Abstract
Purple urine bag syndrome (PUBS) is seen in the prolonged indwelling bladder catheters, and the mechanism of its onset was investigated using low vacuum scanning electron microscopy (LVSEM), which enables us to study the 3D structure of urinary sediments and urine bag walls. The urinary sediment and urine bags of 2 cases of PUBS were observed by LVSEM. The urine was brown turbid urine with a pH of 8.5, and magnesium phosphate stones and granules were observed in the urinary sediment together with Gram-positive and Gram-negative bacilli. Bacteria that moved by Brownian motion were observed with a dark-field microscope. LVSEM showed granular crystals around the bacilli, cocci, or mycelium that adhered to the walls of the bag. Granular crystals were dissolved in chloroform and presumed to be a mixture of the bacterial metabolites indigo blue and indirubin red. LVSEM also detected unusual tubular and honeycomb-like graphene in the urinary sediments, which were derived from the inner layer of the silicon elastomer-coated rubber catheter. LVSEM revealed purple crystals produced by bacteria or fungi attached to the urine bag that caused PUBS.
Keywords: Purple urine bag, Urinary sediment, Bladder catheter, Low vacuum scanning electron microscope
Introduction
Purple urine bag syndrome (PUBS) is rarely observed in the patients with long-term balloon catheter placement [1, 2]. Some bacteria, such as Klebsiella, are thought to breakdown indoxyl sulfate to produce blue indigo and red indirubin pigments [3]. However, it is not completely understood why it is only found in some patients and why the urine itself does not turn purple [4]. Therefore, we tried to elucidate the mechanism of PUBS using low vacuum scanning electron microscopy (LVSEM).
LVSEM is a nonperturbing technology that requires minimal sample preparation [5, 6]. LVSEM using paraffin-embedded slide glass specimens with periodic acid Schiff methenamine silver staining is useful for observing renal biopsy samples to identify electron-dense deposits and changes in the glomerular basement membrane and podocytes [5–8]. Even though the resolution of LVSEM is not sufficient at more than × 10,000 magnification, black and white reversed images of LVSEM images become similar to transmission electron microscopy (TEM) images in the observation of renal biopsy samples [6]. Thus, LVSEM could be a substitute for TEM, for which special preparation and skill, as well as time-consuming processes, are required to obtain images. Moreover, LVSEM has facilitated a new understanding of various types of 3D structures including those observed in regenerated organs, vascular injury thrombus formation, and tumor cells [9].
In the present study, we applied LVSEM in the observation of urinary sediments, urine bags, and bladder catheters to understand the mechanism of purple urine bag syndrome.
Methods
Urine samples (10 mL) from patients with purple urine bag syndrome were centrifuged at 500×g for 5 min. After removing the supernatant by decantation, 200 μL of urinary sediments were stained with 20 μL of Sternheimer stain solution (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) and observed with a dark-field microscope. Some urinary sediments were stained with a Gram staining kit (Fujifilm, Osaka, Japan). The remaining urinary sediments stained with Sternheimer stain solution were washed with 1000 μL of distilled water twice. Fifteen microliters of urinary sediments was mounted on a carbon filter membrane (Nisshin EM, Tokyo, Japan) and observed by LVSEM (Hitachi TM4000 Plus, Tokyo, Japan). The acceleration voltage was fixed at 15 kV with a vacuum of 30 Pa to detect backscattered electrons [6]. Urine samples were collected from urine bags with the approval of the research ethics committee of Dokkyo Medical University (R-2-1).
The walls of the urine bag from cases of purple urine bag syndrome were cut and directly observed by LVSEM. To analyze the properties of the crystals attached to the urine bag, the wall surface of the purple bag was immersed in 1 N hydrochloric acid, 1 N sodium hydroxide, 30% hydrogen peroxide, and 99% chloroform, and the decolorization of the dye and the dissolution of the crystals were observed by LVSEM.
To confirm crystal formation from indoxyl sulfate by the bacteria on the urine bag surface, bacteria on the urine bag surface were cultured by sheep blood agar palate (Nippon Becton Dickinson Company Ltd, Fukushima, Japan) with 250 μmole/L indoxyl sulfate (Cayman Chemical Company, Ann Arber, MI, USA) in a CO2 incubator for 3 days. The surface of agar was transferred to the glass slides by stamping and observed by light microscopy.
A silicone elastomer-coated bladder catheter (Medicon Inc., Osaka, Japan) was cut, and both the inner and outer surfaces of the tube were stained with 1% Ponceau S solution (Olympus Optical Co., Ltd., Tokyo, Japan) for 15 min, washed with distilled water, mounted on a carbon filter, and observed by LVSEM.
Results
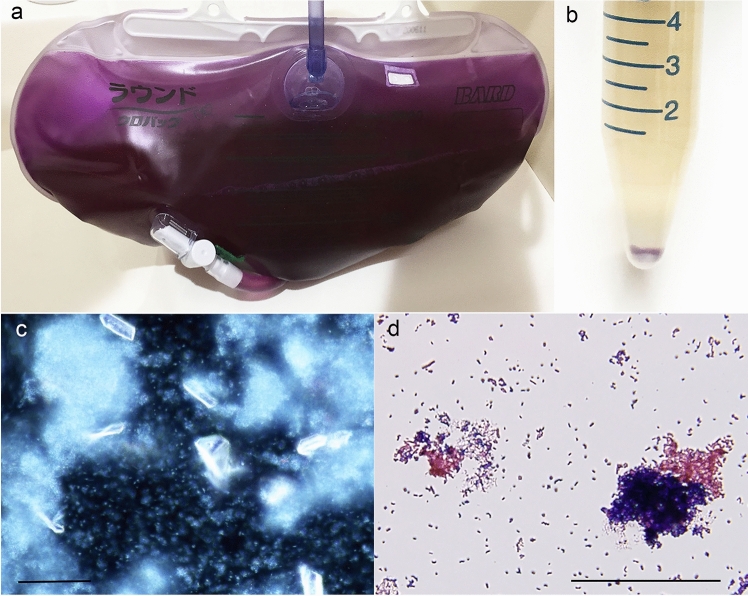
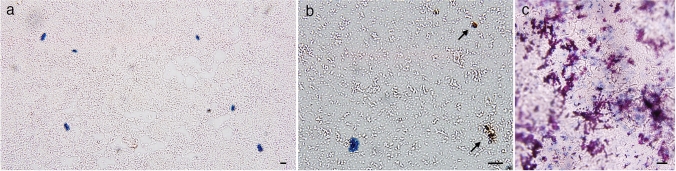
Table 1 shows the clinical background of the two cases of PUBS. In both cases, approximately 1 month after wearing the bladder balloon catheter, urinary tract infections were confirmed by an increase in urinary leukocytes, multiple bacteria, or fungi, but no antibiotics were given. In case 1, the urine bag was purple, but the color of the urine was brown, and when centrifuged, a purple substance is precipitated in the sediment (Fig. 1a, b). The supernatant was discarded, a part of the precipitate was stained with Sternheimer staining, and observed with a dark-field microscope to identify struvite crystals, which are magnesium ammonium phosphate stones, and white glowing coccid and bacilli showed Brownian motion (Fig. 1c). Gram staining of a part of the sediment revealed a mixture of red Gram-negative bacilli and blue Gram-positive cocci (Fig. 1d). In urine culture, the Gram-negative rods were Enterobacter cloacae and Pseudomonas aeruginosa, and the Gram-positive cocci were Streptococcus anginosus (Table 1).
Table 1.
Clinical data of two cases of purple urine bag syndrome
| Case 1 85-yo male |
Case 2 77-yo male |
|
|---|---|---|
| Background disease | Post-renal renal failure with BPH | Myocardial infarction |
| Urinary pH | 8.5 | 5.5 |
| Urinary sediments |
RBC 5–9/HPF WBC 5–9/HPF Bacteria + |
RBC 10–19/HPF WBC 30–49/HPF Bacteria + |
| Urinary culture |
E. cloacae P. aeruginosa S. anginosus |
S. epidermidis C. albicans |
| Blood WBC, CRP | 7300/μL, 5.14 mg/dL | 9900/μL, 1.82 mg/dL |
| Days after inserting the urine bag | 28 days | 32 days |
| Use of antibiotics | No | No |
Fig. 1.
Case 1 of purple urine bag syndrome (a), urine after centrifugation (b), urinary sediments observed with dark-field microscopy (c), and Gram staining of urinary sediments (d). Even though the urine bag is purple (a), and urine itself is brown with purple crystals in the sediments (b) and with more than two species of bacteria (c, d). The bars indicate 50 μm
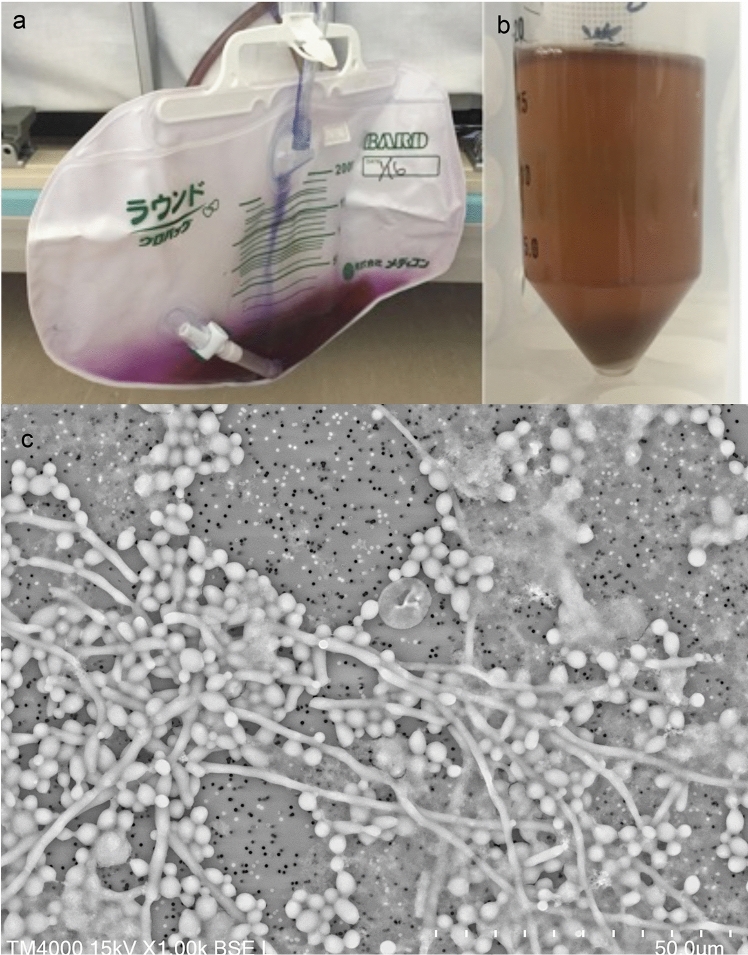
In case 2, the wall of the urine bag was purple, but the urine was brown (Fig. 2a, b). When the urine sediment was observed by LVSEM, fungal hyphae and large spherical yeast-shaped spores were observed, and smaller cocci were observed in the background (Fig. 2c). Urine culture revealed that the fungus was Candida albicans and the background cocci were Staphylococcus epidermidis (Table 1).
Fig. 2.
Case 2 of purple urine bag syndrome (a), urine after centrifugation (b), and urinary sediments observed by LVSEM (c). Urine in the purple bag (a) is not purple (b), and urinary sediments include fungi and bacteria (c). Magnification × 1000 (c). The bars indicate 50 μm
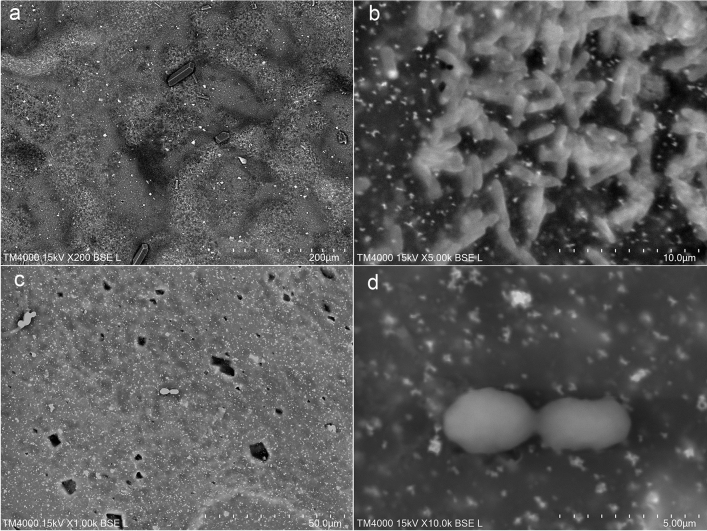
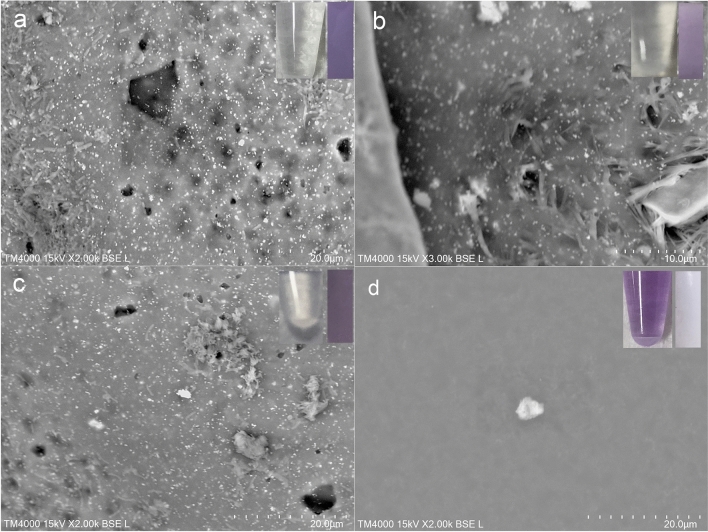
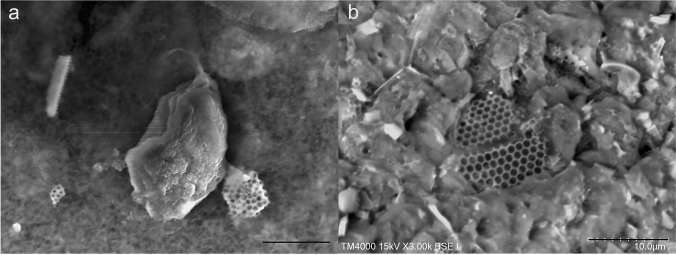
LVSEM observations showed many amorphous crystals on the purple surface of the urine bag. In Case 1, bacilli adhered to the white polyvinyl chloride (PVC) surface of the urine bag, and many amorphous crystals were observed around them (Fig. 3a, b). In Case 2, LVSEM revealed yeast-like spores with large C. albicans adhered to the urine bag, and many irregularly shaped crystals were observed around them (Fig. 3c, d). The purple surface of the urine bag was cut into strips and soaked in 1 N HCL acid solution (Fig. 4a), 1 N NaOH alkali solution (Fig. 4b), 30% hydrogen peroxide (Fig. 4c), or chloroform (Fig. 4d) for 15 min to examine the bleaching of the urine bag and the dissolution of crystals. Chloroform easily decolorized the purple color of the urine bag, dissolved the crystals on the surface, and turned the extract into purple (Fig. 4d). On the other hand, the acidic solution did not elute the purple urine bag at all and granular crystals and bacteria remained on the wall (Fig. 4a). The alkaline solution (Fig. 4b) and the hydrogen peroxide solution (Fig. 4c) slightly dissolved with few bacteria but granular and spindle crystals remained on the wall in 15 min, and finally decolorized after a long time. To confirm that the bacteria on the bag produced colored crystals, bacteria on the urine bag were swabbed and incubated on a blood agar plate with indoxyl sulfate. Crystals with blue (Fig. 5a) and brown color (Fig. 5b) were identified around the bacteria. The crystals produced from indoxyl sulfate by cultured bacteria were not perfectly the same but similar to purple and blue crystals on the lucid polypropylene surface of the urine bag (Fig. 5c).
Fig. 3.
LVSEM observation of the inner surface of the purple urine bags in case 1 (a, b) and case 2 (c, d). Magnification × 200 (a), × 5000 (b), × 1000 (c), and × 10,000 (d) The bars indicate 200 μm (a), 10 μm (b), 50 μm (c), and 5 μm (d).
Fig. 4.
LVSEM observation of the segments of the surface of purple urinary bag in case 1 immersed in 1 N HCl (a), 1 N NaOH (b), 30% H2O2 (c), and chloroform (d). The insertions are the color of the immersion solution (left) and segment of purple urine bag (right) after 15 min. Magnification × 2000 (a, c, d) and × 3000 (b). The bars indicate 20 μm (a, c, d) and 10 μm (b)
Fig. 5.
Light microscopic observation of crystals with blue and brown (arrow) colors formed from indoxyl sulfate via incubated bacteria from the urine bag of case 1 (a, b) and light microscopy of crystals on the lucid polypropylene surface of urine bag (c). The bacteria obtained from urine bags were incubated with indoxyl sulfate, and formed blue crystals (a) and brown crystals (b), which resembled the purple and blue crystals on the surface of urine bags (c). The bars indicate 10 μm (a–c)
LVSEM observations of urine in the bag showed strange honeycomb and tubular structures with spikes, similar to graphene and carbon nanotubes (Fig. 6a). LVSEM observations of the bladder catheter revealed that these structures shed from the luminal wall of the bladder catheter coated with a silicon elastomer to reinforce the rubber tube (Fig. 6b).
Fig. 6.
Urinary sediments (a) and the inner surface of the urinary bladder catheter (b) observed by LVSEM. Graphene and tubular structures (a) in the urinary sediments are derived from the surface of the urinary bladder catheter (b). Magnification × 3000 (a, b). The bars indicate 10 μm (a, b)
Discussion
In this case study, LVSEM observations showed that the urine bag was colored purple by the irregularly shaped crystals produced by the bacteria on the urine bag, even though the urine itself was brown in PUBS.
Relationship between the characteristics of urinary bacteria and purple urine bags
Since purple urine bag syndrome was first reported in 1978 [1], it has been recognized as a complication of urinary tract infections (UTIs) wherein urine bags turn purple, and it occurs predominantly in female, chronically catheterized patients with alkaline urine [2] because of frequent UTIs in these cases. Some bacteria, including Providencia stuartii and Providencia rettgeri, Klebsiella pneumoniae, Proteus mirabilis, Escherichia coli, Enterococcus species, and Morganella morganii, produce sulfatase and phosphatase, which oxidize the uremic toxin indoxyl sulfate to indigo (a blue pigment) and indirubin (a red pigment), and these pigment mixtures react with the urine catheter and bag to produce a striking purple hue [3, 10, 11].
In case 1, E. cloacae and P. aeruginosa are Gram-negative bacilli with flagella and motility, and cannot produce indole. Indole is produced from food-derived tryptophan via a deamination reaction by intestinal bacteria, and is sulfated in the liver to indoxyl sulfate (indican), which is filtered by glomeruli and removed in urine. Enterobacter has an indoxyl phosphatase and produces indigo from indican [3], and P. aeruginosa has alkylsulfatase (SdsA1) [12], which decomposes indoxyl sulfate into indoxyl and further oxidizes it to indigo (blue) and indirubin (red) [10, 11]. S. anginosus is a Gram-positive coccus that has phosphoserine phosphatase, produces H2S, and may be involved in crystal formation [13]. Actually, bacteria on the bag in case 1 can produce blue- and brown-colored crystals from indoxyl sulfate. In Case 2, S. epidermidis and C. albicans were identified in urine cultures. S. epidermidis has phosphatase [14], and C. albicans has sulfatase [15], so indigo and indirubin can be produced from indoxyl sulfate.
Identification of crystals by morphology and solubility
Purple amorphous crystals were found on the surface of the urine bag. Calcium oxalate, uric acid, cystine crystals, and magnesium ammonium phosphate crystals commonly found in urine are denied by morphology, but are also soluble in hydrochloric acid [16, 17]. Atypical phosphates and yellow urates are shown in the form of amorphous granules, but they are rejected, because they are dissolved in hydrochloric acid or acetic acid. Purple crystals are indigotin crystals that are difficult to dissolve in acids and alkalis, but they are soluble in chloroform [18]. This is consistent with the result in this case. Observations by LVSEM clearly showed that the purple color of PUBS was not the color of the liquid phase urine itself, but the color of the crystals that grew around the bacteria attached to the wall of the bag. Purple crystals are also seen in the precipitate of urine after centrifugation (Fig. 1b). The purple color of the urine bag disappeared after treatment with antibiotics [19].
Identification of new structures in urinary sediment by LVSEM
Our study clearly demonstrated novel 3D structures in the urinary crystals by LVSEM. In contrast to conventional SEM [20–22], LVSEM does not require a freeze-drying process; thus, smaller crystals and bacteria are not lost during processing. The strange honeycomb-like structure and tubular structure with windows or spikes were similar to the graphene and carbon nanotubes in the silicon-polymer coating [23]. Stretchable graphene is used for various purposes on rubber structures, including light-emitting diodes (LEDs) [24]. These structures of graphene oxide and graphite also have an antibacterial effect on the tubular membrane [25]. We should be aware that these graphene and tubular structures originated from bladder catheters.
In conclusion, 3D observation of the urine bag by LVSEM demonstrated that bacterial or fungal sulfatase and phosphatase attached to the surface of the urine bag oxidize indoxyl sulfate to indigo and indirubin, producing amorphous purple crystals on the surface of the bag.
Acknowledgements
We thank Ms. Kyoko Mamada, and Mr. Kazumi Akimoto of the Center for Research Support, Dokkyo Medical University, for their support with culture and incubation.
Funding
This work was partially supported by research donations from Dr. Naohiko Kobayashi of the Kobayashi Internal Medicine Clinic (#2020-9).
Declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buist NR. Purple urine bags. Lancet. 1978;1:883–884. doi: 10.1016/S0140-6736(78)90239-8. [DOI] [Google Scholar]
- 2.Kalsi DS, Ward J, Lee R, Handa A. Purple urine bag syndrome: a rare spot diagnosis. Dis Markers. 2017;2017:9131872. doi: 10.1155/2017/9131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microb. 1988;26:2152–2156. doi: 10.1128/jcm.26.10.2152-2156.1988. [DOI] [Google Scholar]
- 4.Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J. 2012;105:446. doi: 10.1097/SMJ.0b013e31825f3984. [DOI] [PubMed] [Google Scholar]
- 5.Inaga S, Kato M, Hirashima S, Munemura C, Okada S, Kameie T, Katsumoto T, Nakane H, Tanaka K, Hayashi K, Naguro T. Rapid three-dimensional analysis of renal biopsy sections by low vacuum scanning electron microscopy. Arch Histol Cytol. 2010;73:113–125. doi: 10.1679/aohc.73.113. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki H, Uozaki H, Tojo A, Hirashima S, Inaga S, Sakuma K, Morishita Y, Fukayama M. Application of low-vacuum scanning electron microscopy for renal biopsy specimens. Pathol Res Pract. 2012;208:503–509. doi: 10.1016/j.prp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Okada S, Inaga S, Kawaba Y, Hanada T, Hayashi A, Nakane H, Naguro T, Kaidoh T, Kanzaki S. A novel approach to the histological diagnosis of pediatric nephrotic syndrome by low vacuum scanning electron microscopy. Biomed Res. 2014;35:227–236. doi: 10.2220/biomedres.35.227. [DOI] [PubMed] [Google Scholar]
- 8.Okada S, Inaga S, Kitamoto K, Kawaba Y, Nakane H, Naguro T, Kaidoh T, Kanzaki S. Morphological diagnosis of alport syndrome and thin basement membrane nephropathy by low vacuum scanning electron microscopy. Biomed Res. 2014;35:345–350. doi: 10.2220/biomedres.35.345. [DOI] [PubMed] [Google Scholar]
- 9.Sawaguchi A, Kamimura T, Yamashita A, Takahashi N, Ichikawa K, Aoyama F, Asada Y. Informative three-dimensional survey of cell/tissue architectures in thick paraffin sections by simple low-vacuum scanning electron microscopy. Sci Rep. 2018;8:7479. doi: 10.1038/s41598-018-25840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ting IW, Wang R, Wu VC, Hsueh PR, Hung KY. Purple urine bag syndrome in a hemodialysis patient. Kidney Int. 2007;71:956. doi: 10.1038/sj.ki.5002117. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Huang HT, Chien CC, Tzeng DS, Lung FW. Purple urine bag syndrome in nursing homes: ten elderly case reports and a literature review. Clin Interv Aging. 2008;3:729–734. doi: 10.2147/CIA.S3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagelueken G, Adams TM, Wiehlmann L, Widow L, Kolmar H, Tummler B, Heinz DW, Schubert WD. The crystal structure of SdsA1, an alkylsulfatase from Pseudomonas aeruginosa, defines a third class of sulfatases. Proc Natl Acad Sci USA. 2006;103:7631–7636. doi: 10.1073/pnas.0510501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizrak M, Yetkin Mizrak O, Celikbilek N, Basar E, Kocaaga M. Purple urine bag syndrome: a rare clinical case. Mikrobiyoloji Bulteni. 2019;53:457–463. doi: 10.5578/mb.68616. [DOI] [PubMed] [Google Scholar]
- 14.Geary C, Stevens M. Detection of phosphatase production by Staphylococcus species—a new method. Med Lab Sci. 1989;46:291–294. [PubMed] [Google Scholar]
- 15.Shimizu MT, Jorge AOC, Unterkircher CS, Fantinato V, Paula CR. Hyaluronidase and chondroitin sulfatase production by different species of Candida. J Med Vet Mycol. 1995;33:27–31. doi: 10.1080/02681219580000061. [DOI] [PubMed] [Google Scholar]
- 16.Barbas C, Garcia A, Saavedra L, Muros M. Urinary analysis of nephrolithiasis markers. J Chromatogr B Anal Technol Biomed Life Sci. 2002;781:433–455. doi: 10.1016/S1570-0232(02)00557-3. [DOI] [Google Scholar]
- 17.Basiri A, Taheri M, Taheri F. What is the state of the stone analysis techniques in urolithiasis? Urol J. 2012;9:445–454. [PubMed] [Google Scholar]
- 18.Hart S, Koch KR, Woods DR. Identification of indigo-related pigments produced by Escherichia-Coli containing a cloned Rhodococcus gene. J Gen Microb. 1992;138:211–216. doi: 10.1099/00221287-138-1-211. [DOI] [Google Scholar]
- 19.Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med. 2005;44:859–861. doi: 10.2169/internalmedicine.44.859. [DOI] [PubMed] [Google Scholar]
- 20.Tawashi R. Size-shape analysis of calcium oxalate crystals in the study of stone formation. Scan Electron Microsc. 1983;1:397–406. [Google Scholar]
- 21.Iwata H, Nishio S, Wakatsuki A, Ochi K, Takeuchi M. Architecture of calcium oxalate monohydrate urinary calculi. J Urol. 1985;133:334–338. doi: 10.1016/S0022-5347(17)48934-8. [DOI] [PubMed] [Google Scholar]
- 22.Iwata H, Iio S, Nishio S, Takeuchi M. Architecture of mixed calcium oxalate dihydrate and monohydrate stones. Scanning Microsc. 1992;6:231–237. [PubMed] [Google Scholar]
- 23.Cavas L, Yildiz PG, Mimigianni P, Sapalidis A, Nitodas S. Reinforcement effects of multiwall carbon nanotubes and graphene oxide on PDMS marine coatings. J Coat Technol Res. 2018;15:105–120. doi: 10.1007/s11998-017-9956-z. [DOI] [Google Scholar]
- 24.Kim RH, Bae MH, Kim DG, Cheng H, Kim BH, Kim DH, Li M, Wu J, Du F, Kim HS, Kim S, Estrada D, Hong SW, Huang Y, Pop E, Rogers JA. Stretchable, transparent graphene interconnects for arrays of microscale inorganic light emitting diodes on rubber substrates. Nano Lett. 2011;11:3881–3886. doi: 10.1021/nl202000u. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]