Abstract
Background
The U.S. Preventive Services Task Force recommends routine population-based screening for drug use, yet screening for opioid use disorder (OUD) in primary care occurs rarely, and little is known about barriers primary care teams face.
Objective
As part of a multisite randomized trial to provide OUD and behavioral health treatment using the Collaborative Care Model, we supported 10 primary care clinics in implementing routine OUD screening and conducted formative evaluation to characterize early implementation experiences.
Design
Qualitative formative evaluation.
Approach
Formative evaluation included taking detailed observation notes at implementation meetings with individual clinics and debriefings with external facilitators. Observation notes were analyzed weekly using a Rapid Assessment Process guided by the Consolidated Framework for Implementation Research, with iterative feedback from the study team. After clinics launched OUD screening, we conducted structured fidelity assessments via group interviews with each site to evaluate clinic experiences with routine OUD screening. Data from observation and structured fidelity assessments were combined into a matrix to compare across clinics and identify cross-cutting barriers and promising implementation strategies.
Key Results
While all clinics had the goal of implementing population-based OUD screening, barriers were experienced across intervention, individual, and clinic setting domains, with compounding effects for telehealth visits. Seven themes emerged characterizing barriers, including (1) challenges identifying who to screen, (2) complexity of the screening tool, (3) staff discomfort and/or hesitancies, (4) workflow barriers that decreased screening follow-up, (5) staffing shortages and turnover, (6) discouragement from low screening yield, and (7) stigma. Promising implementation strategies included utilizing a more universal screening approach, health information technology (HIT), audit and feedback, and repeated staff trainings.
Conclusions
Integrating population-based OUD screening in primary care is challenging but may be made feasible via implementation strategies and tailored practice facilitation that standardize workflows via HIT, decrease stigma, and increase staff confidence regarding OUD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07675-2.
KEY WORDS: opioid use disorder, screening, primary care
BACKGROUND
With rising incidence of, associated mortality resulting from, and effective treatment for opioid use disorder (OUD), urgency exists to identify and link patients with OUD to evidence-based treatment.1 In 2020, there were over 93,000 overdose-related deaths in the USA and a continued steady rise in new OUD diagnoses.2 Effective medications to treat OUD (MOUD) reduce opioid-related mortality and improve quality of life.1,3 Yet access to MOUD has been limited by prior federal policies requiring provider licensing (for buprenorphine) and/or supervised disbursement of medication (for methadone). As a result, only 21% of patients with diagnosed OUD nationally receive MOUD, with lower treatment access among persons with co-occurring mental health conditions.4,5
The continued rise in opioid-related mortality paired with the disparity that only one in five patients with OUD receives care signifies the need for system-level transformation that expands access and integration of MOUD into routine care.4,6 Primary care offers an advantageous setting for population-based OUD screening that reduces barriers to access and links patients to life-saving treatment.6,7 Integrating systematic approaches to behavioral health screening is a priority in many health systems,8,9 and the National Council for Behavioral Health and the U.S. Preventive Services Task Force (USPSTF) both call for integration of screening for drug use disorders, including OUD, in primary care settings.10,11
Prior research has documented that integrating screening for substance use disorders in primary care presents many nuanced challenges related to stigma, workflow challenges, and lack of primary care team familiarity and comfort with addressing substance use.3,12 Since substance use disorders are among the most stigmatized conditions in our society,13 both patients and primary care teams may have perceptions of stigma surrounding disclosure of substance use that influences how screening processes unfold. Prior research evaluating implementation of screening for unhealthy alcohol use found that stigma and discomfort impacted how primary care staff introduced alcohol screening in ways that increased bias and decreased screening sensitivity.12,14,15 There is also increased complexity for drug use screening, as the use of cannabis and opioids may be legal and/or appropriate in certain contexts, but illegal and/or considered a risk for harm in other contexts, with distinctions not always clear to patients or clinical staff.3
Consequently, there is limited evidence on best practices for implementing OUD screening in primary care.3 Implementation strategies like practice facilitation can improve screening uptake and effectiveness;16,17 however, little is known about the unique barriers primary care teams face in integrating OUD screening into routine practice. As part of a multisite, randomized trial, we supported 10 primary care clinics representing 9 healthcare systems in the implementation of routine OUD screening. In this work, we leverage formative evaluation data to characterize the early OUD screening implementation experiences of primary care clinics.18,19 Our objective was to identify barriers and facilitators of OUD screening implementation experienced by diverse primary care clinics so as to inform future implementation efforts in primary care settings.
METHODS
Study Setting
This study was conducted in a national cohort of primary care clinics recruited to evaluate the effectiveness of the Collaborative Care Model (CoCM) for co-occurring mental health disorders and OUD. CoCM, used extensively to support the delivery of behavioral health care in primary care settings,20,21 is a team-based model of measurement-based care where clinical responsibilities are shared between a primary care provider, a behavioral health care manager, and a psychiatrist who offers consultation for psychiatric medication prescribing and management and other psychotherapeutic interventions. Clinics were randomized to implement CoCM for co-occurring OUD and mental health disorders (n = 10) or serve as control sites (n = 10). Clinics in the intervention arm offered patients care for OUD, including medications for OUD and other behavioral supports, as part of their primary care services, and OUD care was delivered collaboratively by the team of primary care provider, behavioral health care manager, and consulting psychiatrist. The 10 clinics randomized to implement CoCM for co-occurring OUD and mental health disorders served as the setting for the present study as Formative Evaluation activities (described below) only occurred within the intervention clinics. Activities took place between July 2020 and July 2021, and all activities were reviewed and approved by the Advarra Institutional Review Board.
To support implementation of the CoCM intervention, clinics identified a local clinical implementation team consisting of a team lead, primary care provider, behavioral health care manager, psychiatric consultant, and additional administrative roles. Clinics were assigned an external practice facilitator from the Advancing Integrated Mental Health Solutions (AIMS) Center at the University of Washington, a trained member of the study team who coached the clinical implementation teams via meetings and trainings (biweekly-monthly). Clinics had approximately 4–8 months of preparation time prior to launch of OUD screening (a critical first step in implementing the CoCM intervention) and were asked to implement the 12 items of the validated NIDA-Modified ASSIST (NMA)22 that screen for risky use of multiple categories of opioids (see Supplemental Material 1). Similar in format to the Patient Health Questionnaire-9 (PHQ-9), the NMA includes initial screening questions, and only patients who screen positive on those questions complete the full measure. As part of training, clinics were provided with an OUD Screening Toolkit which included education, sample workflows, and scripting tools for staff to use when discussing OUD screening. Clinics then received coaching following a practice facilitation model, where each clinic identified implementation approaches that best aligned with their patient population, goals, and available resources. As such, clinics were given flexibility in how they defined the target population and/or frequency of routine screening, as well as how they approached the integration of the NMA into existing workflows, staff roles, and electronic health record (EHR) systems. For example, some clinics elected to integrate the NMA into their patient portal for electronic delivery, whereas other clinics decided to distribute the NMA on paper.
Formative Evaluation Data Collection, Analysis, and Generation of Fidelity Assessment
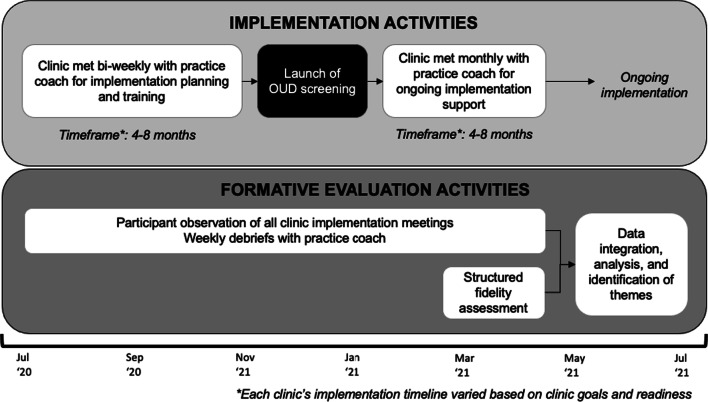
The study team engaged in formative evaluation throughout implementation planning, preparation, and launch of OUD screening (Fig. 1). Formative evaluation—a rigorous ongoing data collection process—aids in identifying factors that influence implementation progress, success, and replicability, including in the context of randomized controlled trials.18,19 For the present study, formative evaluation included taking detailed ethnographic observation notes at all implementation meetings with individual clinics led by the practice facilitator (n = 90 meetings) and weekly internal debriefings with the practice coaches (n = 59 meetings).18 Observation notes were analyzed weekly using a rapid assessment process (RAP), where raw field notes were organized into structured templates guided by the Consolidated Framework for Implementation Research (CFIR) and iteratively reviewed by trained qualitative researchers (EA, EB), formative evaluation lead (EW), and the full investigative team to identify emergent themes.22–25 During ongoing formative evaluation, the team identified challenges to OUD screening across sites, including a wide variation of screening rates and workflows, which warranted further exploration. The team then triangulated these findings with prior research related to substance use disorder (SUD) screening, which informed the development of a structured OUD screening fidelity assessment.
Figure 1.
Flow of study implementation and evaluation activities.
Administration of Structured Fidelity Assessment
Approximately 3–6 months after each clinic had launched OUD screening (depending on each clinic’s individual launch date), we conducted one structured fidelity assessment with each site to systematically evaluate clinic experiences, barriers, and promising strategies to the implementation of routine OUD screening. Structured fidelity assessments involved 1-h group interviews with clinical implementation teams and frontline staff. During assessments, a member of the study team asked open-ended questions about OUD screening processes and workflows while another researcher took typed field notes in Microsoft Word. Interviews walked through a rubric of core workflow steps for patient-reported screening, including (1) how the OUD screener is deployed to the patient, (2) how OUD screener items are collected from the patient, (3) how staff tracked OUD screener completion, (4) how OUD screener scores are reviewed by the clinical team, and (5) how OUD screener scores are documented for future follow-up (see Supplemental Material 2).
Analysis and Triangulation
Data from ethnographic field notes and structured fidelity assessments were combined into a matrix, organized around the core screening workflow steps. Following a RAP,24 trained qualitative researchers (EA, EB) then compared data across clinics and workflow steps to identify common patterns as well as cross-cutting themes, guided by CFIR.25 Iterative analysis with the qualitative lead (EW) continued until no new barriers or facilitators were identified, and findings were confirmed with the full study team and clinical sites.
RESULTS
Clinics (n = 10) were located in the District of Columbia, Idaho, Illinois, Georgia, Massachusetts, Nebraska, Washington, and Wisconsin. Among the 10 clinics, 2 were located in urban areas, 6 in suburban areas, and 2 in rural areas. Two clinics were federally qualified health centers, and four clinics were training sites staffed with residents and/or clinical interns. Three clinics had universal screening for SUDs in place prior to introducing screening for OUD specifically.
Summary of Screening Processes
OUD screening workflows were highly variable across clinics (Table 1). The majority of clinics (8/10) implemented OUD screening for patients on an annual basis; however 2 implemented OUD screening on an “every visit” approach, and several clinics modified their screening frequency during their initial implementation period of the study. All clinics implemented OUD screening for in-person visits, but many struggled to implement OUD screening for telehealth visits due to the need to administer the screener electronically. Eight of the clinics asked patients to complete the OUD screener on paper, and two asked patients to complete it electronically (i.e., via the patient portal or other patient-facing application).
Table 1.
Clinic Characteristics and Screening Practices
| Number of clinics represented | 10 |
| Number of health systems represented | 9 |
| Geographic setting of clinics* | |
| Urban | 2 |
| Suburban | 6 |
| Rural | 2 |
| Clinic setting characteristics | |
| FQHC | 2 |
| Trainee site (residents, interns) | 4 |
| Academic medical center affiliated | 2 |
| Existing SUD screening in place? | |
| Yes | 3 |
| No | 7 |
| Screening frequency | |
| Universal—every visit | 2 |
| Universal—annually | 8 |
| Screening visit formats | |
| In-person visits only | 8 |
| Both in person and telehealth | 2 |
| Primary approach to OUD screening capture | |
| Patient completes on paper | 8 |
| Patient completes electronically (e.g., patient portal or third-party app) | 2 |
| Patient completes via verbal administration with clinic staff | 0 |
*Based on clinic self-description
Barriers to OUD Screening Implementation: Summary
We identified 7 themes characterizing barriers to OUD screening implementation. Themes, described in detail below and organized by CFIR domains, include (1) challenges identifying who/when to screen, (2) the complexity of the screening tool, (3) staff discomfort and/or hesitancies, (4) workflow barriers that decreased screening follow-up, (5) staffing shortages and turnover, (6) discouragement from low screening yield, and (7) clinic-level stigma.
CFIR Domain: Intervention Characteristics
Theme 1: Identifying Who, When, and How Often to Screen Was Complicated
While most clinics envisioned implementing universal OUD screening, they struggled to clearly define what “universal” meant in practice. For example, many clinics decided to incorporate OUD screening into annual wellness visits, yet as they worked with frontline staff to operationalize this, they quickly identified multiple types of wellness visits and were challenged to establish clarity around which visits were appropriate for OUD screening. This was especially felt by clinics working to integrate OUD screening into their EHR. As one project leader described, “[we’ve had] endless conversation about targeting, no one is really satisfied with it, and even if they have tools built into [the patient portal], the problem still comes down to how do you define, or what do you attach the screener to, whether it’s every patient, every visit, or just attached to all annual visits” (clinic C). In some cases, clinics also incorporated targeted screening for patients at heightened risk for OUD, such as those currently prescribed opioids. However, this often led staff to feel unclear about “what exactly is the rule for when a patient gets screened” (clinic A). As a result, clinics often felt a tension between increasing the universality of OUD screening (i.e., every patient, every visit), which simplified the workflow for identifying when screeners were due, and limiting the frequency of OUD screening (i.e., every patient annually) to reduce administrative burdens for both patients and clinical staff.
Theme 2: The NIDA-Modified ASSIST (NMA) Felt Overly Complex and Challenging to Administer
Though staff agreed that when a patient with OUD is identified and linked to treatment, “one patient justifies the whole screening” effort (clinic B), the NMA felt overly complex to clinical staff and some doubted that it accurately identified patients with OUD. Clinical staff felt intimidated by the complexity of scoring the screener and understanding how to help patients identify when they needed to complete the full screener versus just the initial two pre-screening items. Teams also reported that “the way the questions were worded were [sic] very confusing” (clinic D), and that patients often were confused about reporting opioids taken “other than as prescribed.” Some providers acknowledged that even patients taking opioids as prescribed might still experience symptoms of opioid dependence which were important to uncover. As one provider said, “I don’t trust that those are false positives unless I’ve asked the questions, because they could be trying to tell me something” (clinic D).
Domain: Characteristics of Individuals
Theme 3: Staff Expressed Discomfort, Hesitancy, and Uncertainty with OUD Screening Administration and Follow-Up
Staff expressed discomfort in both screening and following up on positive OUD screens. This was evident in efforts to ensure that patients understood they were not being singled out, such as by offering lead-in statements clarifying that clinics were “asking everyone to fill this form out” (clinic I) or stating that the screener was a way to “see if there are more resources that they may need” (clinic J) and part of their efforts “to treat the whole patient” (clinic E). However, some staff were particularly uncomfortable introducing OUD screening to patients. As one medical assistant (MA) said, “I mean, I kind of know what it’s for, but I don’t know how to explain it in full detail, like if a patient asks questions about it” (clinic I). Another implementation team member expressed that “I’m not sure [MAs] even really know what the form is about” (clinic F). Yet another described that “it was hard for the front desk staff to know what to say” when introducing OUD screening (clinic B), and in some clinics, staff felt uncomfortable asking elderly patients about opioid use. At times, staff discomfort was heightened when patients expressed discomfort over completing OUD screening and reported that they “found it very offensive” (clinic E).
Staff also expressed discomfort and uncertainty about how to follow-up on a positive OUD screen. As one primary care provider said, “as a newly waivered PCP, it’s tough to feel empowered and educated to say ‘well I think you have a problem’” (clinic E). Some providers felt uncomfortable documenting OUD in the chart; one stated, “if they score positive, it’s in the progress note, and that’s available and not necessarily confidential” (clinic F); another clinic also noted that providers who were less comfortable with OUD felt “a hesitancy to put that label in the chart” (clinic J).
Domain: Inner Setting
Theme 4: Clinics Struggled to Optimize Workflow and Ensure Screening Provided Opportunity for Follow-Up of Positive Screens
Clinics described frequent and critical workflow breakdowns, especially “a disconnect” (clinic J) between the MA and provider roles around screener follow-up. As one clinic administrator said, “even if we’re doing the [NMA] screener, the follow-up is not happening even if it’s positive” (clinic H). Another clinic described that “the MA’s have not gotten on board with alerting the PCP about a positive NMA yet” (clinic B). Primary care providers expressed the importance of this step, asserting that they “have a lot that they need to keep track of with the EMR, documentation, etc., so they would like the MAs to really bring their attention to the screening tool like directly, like ‘you have to talk to this person about this issue’” (clinic G). However, clinics acknowledged that MA staff may not feel comfortable identifying a positive OUD screen as they “don’t interpret data they just collect it” (clinic D). Another clinic reiterated that “most of the MAs aren’t going to be able to interpret what’s a positive score” (clinic B). As a result of these workflow breakdowns, clinic managers and implementation team leads felt that they needed to be “meticulous” (clinic G) about reviewing charts on a weekly basis to catch missed opportunities for follow-up on potentially positive OUD screens.
Theme 5: Screening Felt Burdensome to Already-Busy Clinics
Almost every clinic noted challenges with staffing shortages and workload pressures on their primary care staff. Clinics described experiences with persistent staff turnover, difficulties hiring due to limited staff with appropriate qualifications, shortened clinical visit times, increased reliance on telehealth during the COVID-19 pandemic, and a growing list of demands on primary care. Clinics felt that the increasing stress on their teams bred resistance from clinical staff about the addition of OUD screening to their work. One provider offered, “the resistance or overwhelming feeling is coming from another screening in a 15 minute or less timeframe” (clinic G). Another clinic described, “MAs are just doing a push back, it’s not that it’s not clear and concise, it’s just push back because they don’t want to do it . . . the MAs feel that they’re overworked, you’re just adding one more thing to their plate for them to do, what does a positive screen mean, what’s in it if for them, what does it mean for them” (clinic B). Several clinics noted similar resistance from providers. As one administrator described, “more and more, even more is going back to the primary care office. There are shortages everywhere. If and where [primary care providers] identify something where they can put up a boundary, that’s one of the last vestiges where they can do that” (clinic G).
Theme 6: The Low Yield from OUD Screening Felt Discouraging
Though OUD prevalence is increasing, routine OUD screening has had low yield in these clinics. Clinics acknowledged that low frequency of positive OUD screens made it more difficult for staff to maintain consistency in their screening processes. For example, many clinics noted that clinical staff would forget the process for flagging a patient with a positive OUD screen so that the PCP and BHCM could provide appropriate follow-up. As one provider noted, “it’s hard for people to remember this referral [for OUD follow-up by other team members] when they’re not doing it very often” (clinic B). Another provider expressed the need to find a “balance of what’s the yield, just in terms of the overhead involved, […] how many patients you upset, efficiencies you lose in your workflow for doing it repeatedly” (clinic F). In these busy clinical settings, the low prevalence of OUD felt disheartening to clinical teams and resulted in questions about the implementation investment required.
Clinics reflected on why OUD screening yield was so low, as their expectations prior to the launch of OUD screening were that routine screening would result in a deluge of patients requiring OUD–related care. For the few clinics in geographic areas with many OUD treatment resources available, clinic teams perceived that patients likely feel that primary care “isn’t the quickest route to Suboxone at this point” (clinic G) and that they may have an easier time getting access to MOUD in a specialized program. In areas where OUD resources were more limited, clinics perceived that patients with OUD may not be aware of the availability of OUD services in primary care. One provider noted, “if you called the hospital main number and said ‘is there any help in the area for opioid addiction’ they wouldn’t even know about this” (clinic E).
Domain: Outer Setting
Theme 7: Stigma May Deter Patients from Disclosing and Seeking OUD Care
Several clinics noted the ways in which societal-level stigma interfered with OUD screening, particularly for patients with a history of prescription opioid use. As one provider noted, “it’s frustrating… everybody’s answering 0, so we’re pretty much having a hard stop there. But I think because so many of our patients we already transitioned to pain management . . . it’s kinda hard, like how do you get them back from pain management?” (clinic E). Another provider hypothesized that stigma and fear are likely preventing patients from seeking care for OUD, saying “a lot of them hate to come in because a lot of them don’t want to talk about it” (clinic B). As clinics reflected on this, they identified potential clinic policies and practices that may reinforce societal stigma for patients with OUD and prescription opioid use. For example, one clinic described that when patients called for early refills of prescription opioids, “the front desk staff would say ‘we need a police report if your opioids went away’” (clinic E), a response intended to instill fear among patients. Another care manager characterized the role of pain contracts, which are often used to create penalties for taking opioids more than prescribed, saying “I think a lot of people are afraid of being honest because it’s a very hard line they draw at that clinic, if you slip up you’re gone, and in our area there’s no one else to go to” (clinic E). A psychiatrist at another clinic echoed this, saying, “maybe some of our screening could actually come out differently if people knew as part of our advertisement that we offer buprenorphine. There’s a lot of reasons not to disclose, but one of the reasons to disclose is if you know treatment is an option” (clinic J).
Exploration of Variance Across Clinics
We secondarily explored the prominence of themes across clinic and OUD screening characteristics. In general, barriers were stronger in rural clinics, where clinics experienced all of the identified themes; suburban and urban clinics experienced many but not all themes. All clinics, even those that delivered the OUD screener electronically, experienced challenges related to screener complexity (theme 2) and workflow breakdowns for screener follow-up (theme 4). However, clinics that used electronic delivery of the screener were less impacted by the barrier of staff discomfort (theme 3), likely due to less staff involvement in the delivery of the screening tool. Interestingly, clinics that had routine SUD screening in place prior to the implementation of OUD screening still experienced barriers related to staff discomfort (theme 3). Two additional patterns emerged: (1) all clinics that experienced challenges with staff discomfort (theme 3) also experienced challenges with clinic-level stigma (theme 7), and (2) all clinics that expressed burden related to primary care workload (theme 5) also experienced discouragement from low yield that resulted from OUD screening (theme 6).
Promising Strategies for OUD Screening Implementation
Lastly, in response to the barriers identified, clinics utilized several promising implementation strategies to support their OUD screening implementation, which were cataloged in formative evaluation field notes and are briefly described in Table 2. Promising strategies included using a more universal screening approach (e.g., every patient, every visit), standardizing workflows via health information technology, reassessing and revising clinic-level policies that reinforce stigma, using audit and feedback approaches, and repeated educational to increase staff knowledge and confidence regarding OUD.
Table 2.
Summary of Barriers and Promising Strategies for OUD Screening Implementation in Primary Care Settings
| CFIR domain | Barriers experienced | Promising strategies |
|---|---|---|
| Intervention characteristics |
• Identifying who, when, and how often to screen for OUD was complicated • The NIDA-Modified ASSIST (NMA) felt overly complex and challenging to administer |
• Utilize a more universal OUD screening approach (e.g., every patient, every visit) to reduce workflow complexity • Use health information technology (e.g., automated reminders) to enhance screening workflow consistency • Identify OUD screening tools that are brief and simple to administer |
| Individual characteristics | • Staff expressed discomfort, hesitancy, and uncertainty with OUD screening administration and follow-up |
• Providing trainings, scripts, and 1:1 coaching for clinical staff of all roles to reduce discomfort and hesitancy around OUD discussions with patients • Providing forums for staff to voice concerns about OUD screening and provision of OUD care • Provide clinical staff with access to OUD experts and/or mentors to address knowledge gaps and provider self-efficacy |
| Inner setting |
• Clinics struggled to optimize workflow and ensure screening provided opportunity for follow-up of positive screens • The low yield from OUD screening felt discouraging • Screening felt burdensome to already-busy clinics |
• Incorporate audit and feedback strategies to increase workflow effectiveness • Clarify clinic goals for OUD screening and the importance of providing life-saving OUD care • Leverage clinical champions (e.g., a waivered primary care provider) to increase staff buy-in for OUD screening |
| Outer setting | • Stigma may deter patients from seeking OUD care in primary care settings |
• Understand external (e.g., local, community) resources for OUD–related care; tailor care to be responsive to patient demand (e.g., reducing wait times, offering alternative treatment approaches) • Advertise the availability of primary care-based OUD care to the broader community • Identify and reduce stigma within clinic policies and practices |
DISCUSSION
We conducted a rigorous formative evaluation of a facilitated effort to implement OUD screening, a recommended but rarely implemented practice, in 10 geographically and structurally diverse primary care clinics. We found that clinics experienced multiple barriers to implementing OUD screening, which ranged from logistical challenges with the instrument and workflow, to disheartenment and resistance, to trickle-down effects of societal-level stigma and clinic-level opioid safety policies (e.g., utilization reviews, patient-provider pain contracts) that reinforce it. Our evaluation also identified several generalizable best practices to meet and overcome these barriers. These practices can support other primary care clinics nationwide in responding to USPSTF’s call for the integration of OUD screening into primary care.3
Findings from the present study are generally consistent with those from prior studies evaluating implementation of screening for unhealthy substance use.6,12,15 Specifically, clinics in this study experienced substantial patient confusion around reporting prescribed opioid medications within the OUD screening tool, often leading to false-positive or false-negative OUD screens that made screening workflows more laborious for clinical staff. Findings suggest the NMA may not be the optimal screening tool to implement in busy primary care settings where OUD expertise is nascent, but instead echo prior work suggesting the need for SUD screening tools that are brief and understandable and enable easy interpretation of screening scores,3 such as those being implemented in Kaiser Permanente Washington.16,17 Findings from this study also echo earlier findings26 in suggesting the value of leveraging health information technology to improve the efficiency and validity of SUD screening administration. For example, the automated administration of OUD screening via the patient portal can reduce the burden on staff to incorporate additional screening activities into clinical visits and may increase the validity of patient responses given the privacy of completing screening measures outside of the clinics. However, more work is needed to understand best practices for HIT tools that alert clinical teams about OUD screener completion and positive OUD screens, as our study demonstrated. Lastly, data from the present study reinforce that even with effective screening tools, primary care staff will need dedicated education, training, and conversation tools (e.g., scripts) that help increase comfort and decrease stigma in their administration of SUD screening.3,12,16,17,26 The barriers that our clinics experienced suggest that implementation teams may have underestimated the degree of training needed to prepare staff for the implementation of OUD care practices, as well as the need to include all staff roles, including paraprofessional and non-clinical staff, in training efforts.
However, the present study is innovative in its explicit focus on OUD screening—an extremely important practice given the availability of life-saving treatment. Routine OUD screening is a practice for which implementation strategies are not well understood, and which—different from other screening practices—has been termed both valuable and imperfect due to a lack of information regarding whether increased screening for drug use can improve patient outcomes.3,27 One particularly complicated barrier that arose was the low frequency of OUD identification, which also differentiates OUD screening from other screening practices. Screening for unhealthy alcohol use, for example, has yielded prevalence rates ranging from 11 to 36%.16,28 Though acutely dangerous (often fatal), the low prevalence of OUD can contribute to poor screening consistency and disheartenment. Learnings from our work identified tension between clinics’ desire to expand screening practices to more universal approaches (i.e., every patient every visit) which made workflows easier for staff to follow and increased likelihood of identifying patients, and the disheartening perspective that OUD screening was low yield. Future studies and/or quality improvement efforts should work to resolve this tension by identifying ways to increase the efficiency of OUD screening such that it reduces burden on staff while simultaneously recognizing that there are opportunity costs to universal screening, especially for a low-yield (but very dangerous) condition.
Clinics also described experiences of multilevel stigma, in part resulting from opioid safety policies that followed initial waves of the opioid epidemic.29–33 Many health systems have implemented systemwide opioid safety initiatives in an effort to counteract harms from rising prescription opioid use, initiatives which can include routine drug utilization reviews, stricter prescription monitoring and authorization requirements, and the use of patient-provider pain contracts. While these initiatives aim to improve safety of care, our data highlight potential limitations of these initiatives, consistent with prior work.34 Specifically, our data suggest these initiatives may also increase stigma and fear surrounding opioid use for both patients and clinical teams, which hinders efforts to implement OUD screening. Expanding OUD screening in primary care will require efforts to dismantle existing stigma and build cultures of acceptance among clinic staff, accounting for a reality in which opioid prescriptions continue to be written while effective treatments for OUD can also be offered. Successful integration of OUD screening into primary care therefore likely necessitates greater attention to patient-centered approaches to OUD screening no matter the route to opioid dependence.35,36
There are several limitations with this work. First, this study represents formative research using primarily qualitative methods, which by nature aim to provide in-depth exploration and may not be generalizable. Our data primarily involved group-based discussions, so future exploration on individual care team perspectives is warranted and planned by this team. Second, though our sample includes a national network of primary care clinics, the sample is small and does not reflect all types of practices. Lastly, these data reflect the early implementation experiences of clinics; more research is needed to associate the implementation strategies and experiences described—such as the varying use of health IT tools or promising implementation strategies—with clinical and implementation outcomes.
CONCLUSION
This is the first study to our knowledge to present barriers to and promising strategies for implementing OUD screening in primary care—a strategy recommended by the USPTSF to combat the opioid epidemic. Future OUD screening implementation efforts in primary care may wish to consider the lessons learned from this study and the potential benefit of brief intuitive screening tools and tailored practice facilitation that standardizes workflows via health information technology, decreases stigma, and increases staff knowledge and confidence regarding OUD.
Supplementary Information
(DOCX 47 kb)
Contributors
N/A.
Funding
This work was supported by the National Institute of Mental Health (NIH/NIMH; grant U014289744). The statements presented in this work are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health.
Declarations
Conflict of Interest
The authors do not have any personal, professional, or financial conflicts of interest to disclose for this work. The authors did not work with or were otherwise influenced by any external sponsors for this work. Dr. Saxon has received travel support from Alkermes, Inc., consulting fees from Indivior, Inc., and royalties from UpToDate, Inc. Anna Ratzliff, MD, PhD receives royalties from Wiley for her book on integrated care.
Footnotes
Prior Presentations: This work was presented as a poster at the 2021 AcademyHealth Conference on the Science of Dissemination and Implementation in Health.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Donroe JH, Bhatraju EP, Tsui JI, Edelman EJ. Identification and management of opioid use disorder in primary care: an update. Curr Psychiatry Rep. 2020;22(5):23. doi: 10.1007/s11920-020-01149-0. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2021. Designed by LM Rossen, A Lipphardt, FB Ahmad, JM Keralis, and Y Chong: National Center for Health Statistics. Accessed at: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 3.Bradley KA, Lapham GT, Lee AK. Screening for drug use in primary care: practical implications of the new USPSTF recommendation. JAMA Intern Med. 2020;180(8):1050. doi: 10.1001/jamainternmed.2019.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapham G, Boudreau DM, Johnson EA, Bobb JF, Matthews AG, McCormack J, Liu D, Samet JH, Saxon AJ, Campbell CI, Glass JE, Rossom RC, Murphy MT, Binswanger IA, Yarborough BJH, Bradley KA, PROUD Collaborative Investigators Prevalence and treatment of opioid use disorders among primary care patients in six health systems. Drug Alcohol Depend. 2020;207:107732. doi: 10.1016/j.drugalcdep.2019.107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manhapra A, Stefanovics E, Rosenheck R. Initiating opioid agonist treatment for opioid use disorder nationally in the Veterans Health Administration: who gets what? Subst Abus. 2020;41(1):110–120. doi: 10.1080/08897077.2019.1640831. [DOI] [PubMed] [Google Scholar]
- 6.Rhee TG, Rosenheck RA. Buprenorphine prescribing for opioid use disorder in medical practices: can office-based out-patient care address the opiate crisis in the United States? Addiction. 2019;114(11):1992–1999. doi: 10.1111/add.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, Grusing S, Devine B, Chou R. Primary care-based models for the treatment of opioid use disorder: a scoping review. Ann Intern Med. 2017;166(4):268–278. doi: 10.7326/M16-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis CC, Boyd M, Puspitasari A, Navarro E, Howard J, Kassab H, Hoffman M, Scott K, Lyon A, Douglas S, Simon G, Kroenke K. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324–335. doi: 10.1001/jamapsychiatry.2018.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347–355. doi: 10.1007/s11136-019-02320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Council for Behavioral Health, 2018 Implementing care for alcohol and other drug use in medical settings: an extension of SBIRT. Available at https://www.thenationalcouncil.org/wp-content/uploads/2018/03/021518_NCBH_ASPTReport-FINAL.pdf
- 11.Crowley RA, Kirschner N, Health, Public Policy Committee of the American College of, P. The integration of care for mental health, substance abuse, and other behavioral health conditions into primary care: executive summary of an American College of Physicians position paper. Ann Intern Med. 2015;163:298–299. [DOI] [PubMed]
- 12.Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, Bradley KA. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J Gen Intern Med. 2015;30(8):1125–1132. doi: 10.1007/s11606-015-3248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schomerus G, Holzinger A, Matschinger H, Lucht M, Angermeyer MC. Public attitudes towards alcohol dependence. Psychiatr Prax. 2010;37(3):111–118. doi: 10.1055/s-0029-1223438. [DOI] [PubMed] [Google Scholar]
- 14.Denvir PM. When patients portray their conduct as normal and healthy: an interactional challenge for thorough substance use history taking. Soc Sci Med. 2012;75(9):1650–1659. doi: 10.1016/j.socscimed.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, Kivlahan DR. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med. 2011;26(3):299–306. doi: 10.1007/s11606-010-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobb JF, Lee AK, Lapham GT, Oliver M, Ludman E, Achtmeyer C, Parrish R, Caldeiro RM, Lozano P, Richards JE, Bradley KA. Evaluation of a pilot implementation to integrate alcohol-related care within primary care. Int J Environ Res Public Health. 2017;14(9):E1030. doi: 10.3390/ijerph14091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards JE, Bobb JF, Lee AK, Lapham GT, Williams EC, Glass JE, Ludman EJ, Achtmeyer C, Caldeiro RM, Oliver M, Bradley KA. Integration of screening, assessment, and treatment for cannabis and other drug use disorders in primary care: an evaluation in three pilot sites. Drug Alcohol Depend. 2019;201:134–141. doi: 10.1016/j.drugalcdep.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, Kimmel B, Sharp ND, Smith JL. The role of formative evaluation in implementation research and the queri experience. J Gen Intern Med. 2006;21(Suppl 2):S1–S8. doi: 10.1111/j.1525-1497.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elwy AR, Wasan AD, Gillman AG, Johnston KL, Dodds N, McFarland C, Greco CM. Using formative evaluation methods to improve clinical implementation efforts: description and an example. Psychiatry Res. 2020;283:112532. doi: 10.1016/j.psychres.2019.112532. [DOI] [PubMed] [Google Scholar]
- 20.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, Dickens C, Coventry P. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huffman JC, Niazi SK, Rundell JR, Sharpe M, Katon WJ. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the academy of psychosomatic medicine research and evidence-based practice committee. Psychosomatics. 2014;55(2):109–122. doi: 10.1016/j.psym.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Drug Abuse. NIDA Drug Screening Tool. Available at: https://www.drugabuse.gov/nmassist/step/0. Accessed September 26, 2021.
- 23.Palinkas LA, Mendon SJ, Hamilton AB. Innovations in mixed methods evaluations. Annu Rev Public Health. 2019;40:423–442. doi: 10.1146/annurev-publhealth-040218-044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beebe J. Rapid assessment process. In: Kempf-Leonard K, editor. Encyclopedia of Social Measurement: Three Volume set. 1 ed. San Diego, CA: Elsevier; 2005. pp. 285–91. [Google Scholar]
- 25.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeely J, Mazumdar M, Appleton N, Bunting AM, Polyn A, Floyd S, Sharma A, Shelley D, Cleland CM. Leveraging technology to address unhealthy drug use in primary care: effectiveness of the Substance use Screening and Intervention Tool (Susit) Subst Abus. Published online September. 2021;29:1–9. doi: 10.1080/08897077.2021.1975868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitz R. Screening for unhealthy drug use: neither an unreasonable idea nor an evidence-based practice. JAMA. 2020;323(22):2263–2265. doi: 10.1001/jama.2019.20152. [DOI] [PubMed] [Google Scholar]
- 28.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 29.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of hiv stigma mechanism measures. AIDS Behav. 2009;13(6):1160. doi: 10.1007/s10461-009-9593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447–454. doi: 10.18553/jmcp.2014.20.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain. 2017;158(5):833–839. doi: 10.1097/j.pain.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 32.Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med. 2007;22(4):485–490. doi: 10.1007/s11606-006-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alenezi A, Yahyouche A, Paudyal V. Interventions to optimize prescribed medicines and reduce their misuse in chronic non-malignant pain: a systematic review. Eur J Clin Pharmacol. 2021;77(4):467–490. doi: 10.1007/s00228-020-03026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agnoli A, Xing G, Tancredi DJ, Magnan E, Jerant A, Fenton JJ. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA. 2021;326(5):411–419. doi: 10.1001/jama.2021.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muncan B, Walters SM, Ezell J, Ompad DC. “They look at us like junkies”: influences of drug use stigma on the healthcare engagement of people who inject drugs in New York City. Harm Reduct J. 2020;17(1):53. doi: 10.1186/s12954-020-00399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tofighi B, Williams AR, Chemi C, Suhail-Sindhu S, Dickson V, Lee JD. Patient barriers and facilitators to medications for opioid use disorder in primary care. Subst Use Misuse. 2019;54(14):2409–2419. doi: 10.1080/10826084.2019.1653324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 47 kb)