Abstract
Long noncoding RNAs (lncRNAs) have been shown to be involved in the development of osteoarthritis. However, the expression, function, and mechanism of DLEU1 in OA development remain largely unclear. The present reference demonstrates that DLEU1 is overexpressed in OA specimens compared to control cartilages. Inflammatory cytokines IL-1β, TNF-α, and IL-6 induce DLEU1 expression in chondrocytes. Ectopic expression of DLEU1 induces chondrocyte proliferation, degradation of ECM, and inflammation mediators such as IL-6, IL-8, and TNF-α secretion. Moreover, we demonstrated that DLEU1 targets miR-671-5p expression in chondrocytes. Overexpression of DLEU1 suppresses miR-671-5p expression in chondrocytes. The expression of miR-671-5p is decreased in OA specimens compared to control cartilages. There is a negative correlation between the expression of miR-671-5p and DLEU1 in OA specimens. Inflammatory mediators IL-1β, TNF-α, and IL-6 suppress miR-671-5p expression in OA specimens. Elevated expression of miR-671-5p suppresses chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators. DLEU1 overexpression promotes chondrocytes proliferation, degradation of ECM, and secretion of inflammation mediators via regulating miR-671-5p. These results suggested that DLEU1 acts as one destructive role in OA development via regulating miR-671-5p.
1. Introduction
Osteoarthritis is one degenerative joint disease characterized by deterioration in cartilage, synovial inflammation, chondrocyte hypertrophy, and subchondral sclerosis [1–6]. Osteoarthritis is one leading account of disability, pain, and working life shortening [7, 8]. Multiple causes such as strain, inflammation, aging, trauma, obesity, and congenital malformation contribute to progression of OA [9–12]. However, no definite treatment is available that could alter its development.
Long noncoding RNAs (lncRNAs) are one category of noncoding RNAs that are longer than 200 nucleotides, which act as a sponge for miRNA and suppress miRNA expression [13–17]. Growing references have showed that aberrant lncRNA expression is correlated with several diseases including cancer, myocardial ischemia, immunity- and inflammation-related disease, intervertebral disc degeneration, and OA [18–22]. For example, Jiang et al. [23] demonstrated that SNHG5 induced chondrocyte proliferation and suppressed apoptosis in osteoarthritis through modulating the H3F3B/miR-10a-5p axis. Zhou et al. [24] found that lncRNA PCAT-1 modulated chondrocyte apoptosis through sponging miR-27b-3p in osteoarthritis. lncRNAs are involved in cell functions such as cell apoptosis, differentiation, proliferation, metabolism, and invasion [25–28]. Recently, a new lncRNA DLEU1 has been confirmed to act crucial roles in pathologic development of renal cell carcinoma, osteosarcoma, glioma, bladder cancer, and cervical cancer [29–33]. However, the expression, function, and mechanism of DLEU1 in OA development remain largely unclear. Recently, Chen et al. showed that circ-PTTG1IP promotes migration, proliferation, inflammatory response, and invasion of fibroblast-like synoviocytes in the rheumatoid arthritis [34]. Furthermore, Ma et al. also found that paeoniflorin inhibited rheumatoid arthritis progression through regulating the circ-FAM120A/MDM4/miR-671-5p axis [35]. Moreover, DLEU1 regulated osteosarcoma cell migration, invasion, and migration through targeting miR-671-5p [31].
We studied the role of DLEU1 in OA. Firstly, we indicated that DLEU1 is overexpressed in OA specimens compared to control cartilages. Inflammatory cytokines IL-1β, TNF-α, and IL-6 induce DLEU1 expression in chondrocytes. Ectopic expression of DLEU1 induces chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators, such as IL-6, IL-8, and TNF-α.
2. Materials and Methods
2.1. Tissue Specimens
OA cartilages were collected from OA cases that underwent TKA (total knee arthroplasty), and control cartilages were obtained from cases due to the fracture of knee joint without rheumatoid arthritis or OA. Each case was given an informed consent, and our study was agreed by the Clinical Ethics Committee of our hospital. The demographic and clinical characteristics of OA patients are indicated in Table 1.
Table 1.
Clinical and demographic characteristics of the study OA and control population.
| Clinical data | OA | Control |
|---|---|---|
| Age (years) | 67.6 ± 4.42 | 43.2 ± 5.24 |
| Male/female (n) | 19/11 | 13/7 |
| Disease duration (years) | 7.93 ± 3.35 | 0.82 ± 0.21 |
| HSS score | 49.87 ± 4.86 | 53.34 ± 4.35 |
| CRP (mg/dl) | 0.46 ± 0.16 | 0.31 ± 0.11 |
| ESR (mm/h) | 10.43 ± 4.56 | 11.35 ± 3.32 |
2.2. Chondrocyte Culture and Transfection
Human chondrocytes were obtained from Shanghai Chinese Academy of Sciences, and these cells were cultured in the RPMI-1640 medium containing antibiotics and fetal bovine serum (FBS). pcDNA-DLEU1 and control and miR-671-5p mimic and scramble mimic were collected from GenePharma. Cell transfection was carried out with using Lipofectamine 2000 (Invitrogen, USA) following the protocol.
2.3. Luciferase Reporter Assay
Fragment of DLEU1 3′-UTR was cloned into downstream of pMIR-REPORT plasmid, named wild-type (WT) DLEU1. To build mutant DLEU1 3′-UTR reporter, seed region of DLEU1 3′-UTR was mutated, named mutated (mut) DLEU1. Cells were cotransfected with miR-671-5p mimic or scramble and mutant DLEU1 3′-UTR or mut-DLEU1 using Lipofectamine 2000 (Invitrogen, USA). Luciferase activity was carried out with luciferase reporter analysis (Promega).
2.4. Cell Viability Assay
Cell viability was detected using CCK-8 reagent (Dojindo, Japan) following the typical protocol. Cells were cultured in the 96-well dish and 10 μl CCK-8 fluids were added into each well. After incubation for additional 3 hours, the absorbance was read with a microplate reader at 450 nm.
2.5. RT-qPCR
Total RNA from chondrocytes or cartilage samples was separated by Trizol kit (Invitrogen, USA) according to the protocol. Real-time qPCR was utilized to determine the expression of miR-671-5p, DLEU1, aggrecan, collagen II, ADAMTS-5, and MMP-3 using SYBRTM Green kit (Applied Biosystems, CA) on ABI7500 PCR System (Applied Biosystems, USA). U6 and GAPDH were performed for internal controls. Results were calculated by utilizing the 2−△△CT way. Primers were indicated as follows: U6 primers (forward, 5′-CTCGCT TCG GCA GCA CA-3′and reverse, 5′-AACGCT TCA CGA ATT TGCGT-3′), DLEU1 (forward 5′-CCAGC CCACA GGCAT TTAGT-3′and reverse, 5′-GTTCC GAGG CTTAA GTGCGA-3′), and GAPDH (forward, 5′-AAGTGGTCGTTGAGGGCAATG-3′and reverse, 5′-CTGGGCTACACTGAGCACC-3′).
2.6. ELISA
The culture supernatant was obtained after chondrocytes were treated. The IL-6, IL-8, and TNF-α concentration in the supernatant was detected with ELISA reagent (R&D Systems, UK) following the standard protocol.
2.7. Statistical Analysis
SPSS 18.0 software (Chicago, IL) was processed for statistical assay. Results were indicated as means ± SD. Student's t-test was applied to detect statistical difference between two groups. Statistically significance was set to p < 0.05.
3. Results
3.1. DLEU1 Is Overexpressed, and miR-671-5p Is Decreased in OA Specimens
The pathology of control cartilages and OA cartilages is shown in Figures 1(a) and 1(b). qRT-PCR assay was carried out to examine DLEU1 expression in control cartilages and OA cartilages. As indicated in Figure 2, DLEU1 is overexpressed in OA specimens compared to control cartilages. Moreover, qRT-PCR assay was carried out to examine miR-671-5p expression in control cartilages and OA cartilages. As indicated in Figure 3(a), miR-671-5p is decreased in OA specimens compared to control cartilages. There is a negative correlation between expression of miR-671-5p and DLEU1 in OA specimens (Figure 3(b)).
Figure 1.
The pathology of control cartilages and OA cartilages is shown in (a) and (b).
Figure 2.
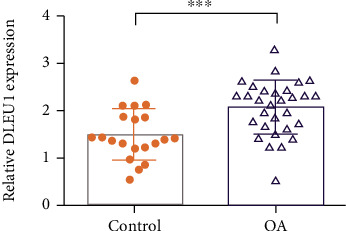
DLEU1 was overexpressed in OA specimens. The DLEU1 expression in the control cartilages and OA cartilages was detected by qRT-PCR assay. GAPDH was used as the internal control. ∗∗∗p < 0.001.
Figure 3.
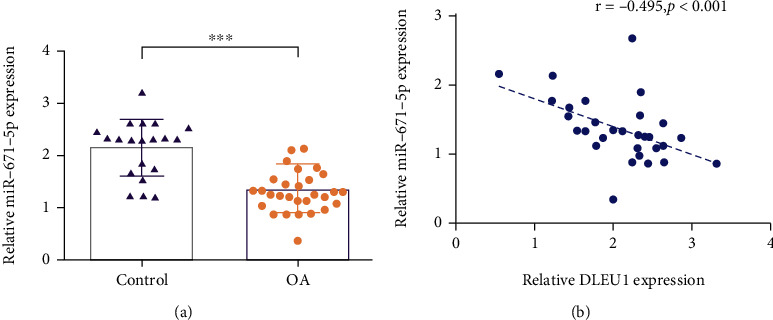
miR-671-5p was decreased in OA specimens. (a) The miR-671-5p expression in the control cartilages and OA cartilages was detected by qRT-PCR assay. (b) There was a negative correlation expression between miR-671-5p and DLEU1 in OA specimens. ∗∗∗p < 0.001.
3.2. Inflammatory Cytokines IL-1β, TNF-α, and IL-6 Induce DLEU1 and Suppress miR-671-5p Expression
We found that inflammatory cytokines IL-1β, TNF-α, and IL-6 increase DLEU1 expression in chondrocytes (Figures 4(a)–4(c)). We indicated that inflammatory cytokines IL-1β, TNF-α, and IL-6 decrease miR-671-5p expression in chondrocytes (Figures 5(a)–5(c)).
Figure 4.
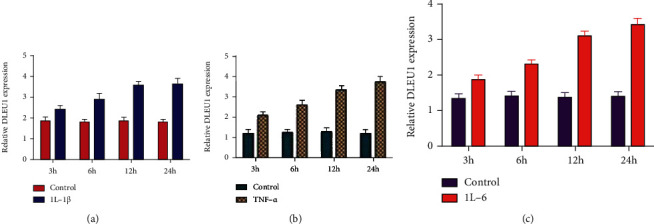
Inflammatory cytokines IL-1β, TNF-α, and IL-6 induced DLEU1 expression. (a) Inflammatory cytokine IL-1β increased DLEU1 expression in chondrocytes. (b) The expression of DLEU1 was measured by qRT-PCR analysis. (c) IL-6 induced DLEU1 expression in chondrocytes.
Figure 5.
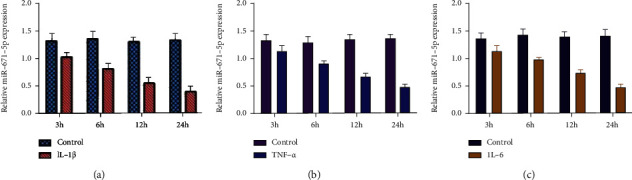
Inflammatory mediators IL-1β, TNF-α, and IL-6 suppressed miR-671-5p expression. (a) The expression of miR-671-5p was detected by qRT-PCR assay. (b) TNF-α suppressed miR-671-5p expression in chondrocytes. (c) IL-6 inhibited miR-671-5p expression in chondrocytes.
3.3. Ectopic Expression of DLEU1 Induces Chondrocyte Proliferation, Degradation of ECM, and Secretion of Inflammation Mediators
The expression of DLEU1 is upregulated in chondrocytes transfected with pcDNA-DLEU1 plasmid (Figure 6(a)). Ectopic expression of DLEU1 promotes cell proliferation in the chondrocytes using CCK-8 assay (Figure 6(b)). Elevated DLEU1 expression suppresses the expression of collagen II (Figure 6(c)) and aggrecan (Figure 6(d)) in chondrocytes. Overexpression of DLEU1 induces ADAMTS-5 (Figure 6(e)) and MMP-3 (Figure 6(f)) expression in chondrocytes. Furthermore, DLEU1 overexpression promotes IL-6 (Figure 6(g)), IL-8 (Figure 6(h)), and TNF-α (Figure 6(i)) expression in chondrocytes using ELISA.
Figure 6.
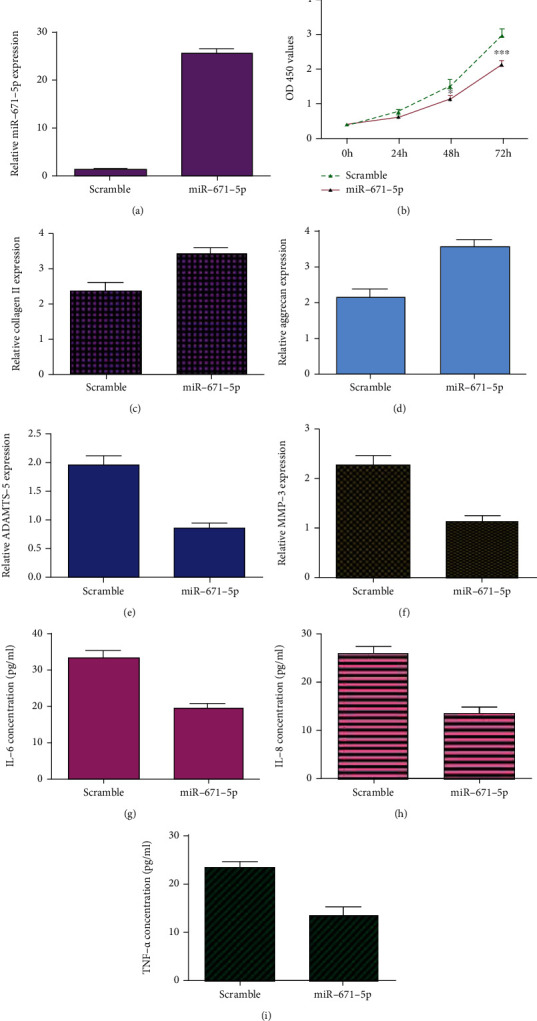
Ectopic expression of DLEU1 induced chondrocyte viability, degradation of ECM, and inflammation mediator secretion. (a) The expression of DLEU1 was measured by qRT-PCR analysis. (b) Ectopic expression of DLEU1 promoted cell viability in the chondrocytes using CCK-8 assay. (c) Elevated expression of DLEU1 suppressed collagen II expression in the chondrocytes. (d) The expression of aggrecan was detected by qRT-PCR assay. (e) Overexpression of DLEU1 induced ADAMTS-5 expression in the chondrocytes. (f) The expression of MMP-3 was measured by qRT-PCR assay. (g) DLEU1 overexpression promoted IL-6 expression in the chondrocytes using ELISA. (F) The expression of IL-8 was measured by ELISA. (i) The expression of TNF-α was measured by ELISA. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.4. Elevated Expression of miR-671-5p Suppresses Chondrocyte Viability, Degradation of ECM, and Secretion of Inflammation Mediators
The expression of miR-671-5p is upregulated in chondrocytes transfected with miR-671-5p mimic (Figure 7(a)). Ectopic expression of miR-671-5p inhibits cell viability in the chondrocytes using CCK-8 assay (Figure 7(b)). Elevated miR-671-5p expression enhances expression of collagen II (Figure 7(c)) and aggrecan (Figure 7(d)) in the chondrocytes. Overexpression of miR-671-5p suppresses ADAMTS-5 (Figure 7(e)) and MMP-3 (Figure 7(f)) expression in the chondrocytes. Furthermore, miR-671-5p overexpression inhibits IL-6 (Figure 7(g)), IL-8 (Figure 7(h)), and TNF-α (Figure 7(i)) expression in the chondrocytes using ELISA.
Figure 7.
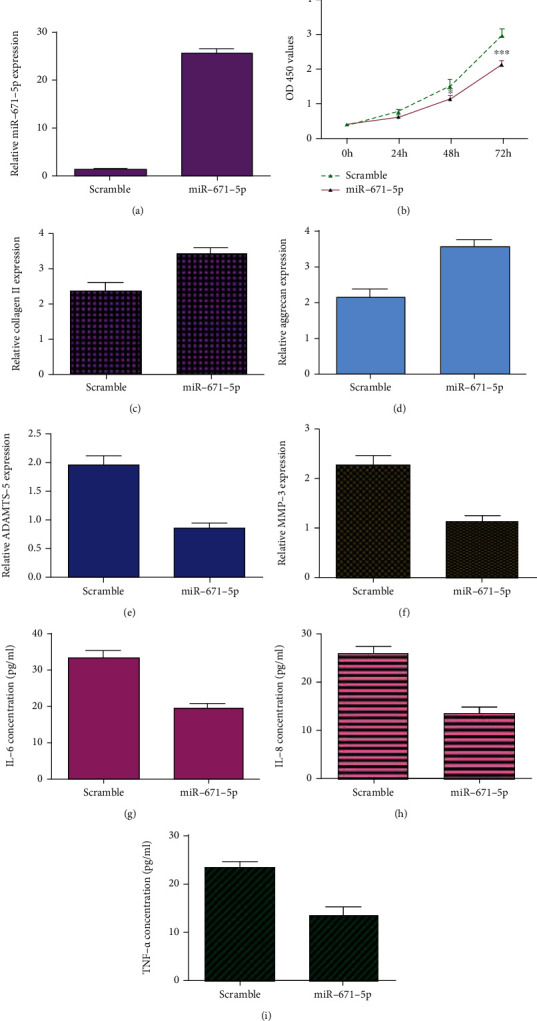
Elevated expression of miR-671-5p suppressed chondrocyte viability, degradation of ECM, and inflammation mediator secretion. (a) The expression of miR-671-5p was measured by qRT-PCR analysis. (b) Ectopic expression of miR-671-5p inhibited cell viability in the chondrocytes using CCK-8 assay. (c) Elevated expression of miR-671-5p enhanced collagen II expression in the chondrocytes. (d) The expression of aggrecan was detected by qRT-PCR assay. (e) Overexpression of miR-671-5p suppressed ADAMTS-5 expression in the chondrocytes. (f) The expression of MMP-3 was measured by qRT-PCR assay. (g) miR-671-5p overexpression inhibited IL-6 expression in the chondrocytes using ELISA. (h) The expression of IL-8 was measured by ELISA. (i) The expression of TNF-α was measured by ELISA. ∗p < 0.05 and ∗∗∗p < 0.001.
3.5. DLEU1 Targets miR-671-5p Expression in Chondrocytes
Following the software (http://starbase.sysu.edu.cn/index.php), miR-671-5p may be a potential combining target gene of the DLEU1 (Figure 8(a)). Luciferase reporter analysis was carried out to study the relationship between miR-671-5p and DLEU1. The data indicated that cotransfection with miR-671-5p mimic and DLEU1-WT decreases luciferase activities when compared to miR-671-5p mimic and DLEU1-mut (Figure 8(b)). In addition, qRT-PCR assay data showed that overexpression of DLEU1 suppresses miR-671-5p expression in chondrocytes (Figure 8(c)).
Figure 8.
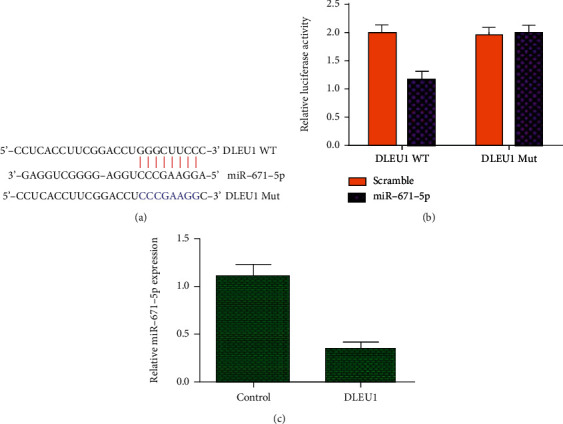
DLEU1 targeted miR-671-5p expression in chondrocytes. (a) Following the software (http://starbase.sysu.edu.cn/index.php), miR-671-5p may be a potential combining target gene of the DLEU1. (b) Luciferase reporter analysis was carried out to study the relationship between miR-671-5p and DLEU1. The data indicated that cotransfection with miR-671-5p mimic and DLEU1-WT decreased luciferase activities when compared to miR-671-5p mimic and DLEU1-mut. (c) Overexpression of DLEU1 suppressed miR-671-5p expression in chondrocytes. ∗∗p < 0.01.
3.6. DLEU1 Overexpression Promotes Chondrocyte Viability, Degradation of ECM, and Secretion of Inflammation Mediators via Regulating miR-671-5p
To learn whether DLEU1 overexpression promoted chondrocyte viability, degradation of ECM, and secretion of inflammation mediators via regulating miR-671-5p, we performed the rescued experiments. We indicated that miR-671-5p overexpression suppresses cell growth in the DLEU1-overexpressing chondrocytes (Figure 9(a)). Elevated expression of miR-671-5p enhances collagen II (Figure 9(b)) and aggrecan (Figure 9(c)) expression in the DLEU1-overexpressing chondrocytes. Overexpression of miR-671-5p suppresses expression of ADAMTS-5 (Figure 9(d)) and MMP-3 (Figure 9(e)) in the DLEU1-overexpressing chondrocytes. Furthermore, miR-671-5p overexpression inhibits expression of IL-6 (Figure 9(f)), IL-8 (Figure 9(g)), and TNF-α (Figure 9(h)) in the DLEU1-overexpressing chondrocytes using ELISA.
Figure 9.
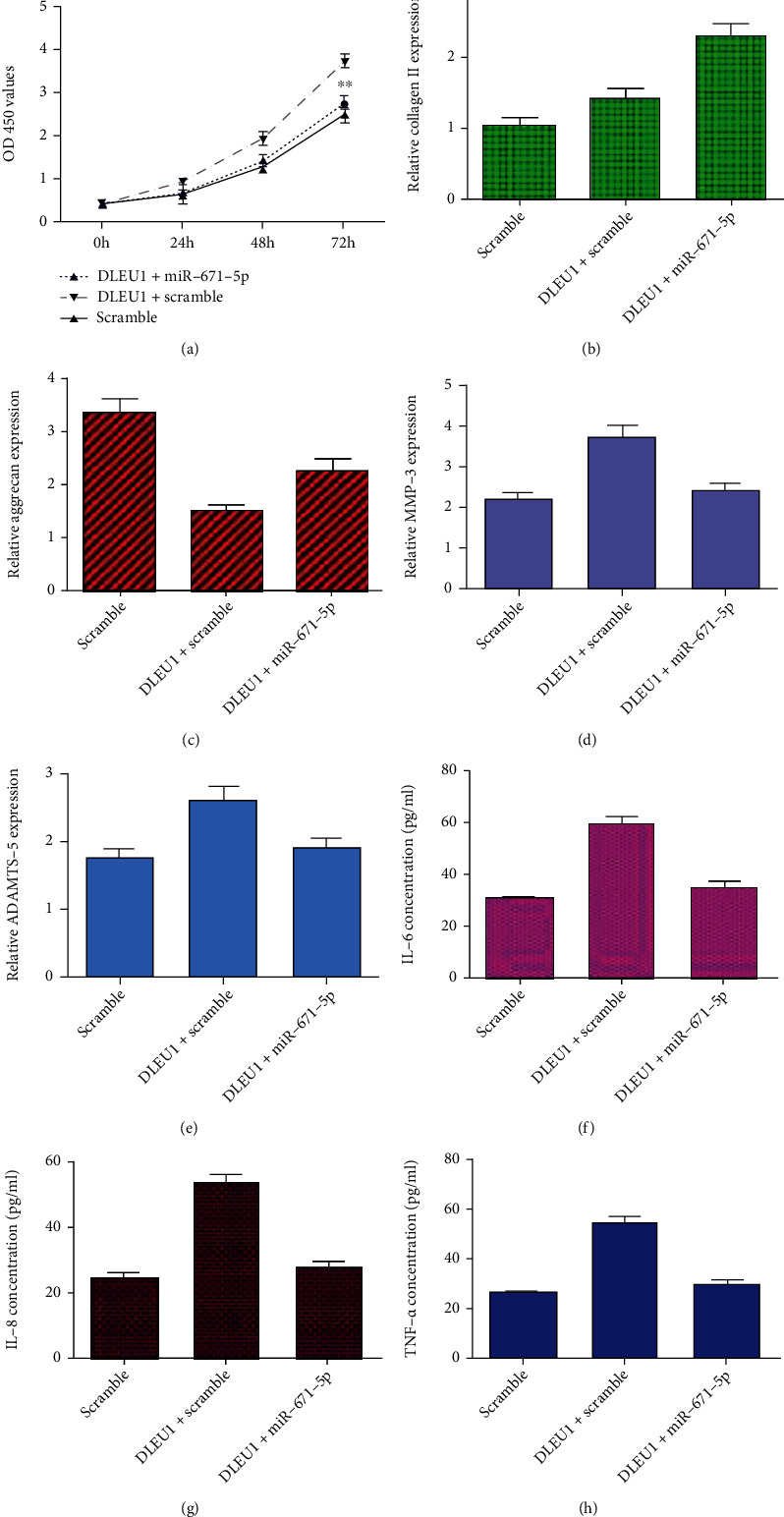
DLEU1 overexpression promoted chondrocyte proliferation, degradation of ECM, and inflammation mediator secretion via regulating miR-671-5p. (a) The cell proliferation was analyzed by CCK-8 assay. (b) The expression of collagen II was detected by qRT-PCR analysis. (c) The expression of aggrecan was detected by qRT-PCR analysis. (d) The expression of ADAMTS-5 was detected by qRT-PCR analysis. (e) The expression of MMP-3 was detected by qRT-PCR analysis. (f) The expression of IL-6 was measured by ELISA. (g) The expression of IL-8 was measured by ELISA. (h) The expression of TNF-α was measured by ELISA. ∗p < 0.05 and ∗∗p < 0.01.
4. Discussion
Emerging reports have supported that lncRNAs are aberrantly expressed in many physiological and pathological procedures, including OA. In this research, we studied the role of DLEU1 in OA. Firstly, we indicated that DLEU1 is overexpressed in OA specimens compared to control cartilages. Inflammatory cytokines IL-1β, TNF-α, and IL-6 induce DLEU1 expression in chondrocytes. Ectopic expression of DLEU1 induces chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators such as IL-6, IL-8, and TNF-α. Moreover, we demonstrated that DLEU1 targets miR-671-5p in chondrocytes. Overexpression of DLEU1 suppresses miR-671-5p expression in chondrocytes. The expression of miR-671-5p is decreased in OA specimens compared to control cartilages. There is a negative correlation between expressions of miR-671-5p and DLEU1 in OA specimens. Inflammatory mediators IL-1β, TNF-α, and IL-6 suppresses miR-671-5p expression in OA specimens. Elevated expression of miR-671-5p suppresses chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators. DLEU1 overexpression promotes chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators via regulating miR-671-5p. These results suggested that DLEU1 acts one destructive role in OA development via regulating miR-671-5p.
Recently, studies have revealed that DLEU1 plays critical roles in the pathologic progression of renal cell carcinoma, glioma, osteosarcoma, cervical cancer, and bladder cancer [29–33]. For instance, DLEU1 was upregulated in glioblastoma and downregulated expression of DLEU1 suppressed glioblastoma cell growth and enhanced cell apoptosis partly via regulating miR-4429 [36]. Yue et al. [29] found that knockdown of DLEU1 suppressed renal cell carcinoma cell growth, invasion, and migration and impaired epithelial mesenchymal transition progression partly through modulating Akt. Chen et al. [31] demonstrated that DLEU1 was upregulated in osteosarcoma cells and specimens. DLEU1 downregulation decreased osteosarcoma cell migration, invasion, and migration. Moreover, DLEU1 was shown to be overexpressed in bladder cancer specimens and overexpression of DLEU1 promoted cell invasion and growth and cisplatin resistance via modulating miR-99b expression [32]. However, the expression, function, and mechanism of DLEU1 in OA development remain largely unclear. In the present reference, we firstly detected the expression of DLEU1 in OA specimens. Our results showed that DLEU1 is overexpressed in OA specimens compared to control cartilages. Inflammatory cytokines IL-1β, TNF-α, and IL-6 induce DLEU1 expression in chondrocytes. Ectopic expression of DLEU1 induces chondrocytes proliferation, degradation of ECM, and inflammation mediators such as IL-6, IL-8, and TNF-α secretion.
Accumulating studies have proved that lncRNA performed as ceRNA to modulate disease development via sponging miRNA [37, 38]. Wang and workmates showed that NEAT1 promoted chondrocyte inflammation and proliferation through regulating miR-181a [39]. Zhu and Jiang [40] showed that PART1 regulated cell apoptosis, proliferation, and degradation of extracellular matrix through modulating miR-373-3p expression. Chen et al. [41] demonstrated that MEG3 alleviated extracellular matrix degradation in chondrocytes via targeting miR-93. Furthermore, DLEU1 regulated osteosarcoma cell migration, invasion, and migration through targeting miR-671-5p [31]. Following the software (http://starbase.sysu.edu.cn/index.php), miR-671-5p may be a potential combining target gene of DLEU1. Luciferase reporter analysis was carried out to study the relationship between miR-671-5p and DLEU1. The data indicated that cotransfection with miR-671-5p mimic and DLEU1-WT decreases luciferase activities when compared to miR-671-5p mimic and DLEU1-mut (Figure 7(b)). In addition, qRT-PCR assay data showed that overexpression of DLEU1 suppresses miR-671-5p expression in chondrocytes. In addition, we showed that the expression of miR-671-5p is decreased in OA specimens compared to control cartilages. There is a negative correlation between expression of miR-671-5p and DLEU1 in OA specimens. Inflammatory mediators IL-1β, TNF-α, and IL-6 suppress miR-671-5p expression in OA specimens. Elevated expression of miR-671-5p suppresses chondrocyte proliferation, degradation of ECM, and inflammation mediator secretion. DLEU1 overexpression promotes chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators via regulating miR-671-5p.
Our results defined that DLEU1 is overexpressed in OA specimens compared to control cartilages. DLEU1 overexpression promotes chondrocyte proliferation, degradation of ECM, and secretion of inflammation mediators via regulating miR-671-5p. These data suggested that DLEU1 acts a destructive role in OA development via regulating miR-671-5p expression.
Contributor Information
Yongxi Liu, Email: xianzhi12@yeah.net.
Jun Liu, Email: liujuntianj@163.com.
Data Availability
No data were used to support this study.
Conflicts of Interest
There is no conflict of interest.
Authors' Contributions
Xiangkun Wu and Shuai Yin are co-first authors.
References
- 1.Jackson M. T., Moradi B., Smith M. M., Jackson C. J., Little C. B. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis & Rhematology . 2014;66(6):1525–1536. doi: 10.1002/art.38401. [DOI] [PubMed] [Google Scholar]
- 2.Iliopoulos D., Malizos K. N., Oikonomou P., Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One . 2008;3(11, article e3740) doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliopoulos D., Malizos K. N., Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Annals of the Rheumatic Diseases . 2007;66(12):1616–1621. doi: 10.1136/ard.2007.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gege C., Bao B., Bluhm H., et al. Discovery and evaluation of a non-Zn chelating, selective matrix metalloproteinase 13 (MMP-13) inhibitor for potential intra-articular treatment of osteoarthritis. Journal of Medicinal Chemistry . 2012;55(2):709–716. doi: 10.1021/jm201152u. [DOI] [PubMed] [Google Scholar]
- 5.Wang G., Zhang Y., Zhao X., Meng C., Ma L., Kong Y. MicroRNA-411 inhibited matrix metalloproteinase 13 expression in human chondrocytes. American Journal of Translational Research . 2015;7(10):2000–2006. [PMC free article] [PubMed] [Google Scholar]
- 6.Su W., Xie W., Shang Q., Su B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. BioMed Research International . 2015;2015:5. doi: 10.1155/2015/356893.356893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radons J., Bosserhoff A. K., Grassel S., Falk W., Schubert T. E. p38MAPK mediates IL-1-induced down-regulation of aggrecan gene expression in human chondrocytes. International Journal of Molecular Medicine . 2006;17(4):661–668. doi: 10.3892/ijmm.17.4.661. [DOI] [PubMed] [Google Scholar]
- 8.Piecha D., Weik J., Kheil H., et al. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflammation Research . 2010;59(5):379–389. doi: 10.1007/s00011-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 9.Li N. G., Shi Z. H., Tang Y. P., et al. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Current Medicinal Chemistry . 2011;18(7):977–1001. doi: 10.2174/092986711794940905. [DOI] [PubMed] [Google Scholar]
- 10.Lago R., Gomez R., Otero M., et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis and Cartilage . 2008;16(9):1101–1109. doi: 10.1016/j.joca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Holt D. W., Henderson M. L., Stockdale C. E., et al. Osteoarthritis-like changes in the heterozygous _sedc_ mouse associated with the HtrA1 -Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis and Cartilage . 2012;20(5):430–439. doi: 10.1016/j.joca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Goldring M. B., Otero M. Inflammation in osteoarthritis. Current Opinion in Rheumatology . 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Li X. Y., Chen X., et al. Emerging roles of long non-coding RNAs in neuropathic pain. Cell Proliferation . 2019;52(1, article e12528) doi: 10.1111/cpr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Y. F., Zhong Y. T., Wu J. J., et al. Long non-coding PANDAR as a novel biomarker in human cancer: a systematic review. Cell Proliferation . 2018;51(1) doi: 10.1111/cpr.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S. B., Fu W., Zhang L. Y., et al. LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKα. Cell Proliferation . 2018;51(1) doi: 10.1111/cpr.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Gao Z., Zhang C., Wu H., Gu R., Jiang R. Retracted: Long non-coding RNA ASBEL promotes osteosarcoma cell proliferation, migration, and invasion by regulating microRNA-21. Journal of Cellular Biochemistry . 2018;119(8):6461–6469. doi: 10.1002/jcb.26671. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J. M., Yin M. N., Peng G., Zhao Y., Peng G., Zhao Y. C. CRNDE: an important oncogenic long non-coding RNA in human cancers. Cell Proliferation . 2018;51(3):p. 51. doi: 10.1111/cpr.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X., Zheng H. Y., Tse G., Zhang L., Wu W. K. K. CASC2: an emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Proliferation . 2018;51(6, article e12506) doi: 10.1111/cpr.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong W., Qu Y., Chen H., Qian J. Insight into long noncoding RNA-miRNA-mRNA axes in myocardial ischemia-reperfusion injury: the implications for mechanism and therapy. Epigenomics . 2019;11(15):1733–1748. doi: 10.2217/epi-2019-0119. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Ao L., Yang J. Long non-coding RNAs in diseases related to inflammation and immunity. Annals of Translational Medicine . 2019;7(18):p. 494. doi: 10.21037/atm.2019.08.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X. B., Lv G. H., Li J., Wang B., Zhang Q. S., Lu C. LncRNA-RP11-296A18.3/miR-138/HIF1A pathway regulates the proliferation ECM synthesis of human nucleus pulposus cells (HNPCs) Journal of Cellular Biochemistry . 2017;118(12):4862–4871. doi: 10.1002/jcb.26166. [DOI] [PubMed] [Google Scholar]
- 22.Chen W. K., Yu X. H., Yang W., et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Proliferation . 2017;50(1, article e12313) doi: 10.1111/cpr.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Pang H., Wu P., Cao Z., Li Z., Yang X. LncRNA SNHG5 promotes chondrocyte proliferation and inhibits apoptosis in osteoarthritis by regulating miR-10a-5p/H3F3B axis. Connective Tissue Research . 2021;62(6):605–614. doi: 10.1080/03008207.2020.1825701. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L., Gu M., Ma X., et al. Long non-coding RNA PCAT-1 regulates apoptosis of chondrocytes in osteoarthritis by sponging miR-27b-3p. Journal of Bone and Mineral Metabolism . 2021;39:139–147. doi: 10.1007/s00774-020-01128-8. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Li X. Y., Chen C., et al. Long non-coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Proliferation . 2018;51(5, article e12483) doi: 10.1111/cpr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ping G., Xiong W., Zhang L., Li Y., Zhang Y., Zhao Y. Silencing long noncoding RNA PVT1 inhibits tumorigenesis and cisplatin resistance of colorectal cancer. American Journal of Translational Research . 2018;10:138–149. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X. F., Hao J. L., Xie T., et al. The BRAF activated non-coding RNA: a pivotal long non-coding RNA in human malignancies. Cell Proliferation . 2018;51(4):p. 51. doi: 10.1111/cpr.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C., Su C., Song Q., Dong F., Yu S., Huo J. LncRNA PICART1 suppressed non-small cell lung cancer cells proliferation and invasion by targeting AKT1 signaling pathway. American Journal of Translational Research . 2018;10:4193–4201. [PMC free article] [PubMed] [Google Scholar]
- 29.Yue G., Chen C., Bai L., et al. Knockdown of long noncoding RNA DLEU1 suppresses the progression of renal cell carcinoma by downregulating the Akt pathway. Molecular Medicine Reports . 2019;20:4551–4557. doi: 10.3892/mmr.2019.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng L., He M., Rao M., Diao J., Zhu Y. Long noncoding RNA DLEU1 aggravates glioma progression via the miR-421/MEF2D axis. Oncotargets and Therapy . 2019;12:5405–5414. doi: 10.2147/OTT.S207542. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Chen X., Zhang C., Wang X. Long noncoding RNA DLEU1 aggravates osteosarcoma carcinogenesis via regulating the miR-671-5p/DDX5 axis. Artificial Cells, Nanomedicine, and Biotechnology . 2019;47(1):3322–3328. doi: 10.1080/21691401.2019.1648285. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Shi B., Dong F., Zhu X., Liu B., Liu Y. Long non-coding RNA DLEU1 promotes cell proliferation, invasion, and confers cisplatin resistance in bladder cancer by regulating the miR-99b/HS3ST3B1 axis. Frontiers in Genetics . 2019;10:p. 280. doi: 10.3389/fgene.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C., Tian X., Zhang J., Jiang L. Long non-coding RNA DLEU1 promotes proliferation and invasion by interacting with miR-381 and enhancing HOXA13 expression in cervical cancer. Frontiers in Genetics . 2018;9:p. 629. doi: 10.3389/fgene.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L., Huang H., Chen L., Xu L., Chen J., Lu Q. circ-PTTG1IP/miR-671-5p/TLR4 axis regulates proliferation, migration, invasion and inflammatory response of fibroblast-like synoviocytes in rheumatoid arthritis. General Physiology and Biophysics . 2021;40(3):207–249. doi: 10.4149/gpb_2021014. [DOI] [PubMed] [Google Scholar]
- 35.Ma J., Meng Q., Zhan J., et al. Paeoniflorin suppresses rheumatoid arthritis development via modulating the circ-FAM120A/miR-671-5p/MDM4 axis. Inflammation . 2021;44(6):2309–2322. doi: 10.1007/s10753-021-01504-0. [DOI] [PubMed] [Google Scholar]
- 36.Liu X., Chen R., Liu L. SP1-DLEU1-miR-4429 feedback loop promotes cell proliferative and anti-apoptotic abilities in human glioblastoma. Bioscience Reports . 2019 doi: 10.1042/bsr20190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan Y., Wu Y. J., Hu J. L., et al. Long noncoding RNA HOTAIR promotes renal cell carcinoma malignancy through alpha-2, 8-sialyltransferase 4 by sponging microRNA-124. Cell Proliferation . 2018;51(6, article e12507) doi: 10.1111/cpr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng L., Ma P., Cai R., Guan Q., Wang M., Jin B. Long noncoding RNA ZEB1-AS1 promotes the tumorigenesis of glioma cancer cells by modulating the miR-200c/141-ZEB1 axis. American Journal of Translational Research . 2018;10:3395–3412. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z., Hao J., Chen D. Long noncoding RNA nuclear enriched abundant transcript 1 (NEAT1) regulates proliferation, apoptosis, and inflammation of chondrocytes via the miR-181a/glycerol-3-phosphate dehydrogenase 1-like (GPD1L) axis. Medical Science Monitor . 2019;25:8084–8094. doi: 10.12659/MSM.918416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y. J., Jiang D. M. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. European Review for Medical and Pharmacological Sciences . 2019;23(19):8175–8185. doi: 10.26355/eurrev_201910_19124. [DOI] [PubMed] [Google Scholar]
- 41.Chen K., Zhu H., Zheng M.-Q., Dong Q.-R. LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 axis. Cartilage . 2019;13:1274S–1284S. doi: 10.1177/1947603519855759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


