Abstract
During recent oceanographic cruises to Pacific hydrothermal vent sites (9°N and the Guaymas Basin), the rapid microbial formation of filamentous sulfur mats by a new chemoautotrophic, hydrogen sulfide-oxidizing bacterium was documented in both in situ and shipboard experiments. Observations suggest that formation of these sulfur mats may be a factor in the initial colonization of hydrothermal surfaces by macrofaunal Alvinella worms. This novel metabolic capability, previously shown to be carried out by a coastal strain in H2S continuous-flow reactors, may be an important, heretofore unconsidered, source of microbial organic matter production at deep-sea hydrothermal vents.
A chemoautotrophic, hydrogen sulfide-oxidizing bacterium from shallow coastal marine waters has been discovered recently that possesses the unusual metabolic capability of excreting elemental sulfur in the form of rigid irregular filaments (0.5 to 2.0 by 20 to 500 μm) (6). These vibrioid microorganisms, now identified as an Arcobacter species (unpublished data), are retained in sulfidic, high-fluid-flow environments by the production of mats of entangled filamentous sulfur that resist turbulence. Visualization of filamentous sulfur in coastal environments and in samples collected from the 9°N deep-sea hydrothermal vents following a volcanic event (4, 6) lead to a hypothesis that microbial filamentous sulfur formation may be a cosmopolitan activity in mixed H2S-O2 environments and under certain circumstances may be an important primary production microbial process in hydrothermal vent ecosystems (6).
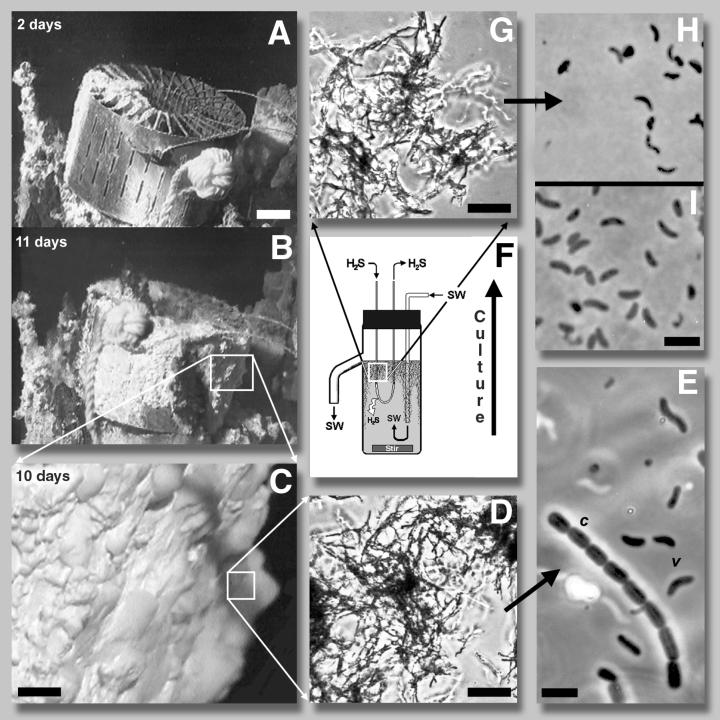
On a recent cruise (December 1997) to the 9°N vent field off the west coast of Mexico (9°46.49′N, 104°16.82′W), support was found for this hypothesis by the observation of the rapid and voluminous in situ formation of filamentous sulfur at an actively flowing warm water hydrothermal vent (vent M; depth, 2,510 m). At this site a titanium ring for Alvinella colonization (TRAC) containing vent chimney rock samples (Fig. 1A) was placed within the active warm water flow (20 to 40°C) to investigate the early recruitment by Alvinella worms. Exposure conditions were similar to those for diffuse-flow warm water vents where primary hydrothermal fluids have mixed with oxygenated seawater, resulting in H2S emission concentrations on the order of 250 to 350 μM (1, 7). The experimental surfaces provided by the TRAC and its enclosed rock samples became the site for a very rapid colonization by H2S-oxidizing, sulfur-filament-producing bacteria. A white filamentous sulfur mat composed of 74% elemental sulfur by dry weight (sea salt-free material analyzed by benzene assay [5]) accumulated on the titanium surfaces to a thickness of ∼0.5 cm in two days and up to 3 cm by 10 to 11 days, nearly completely filling the interior of the ring (Fig. 1A and B). The filamentous sulfur mat (Fig. 1C and D) was tenacious and harbored mostly vibrioid bacteria (0.4 by 1.2 to 2.0 μm) with a morphology (Fig. 1E, cell type v), motility, and surface adhesion behavior very similar to those of the coastal strain (6) (Fig. 1I), as well as smaller numbers of one other significant morphological type, ovoid rods (0.9 by 1.2 to 1.4 μm) arranged in chains of 4 to 10 cells (Fig. 1E, cell type c). These microbial chains may have contributed to mat tenacity by producing some form of cross-linking organic matrix.
FIG. 1.
Microbial filamentous sulfur formation at a 9°N hydrothermal vent site and in shipboard laboratory culture. (A and B) Filamentous sulfur mat formation on the TRAC at vent site M. Bar, 5 cm. (C) Enlarged view of mat material. Bar, 1 cm. (D) Phase photomicrograph of filamentous sulfur mat produced in situ. Bar, 10 μm. (E) Microorganisms contained in the filamentous sulfur mat. Bar, 2 μm. (F) Schematic of continuous culture apparatus for growth of sulfur-filament-forming microbes from hydrothermal vent inocula. H2S, hydrogen sulfide; SW, oxygenated seawater. (G) Phase photomicrograph of filamentous sulfur produced in shipboard laboratory culture. Bar, 10 μm. (H) Microorganisms from shipboard laboratory reactor. (I) Coastal sulfur-filament-producing microorganism (5). Bar, 2 μm.
The mat was also inhabited by a large number of underlying juvenile Alvinella worms that were attached to the TRAC metal and rock sample surfaces by means of the tubes they occupy. It was evident that these metazoans were quickly attracted to the mat-covered TRAC from the surrounding area. Similar studies conducted at somewhat cooler vents (∼25°C) during the HOT 96 expedition (3) revealed no colonization of the TRAC in 13 days by Alvinella worms when the ring was not heavily covered by a filamentous sulfur mat, although at 2 months, the next time point in the study, the device was encrusted with thin grey deposits and heavily colonized by these animals (unpublished data). Though evidence is at the moment circumstantial, it is reasonable to suggest that the rapidly forming filamentous sulfur transformed the new surfaces provided by the TRAC and rock samples into a habitat that attracted and fostered occupation by juvenile alvinellids in record time, 10 to 11 days and probably less.
Shipboard enrichment cultures of sulfur filament-producing bacteria were successfully established in continuous-flow H2S-containing seawater reactors (6) (Fig. 1F) inoculated either from samples of white deposits collected from the surfaces of hydrothermal chimneys or from mat material which had colonized the TRAC. These enrichments were self-perpetuating when an inoculum (∼10%) from a previous enrichment was used, and the filamentous sulfur in these cultures possessed a morphology identical to that which had been produced in situ (compare Fig. 1D and G). The rod-shaped chains of organisms (Fig. 1E, cell type c) were not enriched in the shipboard reactor systems. The resultant shipboard filamentous mat material showed mostly vibrioid cells (Fig. 1H) and had the physical and microscopic properties of material typically obtained in laboratory enrichments of coastal seawater inoculum (6) (i.e., cultured filamentous sulfur flocs more friable than the TRAC mat material). The organisms cultured aboard ship (Fig. 1H) were noticeably smaller (∼0.3 by 1.0 to 1.2 μm) than both the TRAC ring inhabitants (Fig. 1E) and the coastal strain (Fig. 1I). It is not known at present whether the size difference reflects different culture conditions or fixation effects or a difference in phylogeny. Comparative phylogenetic studies are presently under way. Shipboard reactor enrichment samples were incubated at 23°C as a static culture in the presence of H2S, O2, and 0.1 μCi of 14C-bicarbonate ml−1 and analyzed for 14C fixation by the procedures of Tuttle and Jannasch (8). Results showed initial rates of carbon fixation (20.5 nmol of C fixed ml−1 h−1) that were commensurate with those obtained with samples from reactors inoculated with the coastal strain (17.4 ± 9.6 nmol of C fixed ml−1 h−1), indicating that the two populations were similarly autotrophic.
Morphologically identical sulfur filaments have been found in diverse environments, including salt marsh creeks and coastal sediment (6), and in the present study of hydrothermal warm water vents at 9°N. Most recently (May 1998), filamentous sulfur with a morphology that was identical to that in Fig. 1D and G was documented in white, Beggiatoa-free mats overlying hydrothermal sediment cores collected at the Guaymas Basin vent site in the Gulf of California (personal observation). On the basis of recent observations, it appears that microbial filamentous sulfur formation may occur in diverse marine aquatic environments that provide a constant supply of H2S and O2. Under typical continuous-flow conditions of warm water vents, sulfur-filament-producing microbes can be prolific primary colonizers of new surfaces, producing thick mats in just a few days that may in addition create conditions aiding recruitment of rock-surface-dwelling animals such as Alvinella worms. In addition to a continuous low-level release from warm water vents, diking-eruption events may stimulate and release, en masse (2), subsurface-produced sulfur filaments and associated chemolithoautotrophic biomass. Thus, a shallow, nonthermophilic, subsurface biosphere dominated by chemoautotrophic filament-producing H2S oxidizers may be an important component heretofore unconsidered in the overall organic production at deep-sea hydrothermal vent sites.
Acknowledgments
This work was supported by National Science Foundation grant IBN96-30054.
Footnotes
Contribution number 9887 of the Woods Hole Oceanographic Institution.
REFERENCES
- 1.Corliss J B, Dymond J, Gordon L I, Edmond J M, Herzen R P V, Ballard R D, Green K, Williams D, Bainbridge A, Crane K, VanAndel T H. Submarine thermal springs on the Galapagos Rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 2.Delaney J R, Kelley D S, Lilley M D, Butterfield D A, Baross J A, Wilcock W S D, Embley R W, Summit M. The quantum vent of oceanic crustal accretion: impacts of diking at mid-ocean ridges. Science. 1998;281:222–230. [PubMed] [Google Scholar]
- 3.Gaill F, Felbeck H, Desbruyères D, Lallier F, Toulmond A, Alayse A-M, Briand P, Brulport J-P, Caprias J C, Chevaldonnè P, Coail J Y, Cosson R, Crassous P, Delachambre J, Durif C, Echardour L, Hervè G, Hourdez S, Jollivet D, Kerdoncuff J, Kripounoff A, Lechaire J P, Pruski A, Ravaux J, Sarradin P M, Shillito B, Toullec J Y, Arndt C, Fisher C, Lutz R, Childress J. HOT 96 News. InterRidge News. 1996;5:22–24. [Google Scholar]
- 4.Haymon R M, Fornari D J, Von Damm K L, Lilley M D, Perfit M R, Edmond J M, Shanks III W C, Lutz R A, Grebmeier J M, Carbotte S, Wright D, McLaughlin E, Smith M, Beedle N, Olson E. Volcanic eruption of the mid-ocean ridge along the East Pacific Rise crest at 9°45-52′N: direct submersible observations of seafloor phenomena associated with an eruption event in April, 1991. Earth Planet Sci Lett. 1993;119:85–101. [Google Scholar]
- 5.Javor B J, Wilmot D B, Vetter R D. pH-dependent metabolism of thiosulfate and sulfur globules in the chemolithotrophic marine bacterium. Thiomicrospira crunogena. Arch Microbiol. 1990;154:231–238. [Google Scholar]
- 6.Taylor C D, Wirsen C O. Microbiology and ecology of filamentous sulfur formation. Science. 1997;277:1483–1485. [Google Scholar]
- 7.Tunnicliffe V, Juniper S K, deBurgh M E. The hydrothermal vent community on axial seamount, Juan de Fuca Ridge. Bull Biol Soc Wash. 1985;6:453–464. [Google Scholar]
- 8.Tuttle J H, Jannasch H W. Thiosulfate stimulation of microbial dark assimilation of carbon dioxide in shallow marine environments. Microb Ecol. 1977;4:9–25. doi: 10.1007/BF02010426. [DOI] [PubMed] [Google Scholar]