Abstract
INTRODUCTION:
Reduced rectal sensation is involved in the pathophysiology of constipation. The aim of this study was to investigate the effects of transcutaneous electrical acustimulation (TEA) at acupuncture point ST36 on constipation and rectal sensation as well as autonomic functions in patients with constipation and reduced rectal sensation.
METHODS:
In an acute study, anorectal motility and sensation tests were performed in constipation patients (N = 53) who were treated with TEA at ST36 or sham points. In a chronic study, patients (N = 18) underwent 2 weeks of TEA or sham-TEA in a crossover design.
RESULTS:
Chronic TEA increased spontaneous bowel movements (3.72 vs 2.00 per week with sham-TEA, P < 0.0001) and significantly reduced constipation symptoms and increased quality of life in comparison with sham-TEA (P < 0.05). Acute TEA reduced the sensation threshold in response to rectal distention for the urge of defecation and maximum tolerable volume (P < 0.05, vs baseline); chronic TEA reduced the sensation thresholds for first sensation and desire of defecation, and decreased the threshold volume to an elicit rectal anal inhibitory reflex (P < 0.05). Both acute and chronic TEA increased parasympathetic activity (P < 0.05).
DISCUSSION:
TEA at ST36 improves chronic constipation by enhancing rectal sensation possibly mediated by the reinforcement of parasympathetic activity in patients with functional constipation and reported lack/absence of rectal sensation.
INTRODUCTION
Chronic constipation (CC) is a common and symptom-based disorder, and its global prevalence varies from 0.7% to 79% for adults worldwide and is 33.5% in the general population aged 60 years or older (1). It involves 1 or more symptoms, including excessive straining, hard stools, as well as sensations of anorectal obstruction and incomplete evacuation (2). The conventional treatments for constipation include fiber, laxatives, and prokinetic agents (3–5). Biofeedback training, and probiotics have also been used for treating constipation (6–8). However, there are a fair amount of constipation patients whose symptoms are refractory to these conventional and emerging therapies. Accordingly, it is of great significance to develop novel and effective methods for the treatment of constipation.
Defecation is a complex and coordinated process that involves various factors, including colonic contractions, rectal sensation, and anal sphincter relaxation. A reduced rectal sensation or rectal hyposensitivity was regarded as a major pathophysiological characteristic of functional constipation (9–12). In a recent study, rectal hyposensitivity was reported in 25% of 2,876 patients with refractory functional constipation. However, there are no available therapies for treating rectal hyposensitivity except biofeedback training.
Neuromodulation has recently been explored as a novel treatment for constipation, but with limited success. In 62 patients with CC, long-term sacral nerve stimulation (SNS) with an implanted pulse generator improved rectal sensation, reflected as decreased threshold volumes in response to rectal balloon distention for the first constant sensation and desire to defecate (13). Transcutaneous posterior tibial nerve stimulation was noted to ameliorate symptoms in patients with obstructed defecation without anatomic obstruction (14). In previous preliminary studies, we found that transcutaneous neuromodulation at the posterior tibial nerve and the acupuncture point ST36 on the leg improved rectal sensation and reduced the threshold volume to an elicit rectoanal inhibitory reflex (RAIR) and consequently defecation (15). Although rectal sensation and constipation could be improved by neuromodulation according to these findings, the effectiveness has not yet been identified because of the following reasons: (i) Patients enrolled in these studies did not have distinct impaired or reduced rectal sensation; (ii) possible placebo effects could not be ruled out; and (iii) the sample size was small.
Although exact mechanisms involved in rectal hyposensitivity are unclear, we hypothesize that neuromodulation could be a potential therapy for rectal hyposensitivity in patients with functional constipation. Inspired by the applications of neuromodulation in the improvement of rectal sensation, we designed a clinical study to specifically investigate the effect of a noninvasive method of transcutaneous electrical acustimulation (TEA) on rectal sensation in patients with both constipation and reduced rectal sensation. TEA is a combined method of neuromodulation and electroacupuncture. It delivers weak electrical current to certain acupuncture points located near the peripheral nerves through surface electrodes using known parameters to improve autonomic functions. TEA has been shown to be effective in treating various functional gastrointestinal diseases, including gastroesophageal reflux disease, functional dyspepsia, and constipation (15–18). These previous findings have consistently shown TEA-induced improvement in gastrointestinal motility and rectal sensation through the enhancement of parasympathetic activity. However, it is unknown whether TEA is effective in improving both constipation and reduced rectal sensation in patients with CC.
Accordingly, our study aimed at examining (i) the effect of acute TEA on rectal sensation, (ii) the effects of chronic TEA on rectal sensation and constipation, and (iii) mechanisms involving autonomic functions in patients with constipation and reduced rectal sensation.
MATERIALS AND METHODS
Subjects
This study enrolled a total of 62 patients (18–75 years, 16 men and 46 women) with functional constipation according to the Rome IV definition and with reduced rectal sensation. Other inclusion criteria were as follows: (i) Patients had at least 6 months of CC history; (ii) patients underwent colonoscopy or barium enema within 5 years and with negative results; and (iii) patients reported the absence or lack of rectal sensation and a first sensation threshold to ramp distention of the rectum ≥40 mL. Exclusion criteria were as follows: irritable bowel syndrome, inflammatory bowel disease, metabolic, endocrine, psychological distress or neurologic diseases, debilitating illnesses, intestinal surgery, women in gestation or lactation periods, or implanted electronic devices.
The sample size for the chronic study was based on a previous study by Wu et al. (19) and power analysis using the number of spontaneous bowel movements per week as the primary outcome. Seventeen subjects were needed to achieve a convention power of 80 and an alpha value of 0.05. The sample size for the acute study was also based on the previous study (19) and power analysis using the rectal distention threshold for the urge to defecate as the primary outcome. Twenty-five subjects were needed to achieve a conventional power of 80% and an alpha value of 0.05. Accordingly, the number of patients in this study was sufficiently powered.
The Ethical Review Committee of Yinzhou People's Hospital approved the experimental protocol (No. 2017010). Written informed consent was obtained from each patient before this study.
Experimental protocol
A randomized, patient-blinded, sham-controlled clinical study was conducted at Yinzhou People's Hospital. This study, preceded with a 1-week run-in period, consisted of an acute phase and a chronic phase.
In the acute part, patients were treated in 2 randomized sessions on separate days: 30-minute TEA at the acupoint ST36 and 30-minute sham-TEA at a nonacupoint, respectively (Figure 1a). An electrocardiogram (ECG) was evaluated, and anorectal manometry was performed before and after the TEA/sham-TEA treatment.
Figure 1.
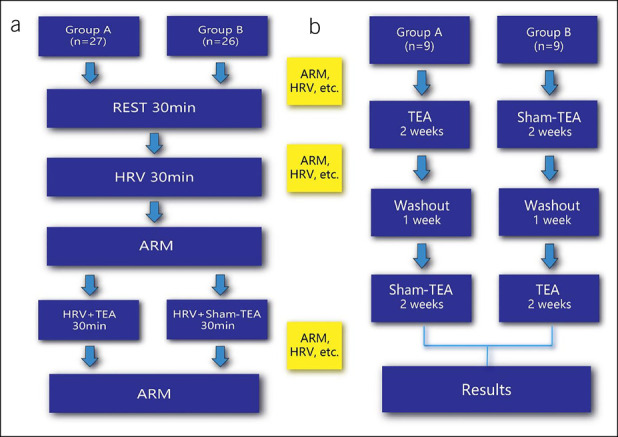
Experimental protocol of the acute part (a) and the chronic part (b). ARM, anorectal manometry; HRV, heart rate variability; TEA, transcutaneous electrical acustimulation.
In the chronic part of this study, a crossover design was used: Patients were randomized to undergo 2-week TEA followed by 2-week sham-TEA (after 1-week washout) or vice versa (Figure 1b). TEA or sham-TEA was performed for 1 hour twice daily, one in the morning after getting up and the other in the evening after dinner (15). Patients were asked for 3 office visits during this study, at the beginning and the ends of each treatment arm. The Patient Assessment of Constipation Symptom (PAC-SYM) questionnaires, the Patient Assessment of Constipation Quality of Life (PAC-QoL) questionnaires, and anorectal manometry were completed during each visit. The patients were asked to keep a bowel habit diary during TEA or sham-TEA, including the frequency of defecation, duration of each defecation, stool quality, and medications used for defecation if any. Lactulose or glycerine enema was permitted as a rescue medication only if patients did not have a spontaneous bowel movement (SBM) for more than 3 days or had severe symptoms such as abdominal bloating.
Transcutaneous electrical acustimulation
Bilateral acupoints ST36 were used in TEA. The location of ST36 was on the anterolateral side of the lower leg, 3 inches under the Dubi and one finger-breadth on the anterior crest of tibia. A pair of electrodes were placed at the bilateral ST36 after skin treatment and connected to a watch-size stimulator (SNM-FDC01; Ningbo Maida Medical Device, Ningbo, China) (Figure 2). Electrical stimulation was performed for 30 minutes in the acute study and 1 hour twice daily in the chronic study with the following parameters: trains on-time of 2 seconds and off-time of 3 seconds, pulse width of 0.5 ms, pulse frequency of 25 Hz, and amplitude of 2–10 mA (at the maximum level tolerated by the patient); these parameters were previously shown to improve gastrointestinal motility (15–17). Sham-TEA was applied at nonacupoints 6–10 cm down and lateral to the ST36 (not on any meridian) using the same electrical stimulation parameters as the TEA (15–17).
Figure 2.
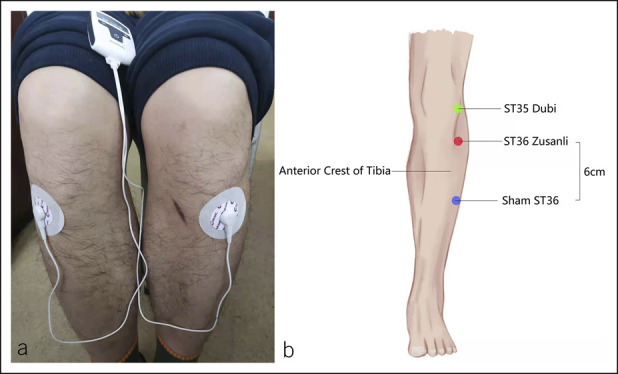
(a) Placement of TEA electrodes at ST36. (b) Location of acupoints for the ST36. TEA, transcutaneous electrical acustimulation.
Anorectal manometry
Enema was used to empty the rectum before the anorectal manometric test. The anorectal manometric test was performed using an eight-channel water-perfused catheter (GAP-08A; Ningbo Maida Medical Device, Ningbo, China), which was inserted into the rectum through the anus. The patient was in a left lateral position during the entire anorectal manometric procedure. Assessments of resting anal sphincter pressure and maximum squeeze pressure, duration of sustained squeeze, rectoanal pressure changes during attempted defecation, and percentage of paradoxical contractions during the attempted defecations were conducted in all patients. Rectal sensation was assessed by gradual balloon distension of the rectum from 10 to 300 mL of air at 10 mL/s. Distention volumes corresponding to the first sensory, desire of defecation, urge of defecation and maximal tolerance were recorded. In this study, first sensation was defined as sensation that was temporary, vague, and unsustainable. Desire to defecate was defined as a desire to defecate with the duration more than 15 seconds. Urge to defecation was defined as continuous and urgent bowel movements accompanied by distension. Maximum tolerable volume was defined as endurable distension to the maximum volume with or without pain (20). Rectoanal inhibitory reflex (RAIR) was elicited by rapid inflation of the balloon with 10, 20, 30, 40, and 50 mL of air in a randomized order. RAIR was considered to exist if there was a 5 mm Hg or more drop in the anal sphincter pressure during the rectal distention; the lowest rectal distention volume that solicited RAIR was noted (21–23).
Outcome measurements
In the acute part of this study, the primary outcome was the improvement in rectal sensation function after the acute TEA. In the chronic part, the primary outcome was the number of weekly SBMs after the treatment. The secondary outcomes were anorectal motility and sensation profiles, PAC-SYM, and PAC-QoL. The PAC-SYM was a 12-item questionnaire which consisted of 3 symptom subscales, abdominal, rectal, and stool, to evaluate the frequency and severity of CC symptoms (24). PAC-QoL was a 28-item questionnaire which consisted of 4 subscales (physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction) to assess the participant's quality of life. Each item was scored on a 5-point Likert-type scale ranging from 0 to 4 (25). The lower scores of the PAC-SYM and PAC-QoL, respectively, indicate less severe symptoms and better quality of life.
Assessment of autonomic functions
The autonomic function was assessed using the spectral analysis of heart rate variability (HRV) derived from 1-channel ECG recorded for 30 minutes at baseline in the fasting state and 30 minutes during TEA or sham-TEA in the acute part of this study and during each visit of the chronic part of this study. The ECG was record from 3 electrodes: the right one on the manubrium sterni, the left one on the fifth rib in the left axillary line, and the ground one on the right chest. A dedicated ECG amplifier (ECG-201; Ningbo Maida Medical Device) was used to record the ECG. We used the software developed in our laboratory to down-sample the ECG signal by monitoring the R-R intervals and perform spectral analysis on the HRV signal. The power spectrum of the HRV signal was divided into a low-frequency band (0.04–0.15 Hz; LF) and a high-frequency band (0.15–0.40 Hz; HF). LF mainly reflects sympathetic activity, and HF only represents parasympathetic activity. The ratio of LF/HF indicates the balance of sympathetic and parasympathetic activities (18,26).
Statistical analysis
All analyses were performed using statistical software SAS9.4 (SAS Institute, Cary, NC). In the acute study, data were shown as mean ± SEM. The paired t test was used to compare the differences in each measurement between baseline and treatment. In the chronic study, the count data were shown as number (percentage) and analyzed using the Fisher exact test. The quantitative data were presented as mean ± SD and compared using the independent samples t test or Wilcoxon test. A MANOVA was performed to evaluate differences between the 2 sequences, periods, and treatments. Statistical significance was set at P < 0.05. χ2 analysis was used to determine the effect of the chronic treatment on the prevalence of patients with an elevated sensation threshold to rectal distention and the prevalence of patients with the number of SBMs per week <3.
RESULTS
Acute study
Sixty patients were enrolled in the acute study to complete 2 sessions of TEA and sham-TEA in a randomized order but 7 of them did not complete the second session.
Effects of acute TEA on anorectal motility and rectal sensation
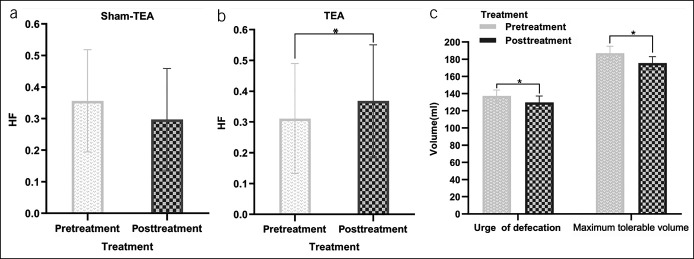
Acute TEA decreased the threshold volume of urge to defecation and maximum tolerance compared with baseline (P < 0.05 for both) (Figure 3c). Sham-TEA exerted no such effects on rectal sensation (Table 1).
Figure 3.
Effects of acute one-time TEA on autonomic nervous function and anorectal sensation. HF: the high-frequency component in the power spectrum of heart rate variability, reflecting parasympathetic activity. (a) Compared with the baseline, sham-TEA did not alter the HF (P > 0.05). (b) TEA significantly increased HF (P < 0.05). (c) TEA decreased the threshold volumes of urge for defecation and maximum tolerance (P < 0.05 for both). TEA, transcutaneous electrical acustimulation.
Table 1.
Anorectal motility and sensation profiles by TEA/sham-TEA in the acute part
| Items | Sham-TEA (N = 26) | TEA (N = 27) | ||||||
| Pretreatment | Posttreatment | t | P | Pretreatment | Posttreatment | t | P | |
| Anal rest pressure (mm Hg) | 63.70 ± 3.35 | 61.57 ± 3.71 | 0.565 | 0.577 | 68.14 ± 5.08 | 69.83 ± 4.67 | −0.423 | 0.676 |
| Maximum squeeze pressure (mm Hg) | 104.35 ± 5.14 | 118.28 ± 7.24 | −1.979 | 0.059 | 120.41 ± 8.55 | 120.16 ± 9.35 | 0.04 | 0.969 |
| Duration of contraction (s) | 19.91 ± 0.75 | 19.56 ± 0.39 | 0.457 | 0.652 | 18.79 ± 0.59 | 18.12 ± 0.53 | 1.110 | 0.277 |
| First sensation (mL) | 54.04 ± 3.40 | 54.04 ± 3.37 | 0.000 | >0.999 | 52.59 ± 2.22 | 50.56 ± 2.27 | 1.009 | 0.322 |
| Desire of defecation (mL) | 92.50 ± 7.63 | 85.96 ± 5.91 | 1.702 | 0.101 | 87.78 ± 3.28 | 83.89 ± 3.42 | 1.162 | 0.256 |
| Urge of defecation (mL) | 124.42 ± 8.71 | 120.58 ± 8.05 | 0.968 | 0.342 | 136.67 ± 7.50 | 128.15 ± 7.64 | 2.281 | 0.031a |
| Maximum tolerable volume (mL) | 164.42 ± 10.67 | 161.15 ± 9.90 | 0.726 | 0.475 | 185.00 ± 8.78 | 174.44 ± 9.41 | 2.128 | 0.043a |
| Paradoxical contraction (%) | 83.85 ± 6.66 | 74.61 ± 7.40 | 1.1 | 0.282 | 72.59 ± 6.72 | 70.49 ± 7.36 | 0.359 | 0.723 |
| Increase in rectal pressure (mm Hg) | 31.08 ± 2.62 | 29.39 ± 2.56 | 0.854 | 0.401 | 27.26 ± 2.70 | 26.92 ± 2.75 | 0.156 | 0.877 |
| RAIR (mL) | 26.54 ± 1.92 | 23.46 ± 2.00 | 1.99 | 0.058 | 24.44 ± 1.45 | 22.59 ± 1.14 | 1.154 | 0.259 |
| Anal sphincter pressure during strain (mm Hg) | 53.71 ± 3.36 | 54.27 ± 3.49 | −0.186 | 0.854 | 54.02 ± 3.75 | 56.43 ± 3.22 | −0.833 | 0.412 |
TEA, transcutaneous electrical acustimulation.
P < 0.05 in the paired t test between pretreatment and posttreatment.
Alterations in autonomic functions with TEA
Acute TEA rather than sham-TEA increased the parasympathetic activity (HF) and decreased the sympathetic activity (LF). TEA increased HF from 0.31 ± 0.03 to 0.37 ± 0.04 (t = −2.537, P = 0.018) (Figure 3b) but decreased LF from 0.69 ± 0.03 to 0.63 ± 0.04 (t = 2.537, P = 0.018 < 0.05). Meanwhile, compared with the baseline, the ratio of LF/HF was significantly decreased from 3.23 ± 0.46 to 2.55 ± 0.43 (t = 2.317, P = 0.029) after acute TEA. No significant difference was seen in HF, LF, or LF/HF between baseline and sham-TEA (Figure 3a).
Chronic study
Twenty-four patients who reported absence or lack of rectal sensation for defecation were enrolled. Six dropped out after 2 weeks of treatment (3 during or after TEA and the other 3 during or after sham-TEA). Four of the 6 patients were allergic to electrodes and the other 2 patients quit the study due to dissatisfaction with the efficacy of the first arm of the treatment. All other patients completed the 5-week study according to the study protocol. The patient information at baseline is summarized in Table 2.
Table 2.
Subject characteristics according to treatment sequence
| Characteristics | TEA-Sham TEA | Sham TEA-TEA | All | P |
| Sex, n (%) | 0.082 | |||
| Male | 0 (0.00) | 4 (44.44) | 4 (22.22) | |
| Female | 9 (100.00) | 5 (55.56) | 14 (77.78) | |
| Age (yr) | 0.860 | |||
| Mean ± SD | 53.00 ± 12.02 | 51.00 ± 18.66 | 52.00 ± 15.26 | |
| 50% (25%, 75%) | 53.00 (46.00, 63.00) | 61.00 (45.00, 64.00) | 58.50 (45.00, 63.00) | |
| Range | 30.00–68.00 | 19.00–66.00 | 19.00–68.00 | |
| BMI (kg/m2) | 0.991 | |||
| Mean ± SD | 21.16 ± 2.57 | 21.15 ± 1.76 | 21.16 ± 2.14 | |
| 50% (25%, 75%) | 20.99 (18.70, 21.93) | 21.74 (19.86, 21.95) | 21.26 (19.53, 21.95) | |
| Range | 18.32–25.95 | 18.34–24.22 | 18.32–25.95 | |
| Duration of constipation (yr) | 0.340 | |||
| Mean ± SD | 5.00 ± 3.35 | 6.78 ± 4.27 | 5.89 ± 3.83 | |
| 50% (25%, 75%) | 5.00 (2.00, 6.00) | 5.00 (5.00, 10.00) | 5.00 (3.00, 10.00) | |
| Range | 1.00–10.00 | 2.00–15.00 | 1.00–15.00 | |
| SBM per week, n (%) | 1.000 | |||
| 1 | 2 (22.22) | 1 (11.11) | 3 (16.67) | |
| 2 | 7 (77.78) | 8 (88.89) | 15 (83.33) | |
| PAC-SYM | 0.397 | |||
| Mean ± SD | 1.92 ± 0.23 | 1.82 ± 0.50 | 1.87 ± 0.38 | |
| 50% (25%, 75%) | 2.08 (1.67, 2.08) | 1.75 (1.58, 1.92) | 1.83 (1.67, 2.08) | |
| Range | 1.58–2.17 | 1.25–2.92 | 1.25–2.92 | |
| PAC-QoL | 0.737 | |||
| Mean ± SD | 1.81 ± 0.28 | 1.74 ± 0.49 | 1.77 ± 0.39 | |
| 50% (25%, 75%) | 1.75 (1.68, 1.89) | 1.89 (1.43, 2.04) | 1.77 (1.50, 2.04) | |
| Range | 1.46–2.29 | 0.96–2.43 | 0.96–2.43 |
BMI, body mass index; PAC-QoL, Patient Assessment of Constipation Quality of Life; PAC-SYM, Patient Assessment of Constipation Symptom; SBM, spontaneous bowel movement; TEA, transcutaneous electrical acustimulation.
Primary outcome.
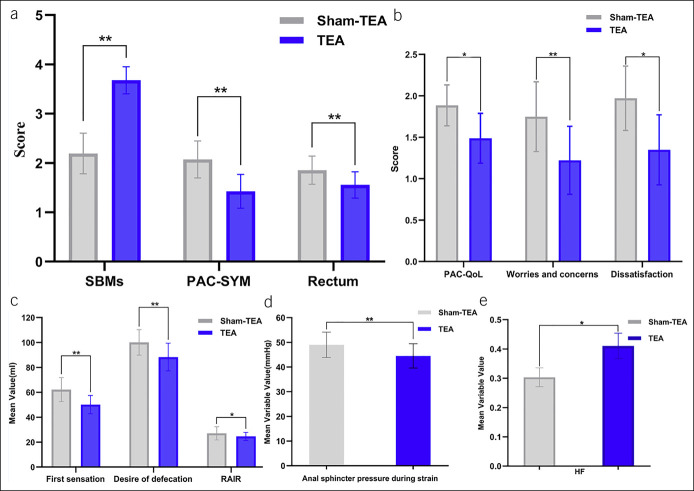
At the end of the treatment, the number of SBMs per week was higher in the TEA group and that in the sham-TEA group (3.72 ± 1.23 vs 2.00 ± 0.77, P < 0.05) (Table 3). The number of patients who had SBMs of 3 or more was significantly increased after 2-week TEA in comparison with sham-TEA (88.89% vs 16.67%, χ2 = 18.836, P < 0.001). The PAC-SYM and PAC-QoL scores were significantly decreased after 2-week TEA compared with those after 2-week sham-TEA (Table 3). The overall PAC-SYM score and the rectum symptom subscore were significantly reduced after 2-week TEA compared with 2-week sham-TEA. The overall PAC-QoL scores, the worries and concerns symptom subscore, and the dissatisfaction symptom subscore were also significantly reduced after 2-week TEA in comparison with 2-week sham-TEA (Figure 4a,b).
Table 3.
Effects of TEA on constipation measured by SBMs per week, PAC-SYM score, and PAC-QoL score in the crossover chronic study
| Items | Sequence | Period | Treatment | |||
| TEA-Sham TEA | Sham TEA-TEA | Period 1 | Period 2 | Sham-TEA | TEA | |
| PAC-SYM | 1.65 ± 0.41 | 1.64 ± 0.51 | 1.66 ± 0.52 | 1.64 ± 0.40 | 1.85 ± 0.44 | 1.45 ± 0.39a |
| Stool | 2.44 ± 0.72 | 2.11 ± 1.16 | 2.14 ± 0.98 | 2.42 ± 0.96 | 2.53 ± 1.05 | 2.03 ± 0.83 |
| Rectum | 1.53 ± 0.54 | 1.64 ± 0.58 | 1.63 ± 0.65 | 1.54 ± 0.45 | 1.80 ± 0.56 | 1.37 ± 0.47a |
| Abdominal | 1.41 ± 0.68 | 1.20 ± 0.63 | 1.26 ± 0.62 | 1.35 ± 0.70 | 1.37 ± 0.67 | 1.24 ± 0.64 |
| PAC-QoL | 1.48 ± 0.38 | 1.61 ± 0.42 | 1.56 ± 0.42 | 1.53 ± 0.40 | 1.63 ± 0.42 | 1.46 ± 0.38a |
| Physical discomfort | 1.55 ± 0.50 | 1.58 ± 0.48 | 1.56 ± 0.51 | 1.57 ± 0.47 | 1.60 ± 0.49 | 1.53 ± 0.49 |
| Psychological discomfort | 1.91 ± 0.55 | 1.62 ± 0.92 | 1.76 ± 0.83 | 1.77 ± 0.71 | 1.78 ± 0.80 | 1.75 ± 0.75 |
| Worries and concerns | 1.29 ± 0.47 | 1.54 ± 0.66 | 1.48 ± 0.63 | 1.35 ± 0.53 | 1.56 ± 0.62 | 1.27 ± 0.50a |
| Dissatisfaction | 1.39 ± 0.55 | 1.89 ± 0.47 | 1.57 ± 0.57 | 1.71 ± 0.57 | 1.79 ± 0.59 | 1.49 ± 0.51a |
| SBMs per week | 2.89 ± 1.32 | 2.83 ± 1.38 | 2.78 ± 1.40 | 2.94 ± 1.30 | 2.00 ± 0.77 | 3.72 ± 1.23a |
PAC-QoL, Patient Assessment of Constipation Quality of Life; PAC-SYM, Patient Assessment of Constipation Symptom; SBM, spontaneous bowel movement; TEA, transcutaneous electrical acustimulation.
P < 0.05 between TEA and sham-TEA.
Figure 4.
Effects of 2-week TEA on SBMs per week, PAC-SYM score, rectal symptom subscore (rectum), PAC-QoL score, anorectal manometry parameters, and autonomic nerve function in the chronic study. (a) Compared with sham-TEA, 2-week TEA significantly increased the SBMs per week (P < 0.0001) and decreased PAC-SYM score (P = 0.007) and rectal symptom score (P = 0.002). (b) TEA reduced the PAC-QoL score (P = 0.022), worry and anxiety score (P = 0.003), and dissatisfaction score (P = 0.028). (c) TEA reduced the first sensory threshold (P = 0.008) and defecation threshold (P = 0.002) as the rectal distention volume required to induce relaxation of the internal anal sphincter (RAIR) (P = 0.017). (d) TEA also reduced the anal sphincter pressure during strain (P = 0.010). (e) TEA increased HF reflecting vagus nerve activity (P = 0.020). PAC-QoL, Patient Assessment of Constipation Quality of Life; PAC-SYM, Patient Assessment of Constipation Symptom; SBM, spontaneous bowel movement; TEA, transcutaneous electrical acustimulation.
Secondary outcome.
Compared with sham-TEA, the rectal sensory function was significantly improved after TEA. The threshold of the rectal distention volume for the first sensation was 51.39 ± 13.70 mL after the TEA treatment and 60.56 ± 14.74 mL after the sham-TEA treatment (P < 0.05). Compared with the sham-TEA treatment, the TEA treatment significantly reduced the threshold for the desire of defecation (84.44 ± 15.99 mL vs 98.61 ± 20.35 mL, P < 0.05). The threshold of the rectal distention for eliciting the RAIR after TEA was found to be significantly decreased in comparison with sham-TEA (18.33 ± 7.07 mL vs 25.56 ± 9.84 mL, P < 0.05). The anal sphincter pressure during strain after 2-week TEA was found to be lower than that after 2-week sham-TEA (45.07 ± 14.58 vs 50.55 ± 14.61, P < 0.05) (Table 4 and Figure 4c,d).
Table 4.
Anorectal sphincter pressure and rectal sensation in the crossover TEA/sham-TEA treatment
| Items | Sequence | Period | Treatment | |||
| TEA-Sham TEA | Sham TEA-TEA | Period 1 | Period 2 | Sham TEA | TEA | |
| Anal rest pressure (mm Hg) | 59.80 ± 21.45 | 66.58 ± 22.09 | 70.29 ± 20.57 | 56.09 ± 21.03 | 62.73 ± 15.84 | 63.66 ± 26.86 |
| Maximum squeeze pressure (mm Hg) | 112.05 ± 34.04 | 114.41 ± 26.39 | 119.64 ± 34.56 | 106.81 ± 24.01 | 112.75 ± 25.61 | 113.71 ± 34.67 |
| Duration of contraction (s) | 19.58 ± 2.42 | 20.64 ± 3.26 | 20.65 ± 2.85 | 19.57 ± 2.88 | 20.31 ± 3.23 | 19.91 ± 2.55 |
| First sensation (mL) | 48.61 ± 10.82 | 63.33 ± 14.75 | 58.61 ± 18.05 | 53.33 ± 10.43 | 60.56 ± 14.74 | 51.39 ± 13.69a |
| Desire of defecation (mL) | 81.94 ± 13.41 | 101.11 ± 20.04 | 96.11 ± 22.53 | 86.94 ± 14.96 | 98.61 ± 20.35 | 84.44 ± 15.99a |
| Urge of defecation (mL) | 136.67 ± 28.70 | 156.67 ± 31.53 | 146.11 ± 29.18 | 147.22 ± 34.31 | 152.22 ± 29.81 | 141.11 ± 32.79 |
| Maximum tolerable volume (mL) | 169.72 ± 31.32 | 198.89 ± 29.03 | 179.44 ± 31.76 | 189.17 ± 34.86 | 189.72 ± 31.41 | 178.89 ± 35.00 |
| Increase in rectal pressure (mm Hg) | 26.71 ± 12.34 | 23.09 ± 10.80 | 27.54 ± 11.99 | 22.27 ± 10.83 | 23.65 ± 10.17 | 26.16 ± 13.00 |
| Paradoxical contraction (%) | 87.78 ± 21.84 | 76.67 ± 35.81 | 87.78 ± 22.90 | 76.67 ± 35.15 | 91.11 ± 15.68 | 73.33 ± 37.57 |
| RAIR (mL) | 21.67 ± 9.23 | 22.22 ± 9.43 | 22.78 ± 9.58 | 21.11 ± 9.00 | 25.56 ± 9.84 | 18.33 ± 7.07a |
| Anal sphincter pressure during strain (mm Hg) | 49.13 ± 15.07 | 46.49 ± 14.53 | 50.15 ± 15.66 | 45.47 ± 13.60 | 50.54 ± 14.61 | 45.07 ± 14.57a |
| RAIR difference | 14.10 ± 6.15 | 25.16 ± 12.01 | 22.54 ± 13.57 | 16.72 ± 6.69 | 21.66 ± 13.73 | 17.59 ± 7.05 |
TEA, transcutaneous electrical acustimulation.
P < 0.05 between TEA and sham-TEA.
Because the definition for the normal sensation threshold to rectal distention is largely dependent on the testing method and race, we did not use any published normative values in the literature; instead, we used our own definitions based on clinical experience as follows: elevated first sensation, if the distention threshold was >50 mL, and elevated desire to defecation, if the distention threshold was >100 mL. Based on these definitions, 13 of 18 patients (72.2%) had an elevated distention threshold for first sensation at baseline; this number was reduced to 7 (or 38.9%) at the end of the chronic TEA treatment (P < 0.004) and 12 (66.7%) at the end of the chronic sham-TEA treatment (P = 0.6). Nine of the 18 patients (50%) showed an elevated distention threshold for desire to defecate; this number was reduced to 2 (11.1%) after chronic TEA (P < 0.0001) and 7 (38.9%) after chronic sham-TEA. Compared with the sham-TEA treatment, the TEA treatment significantly reduced the number of patients with elevated first sensation threshold (38.9% vs 66.7%, P < 0.02) and the number of patients with elevated sensation threshold for desire to defecate (11.1% vs 38.9%, P < 0.02).
Compared with 2-week sham-TEA, 2-week TEA reduced the LF (0.59 ± 0.13 vs 0.68 ± 0.13, P < 0.05) and the ratio of LF/HF (1.86 ± 1.46 vs 2.61 ± 1.47, P < 0.05) but increased the HF (0.41 ± 0.13 vs 0.33 ± 0.13, P < 0.05) (Table 5 and Figure 4e).
Table 5.
Effects of TEA on autonomic functions in the crossover chronic study
| Items | Sequence | Period | Treatment | |||
| TEA-Sham TEA | Sham TEA-TEA | Period 1 | Period 2 | Sham-TEA | TEA | |
| LF | 0.59 ± 0.13 | 0.67 ± 0.13 | 0.64 ± 0.14 | 0.63 ± 0.13 | 0.67 ± 0.15 | 0.59 ± 0.11a |
| HF | 0.41 ± 0.13 | 0.32 ± 0.13 | 0.36 ± 0.14 | 0.37 ± 0.13 | 0.33 ± 0.14 | 0.41 ± 0.11a |
| LF/HF | 1.86 ± 1.46 | 2.61 ± 1.47 | 2.29 ± 1.46 | 2.18 ± 1.56 | 2.76 ± 1.76 | 1.72 ± 0.96a |
HF, high-frequency component, reflecting purely parasympathetic/vagal activity; LF, low-frequency component, reflecting mainly sympathetic activity; TEA, transcutaneous electrical acustimulation.
P < 0.05 between TEA and sham-TEA.
DISCUSSION
In this study, acute TEA resulted in a decrease in rectal sensation thresholds for urge to defecation and maximum tolerance, and the 2-week TEA treatment significantly increased the number of SBMs per week and decreased the overall scores of PAC-SYM and PAC-QoL in constipated patients with reported lack/absence of rectal sensation. Moreover, TEA reduced the rectal distention threshold volume to elicit the RAIR, improved rectal sensation to rectal distention, and reduced anal sphincter pressure during strain. Concurrently, TEA enhanced parasympathetic activity and decreased sympathetic activity, which was believed to play an important role in the improvement of symptoms and rectal sensation.
EA has been used to treat CC in recent years with satisfactory therapeutic effects. Less than 3 SBMs per week is a common definition of constipation, and the number of SBMs per week is used as the primary end point in clinical studies. In a multicenter, randomized, sham-controlled trial with 1,075 participants, EA over an 8-week treatment period significantly increased SBMs in adult patients with CC (27). EA was also reported to improve the SBMs in constipated children (28). In this study, the number of weekly SBMs was significantly increased after the 2-week TEA (without needles), which was in accordance with the previous studies with EA using needles. In addition, the 2-week TEA also significantly decreased the scores of PAC-QoL and PAC-SYM, demonstrating the improvement in constipation symptoms and quality of life in constipated patients with reported absence or lack of rectal sensation.
The receptive relaxation in the normal rectum permits storage of stool ahead of defecation, which indicates mechanical properties of the rectal wall. The interaction of motor and sensory neurons has an effect on these properties (29). Normal defecation commences with responses to the “call to stool”. Rectal sensation refers to the feeling of rectal filling associated with the anal reflexes, which is different from distension of other intestines usually lined with pain (30). When the rectal filling is sufficient to attain awareness of evacuation, the defecation reflex is evoked, which requires the participation of internal and external sphincters and intramural plexus of the rectum and rectum mucosa. There is research indicating that the mechanical properties of the rectal wall and the afferent pathway mediating sensations from the rectum are critical for rectal filling (31). In a previous study, the onset and duration of sensation perception induced by rapid inflation was in accordance with that of the external anal sphincter responses, indicating that rectal sensation is important for maintaining fecal continence by external anal sphincter contractions (32). Rectal sensorimotor response, referring to a transient contractile response of the anal sphincter, is associated with rectal sensory perception in healthy adults, which indicates its role in regulating anorectal sensation and function in the brain-gut interactions (33).The rectum is supplied with visceral afferents and somatic nerves originated from the pudendal nerve (31). Conscious awareness is motivated by afferent information from the rectum through a 3-order neuron chain with the involvement of spino-thalamo-cortical pathway, dorsal posterior insular cortex, ventrocaudal part of the medial dorsal nucleus, and dorsal anterior cingulate cortex (34). Moreover, the guinea pig myenteric ganglia contain a high density of slow adapting, low-threshold mechanoreceptors called rectal intraganglionic laminar nerve endings in the myenteric ganglia of guinea pig are sensitive to mechanical distention (35). Actually, constipation may be partially caused by impairment of the rectal afferent pathway at any level from receptors to the cortex (31,36). As an objective method for the assessment of sensory disorders affecting the afferent neural pathways, the cerebral evoked potential was found to be prolonged in latencies in children with CC and encopresis, suggesting a defect in the afferent pathway from the rectum (37).
Rectal sensory dysfunction involves in the development of functional bowel diseases (22), and rectal hyposensitivity was reported in patients with functional constipation (38,39). In addition, diminished awareness of rectal distension has been shown in metabolic diseases, neurological diseases, and postanorectal surgery. Currently, the significance of reduced rectal sensation has gained more and more attention. Because the rectal sensory thresholds in patients with constipation is higher than that in healthy control subjects, it is especially necessary to seek an effective therapy to reduce sensory threshold volumes and restore the rectal sensation. Rao et al. (40) proposed the biofeedback therapy to improve impaired rectal sensation. However, in a study involving 22 constipated patients with impaired rectal sensation, only 10 patients treated with the biofeedback therapy showed improvement in rectal sensation (11). In the current study, acute TEA decreased sensory threshold volumes for urge to defecate and maximal tolerance. The 2-week TEA reduced the sensory threshold volumes for the first sensation, the desire to defecate, and the maximum tolerance compared with the 2-week sham-TEA.
It has been consistently reported that anal pressure during strain in constipated patients was higher than in healthy subjects (41). In addition, inadequate relaxation and paradoxical contraction of the anal sphincter during defecation are the major causes of functional constipation (20,42). In this study, we also found that the anal sphincter pressure during strain in patients after the 2-week TEA was lower than that after the 2-week sham-TEA. These results showed that TEA might improve the relaxation of anal sphincter during defecation to some extent and contribute to evacuation. The decrease in anal pressure was believed to be attributed to the decrease in internal anal sphincter pressure as the RAIR was improved after 2-week TEA.
The RAIR occurs during transient relaxation of the internal anal sphincter in response to rectal distension or fecal filling and requires intact intramural pathways. The receptor of the RAIR is located in the rectal wall and its modulation depends on the integrity of the autonomic nervous system (43). The RAIR is triggered when all sorts of contents, including water and air in the rectum, reach the upper part of the anal canal (44). The RAIR is used clinically to screen for Hirschsprung disease (45). A number of studies have shown that RAIR is impaired in patients with defecation disorders, diabetes mellitus, postanorectal surgery, and sclerosis. Netinho et al. (46) suggested that the recovery velocity of the resting anal pressure relating to the RAIR in the proximal anal canal in patients with obstructive evacuation was faster than that of normal subjects. Liu et al. (47) reported that the threshold volume for the RAIR was not different between the healthy control group and the constipation group, but the prevalence of impaired RAIR was higher in the constipated group compared with healthy controls. Similarly, Xu et al. (48) reported that a higher volume of distension was required to achieve a relaxation of 50% in constipated patients than that in healthy subjects or patients with fecal incontinence. Moreover, rectal distension at 20, 30, 40, and 50 mL triggered less sphincter relaxation in constipated patients than that in healthy subjects. These results indicated that the quantitative assessment of the RAIR might be useful for the diagnosis of patients with constipation. Currently, there is no standard for the normal range of the RAIR. In this study, a 5 mm Hg or more decrease in the anal sphincter pressure compared with baseline was considered as the presence of the RAIR; otherwise, it was considered as the absence of the RAIR. This study showed that 2-week TEA reduced the volume of rectal distension for eliciting the RAIR in comparison with that after 2-week sham-TEA, which is consistent with a previous study using transcutaneous neuromodulation at both ST36 and tibial nerve (15). These results suggested that chronic TEA might have a clinical benefit in improving the RAIR in constipated patients with impaired sensation.
The autonomic nervous system takes charge of homeostatic regulation of gut functions and makes visceral perception closely related to the central nervous system (49). Autonomic dysfunction occurs in patients with functional gastrointestinal disorders and neurological disorders, such as multiple sclerosis, Parkinson disease, and peripheral neuropathies (50,51). In this study, TEA was found to enhance parasympathetic activity and suppress sympathetic activity, suggesting a possible mechanism involving autonomic functions. Chen et al. (52) reported that an eight-week electroacupuncture treatment increased parasympathetic activity and improved constipation. Several studies from our laboratory showed an improvement in parasympathetic activity with EA in animals (53–55). The parasympathetic nerve modulates the frequency of high-amplitude propagated contraction in the colon, which is crucial for slow transit constipation (56). The autonomic nervous system is also known to play an important role in rectal sensation (19,57). The TEA-induced improvement in autonomic function is believed to play a role in the enhancement of rectal sensation and improvement of constipation in this study.
SNS using an implantable pulse generator has been used for treating constipation with conflicting results; a recent meta-analysis concluded that SNS was effective in treating fecal incontinence but not effective in treating constipation (58). The major issue with SNS for constipation is the selection of appropriate stimulation parameters. In most published clinical studies, the SNS method used for treating constipation was adopted from that used for treating fecal incontinence, which shares no pathophysiological similarities with constipation. Further research is needed to make the SNS therapy effective for constipation. Compared with SNS, the TEA method applied in this study is noninvasive and much less expensive, although it might be cumbersome to administrate the therapy on a daily basis.
This study has certain limitations. The major limitation is the relatively small sample size. Furthermore, measurements of colon motility were absent, such as colon transit and pudendal nerve terminal motor latency.
In conclusion, TEA at ST36 improves functional constipation by improving rectal sensation and anal sphincter functions possibly mediated by the enhancement of parasympathetic activity in patients with functional constipation and reported lack/absence of rectal sensation.
CONFLICTS OF INTEREST
Guarantor of the article: Lin Lin, PhD.
Specific author contributions: Y.X., F.X., L.L., and J.C.: study design. Y.X. and F.X.: data acquisition. Y.X., F.X., and J.C.: data analysis/interpretation. Y.X., L.L., and J.C.: manuscript preparation.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Functional constipation is largely attributed to slow colon transit and dyssynergic defecation.
✓ The role of rectal sensitivity in contributing to the pathophysiology of functional constipation is not established; however, recent studies have reported rectal hyposensitivity in patients with functional constipation.
✓ Functional constipation is currently treated with medications and biofeedback training.
WHAT IS NEW HERE
✓ The noninvasive, home-based, and self-administrated method of transcutaneous electrical acustimulation improved constipation in patients with functional constipation and reported lack of rectal sensation for the desire of defecation.
✓ The transcutaneous electrical acustimulation-induced improvement seemed mediated by the enhancement in rectal sensation and activation of parasympathetic activity.
Footnotes
Ye Xiao and Feng Xu contributed equally to the study.
Contributor Information
Feng Xu, Email: xufengxh19@163.com.
Lin Lin, Email: lin9100@aliyun.com.
Jiande D.Z. Chen, Email: jiandedzchen@gmail.com.
REFERENCES
- 1.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: A systematic review. Best Pract Res Clin Gastroenterol 2011;25:3–18. [DOI] [PubMed] [Google Scholar]
- 2.Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902–6. [DOI] [PubMed] [Google Scholar]
- 3.Suares NC, Ford AC. Systematic review: The effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther 2011;33:895–901. [DOI] [PubMed] [Google Scholar]
- 4.Basilisco G, Coletta M. Chronic constipation: A critical review. Dig Liver Dis 2013;45:886–93. [DOI] [PubMed] [Google Scholar]
- 5.Yiannakou Y, Piessevaux H, Bouchoucha M, et al. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol 2015;110:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao SS. Biofeedback therapy for constipation in adults. Best Pract Res Clin Gastroenterol 2011;25:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zar-Kessler C, Kuo B, Belkind-Gerson J. Botulinum toxin injection for childhood constipation is safe and can be effective regardless of anal sphincter dynamics. J Pediatr Surg 2018;53:693–7. [DOI] [PubMed] [Google Scholar]
- 8.Coccorullo P, Strisciuglio C, Martinelli M, et al. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: A double-blind, randomized, placebo-controlled study. J Pediatr 2010;157:598–602. [DOI] [PubMed] [Google Scholar]
- 9.Rao SS, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol 2010;44:597–609. [DOI] [PubMed] [Google Scholar]
- 10.Gosselink MJ, Schouten WR. Rectal sensory perception in females with obstructed defecation. Dis Colon Rectum 2001;44:1337–44. [DOI] [PubMed] [Google Scholar]
- 11.Chang HS, Myung SJ, Yang SK, et al. Effect of electrical stimulation in constipated patients with impaired rectal sensation. Int J Colorectal Dis 2003;18:433–8. [DOI] [PubMed] [Google Scholar]
- 12.Pucciani F, Ringressi MN. Obstructed defecation: The role of anorectal manometry. Tech Coloproctol 2012;16:67–72. [DOI] [PubMed] [Google Scholar]
- 13.Kamm MA, Dudding TC, Melenhorst J, et al. Sacral nerve stimulation for intractable constipation. Gut 2010;59:333–40. [DOI] [PubMed] [Google Scholar]
- 14.Madbouly KM, Abbas KS, Emanuel E. Bilateral posterior tibial nerve stimulation in the treatment of rectal evacuation disorder: A preliminary report. Dis Colon Rectum 2017;60:311–7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Huang Z, Xu F, et al. Transcutaneous neuromodulation at posterior tibial nerve and ST36 for chronic constipation. Evid Based Complement Alternat Med 2014;2014:560802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng LN, Chen S, Chen JD, et al. Effects of transcutaneous electrical acustimulation on refractory gastroesophageal reflux disease. Evid Based Complement Alternat Med 2016;2016:8246171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji T, Li X, Lin L, et al. An alternative to current therapies of functional dyspepsia: Self-administrated transcutaneous electroacupuncture improves dyspeptic symptoms. Evid Based Complement Alternat Med 2014;2014:832523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Li S, Wang Y, et al. Effects and mechanisms of auricular electroacupuncture on gastric hypersensitivity in a rodent model of functional dyspepsia. PLoS One 2017;12:e0174568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu GJ, Xu F, Sun XM, et al. Transcutaneous neuromodulation at ST36 (Zusanli) is more effective than transcutaneous tibial nerve stimulation in treating constipation. J Clin Gastroenterol 2020;54:536–44. [DOI] [PubMed] [Google Scholar]
- 20.Rao SS, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil 2002;14:553–9. [DOI] [PubMed] [Google Scholar]
- 21.van Duijvendijk P, Slors F, Taat CW, et al. A prospective evaluation of anorectal function after total mesorectal excision in patients with a rectal carcinoma. Surgery 2003;133:56–65. [DOI] [PubMed] [Google Scholar]
- 22.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- 23.Pfefferkorn MD, Croffie JM, Corkins MR, et al. Impact of sedation and anesthesia on the rectoanal inhibitory reflex in children. J Pediatr Gastroenterol Nutr 2004;38:324–7. [DOI] [PubMed] [Google Scholar]
- 24.Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 1999;34:870–7. [DOI] [PubMed] [Google Scholar]
- 25.Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–51. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Xu F, Hu P, et al. Needleless transcutaneous electrical acustimulation: A pilot study evaluating improvement in post-operative recovery. Am J Gastroenterol 2018;113:1026–35. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Yan S, Wu J, et al. Acupuncture for chronic severe functional constipation: A randomized trial. Ann Intern Med 2016;165:761–9. [DOI] [PubMed] [Google Scholar]
- 28.Broide E, Pintov S, Portnoy S, et al. Effectiveness of acupuncture for treatment of childhood constipation. Dig Dis Sci 2001;46:1270–5. [DOI] [PubMed] [Google Scholar]
- 29.Siproudhis L, Bellissant E, Pagenault M, et al. Fecal incontinence with normal anal canal pressures: Where is the pitfall? Am J Gastroenterol 1999;94:1556–63. [DOI] [PubMed] [Google Scholar]
- 30.Bajwa A, Emmanuel A. The physiology of continence and evacuation. Best Pract Res Clin Gastroenterol 2009;23:477–85. [DOI] [PubMed] [Google Scholar]
- 31.Burgell RE, Scott SM. Rectal hyposensitivity. J Neurogastroenterol Motil 2012;18:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut 1990;31:1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Ocampo S, Remes-Troche JM, Miller MJ, et al. Rectoanal sensorimotor response in humans during rectal distension. Dis Colon Rectum 2007;50:1639–46. [DOI] [PubMed] [Google Scholar]
- 34.Brookes SJ, Dinning PG, Gladman MA. Neuroanatomy and physiology of colorectal function and defaecation: From basic science to human clinical studies. Neurogastroenterol Motil 2009;21(Suppl 2):9–19. [DOI] [PubMed] [Google Scholar]
- 35.Lynn PA, Olsson C, Zagorodnyuk V, et al. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 2003;125:786–94. [DOI] [PubMed] [Google Scholar]
- 36.Cheng JF, Li LD, Xu F, et al. Poststroke constipation is associated with impaired rectal sensation. Am J Gastroenterol 2020;115:105–14. [DOI] [PubMed] [Google Scholar]
- 37.Loening-Baucke V, Yamada T. Is the afferent pathway from the rectum impaired in children with chronic constipation and encopresis? Gastroenterology 1995;109:397–403. [DOI] [PubMed] [Google Scholar]
- 38.Yu T, Qian D, Zheng Y, et al. Rectal hyposensitivity is associated with a defecatory disorder but not delayed colon transit time in a functional constipation population. Medicine (Baltimore) 2016;95:e3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollebregt PF, Burgell RE, Hooper RL, et al. Clinical impact of rectal hyposensitivity: A cross-sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol 2021;116:758–68. [DOI] [PubMed] [Google Scholar]
- 40.Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Dig Dis Sci 1997;42:2197–205. [DOI] [PubMed] [Google Scholar]
- 41.Kaur G, Gardiner A, Duthie GS. Rectoanal reflex parameters in incontinence and constipation. Dis Colon Rectum 2002;45:928–33. [DOI] [PubMed] [Google Scholar]
- 42.Bharucha AE, Wald A, Enck P, et al. Functional anorectal disorders. Gastroenterology 2006;130:1510–8. [DOI] [PubMed] [Google Scholar]
- 43.Guinet A, Jousse M, Damphousse M, et al. Modulation of the rectoanal inhibitory reflex (RAIR): Qualitative and quantitative evaluation in multiple sclerosis. Int J Colorectal Dis 2011;26:507–13. [DOI] [PubMed] [Google Scholar]
- 44.Gang Y. What is the desirable stimulus to induce the rectoanal inhibitory reflex? Dis Colon Rectum 1995;38:60–3. [DOI] [PubMed] [Google Scholar]
- 45.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: Review of collective experience. Am J Gastroenterol 2002;97:232–40. [DOI] [PubMed] [Google Scholar]
- 46.Netinho JG, Ayrizono Mde L, Coy CS, et al. Amplitude and recovery velocity of relaxation induced by rectoanal inhibitory reflex and its importance for obstructive evacuation. Arq Gastroenterol 2005;42:19–23. [DOI] [PubMed] [Google Scholar]
- 47.Liu TT, Chen CL, Yi CH. Anorectal manometry in patients with chronic constipation: A single-center experience. Hepatogastroenterology 2008;55:426–9. [PubMed] [Google Scholar]
- 48.Xu X, Pasricha PJ, Sallam HS, et al. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. J Clin Gastroenterol 2008;42:692–8. [DOI] [PubMed] [Google Scholar]
- 49.Tougas G. The autonomic nervous system in functional bowel disorders. Gut 2000;47(Suppl 4):iv78–80; discussion iv87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong L, Leung TWH. Autonomic dysfunction in neurological disorders. Aging-Us 2019;11:1903–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittinger M, Barnert J, Wienbeck M. Autonomic dysfunction and the gastrointestinal tract. Clin Autonom Res 1999;9:75–81. [DOI] [PubMed] [Google Scholar]
- 52.Chen CY, Ke MD, Kuo CD, et al. The influence of electro-acupuncture stimulation to female constipation patients. Am J Chin Med 2013;41:301–13. [DOI] [PubMed] [Google Scholar]
- 53.Jin H, Liu J, Foreman RD, et al. Electrical neuromodulation at acupoint ST36 normalizes impaired colonic motility induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol 2015;309:G368–76. [DOI] [PubMed] [Google Scholar]
- 54.Song J, Yin J, Sallam HS, et al. Electroacupuncture improves burn-induced impairment in gastric motility mediated via the vagal mechanism in rats. Neurogastroenterol Motil 2013;25:807–e635. [DOI] [PubMed] [Google Scholar]
- 55.Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol 2010;298:G563–70. [DOI] [PubMed] [Google Scholar]
- 56.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47:861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta V, Sheffield D, Verne GN. Evidence for autonomic dysregulation in the irritable bowel syndrome. Dig Dis Sci 2002;47:1716–22. [DOI] [PubMed] [Google Scholar]
- 58.Southwell BR. Electro-neuromodulation for colonic disorders-review of meta-analyses, systematic reviews, and RCTs. Neuromodulation 2020;23:1061–81. [DOI] [PubMed] [Google Scholar]