Abstract
Background:
Development of a diverse T-cell receptor β (TRB) repertoire is associated with immune recovery following hematopoietic cell transplantation (HCT) for severe combined immunodeficiency (SCID). High-throughput sequencing of the TRB repertoire allows evaluation of clonotype dynamics during immune reconstitution.
Objectives:
We investigated whether longitudinal analysis of the TRB repertoire would accurately describe T-cell receptor diversity and illustrate the quality of T-cell reconstitution following HCT or gene therapy for SCID.
Methods:
We used high-throughput sequencing to study composition and diversity of the TRB repertoire in 27 infants with SCID at 3, 6, and 12 months and yearly posttreatment(s). Total RNA from peripheral blood was used as template to amplify TRB rearrangements.
Results:
TRB sequence analysis showed poor diversity at 3 months, followed by significant improvement by 6 months after cellular therapies. Kinetics of development of TRB diversity were similar in patients with a range of underlying gene defects. However, in patients with RAG and DCLRE1C defects, HCT with no conditioning or immune suppression only resulted in lower diversity than did HCT with conditioning. HCT from a matched donor correlated with higher diversity than did HCT from a mismatched donor. Naive CD4+ T-cell count at 6 months post-HCT correlated with higher TRB diversity. A Shannon index of diversity of 5.2 or lower 3 months after HCT predicted a need for a second intervention.
Conclusions:
TRB repertoire after hematopoietic cell therapies for SCID provides a quantitative and qualitative measure of diversity of T-cell reconstitution and permits early identification of patients who may require a second intervention.
Keywords: Complementarity determining region 3 (CDR3), hematopoietic cell transplantation, high-throughput sequencing, immune reconstitution, severe combined immunodeficiency (SCID), T-cell receptor (TCR), T-cell receptor β (TRB) diversity, T-cell receptor β (TRB) repertoire
INTRODUCTION
Severe combined immunodeficiency (SCID) comprises a heterogeneous group of genetic disorders characterized by impaired T-cell development, resulting in profound T-cell lymphopenia and lack of adaptive immune responses.1–3 Allogeneic hematopoietic cell transplantation (HCT) or gene therapy (GT) can fully correct the T-cell deficiency of SCID.1,4,5 Key features associated with favorable outcome are the speed and robustness of immune reconstitution post-HCT.5–7 A diverse T-cell repertoire is an essential feature of adequate immune function after HCT for SCID. Various methods have been proposed to ascertain T-cell reconstitution, including enumeration of total and naive T cells, quantification of T-cell receptor excision circles as a measure of thymopoiesis, flow-cytometric analysis of T-cell receptor (TCR) β Variable (V) gene family expression, and spectratyping of TCR β (TRB) complementarity determining region 3 (CDR3) rearranged products. However, these assays do not adequately capture the diversity of the T-cell repertoire, and more precise methods are needed to better understand the clonotypic dynamics of immune reconstitution post-HCT. With the advent of high-throughput sequencing (HTS), it is possible to analyze in great detail the richness, diversity, and clonality of TRB-CDR3 products, which are responsible for peptide recognition by the T-cell antigen receptor complex.8 Such analysis may provide a quantitative and qualitative measure of immune reconstitution following cellular therapies.9
We hypothesized that the degree of TRB diversity obtained using HTS may constitute an early biomarker predictive of long-term immune reconstitution. To test this hypothesis, we performed longitudinal HTS measurement of the TRB repertoire following HCT or GT for SCID and correlated the results with need for second interventions and total and naive CD4+ T-cell counts. We also studied the relationship of SCID genotype, conditioning regimen, and donor type with TCR diversity post-HCT. Finally, because autoimmune manifestations (particularly autoimmune hemolytic anemia) are common in the first months after HCT,10 we interrogated the TRB repertoire for molecular signatures of self-reactivity at various time points after HCT.
We studied 27 children with SCID who received HCT (n = 24) or GT (n = 3) as first line of definitive therapy between 2010 and 2017, and from whom samples were available for TRB repertoire analysis. All were enrolled with informed consent (as approved by the central UCSF Institutional Review Board or institutional review boards at individual enrolling sites) in Protocol 6901 (www.clinicaltrials.gov NCT01186913) of the Primary Immune Deficiency Treatment Consortium, a prospective multicenter observational study evaluating treatment outcomes of patients with SCID. Table I lists the patients’ genotypes, type of cell therapy (with second treatments if given), total and naive CD4+ T-cell counts 6 months after treatment (compared to age matched reference range11), and time points of samples studied. Of 28 total HCT treatments, 10 were from 8/8 HLA-antigen (Ag) matched related or unrelated donors, whereas 18 were from 1 or more HLA-Ag mismatched donors. All patients who received allogeneic HCT developed full donor T-cell chimerism. The composition and diversity of the TRB repertoire was determined pre-HCT and at 3, 6, and 12 months and then yearly posttreatment(s) up to 4 years. Treatment was considered unsuccessful when requiring a second intervention (either HCT or GT). Multiple treatments in the same subject were considered as independent events. Four patients required a second HCT; 2 others received HCT followed by GT, for a total of 33 treatments for the entire cohort. HTS of TRB repertoire was performed after 31 such treatments (Table I). One patient required a boost of PBSCs after the first sample collection at 3 months due to slow T-cell reconstitution. Mean age at the first treatment was 98 days (range, 24–240 days). All patients had a minimum follow-up of 4 years.
TABLE I.
Patients, with cell therapies received and samples studied for TRB diversity
| Patient | Sex | Genotype | Treatment type (HCT/GT) | Time points studied (months posttreatment) | Donor type* | Conditioning regimen | CD4+ T cells/mm3 6 mo posttreatment* | CD4+ naive T cells/mm3 6 mo posttreatment* |
|---|---|---|---|---|---|---|---|---|
| 1 | F | RAG | HCT(a)† | 12, 24, 36 | MMRD | None | 183 | 0 |
| HCT(b) | ND | MMRD | MAC | 65 | 6 | |||
| 2 | F | RAG | HCT | 12, 36, 48 | URD | RIC | 576 | 65 |
| 3 | M | RAG | HCT | 24, 36, 48 | mmURD | MAC | 281 | 132 |
| 4 | M | RAG | HCT | 12, 36, 48 | MSD | None | 203 | 107 |
| 5 | F | RAG | HCT† | 6, 24, 36, 48 | MMRD | RIC | 1242 | 384 |
| 6 | F | RAG | HCT | 6, 12, 24, 36 | URD | RIC | 304 | 119 |
| 7 | M | RAG | HCT | 6, 12, 24 | URD | RIC | 912 | 511 |
| 8 | F | RAG | HCT | 6, 12 | URD | RIC | 194 | ND |
| 9 | F | DCLRE1C | HCT(a)† | 12, 24, 36, 48 | MMRD | None | 281 | 3 |
| GT(b) | ND | GT | RIC | 252 | 15 | |||
| 10 | F | DCLRE1C | HCT(a) | 3, 6, 12, 24, 36 | MMRD | RIC | 350 | 8 |
| HCT(b) | 3, 6, 24 | MMRD | RIC | 427 | 85 | |||
| 11 | F | DCLRE1C | HCT | 12 | MMRD | None | 161 | 1 |
| 12 | M | IL2RG | HCT(a)† | 12, 24, 36 | MMRD | None | 20 | 11 |
| GT(b) | 3, 6, 12 | GT | RIC | 187 | 60 | |||
| 13 | M | IL2RG | HCT(a) | 3 | MMRD | IS | Second therapy ‡ | Second therapy |
| HCT(b) | 6, 24 | mmURD | RIC | 570 | ND | |||
| 14 | M | IL2RG | HCT | 12, 36, 48 | MMRD | None | 627 | 74 |
| 15 | M | IL2RG | GT | 3 | GT | RIC | 1949 | 779 |
| 16 | M | IL2RG | HCT | 3, 6, 48 | URD | IS | >500 | 375 |
| 17 | M | IL2RG | HCT | 6, 12, 24, 36, 48 | MMRD | IS | 1417 | 879 |
| 18 | M | IL2RG | HCT | 3, 6, 12 | MMRD | IS | 920 | 626 |
| 19 | M | IL2RG | GT | 3, 6, 12 | GT | RIC | 1050 | 726 |
| 20 | M | IL2RG | GT | 3, 6 | GT | RIC | 2039 | 1305 |
| 21 | M | IL2RG | HCT† | 3, 12, 24, 36, 48 | MMRD | None | 289 | 87 |
| 22 | F | IL7R | HCT | 24 | URD | IS | 203 | 3 |
| 23 | F | IL7R | HCT | 6, 12, 24, 48 | MMRD | None | 1086 | 478 |
| 24 | F | IL7R | HCT (Boost) § | 3, 6, 12 | MMRD | None | 2291 | 226 |
| 25 | F | IL7R | HCT | 6, 12, 24, 36 | URD | IS | 217 | 91 |
| 26 | F | JAK3 | HCT | 3, 6 | MMRD | IS | 1296 | ND |
| 27 | M | BCL11B | HCT(a) | 3, 6 | URD | IS | Second therapy ‡ | Second therapy |
| HCT(b) | 36 | URD | RIC | 1120 | 44 |
Multiple cell therapies are indicated by letters following the treatment type.
F, Female; IS, immune suppression; M, male; MAC, myeloablative conditioning; MMRD, mismatched related donor; mmURD, mismatched unrelated donor; MSD, matched sibling donor; ND, not done; RIC, reduced-intensity conditioning; URD, unrelated (HLA-matched) donor.
Lower end of 10%–90% of healthy children aged 1–2 y 1300/mm3 for CD4; 950 for CD4/CD45RA/CD62L.11
Autoimmune complications.
Value not available if second treatment occurred within 6 mo of initial treatment.
Boost, additional cells from previous donor source administered with no conditioning between 3 and 6 mo post-HCT.
The TCR library preparation, adapted from Zvyagin et al,12 used total RNA from lysed peripheral blood (PAXgene RNA tubes; RNeasy Mini Kit, Qiagen, Inc, Germantown, Md). At least 200 ng of RNA was reverse transcribed (SMARTer PCR cDNA Synthesis Kit, Takara Bio, Inc, Mountain View, Calif) with unique molecular identifier barcodes incorporated into 5′ primers for amplification to permit identification and quantification of the TRB repertoire. PCR products were pooled, purified (DNA Clean & Concentrator-5, Zymoresearch, Irvine, Calif), and sequenced to obtain 150 bp paired end reads (Illumina HiSeq2500). Raw sequences were filtered for productive rearrangements and analyzed for V, D, and J gene composition (IMGT HighV-QUEST software). The VDJ statistics file (PAST program) was used to calculate a Shannon [H] entropy index to measure repertoire diversity, and a Simpson [1-D] index of unevenness, measuring inequality in the relative representation of individual sequences in a given sample. TCR repertoire diversity was illustrated by hierarchical tree maps, using iRepertoire software, with each dot representing a unique CDR3 sequence and the size of each dot corresponding to the frequency of that sequence in the total population of sequences obtained. The differences between groups were analyzed using 2-way ANOVA (for multiple comparisons), with statistical significance indicating a 95% CI Correlation was measured by Spearman rank correlation coefficient (Rs). Statistical analysis was performed using Prism Software or custom script (R environment, version 3.3.2) to calculate the Cysteine index.13
RESULTS AND DISCUSSION
Severe restriction of repertoire diversity as measured by the Shannon [H] and the Simpson [1-D] indices and clonotypic expansions were observed 3 months after cellular therapy, followed by significant improvement by 6 months (P < .01) (Fig 1, A-C). Among all patients who received HCT, whether conditioned or nonconditioned, diversity and clonality improved more rapidly following successful as compared with unsuccessful HCT (Fig 1, D), consistent with the observation that a diverse repertoire with clone evenness is a key feature of successful immune reconstitution.8 A second intervention (allogenic HCT or GT) was needed only in patients having a 3-month H index of 5.2 or lower; none of the patients with an H index above 5.2 at 3 months required a second treatment (P < .0001; Fig 1, D). Significant correlation (Rs = 0.89; P = .001) was observed at 6 months posttreatment between the H index and the total number of circulating naive CD4+ T cells (Fig 1, E), a known biomarker predictive of the quality of immune reconstitution.5 Analysis of the association of genotype and immune reconstitution following HCT revealed similar kinetics of development of TCR diversity in 12 patients with IL2RG, IL7R, and JAK3 gene defects in cytokine receptor signaling as compared with 11 patients with RAG and DCLRE1C defects in V(D)J recombination (Fig 1, F). In the latter group of treatments, however, HCT with no conditioning or immune suppression only (4 of 12) was associated with persistently lower diversity than HCT with myeloablative or reduced-intensity conditioning (8 of 12; P < .01) (Fig 2, A), while in the IL2RG/IL7R/JAK3 group (Fig 2, B) all 12 patients who received HCT with immune suppression only achieved normal diversity. Competition between autologous stem and progenitor cells versus donor-derived cells up to the double-positive stage of T-cell differentiation may have contributed to poorer immune reconstitution in the patients with RAG deficiency who did not receive chemotherapy, whereas the block in T-cell development occurs earlier in patients with IL2RG, IL7R, and JAK3 defects.14 Furthermore, in RAG and DCLRE1C V(D)J recombination defects, activated and hypercytotoxic natural killer cells may resist engraftment.15
FIG 1.
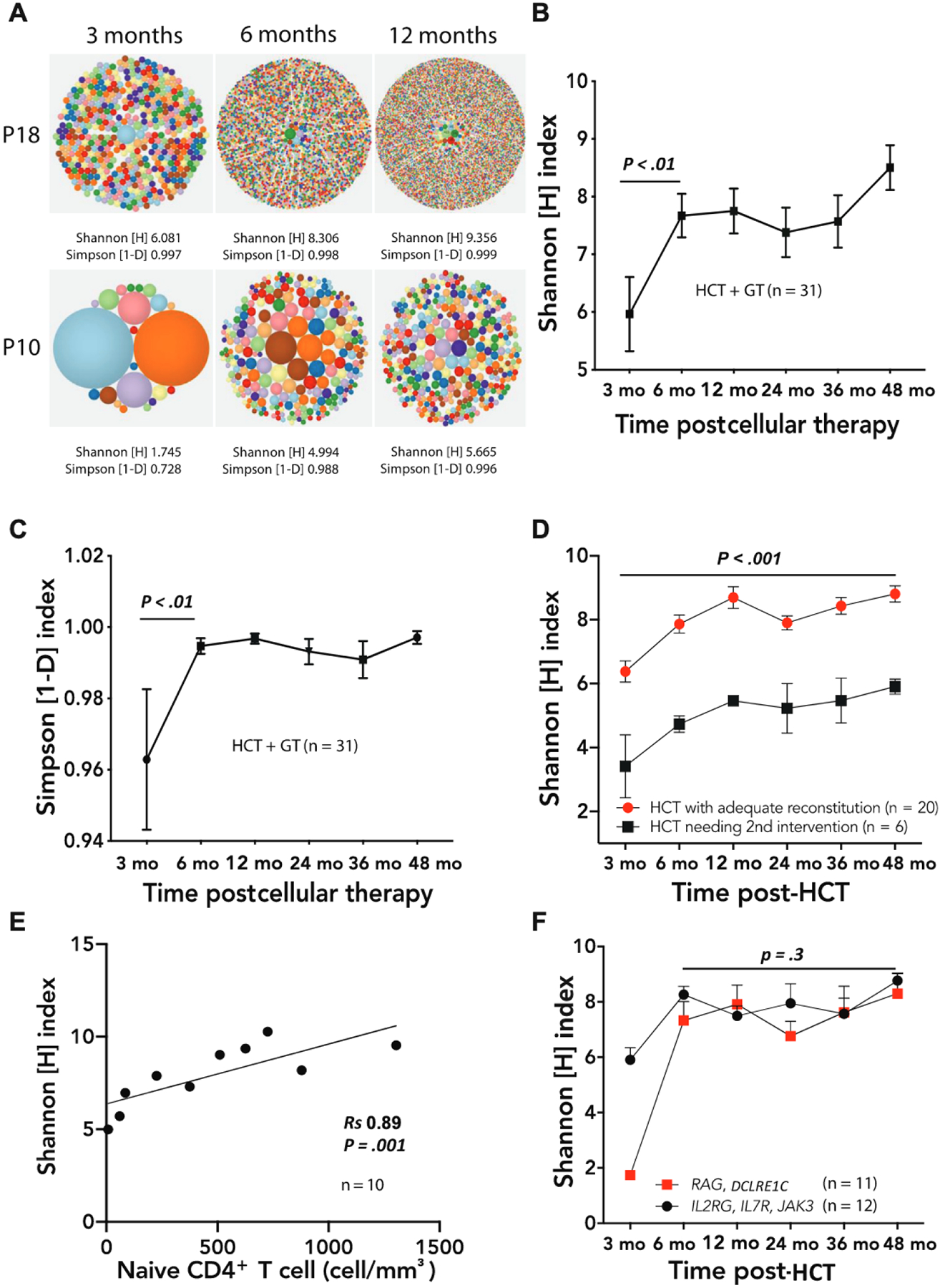
A, Representative hierarchical tree maps showing increasing TRB repertoire diversity following successful HCT in patient 18 (P18, top row) and failed HCT in patient 10, treatment A (P10, bottom row). (B) Increasing Shannon [H] index of TRB diversity and (C) Simpson [1-D] index of clonality in all patient cellular treatments over time. The P values refer to differences between 3 and 6 months post-HCT. D, Persistently lower Shannon diversity scores following cellular treatments that ultimately needed another treatment (black squares) compared with those that were successful (red circles); differences were apparent as soon as 3 months after cellular treatments. The P value refers to the differences between the 2 groups considering all time points. E, Correlation (Spearman rank coefficient Rs) between naive CD4+ T-cell number and Shannon [H] index of diversity 6 months after a cellular treatment. F, Relationship between TCR diversity and genotype groupings (IL2RG, IL7R, and JAK3, black circles; RAG and DCLRE1C, red squares). The number of samples available at the 3-month time point was insufficient to assess whether the difference between the 2 groups was significant (only 1 sample was available for the RAG/DCLRE1C group at 3 months). In Fig 1, B-D and F, symbols and bars correspond to mean ± SEM.
FIG 2.
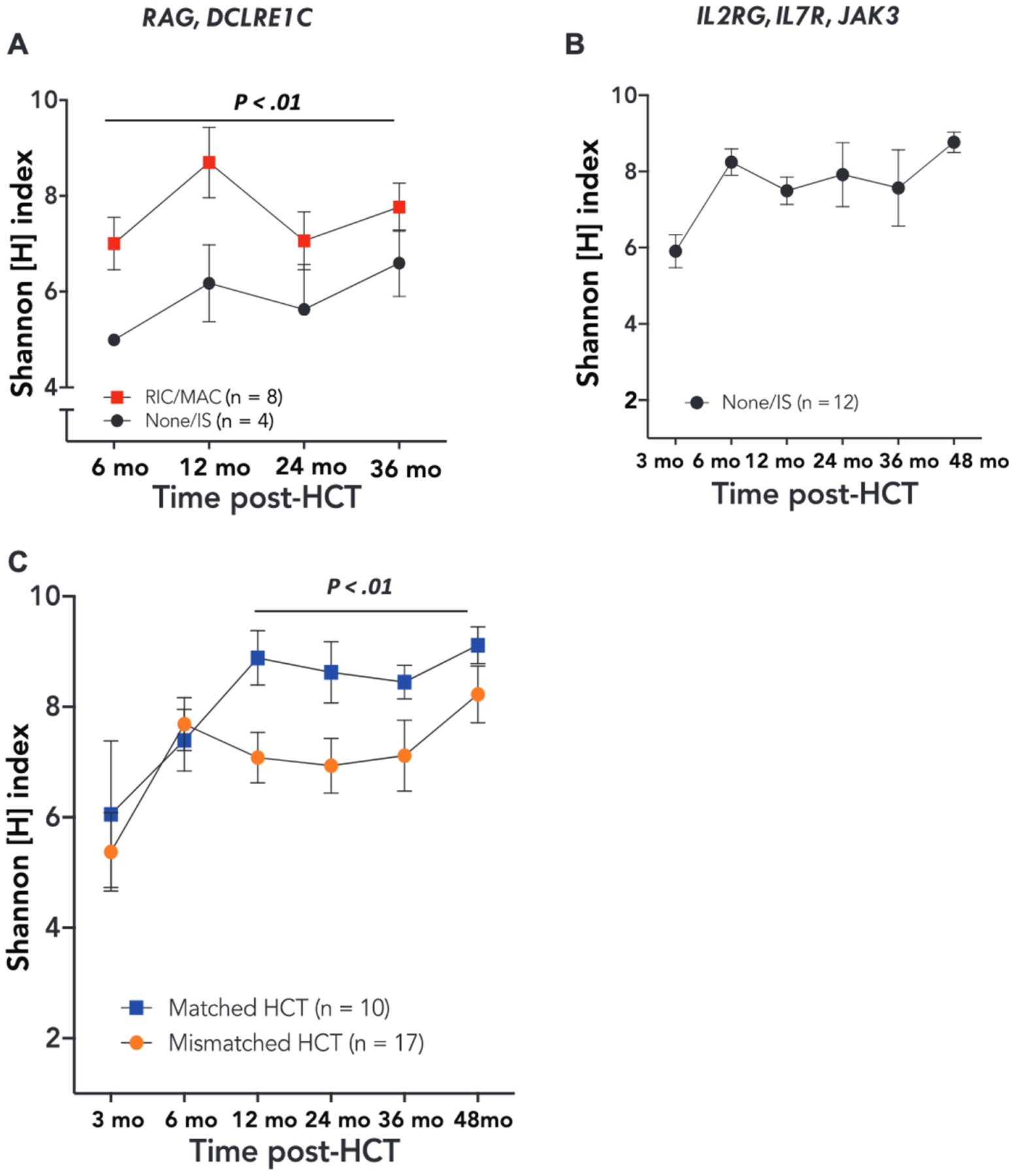
A, Relationship between conditioning and TRB diversity for patients with V(D)J recombination defects, with P value showing the difference between the 2 groups considering all time points. B, TRB diversity following unconditioned HCT for cytokine receptor defects (data were available for comparison of conditioning only from 6 months and beyond; see Table I). C, Relationship between donor HLA match and TRB diversity. Symbols and bars correspond to mean ± SEM.
Starting at 12 months post-HCT, T cells derived from a matched donor had higher diversity than those from a mismatched donor (P = .01) (Fig 2, C). This finding may reflect more frequent use of reduced-intensity conditioning/myeloablative conditioning regimens in recipients of matched donor HCT as compared with mismatched HCT (5 of 10 vs 5 of 17) and is consistent with the superior T-cell reconstitution observed after conditioned HCT in a large cohort of patients with SCID.5 In addition, a CD4+ T-cell count of more than 500 cells/mm3 or a naive CD4+ T-cell count of more than 200 cells/mm3 at 6 months post-HCT correlated with higher TRB diversity at 24 and 36 months post-HCT (P < .01) (Fig 3, A and B), confirming previous observations that CD4+ and CD45RA+CD4+ T-cell counts at 6 months after HCT are predictive of sustained immune reconstitution.5 Finally, a qualitative analysis showed that the TRB repertoire 3 months post-HCT in all 27 patients with SCID, including 5 who experienced autoimmune complications within 4 months after HCT (Table I), was enriched (P < .01) for unique reads containing cysteine residues at the apex of the CDR3 (Fig 3, C), a biomarker of self-reactivity.13 The occurrence of this molecular signature in the early T-cell repertoire following treatment for SCID may help to explain the increased risk of autoimmunity known to occur in these patients.10
FIG 3.
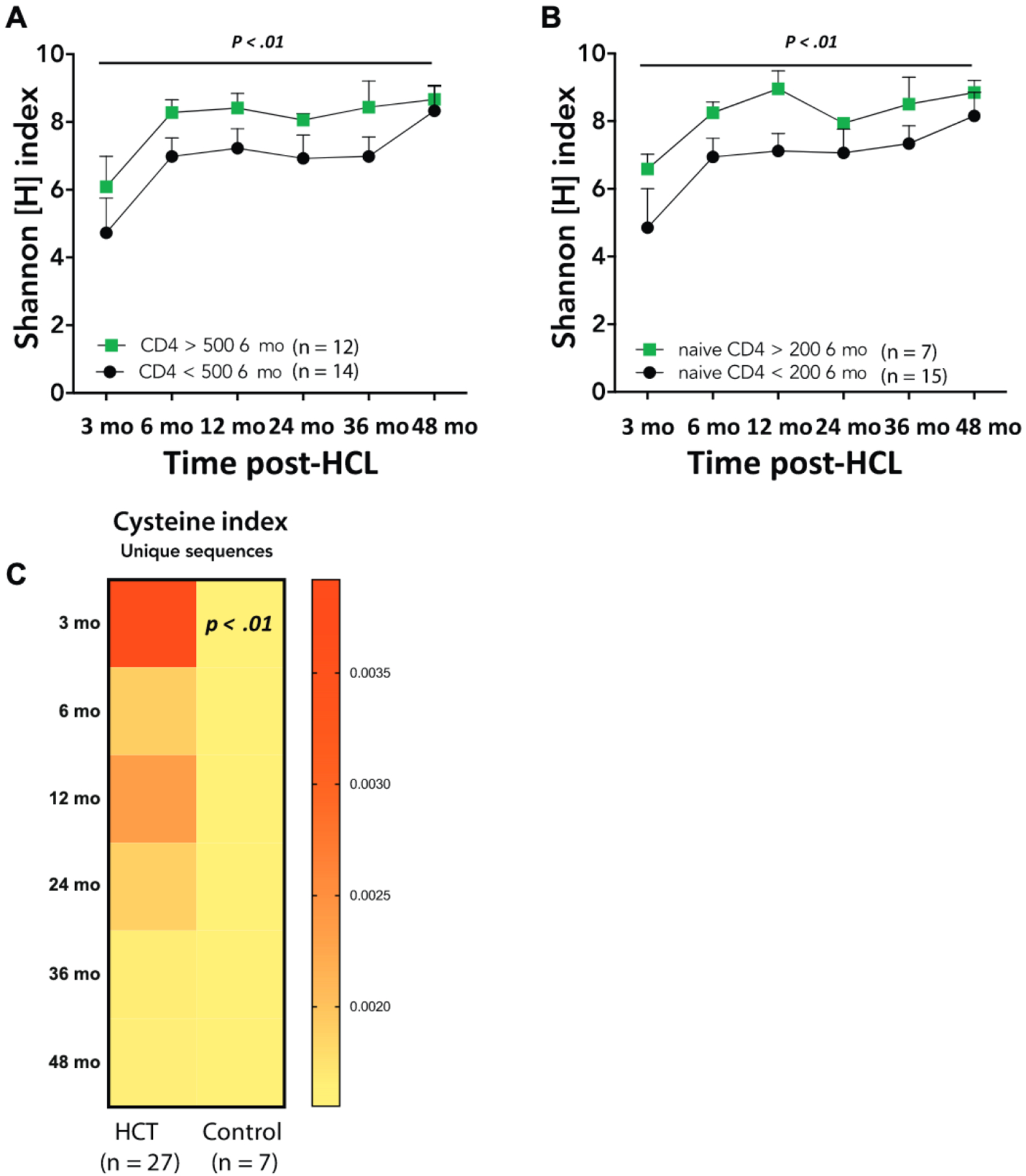
A and B, Correlation between (Fig 3, A) TRB diversity and number of CD4+ T cells/μL, and (Fig 3, B) TRB diversity and CD45RA+/CD4+ naive T cells/μL at 6 months post-HCT.5 Symbols and bars correspond to mean ± SEM; P values refer to the difference between the 2 groups considering all time points. C, Heatmap representing Cysteine index at indicated times after HCT in all patients (n = 27); data are compared with Cysteine index of healthy pediatric controls (n = 7, mean age 15 months). The P value refers to difference between patients and controls at the 3-month time point.
This study represents the first attempt to apply HTS to measure TCR clonotypic dynamics in a cohort of 27 infants undergoing HCT or GT for SCID. The results show that analysis of TRB diversity and composition permits a detailed assessment of T-cell immune reconstitution in response to these therapies. In particular, we have obtained preliminary evidence that a restricted TRB diversity at 3 months post-HCT may identify patients at risk for failure of immune reconstitution, thus prompting a second intervention without delay. This study has limitations, including a small sample size, which did not allow adjustment for confounders such as infections, graft versus host disease, and age at the time of HCT5; confinement of TCR analysis to TRB only; and unavailability of samples for some of the time points. Nonetheless, our study confirms that treatment strategies ought to be customized to SCID genotype to optimize immune reconstitution. If confirmed in large-scale prospective studies, these data support incorporating HTS of TCR repertoire as a valuable biomarker following cellular therapy for SCID.
Clinical implications:
HTS of the TRB repertoire after HCT for SCID leads to early indentification of patients who may benefit from a second intervention.
Acknowledgments
We appreciate the careful technical assistance of Melanie Dela Cruz and Stephen Chow, and we thank the patients, investigators, and staff of the Primary Immune Deficiency Treatment Consortium for their enthusiasm and commitment to this research.
The Primary Immune Deficiency Treatment Consortium is funded by the National Institute of Allergy and Infectious Diseases (NIAID; grant no. U54-AI082973) and the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health (NIH), Bethesda, Md. Additional funding has come from the Division of Intramural Research, NIAID, NIH (grant no. 1 ZIA AI001222-02 to L.D.N.) and the California Institute of Regenerative Medicine (grant no. CLIN2-10830 to M.J.C. and grant no. CLIN2-09504 to Dr Gottschalk, supporting gene therapy trials for DCLRE1C and IL2RG SCID). J.M.P received further support from the Lisa and Douglas Goldman Fund.
Abbreviations used
- CDR3
Complementarity determining region 3
- GT
Gene therapy
- HCT
Hematopoietic cell transplantation
- HTS
High-throughput sequencing
- SCID
Severe combined immunodeficiency
- TCR
T-cell receptor
- TRB
TCR β
Footnotes
Disclosure of potential conflict of interest: M. J. Cowan, C. C. Dvorak, L. D. Notarangelo, and J. M. Puck declare receipt of royalty payments from UpToDate. M. J. Cowan is on Scientific Advisory Board and owns stock in Homology Medicine, Inc. The spouse of J. M. Puck is employed by and holds stock in Invitae, Inc. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med 2014;371:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol 2014;133:1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangye S, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2020;40:24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ, Veys P, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol 2010;126:602–10. [DOI] [PubMed] [Google Scholar]
- 5.Haddad E, Logan BR, Griffith LM, Buckley RH, Parrot RE, Prockop SE, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood 2018;132:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2015;2:e194–203. [DOI] [PubMed] [Google Scholar]
- 7.Lev A, Simon AJ, Bareket M, Bielorai B, Hutt D, Amariglio N, et al. The kinetics of early T and B cell immune recovery after bone marrow transplantation in RAG-2-deficient SCID patients. PLoS One 2012;7:e30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gkazi AS, Margetts BK, Attenborough T, Mhaldien L, Standing JF, Oakes T, et al. Clinical T cell receptor repertoire deep sequencing and analysis: an application to monitor immune reconstitution following cord blood transplantation. Front Immunol 2018;9:2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol 2006;24:419–66. [DOI] [PubMed] [Google Scholar]
- 10.Horn B, Viele M, Mentzer W, Mogck N, DeSantes K, Cowan M. Autoimmune hemolytic anemia in patients with SCID after T cell-depleted BM and PBSC transplantation. Bone Marrow Transplant 1999;24:1009–13. [DOI] [PubMed] [Google Scholar]
- 11.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003;112:973–80. [DOI] [PubMed] [Google Scholar]
- 12.Zvyagin I, Mamedov I, Tatarinova O, Komech E, Kurnikova E, Boyakova E, et al. Tracking T-cell immune reconstitution after TCRαβ/CD19-depleted hematopoietic cells transplantation in children. Leukemia 2017;31:1145–53. [DOI] [PubMed] [Google Scholar]
- 13.Daley SR, Koay HF, Dobbs K, Bosticardo M, Wirasinha RC, Pala F, et al. Cysteine and hydrophobic residues in CDR3 serve as distinct T-cell self-reactivity indices. J Allergy Clin Immunol 2019;144:333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosticardo M, Pala F, Calzoni E, Delmonte OM, Dobbs K, Gardner CL, et al. Artificial thymic organoids represent a reliable tool to study T-cell differentiation in patients with severe T-cell lymphopenia. Blood Adv 2020;4:2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs K, Tabellini G, Calzoni E, Patrizi O, Martinez P, Giliani SC, et al. Natural killer cells from patients with Recombinase-Activating Gene and non-homologous end joining gene defects comprise a higher frequency of CD56bright NKG2A+++ cells, and yet display increased degranulation and higher perforin content. Front Immunol 2017;8:798. [DOI] [PMC free article] [PubMed] [Google Scholar]


