be prospectively validated in an independent cohort. Because pANCA positivity is typical for the ulcerative colitis (UC)-like phenotype, and anti-TNF effectivity is generally lower in UC than in CD (9,12), it remains unclear whether the abovementioned serological combination could be a potential proxy marker of distinct disease phenotype of CD with lower sensitivity to anti-TNF treatment.
Based on the results of a network meta-analysis focused on the side effects of anti-TNF, the relative safety profiles of ADA and IFX seem to be comparable (55). In our study, SAE rates were low, did not differ between both groups in the mixed model, and led only occasionally to treatment cessation (only 1 patient in each group), supporting the current opinion that anti-TNF treatment is safe in pediatric CD. Neither a recently published nationwide cohort study among 2018 pediatric IBD patients revealed any association between anti-TNF use and the risk of serious infections (56,57).
Proactive TDM was not performed in our study; thus, limited data did not allow us to fully evaluate the predictive value of PK regarding anti-TNF response. However, data available from selected visits have revealed median levels of both ADA and IFX in the range of recent recommendations in pediatric patients (11). Thus, it is unlikely that our patients were underdosed and that the physicians' approach was affected by this phenomenon. ATI formation was very rare but the transient presence of ATI could have been overlooked due to the reactive TDM approach. Recent studies identified various predictors of IFX levels, such as the presence of ATI, serum albumin concentration, concomitant IMM therapy, body weight, and sex (58). Owing to the scarcity of PK data in our study, we did not intend to identify any predictors of PK in our patients.
The approach to concomitant IMM therapy differs among pediatric IBD centers. In the European Union, AZA is used in most patients; by contrast, in the United States, use of MTX or anti-TNF monotherapy is more popular (39,59). Despite conflicting data, combination therapy is still considered useful (13,60–63), and most pediatric centers use it for at least 6 months from the onset of anti-TNF treatment. In accordance with recent data and current guidelines, IMM has been used less frequently in patients treated with ADA compared with patients treated with IFX (11,64). In our study, IMM treatment was not identified as a strong predictor of relapse on both univariate and multivariate analyses. These results could be affected by the high rate of concomitant IMM treatment in both groups. Owing to limited data, we could not analyze the possible effect of IMM on anti-TNF PK.
In addition to efficacy and safety, cost may be an important factor when selecting an appropriate first-line anti-TNF therapy. In some studies, ADA seems to be less costly than IFX; in others, the opposite seems to be true (65–68). Moreover, biosimilars coming to the market have changed the scenario significantly (69,70). Financial issues may be strongly dependent on the local situation, and other aspects such as quality of life should be considered when selecting an appropriate biological treatment.
Our data show that both ADA and IFX seem to demonstrate comparable efficacy and safety in pediatric CD patients naive to biologics. This study included a relatively small sample size compared with adult trials, preventing us from drawing strong conclusions. Despite its prospective design, some data were missing, and PK data were not available at all time points because TDM was not applied proactively during the study. Results in ADA-treated patients may have been influenced by a lack of adherence to therapy, which we were unable to evaluate. Conversely, this is the first pediatric study using propensity score matching with effective pairing (no dropouts), a prospective design, and a long duration of follow-up. Data comparing both anti-TNFs should be considered with caution in the future because these are derived from the traditional step-up approach. Because the top-down strategy (at least for IFX) may become preferable in children based on recent data (11,71), further research on the efficacy of various biologics as first-line treatment immediately after diagnosis must be performed.
CONFLICTS OF INTEREST
Guarantor of the article: Jiri Bronsky, MD, PhD.
Specific author contributions: J.B.: study design, data analysis, writing of the manuscript, and project supervision. I.C., D.K., T.L., K.M., K.P., M.S., and K.Z.: patient recruitment, data collection, and revision of manuscript draft; O.H.: study design, patient recruitment, data collection, statistics, and revision of manuscript draft.
Financial support: This work was supported by the Ministry of Health, Czech Republic, for conceptual development of research organizations (00064203, University Hospital Motol, Prague, Czech Republic), GAUK 2120248, and research grant from the Working Group for Paediatric Gastroenterology and Nutrition of the Czech Paediatric Society.
Potential competing interests: J.B.: lectures/congress fees/consultancy (outside submitted work)–MSD, AbbVie, Nutricia, Nestlé, Ferring, Biocodex, and Walmark; T.L.: lectures/congress fees/consultancy (outside submitted work)—Nutricia, Ferring, and Biocodex; K.M.: lectures/congress fees/consultancy (outside submitted work)—AddVie and Takeda; K.Z: lectures/congress fees/consultancy (outside submitted work)—Nutricia and Nestlé; O.H.: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, Nestlé, Ferring, and Falk; I.C., D.K., K.P., and M.S. report no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Both adalimumab (ADA) and infliximab (IFX) are effective and safe in the treatment of pediatric Crohn's disease.
WHAT IS NEW HERE
✓ This is the first prospective observational study comparing ADA and IFX in pediatric Crohn's disease.
✓ Propensity score matching did not reveal substantial differences in efficacy or safety between ADA and IFX.
✓ The ASCA−/pANCA+ combination is a strong predictor of treatment escalation.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Editage (www.editage.com) for English language editing.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A798, http://links.lww.com/CTG/A799, http://links.lww.com/CTG/A800
Contributor Information
Ivana Copova, Email: ivana.copova@fnmotol.cz.
Denis Kazeka, Email: denis.kazeka@fnmotol.cz.
Tereza Lerchova, Email: tereza.lerchova@fnmotol.cz.
Katarina Mitrova, Email: katarina.mitrova@fnmotol.cz.
Kristyna Pospisilova, Email: potuznikovakris@gmail.com.
Miroslava Sulovcova, Email: mirkasulovcova@gmail.com.
Kristyna Zarubova, Email: zarubova.kristyna@gmail.com.
Ondrej Hradsky, Email: ondrej.hradsky@fnmotol.cz.
REFERENCES
- 1.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology 2007;132:863–6; quiz 1165-6. [DOI] [PubMed] [Google Scholar]
- 2.Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology 2012;143:365–e2. [DOI] [PubMed] [Google Scholar]
- 3.Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: A network meta-analysis. Gastroenterology 2015;148:344–5, e5; quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Reynaert C, Su AL, et al. Efficacy and safety of infliximab in pediatric Crohn disease: A systematic review and meta-analysis. Can J Hosp Pharm 2019;72:227–38. [PMC free article] [PubMed] [Google Scholar]
- 5.Nuti F, Civitelli F, Bloise S, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-alpha therapy in a paediatric Crohn's disease cohort. J Crohns Colitis 2016;10:5–12. [DOI] [PubMed] [Google Scholar]
- 6.Nuti F, Viola F, Civitelli F, et al. Biological therapy in a pediatric Crohn disease population at a referral center. J Pediatr Gastroenterol Nutr 2014;58:582–7. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: First- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment Pharmacol Ther 2018;48:394–409. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Garg SK, Pardi DS, et al. Comparative efficacy of biologic therapy in biologic-naïve patients with Crohn disease: A systematic review and network meta-analysis. Mayo Clin Proc 2014;89:1621–35. [DOI] [PubMed] [Google Scholar]
- 9.Romeo AC, Ventimiglia M, Dipasquale V, et al. Effectiveness and safety of biologics in pediatric inflammatory bowel disease: Real-life data from the Sicilian network. Clin Res Hepatol Gastroenterol 2020;44:223–9. [DOI] [PubMed] [Google Scholar]
- 10.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ecco/espghan on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179–207. [DOI] [PubMed] [Google Scholar]
- 11.van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn's disease: An ECCO-ESPGHAN guideline update. J Crohns Colitis 2020. doi: 10.1093/ecco-jcc/jjaa161. [DOI] [PubMed] [Google Scholar]
- 12.Bank S, Andersen PS, Burisch J, et al. Effectiveness of anti-tumour necrosis factor-alpha therapy in Danish patients with inflammatory bowel diseases. Dan Med J 2015;62:A4994. [PubMed] [Google Scholar]
- 13.Cosnes J, Sokol H, Bourrier A, et al. Adalimumab or infliximab as monotherapy, or in combination with an immunomodulator, in the treatment of Crohn's disease. Aliment Pharmacol Ther 2016;44:1102–13. [DOI] [PubMed] [Google Scholar]
- 14.Kestens C, van Oijen MG, Mulder CL, et al. Adalimumab and infliximab are equally effective for Crohn's disease in patients not previously treated with anti-tumor necrosis factor-alpha agents. Clin Gastroenterol Hepatol 2013;11:826–31. [DOI] [PubMed] [Google Scholar]
- 15.Lehtola E, Haapamaki J, Farkkila MA. Outcome of inflammatory bowel disease patients treated with TNF-alpha inhibitors: Two-year follow-up. Scand J Gastroenterol 2016;51:1476–81. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Sylwestrzak G, Ruggieri AP, et al. Intravenous versus subcutaneous anti-TNF-alpha agents for Crohn's disease: A comparison of effectiveness and safety. J Manag Care Spec Pharm 2015;21:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn's disease. Clin Gastroenterol Hepatol 2014;12:811–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil SA, Rustgi A, Langenberg P, et al. Comparative effectiveness of anti-TNF agents for Crohn's disease in a tertiary referral IBD practice. Dig Dis Sci 2013;58:209–15. [DOI] [PubMed] [Google Scholar]
- 19.Preda C, Fulger L, Gheorghe L, et al. Adalimumab and infliximab in Crohn's disease–real life data from a national retrospective cohort study. Curr Health Sci J 2016;42:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varma P, Paul E, Huang C, et al. A retrospective comparison of infliximab versus adalimumab as induction and maintenance therapy for Crohn disease. Intern Med J 2016;46:798–804. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama K, Yamazaki K, Katafuchi M, et al. A retrospective claims database study on drug utilization in Japanese patients with Crohn's disease treated with adalimumab or infliximab. Adv Ther 2016;33:1947–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorzi F, Zuzzi S, Onali S, et al. Efficacy and safety of infliximab and adalimumab in Crohn's disease: A single centre study. Aliment Pharmacol Ther 2012;35:1397–407. [DOI] [PubMed] [Google Scholar]
- 23.Doecke JD, Hartnell F, Bampton P, et al. Infliximab vs. adalimumab in Crohn's disease: Results from 327 patients in an Australian and New Zealand observational cohort study. Aliment Pharmacol Ther 2017;45:542–52. [DOI] [PubMed] [Google Scholar]
- 24.Narula N, Kainz S, Petritsch W, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-alpha naive Crohn's disease. Aliment Pharmacol Ther 2016;44:170–80. [DOI] [PubMed] [Google Scholar]
- 25.Benmassaoud A, Al-Taweel T, Sasson MS, et al. Comparative effectiveness of infliximab versus adalimumab in patients with biologic-naive Crohn's disease. Dig Dis Sci 2018;63:1302–10. [DOI] [PubMed] [Google Scholar]
- 26.Olivera P, Thiriet L, Luc A, et al. Treatment persistence for infliximab versus adalimumab in Crohn's disease: A 14-year single-center experience. Inflamm Bowel Dis 2017;23:976–85. [DOI] [PubMed] [Google Scholar]
- 27.Tursi A, Elisei W, Picchio M, et al. Effectiveness and safety of infliximab and adalimumab for ambulatory Crohn's disease patients in primary gastroenterology centres. Eur J Intern Med 2014;25:485–90. [DOI] [PubMed] [Google Scholar]
- 28.Di Domenicantonio R, Trotta F, Cascini S, et al. Population-based cohort study on comparative effectiveness and safety of biologics in inflammatory bowel disease. Clin Epidemiol 2018;10:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung YS, Han M, Park S, et al. Biologic use patterns and predictors for non-persistence and switching of biologics in patients with inflammatory bowel disease: A nationwide population-based study. Dig Dis Sci 2020;65:1436–44. [DOI] [PubMed] [Google Scholar]
- 30.Ananthakrishnan AN, Cagan A, Cai T, et al. Comparative effectiveness of infliximab and adalimumab in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2016;22:880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaniewska M, Rosołowski M, Rydzewska G. Efficacy, tolerability, and safety of infliximab biosimilar in comparison to originator biologic and adalimumab in patients with Crohn disease. Pol Arch Intern Med 2019;129:484–9. [DOI] [PubMed] [Google Scholar]
- 32.Ma C, Huang V, Fedorak DK, et al. Crohn's disease outpatients treated with adalimumab have an earlier secondary loss of response and requirement for dose escalation compared to infliximab: A real life cohort study. J Crohns Colitis 2014;8:1454–63. [DOI] [PubMed] [Google Scholar]
- 33.Riis A, Martinsen TC, Waldum HL, et al. Clinical experience with infliximab and adalimumab in a single-center cohort of patients with Crohn's disease. Scand J Gastroenterol 2012;47:649–57. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of anti-tumor necrosis factor agents in biologic-naive patients with Crohn's disease. Clin Gastroenterol Hepatol 2016;14:1120–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inokuchi T, Takahashi S, Hiraoka S, et al. Long-term outcomes of patients with Crohn's disease who received infliximab or adalimumab as the first-line biologics. J Gastroenterol Hepatol 2019;34:1329–36. [DOI] [PubMed] [Google Scholar]
- 36.Taxonera C, Robledo P, Rodríguez A. Treatment persistence during therapeutic sequences with adalimumab and infliximab in the treatment of Crohn's disease. Rev Esp Enferm Dig 2017;109:690–3. [DOI] [PubMed] [Google Scholar]
- 37.Gomollón F, Dignass A, Annese V, et al. 3rd european evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 38.Aardoom MA, Veereman G, de Ridder L. A review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci 2019;20:2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronsky J, de Ridder L, Ruemmele FM, et al. Diagnostic and therapeutic approach in paediatric inflammatory bowel diseases: Results from a clinical practice survey. J Pediatr Gastroenterol Nutr 2019;68:676–83. [DOI] [PubMed] [Google Scholar]
- 40.Penagini F, Cococcioni L, Pozzi E, et al. Biological therapy in pediatric age. Pharmacol Res 2020;161:105120. [DOI] [PubMed] [Google Scholar]
- 41.Mogilevski T, Sparrow MP. Infliximab versus adalimumab in patients with biologic-naive Crohn's disease: Is the difference real? Dig Dis Sci 2018;63:1094–6. [DOI] [PubMed] [Google Scholar]
- 42.Srinivas NR. Letter: Comparative safety and efficacy of infliximab vs Adalimumab in Crohn's disease–should one consider disease location? Aliment Pharmacol Ther 2016;44:771–2. [DOI] [PubMed] [Google Scholar]
- 43.Tursi A, Elisei W, Picchio M, et al. Letter: Infliximab vs. adalimumab in treating ambulatory perianal fistulising Crohn's disease. Aliment Pharmacol Ther 2014;40:218–20. [DOI] [PubMed] [Google Scholar]
- 44.van Rheenen H, van Rheenen PF. Long-term efficacy of anti-tumor necrosis factor agents in pediatric luminal Crohn's disease: A systematic review of real-world evidence studies. Pediatr Gastroenterol Hepatol Nutr 2020;23:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: Loss of response and requirement of anti-TNFalpha dose intensification in Crohn's disease. J Gastroenterol 2017;52:535–54. [DOI] [PubMed] [Google Scholar]
- 46.Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: From basic science to clinical practice. J Crohns Colitis 2020;14:694–709. [DOI] [PubMed] [Google Scholar]
- 47.Ricciuto A, Aardoom M, Orlanski-Meyer E, et al. Predicting outcomes in pediatric Crohn's disease for management optimization: Systematic review and consensus statements from the pediatric inflammatory bowel disease-ahead program. Gastroenterology 2021;160:403–36.e26. [DOI] [PubMed] [Google Scholar]
- 48.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: A multicentre inception cohort study. Lancet 2017;389:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caviglia GP, Rosso C, Stalla F, et al. On-treatment decrease of serum interleukin-6 as a predictor of clinical response to biologic therapy in patients with inflammatory bowel diseases. J Clin Med 2020;9:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gole B, Potočnik U. Pre-treatment biomarkers of anti-tumour necrosis factor therapy response in Crohn's disease-a systematic review and gene ontology analysis. Cells 2019;8:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mateos B, Saez-Gonzalez E, Moret I, et al. Plasma oncostatin-m, TNF-alpha, IL-7 and IL-13 network predicts Crohn's disease response to infliximab, as assessed by calprotectin log-drop. Dig Dis 2020;39:1–9. [DOI] [PubMed] [Google Scholar]
- 52.Salvador-Martin S, Bossacoma F, Pujol-Muncunill G, et al. Genetic predictors of long-term response to antitumor necrosis factor agents in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2020;71:508–15. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Shen J, Zheng Q. Development of a clinical model to predict secondary non-response of infliximab treatment in Crohn's disease. Int J Colorectal Dis 2020;35:2019–26. [DOI] [PubMed] [Google Scholar]
- 54.Zorlu O, Bülbül Başkan E, Yazici S, et al. Predictors of drug survival of biologic therapies in psoriasis patients. J Dermatolog Treat 2020;33:437–42. [DOI] [PubMed] [Google Scholar]
- 55.Mocko P, Kawalec P, Pilc A. Safety profile of biologic drugs in the therapy of Crohn disease: A systematic review and network meta-analysis. Pharmacol Rep 2016;68:1237–43. [DOI] [PubMed] [Google Scholar]
- 56.Ruemmele FM. Safety of anti-TNF biologics in paediatric inflammatory bowel disease. Lancet Gastroenterol Hepatol 2019;4:813–5. [DOI] [PubMed] [Google Scholar]
- 57.Wintzell V, Svanström H, Melbye M, et al. Use of tumour necrosis factor-α inhibitors and the risk of serious infection in paediatric inflammatory bowel disease in Denmark: A nationwide cohort study. Lancet Gastroenterol Hepatol 2019;4:845–53. [DOI] [PubMed] [Google Scholar]
- 58.Santacana E, Rodríguez-Alonso L, Padullés A, et al. Predictors of infliximab trough concentrations in inflammatory bowel disease patients using a repeated-measures design. Ther Drug Monit 2020;42:102–10. [DOI] [PubMed] [Google Scholar]
- 59.Church PC, Hyams J, Ruemmele F, et al. The continental divide: Anti-TNF use in pediatric IBD is different in north America compared to other parts of the world. Can J Gastroenterol Hepatol 2018;2018:3190548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peyrin-Biroulet L, Salleron J, Filippi J, et al. Anti-TNF monotherapy for Crohn's disease: A 13-year multicentre experience. J Crohns Colitis 2016;10:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 62.Dhillon AS, Harris AW. Infliximab vs adalimumab for Crohn's disease. Clin Gastroenterol Hepatol 2015;13:210. [DOI] [PubMed] [Google Scholar]
- 63.Osterman MT. Reply: To PMID 23811254. Clin Gastroenterol Hepatol 2015;13:210–1. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: A prospective, randomized trial. J Crohns Colitis 2016;10:1259–66. [DOI] [PubMed] [Google Scholar]
- 65.Aliyev ER, Hay JW, Hwang C. Cost-effectiveness comparison of ustekinumab, infliximab, or adalimumab for the treatment of moderate-severe Crohn's disease in biologic-naive patients. Pharmacotherapy 2019;39:118–28. [DOI] [PubMed] [Google Scholar]
- 66.Choi GK, Collins SD, Greer DP, et al. Costs of adalimumab versus infliximab as first-line biological therapy for luminal Crohn's disease. J Crohns Colitis 2014;8:375–83. [DOI] [PubMed] [Google Scholar]
- 67.Sussman DA, Kubiliun N, Mulani PM, et al. Comparison of medical costs among patients using adalimumab and infliximab: A retrospective study (compairs). Inflamm Bowel Dis 2012;18:2043–55. [DOI] [PubMed] [Google Scholar]
- 68.Yu AP, Johnson S, Wang ST, et al. Cost utility of adalimumab versus infliximab maintenance therapies in the United States for moderately to severely active Crohn's disease. Pharmacoeconomics 2009;27:609–21. [DOI] [PubMed] [Google Scholar]
- 69.Rencz F, Gulácsi L, Péntek M, et al. Cost-utility of biological treatment sequences for luminal Crohn's disease in europe. Expert Rev Pharmacoecon Outcomes Res 2017;17:597–606. [DOI] [PubMed] [Google Scholar]
- 70.Cozijnsen MA, Samsom JN, de Ridder L. Anti-tumour necrosis factor therapy for paediatric Crohn's disease: Improved benefits through treatment optimisation, deeper understanding of its risks, and reduced costs due to biosimilar availability. Paediatr Drugs 2018;20:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jongsma MME, Aardoom MA, Cozijnsen MA, et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: An open-label multicentre randomised controlled trial. Gut 2020;71:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.