Abstract
Background
In people with mild asthma poor adherence to regular therapy is common and increases the risk of exacerbations, morbidity and mortality. The use of fixed-dose combination inhalers containing an inhaled corticosteroid (ICS) and a fast-acting β2-agonist (FABA) is established in moderate asthma, but they may also have potential utility in mild asthma.
Objectives
To evaluate the efficacy and safety of single combined FABA/ICS inhaler only used as needed in people with mild asthma.
Design and setting
Cochrane meta-analysis of available trial data.
Participants
Children aged 12+ and adults with mild asthma.
Search methods
We searched the Cochrane Airways Trials Register, Cochrane Central Register of Controlled Trials, MEDLINE and Embase, ClinicalTrials.gov and the WHO trials portal on 19 March 2021.
Interventions
A single fixed-dose FABA/ICS inhaler used as required compared with no treatment, placebo, short-acting beta agonist (SABA) as required, regular ICS with SABA as required, regular fixed-dose combination ICS/long-acting beta agonist (LABA), or regular fixed-dose combination ICS/FABA with as required ICS/FABA. We included randomised controlled trials (RCTs) and cross-over trial. We excluded trials shorter than 12 weeks. We included full texts, abstracts and unpublished data.
Data collection and analysis
We used Cochrane’s standard methodological procedures and applied the GRADE approach to assess the evidence.
Main outcome measures
We included six studies from which 9657 participants contributed to the meta-analyses. All used dry powder budesonide and formoterol as the combination inhaler. Two studies included children aged 12+ years and two studies were open-label.
FABA/ICS as-required versus FABA as-required
Compared with as-required FABA alone, as-required FABA/ICS reduced exacerbations requiring systemic steroids (OR 0.45, 95% CI 0.34 to 0.60, 2 RCTs, 2997 participants, high-certainty evidence), equivalent to 109 people out of 1000 in the FABA alone group experiencing an exacerbation requiring systemic steroids, compared with 52 (95% CI 40 to 68) out of 1000 in the FABA/ICS as-required group. FABA/ICS as required may also reduce the odds of an asthma-related hospital admission or emergency department or urgent care visit (OR 0.35, 95% CI 0.20 to 0.60, 2 RCTs, 2997 participants, low-certainty evidence). Changes in asthma control were small and less than the minimal clinically important difference (MCID). FABA/ICS as required was associated with reductions in fractional exhaled nitric oxide, probably reducing the odds of an adverse event (OR 0.82, 95% CI 0.71 to 0.95) and may reduce total systemic steroid dose (mean difference (MD) −9.90, 95% CI −19.38 to −0.42).
FABA/ICS as required versus regular ICS plus FABA as required
There may be little or no difference in the number of people with asthma exacerbations requiring systemic steroids with FABA/ICS as required compared with regular ICS (OR 0.79, 95% CI 0.59 to 1.07, 4 RCTs, 8065 participants, low-certainty evidence), equivalent to 81 people out of 1000 in the regular ICS plus FABA group experiencing an exacerbation requiring systemic steroids, compared with 65 (95% CI 49 to 86) out of 1000 in the FABA/ICS as-required group. The odds of an asthma-related hospital admission or emergency department or urgent care visit may be reduced in those taking FABA/ICS as required (OR 0.63, 95% CI 0.44 to 0.91, 4 RCTs, 8065 participants, low-certainty evidence). Changes in asthma control were small and less than MCID. Adverse events and total systemic corticosteroid doses were similar between groups. FABA/ICS as required was likely associated with less average daily exposure to ICS than those on regular ICS (MD −154.51 mcg/day, 95% CI −207.94 to −101.09).
Conclusions
FABA/ICS as required is clinically effective in adults and adolescents with mild asthma and reduced exacerbations, hospital admissions or unscheduled healthcare visits and exposure to systemic corticosteroids and probably reduces adverse events compared with FABA as required alone. FABA/ICS as required is as effective as regular ICS and reduced asthma-related hospital admissions or unscheduled healthcare visits, and average exposure to ICS, and is unlikely associated with increased adverse events.
Keywords: asthma, evidence-based practice, respiratory tract diseases, primary healthcare
Summary box.
What is already known about this subject?
Poor adherence to inhaled corticosteroids (ICS) in mild asthma is associated with preventable exacerbations.
What are the new findings?
This meta-analysis of five randomised controlled trials enrolling 9657 participants found symptom-driven, as-required use of fast-acting β2-agonist (FABA)/ICS compared with reliever-only treatment reduced severe exacerbations requiring tablet steroids and rates of emergency admission to hospital with asthma symptoms. Symptom-driven, as-required use of FABA/ICS compared with regular daily ICS led to similar rates of severe exacerbations but lower rates of hospital admission and lower total ICS dose.
How might it impact clinical practice in the foreseeable future?
These findings support changes in guidelines away from the use of short-acting β2-agonists alone in mild asthma. Use of as-required FABA/ICS is a therapeutic alternative to maintenance ICS in mild asthma, associated with reduced hospital admissions for asthma and reduced average daily exposure to ICS.
Background and objectives
Between 45% and 70% of the 350 million people worldwide living with asthma have mild disease,1–3 and yet continue to suffer intermittent severe asthma attacks requiring oral steroids or hospital admission, and in some cases leading to asthma-related deaths. Inhaled corticosteroids (ICS) are effective in achieving disease control and reducing mortality,4 but intermittence of symptoms in mild asthma often leads to poor inhaler adherence,5 with consequent risk of exacerbations. A new treatment approach being considered in mild asthma is the use of single combined (fast-acting β2-agonist (FABA) plus an ICS) inhaler only used as needed according to symptoms, which may increase adherence in those at greatest risk of exacerbations. This Cochrane Review aims to summarise the data on efficacy and safety of single combined FABA/ICS inhaler used only as needed in people with mild asthma, to guide clinicians and policy makers in decision making.
The mainstay of asthma therapy is treatment with inhaled FABA, typically taken as required to relieve bronchospasm, and ICS as regular preventive therapy. Although ICS are very effective in reducing severe asthma exacerbations and asthma deaths4 6 the intermittent nature of symptoms in mild asthma, the slower perceived response to ICS and concerns about steroid-related side effects frequently lead to poor adherence to regular ICS.5 7 In the UK the majority of asthma deaths occur in those considered to have mild or moderate asthma,8 with over-reliance on reliever medication7 and poor adherence to preventer ICS considered to be a main cause for an increase in risk of exacerbations in people with mild asthma.9
Fixed-dose combination inhalers containing both a steroid and a FABA in the same device, used as both maintenance and reliever therapy simplify inhaler regimens and ensure symptomatic relief is accompanied by preventative therapy. Their use is established in moderate asthma,10 but they may also have potential utility in mild asthma. Globally, prevalence of mild asthma is estimated to be between 45% and 70% of all patients diagnosed with asthma.1 2 We assessed the efficacy and safety of single combined FABA/ICS inhaler used only as needed in people with mild asthma. We compared this with two current approaches to treatment: use only of a FABA when needed, or use of a FABA when needed on top of regular-maintenance ICS.
Several clinical trials of as-required fixed-dose combination inhalers have been reported in recent years, and have led to a significant change in an international guideline,11 which now recommends fixed-dose ICS/FABA as first-line therapy for mild asthma, where the previous guideline recommended use of short-acting β agonist (SABA) only. We aimed to provide an objective, global review of the available evidence to inform decision makers, clinicians and people with asthma.
Methods
We followed a published protocol in The Cochrane Library. We included randomised controlled trials (RCTs) and cross-over trials with at least a 1-week washout period. We included studies of a single fixed-dose FABA/ICS inhaler used as required compared with no treatment, placebo, SABA as required, regular ICS with SABA as required, regular fixed-dose combination ICS/long-acting β agonist, or regular fixed-dose combination ICS/FABA with as-required ICS/FABA. We planned to include cluster-randomised trials if the data had been or could be adjusted for clustering. We excluded trials shorter than 12 weeks. We included full texts, abstracts and unpublished data.
We included adults and children (age 6 years and older) with a diagnosis of mild asthma as defined by Global INitiative for Asthma (GINA) 2019.11 We excluded participants with chronic obstructive pulmonary disease or other respiratory comorbidity. We excluded participants receiving regular moderate or high-dose ICS, defined as ≥300 mcg per day of beclomethasone equivalent.
We assessed the following primary outcomes: 1. Exacerbations requiring systemic steroids; 2. Hospital admissions/emergency department or urgent care visits for asthma; 3. Measures of asthma control: in order of preference Asthma Control Questionnaire (ACQ), asthma control test, symptom-free days.
We assessed the following secondary outcomes: 1. Measures of lung physiology: in order of preference postbronchodilator; forced expieratroy volume in 1 second (FEV1), postbronchodilator peak expiratory flow rate (PEFR), fractional exhaled nitric oxide (FeNO), then other measures; 2. Quality of life measures, preferably Asthma Quality of Life Questionnaire, then the Short Form 36. 3. Adverse events/side effects; 4. Total inhaled steroid dose; 5. Total systemic corticosteroid dose; 6. Mortality.
Data collection and analysis
We searched the Cochrane Airways Trials Register, Cochrane Central Register of Controlled Trials, Medical Literature Analysis and Retrieval System Online (MEDLINE) and Embase, ClinicalTrials.gov and the WHO trials portal. We used Cochrane’s Screen4Me workflow to help assess search results and followed a prepublished protocol. Four authors screened titles and abstracts independently using Rayyan, with each abstract screened by at least two review authors. We contacted trial authors for further information and requested details regarding the possibility of unpublished trials. The most recent search was conducted on 19 March 2021. Two review authors independently extracted outcome data from included studies. Bias was assessed using V.5.1 of the Cochrane Handbook for Systematic Reviews of Interventions, assessing the following domains: 1. Random sequence generation, 2. Allocation concealment, 3. Blinding of participants and personnel, 4. Blinding of outcome assessment, 5. Incomplete outcome data, 6. Selective outcome reporting, 7. Other bias.
We analysed dichotomous data as ORs or rate ratios and continuous data as mean difference (MD). We reported 95% CIs. We used Cochrane’s standard methodological procedures of meta-analysis. We intended to assess publication bias. We applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarise results and to assess the overall certainty of evidence.
Main results
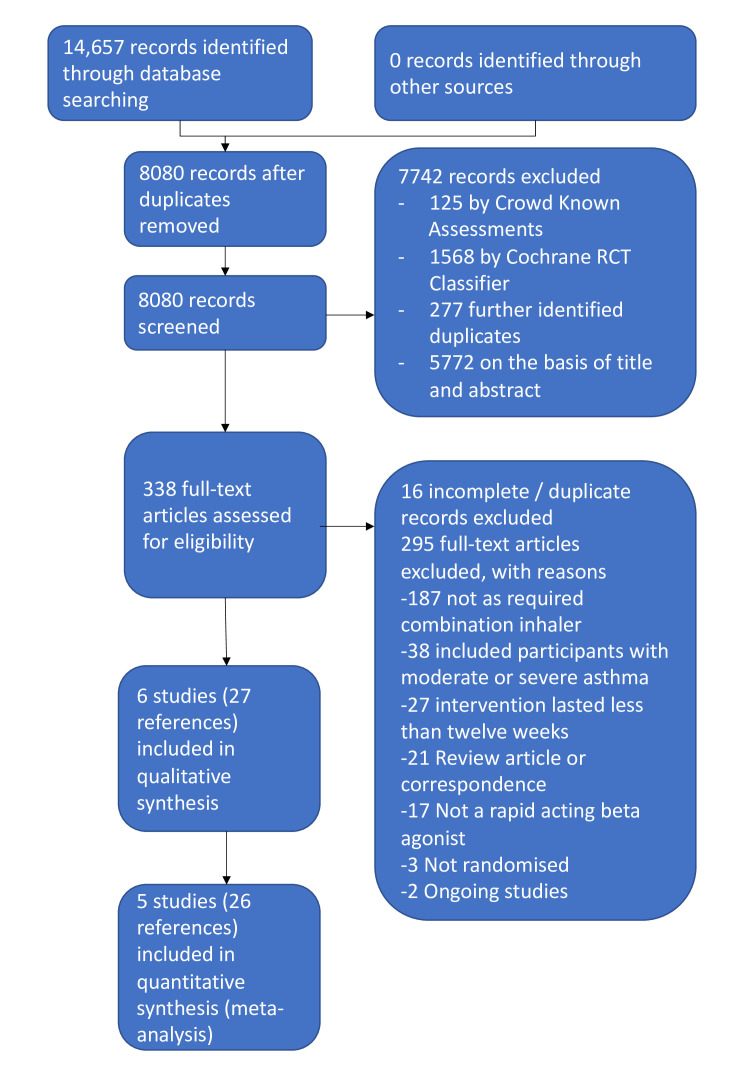
We identified 14 657 records in our literature searches (figure 1). We included six studies of which five contributed results to the meta-analyses.12–16 Four studies were large, multinational studies from the same research group which used budesonide 200 mcg and formoterol 6 mcg in a dry powder formulation as the combination inhaler. Comparator fast-acting bronchodilators included terbutaline and formoterol. Two studies included children aged 12+ years and adults; two studies were open label. A total of 9657 participants was included, with a mean age of 36–43 years; 2.3%–11% were current smokers.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. RCT, randomised controlled trial.
Two studies were open label (Novel START13 and PRACTICAL)14 and were judged as high risk of bias in this domain, but all studies were otherwise of low risk of bias in other domains. Four of the RCTs were funded by AstraZeneca.
FABA/ICS as required versus FABA as required
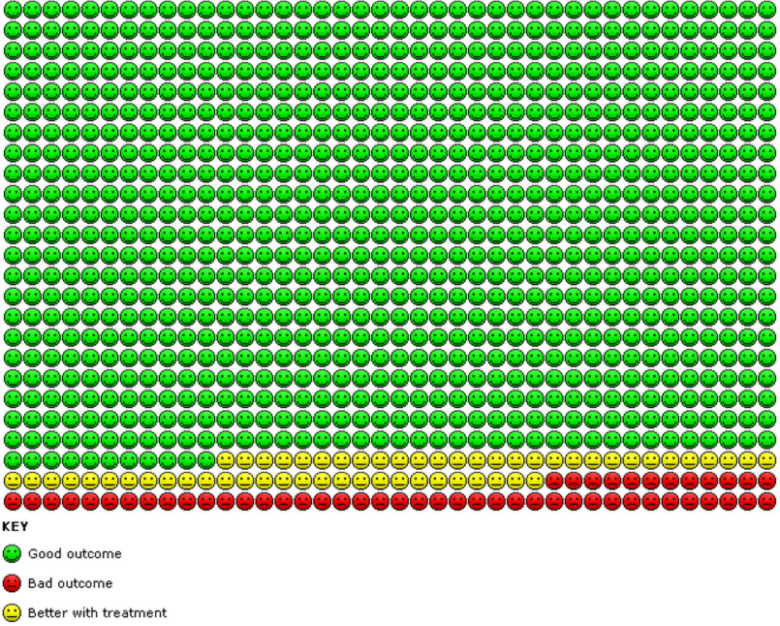
Results for this comparison are presented in the Summary of findings 1 (table 1). We found evidence from two trials (Novel START,13 SYGMA 1)15 that compared with as-required β-agonists alone, as-required FABA/ICS significantly reduced the number of asthma exacerbations requiring systemic steroids over a 52-week period (OR 0.45, 95% CI 0.34 to 0.60; n=2997, high-certainty evidence). This is equivalent to 109 people out of 1000 in the FABA alone group experiencing an exacerbation requiring systemic steroids, compared with 52 (95% CI 40 to 68) out of 1000 in the FABA/ICS as-required group (represented graphically in figure 2).
Table 1.
Summary of findings 1. As-required FABA/ICS inhalers compared with as-required FABA inhalers for mild asthma
| As‐required FABA/ICS inhalers compared with as‐required FABA inhalers for mild asthma | ||||||
|
Patient or population: Mild asthma Setting: Community Intervention: As‐required FABA/ICS inhalers Comparison: As‐required FABA inhalers | ||||||
| Outcomes | Anticipated absolute effects$ (95% CI) |
Relative effect
(95% CI) |
Number of participants
(studies) |
Certainty of the evidence
(GRADE) |
Comments | |
| Risk with as-required FABA inhalers | Risk with as-required FABA/ICS inhalers | |||||
| Asthma exacerbation requiring systemic steroid follow‐up: 52 weeks | 109 per 1000 | 52 per 1000 (40 to 68) |
OR 0.45, 95% CI 0.34 to 0.60 | 2997 (2 RCTs) |
⊕⊕⊕⊕ HIGH*† |
People with mild asthma treated with combined inhalers have substantially fewer exacerbations requiring systemic steroid than those treated with FABA alone. |
| Hospital admission, ED and urgent care visits follow‐up: 52 weeks | 34 per 1000 | 12 per 1000 (7 to 21) |
OR 0.35, 95% CI 0.20 to 0.60 | 2997 (2 RCTs) |
⊕⊕⊝⊝ LOW†‡ |
People with mild asthma treated with combined inhalers probably have substantially fewer exacerbations requiring hospital admission, ED attendance or urgent care visit than those treated with FABA alone. |
| Asthma control follow‐up: 52 weeks Lower scores=better control |
Mean baseline ACQ‐5 ranged from 1.1 to 1.61 | MD −0.15, 95% CI −0.20 to −0.10 | ‐ | 2859 (2 RCTs) |
⊕⊕⊕⊝ MODERATE† |
MCID for ACQ‐5 is 0.5. A third study reported no difference in asthma symptom scores between the two arms. |
| Inhaled steroid dose assessed with: mean daily inhaled steroid dose, μg beclomethasone equivalent follow‐up: 52 weeks | The mean inhaled steroid dose was 18.7 µg beclomethasone | MD 76.50 µg beclomethasone higher (69.40 higher to 83.60 higher) | ‐ | 2554 (2 RCTs) |
⊕⊕⊕⊝ MODERATE† |
People with mild asthma treated with a combined inhaler have a higher daily inhaled steroid dose than those treated with a FABA alone. |
| Total systemic steroid dose assessed with: mg prednisolone total over 52 weeks follow‐up: 52 weeks | The mean total systemic steroid dose was 17.4 mg prednisolone | MD 9.90 mg prednisolone lower (19.38 lower to 0.42 lower; participants=443) | ‐ | 443 (1 RCT) |
⊕⊕⊝⊝ LOW†§ |
Total systemic steroid dose was similar and small in both: those given combined inhalers and those given FABA alone. |
| Adverse events follow‐up: 52 weeks | 486 per 1000 | 437 per 1000 (402 to 473) |
OR 0.82 (0.71 to 0.95) |
3002 (2 RCTs) |
⊕⊕⊕⊝ MODERATE† |
Slightly fewer adverse events occurred in those taking combination inhalers compared with those taking FABA alone. |
| $The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
|
GRADE Working Group grades of evidence
High certainty ⊕⊕⊕⊕: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty ⊕⊕⊕: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty ⊕⊕: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty ⊕: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
*Upgraded as large effect (OR <0.5) with fairly tight CIs.
†Downgraded as included open label study.
‡Downgraded as based on a small number of events.
§Downgraded as based on one study with a relatively small number of participants.
ACQ-5, Asthma Control Questionnaire‐5; ED, emergency department; FABA, fast‐acting β₂‐agonist; ICS, inhaled corticosteroid; MCID, minimum clinically important difference; MD, mean difference; RCT, randomised controlled trial.
Figure 2.
In the FABA as-required group, 109 people out of 1000 had exacerbations requiring systemic steroids over 52 weeks, compared with 52 (95% CI 40 to 68) out of 1000 in the FABA/ICS as-required group. FABA, fast-acting β2-agonist; ICS, inhaled corticosteroid.
We found a reduction in the odds of hospital admission or emergency department or urgent care visit for asthma in participants given as-required FABA/ICS compared with as-required FABA alone (OR 0.35, 95% CI 0.20 to 0.60; n=2997, low-certainty evidence).
Compared with as-required FABA alone, any changes in asthma control or spirometry, though favouring as-required FABA/ICS, were small and less than the minimal clinically important differences (MCIDs). We did not find evidence of differences in asthma-associated quality of life or mortality. For other secondary outcomes FABA/ICS as required was associated with reductions in FeNO, probably reducing the odds of an adverse event (OR 0.82, 95% CI 0.71 to 0.95, 2 RCTs, 3002 participants, moderate-certainty evidence) and may reduce total systemic steroid dose (MD −9.90, 95% CI −19.38 to −0.42, 1 RCT, 443 participants, low-certainty evidence), with an increase in the daily inhaled steroid dose (MD 77 mcg beclomethasone equivalent/day, 95% CI 69 to 84, 2 RCTs, 2554 participants, moderate-certainty evidence).
We did not find a clear difference in serious adverse events in the three trials reporting this outcome, though CIs were wide (OR 1.31, 95% CI 0.50 to 3.46; n=3095). In the two trials reporting all adverse events, the odds of an adverse event were 18% lower in the as-required FABA/ICS group compared with the as-required FABA group (OR 0.82, 95% CI 0.71 to 0.95; n=3002, moderate-certainty evidence).
There was no difference in mortality observed, but this was based on a single death in the three studies, so no conclusions could be drawn about mortality differences.
FABA/ICS as required versus regular ICS plus FABA as required
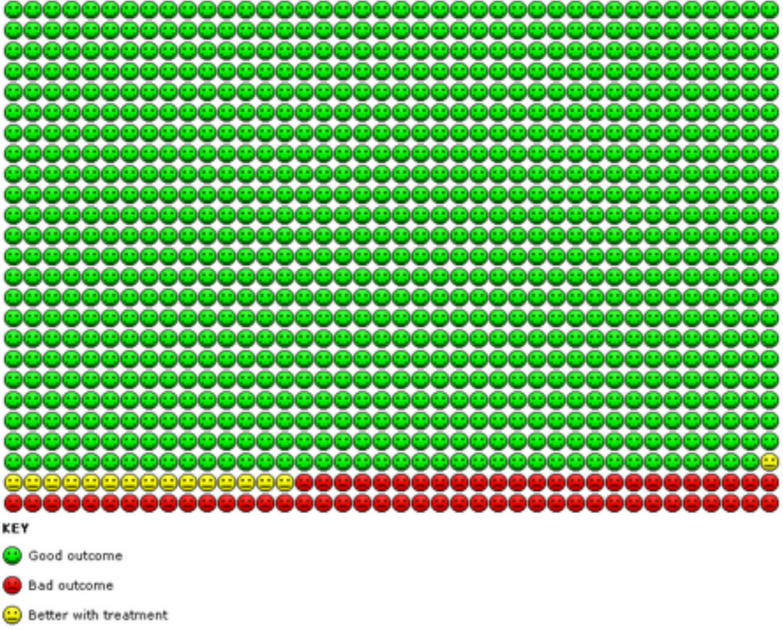
Results for this comparison are presented in the Summary of findings (table 2). We found evidence based on four studies13–16 that the odds of an asthma exacerbation requiring systemic steroids were reduced in participants treated with as-required FABA/ICS compared with regular ICS, but CIs include no difference (OR 0.79, 95% CI 0.59 to 1.07; n=8065, low-certainty evidence). This is equivalent to 81 people out of 1000 in the regular ICS plus FABA group experiencing an exacerbation requiring systemic steroids, compared with 65 (95% CI 49 to 86) out of 1000 in the FABA/ICS as-required group (represented graphically in figure 3). There were fewer exacerbations of asthma requiring either hospital admission or a visit to an emergency department or urgent care clinic in participants taking as-required FABA/ICS compared with regular ICS (OR 0.63, 95% CI 0.44 to 0.91; n=8065, low-certainty evidence).
Table 2.
Summary of findings 2. As-required FABA/ICS inhalers compared with regular inhaled steroids for mild asthma
| As‐required FABA/ICS inhalers compared with regular inhaled steroid for mild asthma | ||||||
|
Patient or population: Mild asthma Setting: Community Intervention: As‐required FABA/ICS inhalers Comparison: Regular inhaled steroid | ||||||
| Outcomes | Anticipated absolute effects$ (95% CI) |
Relative effect
(95% CI) |
Number of participants
(studies) |
Certainty of the evidence
(GRADE) |
Comments | |
| Risk with regular inhaled steroid | Risk with as-required FABA/ICS inhalers | |||||
| Exacerbations requiring systemic steroid follow‐up: 52 weeks | 81 per 1000 | 65 per 1000 (49 to 86) |
OR 0.79 (0.59 to 1.07) | 8065 (4 RCTs) |
⊕⊕⊝⊝ LOW*† |
Exacerbations requiring systemic steroid occurred less frequently in those treated with as-required combination inhalers than those treated with regular inhaled steroids, but the 95% CI includes no difference. |
| Hospital admission, ED and urgent care visits follow‐up: 52 weeks | 19 per 1000 | 12 per 1000 (8 to 17) |
OR 0.63 (0.44 to 0.91) | 8065 (4 RCTs) |
⊕⊕⊝⊝ LOW*‡ |
Fewer hospital admissions, ED attendances and urgent care visits occurred in those treated with as-required combination inhalers compared with regular inhaled steroids. |
| Asthma control assessed with: ACQ‐5, follow‐up: 52 weeks. Lower scores indicate better asthma control |
The mean asthma control was −0.467 points, change from baseline | MD 0.12 points higher (0.09 higher to 0.15 higher) |
‐ | 7382 (4 RCTs) |
⊕⊕⊕⊕ HIGH |
ACQ‐5 fell slightly more compared with baseline in those treated with regular inhaled steroids than those treated with combination inhalers. MCID for ACQ‐5 is 0.5 points. |
| Inhaled steroid dose assessed with: mean daily dose in μg, beclomethasone equivalent follow‐up: 52 weeks | The mean inhaled steroid dose was 257.8 µg beclomethasone equivalent per day | MD 154.51 µg/day lower (207.94 lower to 101.09 lower) | ‐ | 7180 (4 RCTs) |
⊕⊕⊕⊝ MODERATE* |
Those treated with as-required combination inhalers had a lower average daily inhaled steroid dose than those treated with a regular inhaled steroid. |
| Total systemic steroid dose assessed with: mean cumulative dose of prednisolone over the course of the trial in mg follow-up: 52 weeks | The mean total systemic steroid dose was 20.97 mg prednisolone | MD 7 mg prednisolone lower (13.97 lower to 0.03 lower) | ‐ | 1330 (2 RCTs) |
⊕⊕⊕⊝ MODERATE* |
Total systemic steroid exposure was similar and low in those treated with regular inhaled steroid and those treated with as-required combination inhalers. |
| Adverse events assessed with: Participants experiencing at least one adverse event follow‐up: 52 weeks | 493 per 1000 | 482 per 1000 (443 to 525) |
OR 0.96 (0.82 to 1.14) |
8072 (4 RCTs) |
⊕⊕⊕⊝ MODERATE* |
The proportion of participants experiencing at least one adverse event was similar in those treated with combination inhalers and those with regular inhaled steroid. |
| $ The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
|
GRADE Working Group grades of evidence
High certainty ⊕⊕⊕⊕: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty ⊕⊕⊕: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty ⊕⊕: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty ⊕: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
*Downgraded as included open label studies.
†Downgraded as heterogeneity between trials at low risk of bias in all domains and those at high risk in at least one domain.
‡Downgraded as based on a relatively small number of events.
ACS-5, Asthma Control Questionnaire‐5; ED, emergency department; FABA, fast‐acting β₂‐agonist; ICS, inhaled corticosteroid; MCID, minimum clinically important difference; MD, mean difference; RCT, randomised controlled trial.
Figure 3.
In the regular ICS group 81 people out of 1000 had exacerbations requiring systemic steroids over 52 weeks, compared with 65 (95% CI 49 to 86) out of 1000 in the FABA/ICS as-required group. FABA, fast-acting β2-agonist; ICS inhaled corticosteroid.
When assessing ACQ-5 data we found a statistical advantage to regular ICS compared with as-required FABA/ICS but the absolute differences were small (MD 0.12, 95% CI 0.09 to 0.15; participants=7382) and less than the MCID, which for ACQ-5 is 0.5 points. Compared with regular ICS, any changes in asthma control, spirometry, peak flow rates or asthma-associated quality of life, though favouring regular ICS, were small and less than the MCIDs. Adverse events, serious adverse events, total systemic corticosteroid dose and mortality were similar between groups, although deaths were rare, so CIs for this analysis were wide. We found moderate-certainty evidence from four trials involving 7180 participants that FABA/ICS as required was likely associated with less average daily exposure to ICS than regular ICS (MD −154.51 mcg/day, 95% CI −207.94 to −101.09).
In preplanned sensitivity analyses, excluding the two open-label studies13 14 did not alter the direction of effect in any of the primary outcomes, neither did use of a fixed-effects rather than a random-effects model.
Discussion and conclusions
We found moderate-certainty evidence to high-certainty evidence that as-required fixed-dose FABA/ICS is clinically effective in adults and adolescents with mild asthma. Their use instead of FABA as required alone reduced exacerbations, hospital admissions or unscheduled healthcare visits, and exposure to systemic corticosteroids, and probably reduced adverse events. As exacerbations are responsible for the majority of morbidity, mortality and the economic costs of asthma, this would support recent changes in international guidelines (GINA 2019) away from the use of SABA alone in mild asthma.
Furthermore, use of as-required FABA/ICS is as effective as regular ICS, and is associated with a reduction of asthma-related hospital admissions or unscheduled healthcare visits, while reducing the average daily exposure to ICS, without any evidence of an increase in adverse events. As mild asthma is heterogeneous, with eosinophilic airway inflammation— linked to the greatest benefit from ICS—present in only a quarter of individuals,17 these dual benefits are likely to be achieved by a symptom-driven therapy reducing intentional and unintentional use of FABA in monotherapy in these individuals. We now recognise distinct asthma phenotypes.18 Those with type-2 high disease have steroid-responsive inflammation associated with high peripheral blood and sputum eosinophil counts, and high FeNO, and are at risk of exacerbations. As it is hard to distinguish between type-2 high and type-2 low disease in primary care, the symptom-driven approach effectively enables those with symptomatic type-2 high disease to self-titrate their therapy in line with the level of underlying steroid-responsive inflammation.
We believe these studies are representative of adults with mild asthma in the real world, with broad inclusion criteria, with only two of the studies that contributed data requiring reversibility as an inclusion criterion, the others depending on self-report of physician-diagnosed asthma. Participants had mean age 36–43 years, a mild deficit in baseline lung function (prebronchodilator FEV1 84%–90%) and included current smokers (2.3%–11% of participants), and those with a range of preceding annual exacerbation rates (5.5%–22%). These results are therefore likely to be generalisable to populations with mild asthma in primary care.
Using the GRADE system, we judged the certainty of the evidence per outcome for main comparisons—those related to rates of exacerbations—to be low (with the exception of exacerbations requiring systemic steroid in the as-required FABA/ICS vs as-required FABA comparison). This judgement may be overly conservative, as the results are based solely on relevant, well-designed RCTs. The methodological quality was otherwise good for the included trials; they were conducted in applicable populations, examining outcomes of direct relevance to participants, with low–moderate heterogeneity across studies, and with consistent findings between studies, including between blinded and unblinded studies.
Our findings are consistent with data from a 2007 double-blind RCT which showed as-required beclomethasone-salbutamol 250/100 mcg in a single inhaler was as effective as regular use of inhaled beclomethasone 250 mcg twice daily and more effective than as-required salbutamol alone in preventing exacerbations and improving morning PEFR.19 That study was judged as at low risk of bias, but was excluded because 31.6% of participants were receiving regular ICS, with a mean dose of 460 mcg/day.
These findings support as-required use of FABA/ICS in a fixed dose combination inhaler as superior to SABA alone, and as a therapeutic alternative to maintenance ICS in mild asthma, could reduce the number of severe asthma attacks. This is important as asthma is a major cause of time off work, economic costs and chronic ill-health, and it remains a largely preventable cause of death for 400 000 people per year globally. This approach also simplifies treatment regimens and could reduce contradictory or ambiguous messages to people with asthma. It would support adoption of this strategy in current guidelines. However, cost frequently limits the availability of these inhalers in many low-income and middle-income countries, who rely heavily on reliever therapies or tablet steroids instead. Further pragmatic studies and healthcare cost assessments in such countries are needed to support equitable access to affordable quality-assured asthma medicines.
The implementation of these findings may depend on differing health economic assessments, differing healthcare infrastructures and population-specific factors in different settings globally. All the data are derived from studies of dry powder formulations and may not necessarily apply to pressurised metred-dose inhalers. Further research is needed to explore use of FABA/ICS as required in children under 12 years of age, use of other FABA/ICS preparations, health economic factors and long-term outcomes beyond 52 weeks.
Acknowledgments
The authors thank the Cochrane Airways Editorial team, particularly Rebecca Fortescue, Emma Dennett and Emma Jackson for their help defining the clinical question and methods and Liz Stovold for the search strategy. The authors also thank Adnan Chaudhry, Gareth Hynes and Hannah Bruguier for contributions to screening abstracts and data extraction. The authors and Airways' Editorial Team thank the following peer reviewers and patient reviewers for their time and comments: Kevin Mortimer (UK), Refiloe Masekala (South Africa), Hebatullah Abdulazeem (Germany), Chen-Yuan Chiang (France), Amnuayporn Apiraksakorn (Thailand) and Sarah Hodgkinson (UK). The Background and Methods sections of this review were based on a standard template used by Cochrane Airways.
Footnotes
Twitter: @iainxingham, @HinksLab
Contributors: SR and TSCH wrote the protocol Background. IC wrote the Methods section based on a standard Cochrane template with contributions from all the authors. IC, ST, SR, MG, FY, AF and TSCH screened abstracts and full texts. IC, ST, SR, MG, FY, AF, TSCH, RR, PW and EO’B performed data extraction. RR and TSCH wrote the lay summary. IC performed the meta-analysis and TSCH wrote the discussion with contributions from all authors.
Funding: This project was funded by the National Institute for Health Research (NIHR) (Cochrane Incentive Awards 2019 (NIHR130687)) and the Wellcome Trust (211050/Z/18/Z).
Disclaimer: Summary based on Cochrane Database of Systematic Reviews 2021 May 4;5(5):CD013518. doi: 10.1002/14651858.CD013518.pub2.). Cochrane reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Library should be consulted for the most recent version of the review.
Competing interests: IC has been involved in recruitment for a GlaxoSmithKline-sponsored trial of inhaled nemiralisib for COPD, but did not directly receive funding for this. ST reports money for travel from Novartis in 2019 for an educational event. SR is undertaking a PhD supported by an unrestricted research grant from AstraZeneca. He has attended educational events sponsored by AstraZeneca (2019). TSCH has received research funding from the Wellcome Trust, NIHR, the Beit Guardians; has received speaker fees from AstraZeneca, Boehringer Ingelheim; his research team have received funding from Sanofi.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
Data sharing is not applicable as no data sets were generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 2004;114:40–7. 10.1016/j.jaci.2004.04.042 [DOI] [PubMed] [Google Scholar]
- 2. Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007;62:591–604. 10.1111/j.1398-9995.2007.01394.x [DOI] [PubMed] [Google Scholar]
- 3. Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J 2010;17:74–80. 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332–6. 10.1056/NEJM200008033430504 [DOI] [PubMed] [Google Scholar]
- 5. Taylor A, Chen L-C, Smith MD. Adherence to inhaled corticosteroids by asthmatic patients: measurement and modelling. Int J Clin Pharm 2014;36:112–9. 10.1007/s11096-013-9862-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddel HK, Busse WW, Pedersen S, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the start study. Lancet 2017;389:157–66. 10.1016/S0140-6736(16)31399-X [DOI] [PubMed] [Google Scholar]
- 7. Kuprys-Lipinska I, Kolacinska-Flont M, Kuna P. New approach to intermittent and mild asthma therapy: evolution or revolution in the GINA guidelines? Clin Transl Allergy 2020;10:19. 10.1186/s13601-020-00316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Physicians RCo . Why asthma still kills: the National review of asthma Neaths (NRAD) Confidential enquiry report. London, 2014. [Google Scholar]
- 9. Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015;45:396–407. 10.1183/09031936.00075614 [DOI] [PubMed] [Google Scholar]
- 10. Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev 2013:CD007313. 10.1002/14651858.CD007313.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asthma GIf . Global strategy for asthma management and prevention (2019 update), 2019. [Google Scholar]
- 12. Haahtela T, Tamminen K, Malmberg LP, et al. Formoterol as needed with or without budesonide in patients with intermittent asthma and raised no levels in exhaled air: a soma study. Eur Respir J 2006;28:748–55. 10.1183/09031936.06.00128005 [DOI] [PubMed] [Google Scholar]
- 13. Beasley R, Holliday M, Reddel HK, et al. Controlled trial of Budesonide-Formoterol as needed for mild asthma. N Engl J Med 2019;380:2020–30. 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 14. Hardy J, Baggott C, Fingleton J, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (practical): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019;394:919–28. 10.1016/S0140-6736(19)31948-8 [DOI] [PubMed] [Google Scholar]
- 15. O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined Budesonide-Formoterol as needed in mild asthma. N Engl J Med 2018;378:1865–76. 10.1056/NEJMoa1715274 [DOI] [PubMed] [Google Scholar]
- 16. Bateman ED, Reddel HK, O'Byrne PM, et al. As-Needed Budesonide-Formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018;378:1877–87. 10.1056/NEJMoa1715275 [DOI] [PubMed] [Google Scholar]
- 17. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med 2019;380:2009–19. 10.1056/NEJMoa1814917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hinks TSC, Levine SJ, Brusselle GG. Treatment options in type-2 low asthma. Eur Respir J 2021;57:2000528. 10.1183/13993003.00528-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007;356:2040–52. 10.1056/NEJMoa063861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no data sets were generated and/or analysed for this study.