Abstract
Background:
Collagen-rich fibrous septae and subcutaneous adipose protrusions play a role in cellulite pathophysiology. Collagenase clostridium histolyticum-aaes (CCH-aaes) injection causes enzymatic release of septae to resolve cellulite depressions and create a skin smoothing effect. This analysis pooled data from two identically designed, phase-3, randomized, double-blind, placebo-controlled studies to examine the efficacy and safety of CCH-aaes.
Methods:
Adult women with moderate/severe cellulite (3–4 on Clinician Reported Photonumeric Cellulite Severity Scale and Patient Reported Photonumeric Cellulite Severity Scale) on the buttocks received up to three treatment sessions (Days 1, 22, and 43) of subcutaneous CCH-aaes 0.84 mg or placebo per treatment area. Composite and individual component response (≥2-level or ≥1-level improvement from baseline in Patient Reported Photonumeric Cellulite Severity Scale and/or Clinician Reported Photonumeric Cellulite Severity Scale) and additional patient-reported outcomes were determined at Day 71.
Results:
Analysis included 424 CCH-aaes−treated and 419 placebo-treated women. CCH-aaes−treated women were 5.9 times more likely than placebo-treated women to be ≥2-level composite responders at Day 71 (odds ratio [95% confidence interval], 5.9 [2.2–15.4]; P < 0.001). A significantly greater percentage of CCH-aaes−treated women versus placebo-treated women were ≥1-level composite responders at Day 71 (39.4% versus 14.6%; P < 0.001). Subgroup analyses indicated no apparent impact of Fitzpatrick skin type category and baseline cellulite severity (moderate/severe) on CCH-aaes efficacy. An inverse relationship between age and CCH-aaes response was observed in those with a body mass index less than 32 kg per m2. The most common adverse events with CCH-aaes were injection-site bruising and injection-site pain.
Conclusion:
CCH-aaes treatment significantly improved moderate-to-severe buttock cellulite appearance and was generally well tolerated.
Takeaways
Question: Is collagenase clostridium histolyticum-aaes (CCH-aaes) effective and safe for treatment of buttock cellulite?
Findings: A pooled analysis of two trials demonstrated that CCH-aaes was significantly more efficacious than placebo for moderate-to-severe buttock cellulite in women. CCH-aaes was efficacious across Fitzpatrick skin type categories. Older age and higher body mass index may attenuate maximum response for moderate-to-severe buttock cellulite. Injection-site bruising and injection-site pain were the most common adverse events with CCH-aaes; the overall CCH-aaes safety profile was not affected in women subgrouped by baseline characteristics.
Meaning: CCH-aaes treatment improved the appearance of moderate-to-severe buttock cellulite in women and was generally well tolerated.
INTRODUCTION
Cellulite is a condition characterized by alterations in skin topography, frequently described as dimpling, in affected areas.1,2 Cellulite affects between 80% and 98% of postpubertal women,2,3 and although not completely understood,4,5 the pathophysiology of cellulite is multifactorial. Both collagen-rich fibrous septae and associated protrusions of subcutaneous adipose tissue play a role.4,6–8 Collagenase clostridium histolyticum-aaes (CCH-aaes; Qwo, Endo Aesthetics‚ LLC, Malvern, Pa.) was approved by the US Food and Drug Administration in 2020 for the treatment of moderate-to-severe cellulite in the buttocks of adult women.9 CCH-aaes is composed of two purified bacterial collagenases [AUX-I and AUX-II (clostridial class I and II collagenases)] that hydrolyze Types I and III collagen with high specificity.9,10 The CCH-aaes mechanism of action is attributed to enzymatic subcision and remodeling. Injection of CCH-aaes in areas with cellulite-related contour alterations causes enzymatic release of pathogenic collagen-rich septae to resolve cellulite-associated depressions and create a skin smoothing effect.11 The mechanism of enzymatic subcision and remodeling with CCH-aaes is supported by human abdominal and porcine tissue histology data, which have shown that CCH-aaes lyses mature collagen-rich septae and also stimulates neocollagenesis (ie, remodeling) and reorganization of subcutaneous adipose tissue into smaller and more homogenous fat lobules.12
CCH-aaes acts locally at the site of injection, and single-dose pharmacokinetic data have shown no quantifiable levels of circulating CCH-aaes, supporting a lack of systemic exposure following CCH-aaes injection.13 As part of the clinical development program of CCH-aaes for cellulite, two phase-2 and two phase-3 studies have demonstrated that CCH-aaes injection is efficacious for improving cellulite appearance on buttocks and is generally well tolerated.11,14,15
Although data for the two phase-3 trials [Randomized Evaluation of Cellulite Reduction by Collagenase Clostridium Histolyticum (RELEASE)-1 and RELEASE-2] have been reported separately,14 a pooled analysis was conducted to further evaluate the efficacy and safety of CCH-aaes for buttock cellulite, including subanalyses based on baseline demographics, to determine the potential impact of patient-related factors on CCH-aaes treatment response.
MATERIALS AND METHODS
Population and Study Design
A pooled analysis was conducted of data from two identically designed, phase-3, randomized, double-blind, placebo-controlled, multicenter studies (ClinicalTrials.gov identifiers: NCT03428750 and NCT03446781). Study design, inclusion and exclusion criteria, and treatment have been previously described.14 Healthy adult, nonpregnant, nonlactating women with moderate-to-severe cellulite [rating, 3 or 4 on Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS)]11 on both buttocks were eligible if, in the areas to be evaluated, participants were not currently receiving treatment for cellulite or did not receive cryolipolysis, implants, injectables, laser treatment, liposuction, radiofrequency treatment, or surgery for cellulite within the previous 12 months. (See figure, Supplemental Digital Content 1, which displays the Patient Reported Photonumeric Cellulite Severity Scale and Clinician Reported Photonumeric Cellulite Severity Scale for assessing cellulite severity for the buttocks. © 2017 Auxilium Pharmaceuticals, LLC. All rights reserved. Used with permission from Endo Pharmaceuticals, Inc. http://links.lww.com/PRSGO/C21.)
Women were excluded if they had a coagulation disorder, history of keloidal scarring or abnormal wound healing, or evidence or history of malignancy (exception, excised basal-cell carcinoma), unless there had been no recurrence during the previous 5 years. Additional exclusion criteria included women in whom the areas to be treated had severe skin laxity, flaccidity, or sagging, had an active infection or inflammation, or had a tattoo or mole located within 2 cm of the injection site.
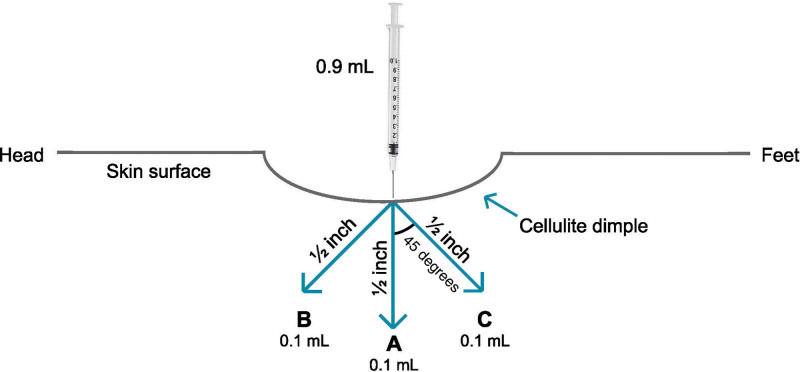
On Day 1 (baseline), one buttock was randomly assigned as the target for the primary efficacy endpoint evaluation, with the other being classified as the nontarget buttock. Women were randomly assigned to receive CCH-aaes (0.84 mg) or placebo [same components (excluding collagenase clostridium histolyticum) and diluent] administered subcutaneously in each buttock, with up to 12 dimples treated (12 injections; one or more injection for single dimple, at discretion of investigator, depending on dimple size) in each buttock per session. Both the random assignment of target buttock and the random assignment to study drug were conducted in a double-blinded manner. Using the same injection technique for all dimples, each injection dose (0.07 mg of CCH-aaes 0.23 mg/mL or placebo) was delivered as an injection of three 0.1-mL aliquots (Fig. 1). (See Video 1 [online], which displays the injection technique. Video provided with permission from Endo Pharmaceuticals, Inc. © 2020. All rights reserved.)
Fig. 1.
Injection technique. Each injection was administered as three 0.1-mL aliquots (0.3-mL total per injection). The depth of each aliquot equaled the treatment needle length of 0.5 inches (from needle tip to the needle hub/base, without downward pressure). The initial aliquot of the injection was administered with the needle perpendicular to the skin surface. For the second and third aliquots, the needle was withdrawn slightly and oriented 45 degrees toward the head and 45 degree toward the feet off the perpendicular axis. The syringe was loaded with 0.9 mL to allow for three injections per syringe. Reprinted with permission from Endo Pharmaceuticals‚ Inc. © 2020. All rights reserved.
Video 1. This video displays the injection technique. Video provided with permission from Endo Pharmaceuticals Inc. © 2020. All rights reserved.
Participants received up to three treatment sessions (Days 1, 22, and 43). As described previously, standardized digital photographic procedures were followed for evaluation of cellulite severity by the patient; live assessments were conducted by investigators.14
Assessments
Scales
The five-level (0 = no cellulite; 4 = severe cellulite) CR-PCSS (live assessment) and PR-PCSS (digital image assessment) were used to rate cellulite severity by the investigator and patient, respectively.11 Four patient-reported outcome measures were included to assess the visual and emotional impact of cellulite: the Subject Global Aesthetic Improvement Scale [S-GAIS (digital images)], Patient Reported Cellulite Impact Scale [PR-CIS (digital images)], Subject Satisfaction with the Cellulite Treatment Assessment (SSCTA), and the Subject Self-Rating Scale (SSRS). The S-GAIS is a seven-level scale ranging from +3 (“very much improved”) to −3 (“very much worse”). The PR-CIS encompasses a six-item questionnaire (domains of happy, bothered, self-conscious, embarrassed, looking older, or looking overweight or out of shape), with each item rated from 0 (“not at all”) to 10 (“extremely”).14 SSCTA is a five-level scale ranging from +2 (“very satisfied”) to −2 (“very dissatisfied”).14 The SSRS is a seven-level scale ranging from 0 (“extremely dissatisfied”) to 6 (“extremely satisfied”). Safety evaluations included treatment-emergent adverse event (AE) monitoring and clinical laboratory testing.
Analyses
The primary efficacy endpoint was the percentage of ≥2-level composite responders (women with improvement from baseline of ≥2 levels for both the CR-PCSS and PR-PCSS) in the target buttock at Day 71. Secondary efficacy endpoints included target buttock assessments at Day 71: the percentage of ≥1-level composite responders (women with improvement from baseline of ≥1 level for both the CR-PCSS and PR-PCSS scale), the percentage of women with ≥2-level or ≥1-level improvement in the CR-PCSS or in the PR-PCSS, and the percentage of women with a ≥2-level or ≥1-level improvement in the S-GAIS. Changes from baseline in PR-CIS score, SSCTA rating, and SSRS rating at Day 71 were also assessed. Subgroup analyses of select demographic and baseline characteristics were also conducted.
Statistics
All participants randomly assigned to treatment who received one or more injection of study medication were included in the intent-to-treat population; all patients who received one or more injection of study medication were included in the safety population. Responder data were analyzed using a Cochran Mantel Haenszel test, adjusted for study and analysis center. P values or odds ratio and 95% confidence interval for between-treatment comparisons were generated. Women with missing efficacy data at Day 71 were considered nonresponders. Change from baseline in PR-CIS score at day 71 was evaluated using analysis of covariance, with a factor of treatment group, analysis center, and study adjusting for baseline scores. Statistical analysis was performed using SAS statistical software package, version 9.3 or higher (SAS Institute‚ Inc., Cary, N.C.).
RESULTS
Study Population
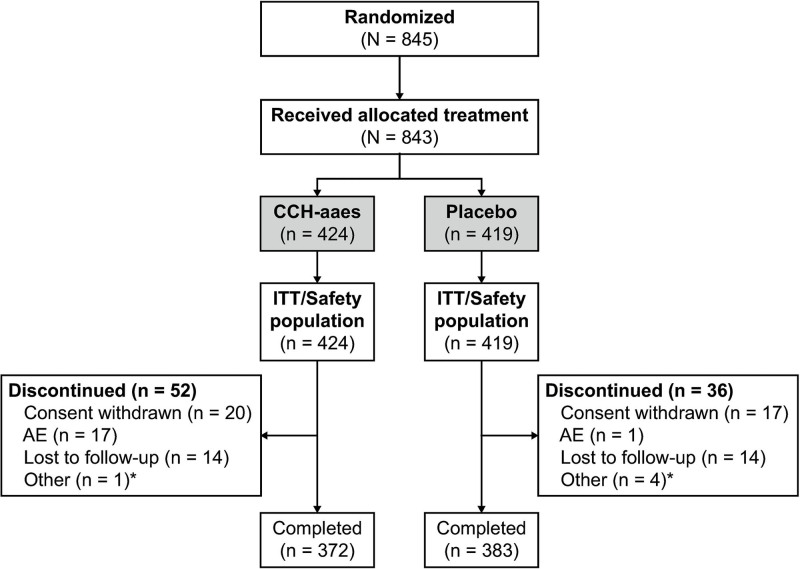
A total of 843 women [CCH-aaes (n = 424); placebo (n = 419)] were included in the pooled intent-to-treat and safety populations, with most (89.6%) completing the studies (Fig. 2). Demographic and baseline characteristics were similar between the treatment groups (Table 1). Baseline cellulite severity was rated by investigators (via CR-PCSS) as moderate for ~61% and as severe for ~39% of target buttocks in both treatment groups (Table 1).
Fig. 2.
Patient disposition. *Protocol nonadherence or administrative. ITT, intent to treat.
Table 1.
Demographic and Baseline Characteristics
| Parameter | CCH-aaes (n = 424) | Placebo (n = 419) |
|---|---|---|
| Mean age ± SD, y (range) | 47.8 ± 10.5 (20–78) | 45.8 ± 10.5 (18–72) |
| Age group, n (%), y | ||
| <35 | 46 (10.8) | 61 (14.6) |
| 35–44 | 106 (25.0) | 122 (29.1) |
| 45–64 | 248 (58.5) | 221 (52.7) |
| ≥65 | 24 (5.7) | 15 (3.6) |
| Race, n (%) | ||
| White | 336 (79.2) | 325 (77.6) |
| Black | 76 (17.9) | 75 (17.9) |
| Other | 12 (2.8) | 19 (4.5) |
| BMI category, n (%) | ||
| Underweight/normal (<25 kg/m2) | 81 (19.1) | 84 (20.1)* |
| Overweight (25 to <30 kg/m2) | 143 (33.7) | 123 (29.4)* |
| Obese (≥30 kg/m2) | 200 (47.2) | 211 (50.5)* |
| Fitzpatrick scale category, n (%) | ||
| I (pale white) | 11 (2.6) | 12 (2.9) |
| II (fair) | 124 (29.2) | 102 (24.3) |
| III (darker white) | 119 (28.1) | 139 (33.2) |
| IV (light brown) | 93 (21.9) | 82 (19.6) |
| V (brown) | 48 (11.3) | 45 (10.7) |
| VI (dark brown) | 29 (6.8) | 39 (9.3) |
| Baseline CR-PCSS level, n (%)† | ||
| 3 (moderate) | 257 (60.6) | 259 (61.8) |
| 4 (severe) | 167 (39.4) | 160 (38.2) |
| Baseline PR-PCSS level, n (%)† | ||
| 3 (moderate) | 179 (42.2) | 168 (40.1) |
| 4 (severe) | 245 (57.8) | 251 (59.9) |
*Data missing for one patient in the placebo group (n = 418).
†Target buttock.
Responder Analysis
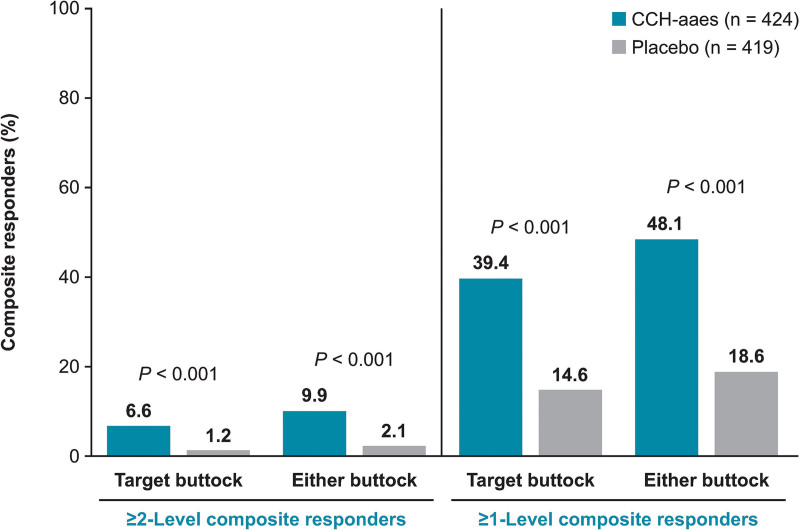
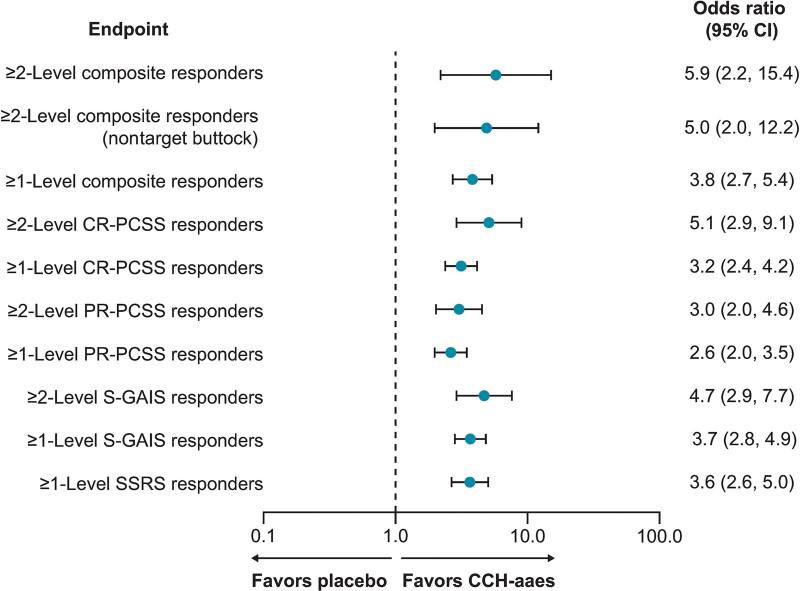
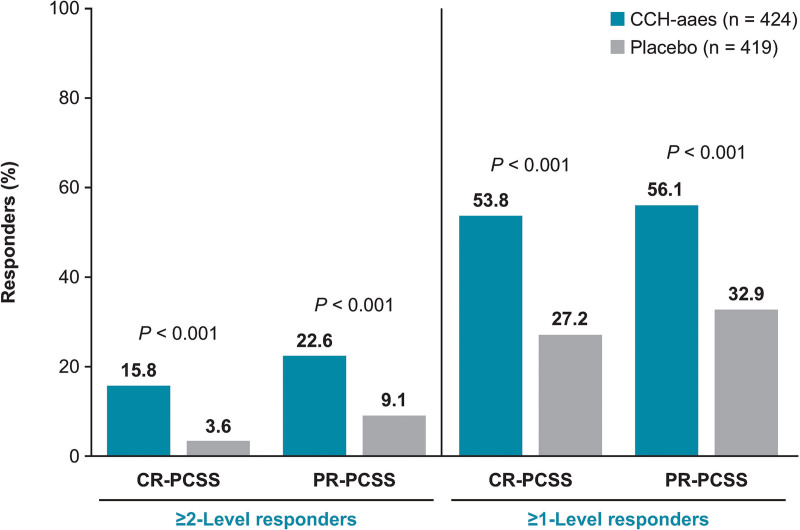
A significantly larger percentage of women treated with CCH-aaes were ≥2-level composite responders for the target buttock (primary efficacy endpoint) or either buttock [ie, at least 1 buttock, either left or right (target or nontarget)] versus placebo-treated women at Day 71 (P < 0.001 for both; Fig. 3). Women treated with CCH-aaes were 5.9 times as likely as placebo-treated women to be a ≥2-level composite responder at Day 71 for the target buttock (OR [95% CI], 5.9 [2.2–15.4]) and 5.0 times as likely in the nontarget buttock (OR [95% CI], 5.0 [2.0–12.2]; Fig. 4). Photographic images show a two-level composite responder with improvement in skin topography at Day 71 after treatment with CCH-aaes compared with baseline. (See figure, Supplemental Digital Content 2, which displays the photographs of a 41-year-old (body mass index [BMI], 29.8 kg/m2; Fitzpatrick Skin Type [FST], IV) with a 2-level composite response* to CCH-aaes in the target buttock. *Indicates 2-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †Indicates 28 days after last CCH-aaes injection. Reprinted with permission from Endo Pharmaceuticals, Inc. © 2021. All rights reserved. http://links.lww.com/PRSGO/C22.) (See Video 2 [online], which displays the rotating images of a 41-year-old (BMI, 29.8 kg/m2; FST, IV) with a 2-level composite response* to CCH-aaes in the target buttock. *Indicates 2-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †Indicates 28 days after last CCH-aaes injection. Video provided with permission from Endo Pharmaceuticals, Inc. © 2021. All rights reserved.)
Fig. 3.
Percentage of ≥2-level composite responders* and ≥1-level composite responders† at Day 71. *Individuals with ≥2-level improvement from baseline in both CR-PCSS and PR-PCSS ratings. †Individuals with ≥1-level improvement from baseline in both CR-PCSS and PR-PCSS ratings.
Fig. 4.
Forest plot of responders at Day 71. Composite and individual CR-PCSS and PR-PCSS response data are for target buttock, unless otherwise indicated. CI, confidence interval.
Video 2. Two-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †28 days after last CCH-aaes injection. BMI, body mass index, CCH-aaes, collagenase clostridium histolyticum-aaes; CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; FST, Fitzpatrick Skin Type; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale. Video provided with permission from Endo Pharmaceuticals Inc. © 2021. All rights reserved.
For the individual components of the composite endpoint, a significantly larger percentage of women treated with CCH-aaes had a ≥2-level improvement in either rating at Day 71 (Fig. 5). At Day 71, 11 (3.0%) women treated with CCH-aaes and 18 (4.7%) women treated with placebo had ≥1-level CR-PCSS increase (worsening) in cellulite severity in the target buttock compared with baseline. For the PR-PCSS, 7 (1.9%) women treated with CCH-aaes and 15 (3.9%) women treated with placebo had ≥1-level increase (worsening) in cellulite severity in the target buttock compared with baseline. Women treated with CCH-aaes were 5.1 times more likely to be a ≥2-level CR-PCSS responder and 3.0 times more likely to be a ≥2-level PR-PCSS responder than placebo-treated women (Fig. 4).
Fig. 5.
Percentage of ≥2-level and ≥1-level CR-PCSS responders* and ≥2-level and ≥1-level PR-PCSS responders† at Day 71. *Individuals with ≥2-level or ≥1-level improvement from baseline in CR-PCSS rating. †Individuals with ≥2-level or ≥1-level improvement from baseline in PR-PCSS rating.
In addition, significantly more women treated with CCH-aaes were ≥1-level composite responders at Day 71 compared with placebo for the target buttock or either (target/nontarget) buttock (P < 0.001 for both; Fig. 3), and they were 3.8 times as likely as placebo-treated women to be a ≥1-level composite responder at Day 71 for the target buttock (Fig. 4). Photographic images show the improvement in skin topography observed in a 1-level composite responder at Day 71 after treatment with CCH-aaes compared with baseline. (See figure, Supplemental Digital Content 3, which displays the photographs of a 47-year-old (BMI, 23.8 kg/m2; FST, IV) with a 1-level composite response* to CCH-aaes in the target buttock. *Indicates 1-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †Indicates 28 days after last CCH-aaes injection. Reprinted with permission from Endo Pharmaceuticals, Inc. © 2021. All rights reserved. http://links.lww.com/PRSGO/C23) (See Video 3 [online], which displays the rotating images of a 47-year-old (BMI, 23.8 kg/m2; FST, IV) with a 1-level composite response* to CCH-aaes in the target buttock. *Indicates 1-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †Indicates 28 days after last CCH-aaes injection. Video provided with permission from Endo Pharmaceuticals, Inc. © 2021. All rights reserved.)
Video 3. With a 1-Level Composite Response* to CCH-aaes in the Target Buttock. ©1-level improvement from baseline at day 71 in both the CR-PCSS and PR-PCSS. †28 days after last CCH-aaes injection. BMI, body mass index, CCH-aaes, collagenase clostridium histolyticum-aaes; CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; FST, Fitzpatrick Skin Type; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale. Video provided with permission from Endo Pharmaceuticals Inc. © 2021. All rights reserved.
For the individual components of the composite endpoint, a significantly larger percentage of women treated with CCH-aaes had a ≥1-level improvement in either rating at Day 71 (Fig. 5). Women treated with CCH-aaes were 3.2 times as likely to be a ≥1-level CR-PCSS responder and 2.6 times more likely to be a ≥1-level PR-PCSS responder when compared with placebo-treated women (Fig. 4). Mean PR-PCSS and CR-PCSS ratings showed progressively greater improvements over time compared with baseline during each of the three treatments with CCH-aaes versus placebo. [See figure, Supplemental Digital Content 4, which displays the mean (standard error) in the PR-PCSS (A) and CR-PCSS (B) over time (target buttock). http://links.lww.com/PRSGO/C24.]
Subgroup Responder Analysis
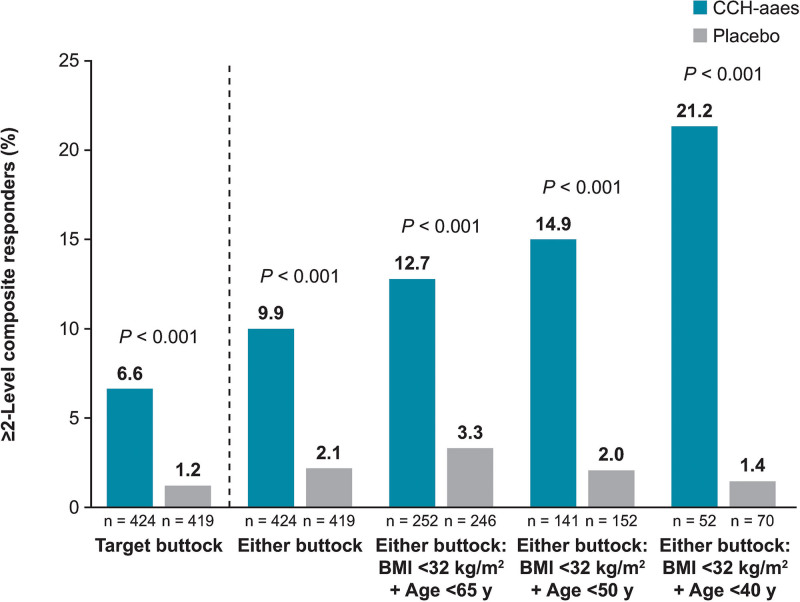
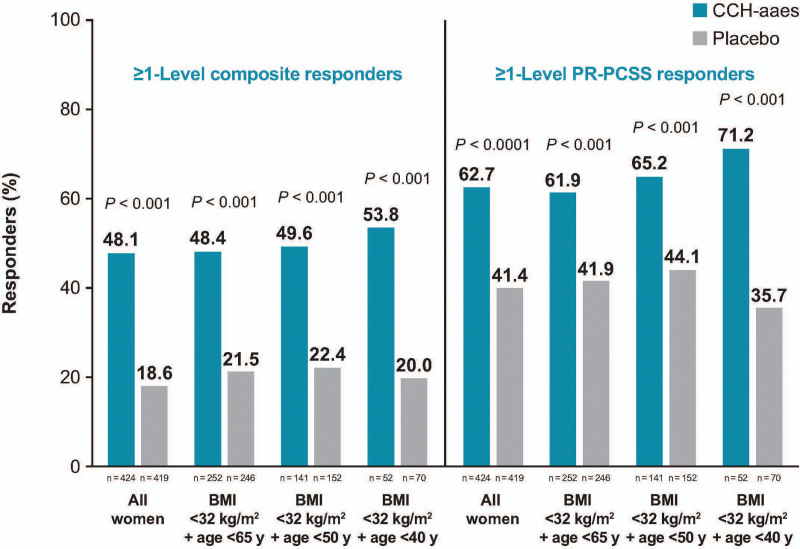
The influence of age, BMI, FST category, and baseline cellulite severity score (target buttock) on ≥2-level composite response at Day 71 was examined, with these analyses supporting the efficacy of CCH-aaes treatment across subgroups (Table 2). Numeric trends favoring CCH-aaes versus placebo were observed across the subgroups analyzed, including no differences in response to CCH-aaes treatment in women with a lighter skin type (FST category I–III) versus a darker skin type (FST category IV–VI). When participants were further subgrouped by both age and BMI, higher response rates were observed in those with a lower age and BMI less than 32 kg per m2 (Fig. 6). In addition, among women treated with CCH-aaes who had a BMI less than 32 kg per m2, there was a trend toward a higher percentage of ≥1-level composite responders (CR-PCSS and PR-PCSS) and ≥1-level PR-PCSS responders at Day 71 with lower age (either buttock; Fig. 7).
Table 2.
Percentage of ≥2-Level Composite Responders* at Day 71, Subgrouped by Baseline Characteristics
| Baseline Characteristic | CCH-aaes, % (n/n) | Placebo, % (n/n) |
|---|---|---|
| Overall population | 6.6 (28/424) | 1.2 (5/419) |
| Age category | ||
| <45 y | 7.9 (12/152) | 0 (0/183) |
| 45–64 y | 6.5 (16/248) | 2.3 (5/221) |
| ≥65 y | 0 (0/24) | 0 (0/15) |
| BMI category | ||
| Underweight/normal (<25 kg/m2) | 12.3 (10/81) | 1.2 (1/84) |
| Overweight (25 to <30 kg/m2) | 9.1 (13/143) | 1.6 (2/123) |
| Obese (≥30 kg/m2) | 2.5 (5/200) | 0.9 (2/211) |
| Fitzpatrick scale category | ||
| I–III (pale white, fair, darker white) | 6.7 (17/254) | 0.8 (2/253) |
| IV–VI (light brown, brown, dark brown) | 6.5 (11/170) | 1.8 (3/166) |
| CR-PCSS level† | ||
| 3 (moderate) | 7.8 (20/257) | 1.2 (3/259) |
| 4 (severe) | 4.8 (8/167) | 1.3 (2/160) |
| PR-PCSS level† | ||
| 3 (moderate) | 7.8 (14/179) | 1.2 (2/168) |
| 4 (severe) | 5.7 (14/245) | 1.2 (3/251) |
*Individuals with ≥2-level improvement from baseline in both CR-PCSS and PR-PCSS ratings in the target buttock.
†Target buttock.
Fig. 6.
Percentage of ≥2-level composite responders* at Day 71, subgrouped by age and BMI. *Individuals with ≥2-level improvement from baseline in both CR-PCSS and PR-PCSS ratings.
Fig. 7.
Percentage of ≥1-level composite responders* and ≥1-level PR-PCSS responders at Day 71 for either buttock, subgrouped by age and BMI. *Individuals with ≥2-level improvement from baseline in both CR-PCSS and PR-PCSS ratings.
Additional PRO Measures
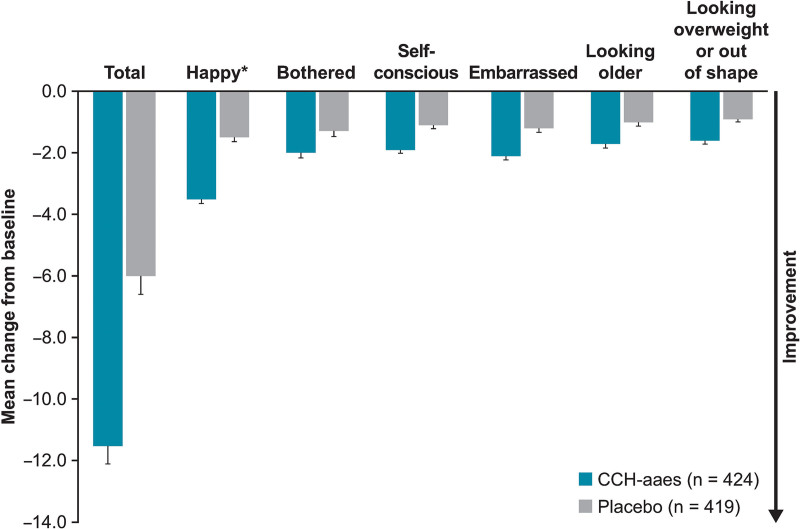
A significantly larger percentage of women treated with CCH-aaes had a ≥1-level improvement in S-GAIS versus those treated with placebo (61.6% versus 30.5%; P ≤ 0.001) or a ≥2-level improvement in S-GAIS versus those treated with placebo (20.5% versus 5.3%; P < 0.001). Women treated with CCH-aaes were 4.7 times or 3.7 times as likely to be ≥2-level or ≥1-level S-GAIS responders, respectively, compared with placebo-treated women (Fig. 4). In addition, 45.3% of women treated with CCH-aaes were at least “slightly satisfied” (1-level improvement [SSRS responders]) at Day 71, according to the SSRS, compared with 18.9% of placebo-treated women (P < 0.001) and were 3.6 times as likely to have this level of improvement (Fig. 4). Based on the SSCTA, a higher percentage of women treated with CCH-aaes were “very satisfied” or “satisfied” with treatment at Day 71 versus those treated with placebo (49.1% [193/393] versus 19.4% [75/386]). Women treated with CCH-aaes had a significantly greater mean improvement from baseline in PR-CIS total score at Day 71 compared with placebo-treated women (-11.7 versus -6.2; Δ-5.4 [95% CI, -7.0 to -3.9]; P < 0.001). For all six of the individual domains of the PR-CIS, a greater numeric improvement from baseline was observed in women treated with CCH-aaes than women treated with placebo (Fig. 8).
Fig. 8.
Mean improvement from baseline at Day 71 for PR-CIS total and six individual domains. *Score related to happiness was reversed by subtracting the participant’s reported assessment from 10 to make it directionally consistent with the other questions. Total score data are least squares mean (standard error) and individual domain data are mean (standard error).
Safety
CCH-aaes treatment for cellulite was generally well tolerated (Table 3). Discontinuations from the study due to an AEs occurred in 4.0% of women in the CCH-aaes group and 0.2% of women in the placebo group. Most (88.2%) of CCH-aaes treated women experienced one or more AE; approximately twice the percentage reported in the placebo group (Table 3). The most common AEs with CCH-aaes treatment were injection-site-related, with injection-site bruising, injection-site pain, and injection-site nodule being the most common AEs (Table 3). Four times as many women treated with CCH-aaes versus placebo had injection-site bruising. Most (92.6%) AEs in the CCH-aaes group were mild to moderate in intensity, and the overall AE occurrence decreased when assessed by treatment session. Most treatment-related AEs were transient, with a mean duration of treatment-related AEs of 14.7 days (median, 9.0 days). There was a decrease over time in the duration of AEs when assessed by treatment session. During the first treatment session, 53.5% and 83.3% of AEs observed in the CCH-aaes group resolved within 14 and 21 days, respectively. During the second treatment session, 77.6% and 89.2% of AEs observed resolved within 14 and 21 days, respectively. During the third treatment session, 74.7% and 84.9% of AEs observed resolved within 14 and 21 days, respectively. No safety signals or concerns in women treated with CCH-aaes were identified when subgrouped by age, BMI, ethnicity, FST categories, and race.
Table 3.
Adverse Events*
| Women With an AE, n (%) | CCH-aaes (n = 424) | Placebo (n = 419) |
|---|---|---|
| ≥1 AE AE leading to study discontinuation ≥1 treatment-related AE ≥1 serious AE Highest intensity AE experienced Mild Moderate Severe |
374 (88.2) 17 (4.0) 368 (86.8) 1 (0.2)†n = 374147 (39.3) 154 (41.2) 73 (19.5) |
169 (40.3) 1 (0.2) 128 (30.5) 1 (0.2) n = 169127 (75.1) 41 (24.3) 1 (0.6) |
| Most common AEs‡ | ||
| Injection-site bruising§ | 358 (84.4) | 88 (21.0) |
| Injection-site pain¶ | 204 (48.1) | 37 (8.8) |
| Injection-site nodule# | 141 (33.3) | 3 (0.7) |
| Injection-site pruritus | 62 (14.6) | 4 (1.0) |
| Injection-site erythema | 36 (8.5) | 21 (5.0) |
| Injection-site discoloration | 33 (7.8) | 2 (0.5) |
| Injection-site swelling** | 29 (6.8) | 1 (0.2) |
| Injection-site warmth | 14 (3.3) | 0 (0.0) |
| Injection-site induration | 10 (2.4) | 5 (1.2) |
*Based on physical examination; no histologic confirmation.
†Individual experienced AEs of hypokalemia, hypotension, and syncope, which were not considered by the investigator to be treatment related.
‡Occurring in 2% or more of women in the CCH-aaes group and at a greater incidence versus the placebo group.
§Includes preferred terms of injection-site bruising, injection-site hematoma, and injection-site hemorrhage (refers to verbatim term injection-site ecchymosis).
¶Includes preferred terms of injection-site pain and injection-site discomfort.
#Includes preferred terms of injection-site nodule and injection-site mass.
**Includes preferred terms of injection-site swelling and injection-site edema.
DISCUSSION
The pooled analysis of the RELEASE-1 and RELEASE-2 studies demonstrated that CCH-aaes injection was significantly more efficacious than placebo for the treatment of moderate-to-severe cellulite in the buttocks of adult women. Subgroup analyses (eg, FST) supported the efficacy of CCH-aaes for the treatment of cellulite compared with placebo, although some subgroups were small in size. Interestingly, there were apparent differences in response rates for the primary efficacy endpoint within the CCH-aaes treatment group as age and BMI increased. In addition, the results from the post hoc analyses of BMI and age revealed a trend of higher response rates directly related to age when nested within a criterion of BMI less than 32 kg per m2. These results suggest that patient selection will be important for optimal response to CCH-aaes treatment. The variations in response rate may be due to the inverse relationship reported between age and elasticity16,17 and the potential impact of BMI on skin elasticity.18 Overall, these data suggest that maximum response to CCH-aaes may be attenuated by older patient age and a higher BMI.
Previous analyses have shown that a PR-PCSS score change greater than or equal to one is clinically meaningful based on anchoring analyses with S-GAIS, thus tying PR-PCSS improvement to clinically meaningful change and establishing support for the rating interpretation.19 A total of 56.1% of women treated with CCH-aaes in the current pooled analysis had a ≥1-level improvement in PR-PCSS and 61.6% had a ≥1-level improvement in S-GAIS for the target buttock. In addition, PR-CIS individual domain score improvements from baseline indicated that there was a lower overall emotional and visual impact of cellulite in women treated with CCH-aaes versus those treated with placebo.
Strengths of the current analysis include the large number of women treated (ie, >400 per arm) and the study design of the RELEASE-1 and RELEASE-2 trials (identically designed, randomized, double-blind, placebo controlled). Combining safety outcomes in the pooled analysis did not identify any additional safety signals. CCH-aaes injection was generally well tolerated. Adverse events were transient, and the incidence, intensity, and duration of AEs were generally reduced during each subsequent treatment session. Most AEs in women treated with CCH-aaes were injection-site reactions, most commonly injection-site bruising, that were primarily mild to moderate in intensity. The overall safety profile of CCH-aaes (eg, incidence of AEs) was not affected when subgrouped by demographic and baseline characteristics (eg, age, BMI, FST, or baseline cellulite severity).
Limitations of the pooled analysis mirror those of the individual studies, including the stringent criterion of the primary efficacy endpoint (ie, ≥2-level composite response), as this outcome would be difficult to achieve in clinical practice, and only moderate-to-severe cellulite on the buttocks was treated. In addition, for the pooled analysis, the studies were not powered for statistical comparisons of pooled CCH-aaes versus placebo responses, and statistical comparisons were not performed for the subgroups analyzed. As well, given the trial exclusion criterion of mild cellulite severity in order to detect relatively large improvements post-treatment [primary endpoint (≥2-level composite responders)], the demographic and baseline characteristics of the overall population may not represent the full range of women who might seek treatment in clinical practice. Also, the CCH-aaes AE profile (eg, frequency of injection-site bruising) may impact patient willingness to use of CCH-aaes for the treatment of buttock cellulite. A trial (ClinicalTrials.gov identifier: NCT04677712) is ongoing to evaluate post-treatment techniques to help mitigate injection-site bruising, the most commonly reported AE in the current pooled analysis.
CONCLUSIONS
This pooled analysis (N = 843) of two identically designed, randomized, double-blind, placebo-controlled studies demonstrated that CCH-aaes injection was efficacious and safe for the treatment of buttock cellulite in women. Subgroup analyses supported CCH-aaes treatment, as shown by a response in all subgroups analyzed. Data also suggest that older age and higher BMI may attenuate maximum response to CCH-aaes treatment of moderate-to-severe cellulite in the buttock.
ACKNOWLEDGMENTS
The studies were conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and ethical principles of the Declaration of Helsinki. The protocols were approved by a central institutional review board (Advarra, Columbia, Md.), and all participants provided written informed consent. Technical editorial and medical writing assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, Synchrony Medical Communications, LLC, West Chester, Pennsylvania, and Ginny Vachon, PhD, Principal Medvantage, LLC, Atlanta, Georgia. Funding for this assistance was provided by Endo Pharmaceuticals, Inc., Malvern, Pennsylvania.
Supplementary Material
Footnotes
Published online 25 May 2022.
These data were presented in part at the Orlando Dermatology, Aesthetic & Surgical Conference 2020, January 17–20, 2020, Orlando, Fla.; at Maui Derm for Dermatologists 2020, January 25-–29, 2020, Maui, Hawaii; and Vegas Cosmetic Surgery & Aesthetic Dermatology [Virtual], September 24–27, 2020.
Disclosure: Lawrence S. Bass reports serving as a consultant and investigator for Cynosure and an investigator for Merz North America. Joely Kaufman-Janette reports serving on advisory boards for Allergan plc (acquired by AbbVie, Inc.), Bonti, Inc. (acquired by Allergan plc), Endo Pharmaceuticals, Inc., Evolus, Inc., and Galderma; and receiving payment for research from Allergan plc, Croma-Pharma GmbH, Endo Pharmaceuticals, Inc., Evolus, Inc., Galderma, Merz North America, Revance Therapeutics, Inc., and Teoxane Laboratories. John H. Joseph reports serving as a clinical investigator and consultant for Endo Pharmaceuticals, Inc. Michael S. Kaminer reports serving as a consultant for Arctic Fox, LLC, ExploraMed, and Soliton, Inc. James Clark reports serving as an investigator for Allergan (acquired by AbbVie, Inc.), Astellas Pharma US, Inc., Biohaven Pharmaceuticals, Endo Pharmaceuticals, Inc., Feelmore Labs, NeuroValens, Noven Pharmaceuticals, Inc., Samumed, LLC, Satsuma Pharmaceuticals, Inc., Teva Pharmaceutical Industries, Ltd., Tonix Pharmaceuticals Holding Corp., Vibrant Pharma, Inc., and Virios Therapeutics, Inc. Sabrina G. Fabi reports receiving research grants from Allergan (acquired by AbbVie, Inc.), Bausch Health Companies, Inc., Galderma Laboratories, L.P., Merz North America, Inc., and Revance Therapeutics, Inc.; and being a speaker and serving as a consultant for Allergan (AbbVie, Inc.), Bausch Health Companies, Inc., Galderma Laboratories, L.P., and Merz North America, Inc. Michael H. Gold reports being a consultant for Aerolase, Alastin, Allergan (acquired by AbbVie, Inc.), Alma Lasers, Almirall, BioFrontera, Candela Healthcare, Clarify MD, Croma-Pharma, DefenAge, Dermira, Inc., EndyMed Medical, Inc., Essence Novel, Galderma Laboratories, L.P., Hugel, Inc., IntraDerm Invasix/InMode, Johnson & Johnson, Joylux, Lumenis, Ltd., Merz, MTF, NanoPass Technologies, Ltd., Neauvia, Novartis, Pierre Fabre/Glytone, Revision, Sensus, Senté, Inc., Skinbetter Science, Sofregen Medical, Inc., Stratpharma, Suneva Medical, Viviscal, and Zimmer. Bruce E. Katz reports being a consultant for AbbVie, Inc., Bausch Health Companies, Inc., Aerolase Corp., Allergan (acquired by AbbVie, Inc.), BTL Aesthetics, Carestream Health, Cynosure, Deka, Galderma Laboratories, L.P., InMode, Merz North America, Inc., Pulse Biosciences, and Suneva Medical. Kappa Peddy reports serving as a clinical investigator for Aclaris Therapeutics, Inc., Athenex, Inc., Bayer AG, Follica, Inc., Novan, Inc., and Sol-Gel Technologies. Joel Schlessinger reports having no additional disclosures. V. Leroy Young reports serving as an investigator for Alder Biopharmaceuticals, Allergan plc (acquired by AbbVie, Inc.), Amgen, Inc., Ardea Biosciences, Boehringer Ingelheim, Celgene Corporation, Daiichi Sankyo Co., Ltd., DalCor Pharmaceuticals, Derma Sciences, Inc., Eisai Co., Ltd., Eli Lilly and Company, Endo Pharmaceuticals, Inc., Evidera, Evolus, Inc., Halscion, Inc., Janssen Pharmaceuticals, Inc., Kythera Biopharmaceuticals, MedImmune, Merck & Co., Inc., Neothetics, Inc., Pfizer, Inc., RXi Pharmaceuticals, Sanofi, Takeda Pharmaceuticals U.S.A., Inc., and Therakos, Inc.; serving as a consultant for AirXpanders, Inc., miRagen Therapeutics, Regeneron Pharmaceuticals, Inc., and Sanofi; and receiving registration and travel expenses from Endo Pharmaceuticals, Inc. David Hurley and Saji Vijayan are employees of Endo Pharmaceuticals, Inc. Michael P. McLane, Genzhou Liu, and Matthew W. Davis are former employees of Endo Pharmaceuticals, Inc. Mitchel P. Goldman reports receiving research grants from Allergan (acquired by AbbVie, Inc.), Bausch Health Companies, Inc., Galderma Laboratories, L.P., Merz North America, Inc., and Revance Therapeutics, Inc.; and being a speaker and serving as a consultant for Allergan (acquired by AbbVie, Inc.), Bausch Health Companies, Inc., and Galderma Laboratories, L.P. This study was supported by Endo Pharmaceuticals, Inc., Malvern, Pennsylvania.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161–168. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol. 2017;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avram MM. Cellulite: a review of its physiology and treatment. J Cosmet Laser Ther. 2004;6:181–185. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph C, Hladik C, Hamade H, et al. Structural gender dimorphism and the biomechanics of the gluteal subcutaneous tissue: implications for the pathophysiology of cellulite. Plast Reconstr Surg. 2019;143:1077–1086. [DOI] [PubMed] [Google Scholar]
- 5.Sadick N. Treatment for cellulite. Int J Womens Dermatol. 2019;5:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass LS, Kaminer MS. Insights into the pathophysiology of cellulite: a review. Dermatol Surg. 2020;46 Suppl 1:S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hexsel DM, Abreu M, Rodrigues TC, et al. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35:1471–1477. [DOI] [PubMed] [Google Scholar]
- 8.Piérard GE, Nizet JL, Piérard-Franchimont C. Cellulite: from standing fat herniation to hypodermal stretch marks. Am J Dermatopathol. 2000;22:34–37. [DOI] [PubMed] [Google Scholar]
- 9.QWO (collagenase clostridium histolyticum-aaes) for injection for subcutaneous use. Package insert. Malvern, Pa.: Endo Aesthetics, LLC; 2020. [Google Scholar]
- 10.French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681–687. [DOI] [PubMed] [Google Scholar]
- 11.Sadick NS, Goldman MP, Liu G, et al. Collagenase clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite): a randomized trial. Dermatol Surg. 2019;45:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shridharani SM. Injectable cellulite treatment—collagenase clostridium histolyticum-aaes effect on tissue histology. Paper presented at: Vegas Cosmetic Surgery & Aesthetic Dermatology [Virtual]; September 24–27, 2020. [Google Scholar]
- 13.Bhatia AC, McLane MP, Priestley T, et al. Human pharmacokinetics and safety of subcutaneous collagenase clostridium histolyticum in women. J Drugs Dermatol. 2020;19:852–856. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman-Janette J, Joseph JH, Kaminer MS, et al. Collagenase clostridium histolyticum-aaes for the treatment of cellulite in women: results from two phase 3 randomized, placebo-controlled trials. Dermatol Surg. 2021;47:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman-Janette JA, Bass LS, Xiang Q, et al. Efficacy, safety, and durability of response of collagenase clostridium histolyticum-aaes for treating cellulite. Plast Reconstr Surg Glob Open. 2020;8:e3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czekalla C, Schönborn KH, Döge N, et al. Impact of body site, age, and gender on the collagen/elastin index by noninvasive in vivo vertical two-photon microscopy. Skin Pharmacol Physiol. 2017;30:260–267. [DOI] [PubMed] [Google Scholar]
- 17.Shin JW, Kwon SH, Choi JY, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:E2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezure T, Amano S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp Dermatol. 2015;24:924–929. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JL, Sadick NS, Kirby MT, et al. Development and validation clinician and patient reported photonumeric scales to assess buttocks cellulite severity. Dermatol Surg. 2020;46:1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.