Abstract
Purpose
Teprotumumab, a specific blocking antibody to the insulin like growth factor 1 receptor, significantly reduced proptosis in patients with thyroid eye disease (TED) in recent clinical trials. Given its specificity, we expect it to demonstrate greater efficacy on the worse affected orbit, in patients with asymmetric TED. Herein, we investigate the differential impact of teprotumumab on the orbits of such patients.
Methods
In this pooled analysis of patients who were enrolled in the recent phase 2 (NCT01868997) and phase 3 (NCT03298867) trials, all patients with asymmetric TED (difference in exophthalmometry of ≥3 mm) were screened for eligibility. The primary outcomes of the trials, proptosis, diplopia and Clinical Activity Score (CAS) response, were evaluated in both orbits of patients who had received treatment or placebo, to examine the differential response from baseline to week 24.
Results
From a pooled group of 84 patients randomised to receive teprotumumab and 87 randomised to placebo, 10 (12%) and 12 (14%), respectively, met the inclusion criteria. The teprotumumab-treated patients demonstrated significant reductions in proptosis, CAS and diplopia in both orbits of each patient and this was not seen with placebo. The reduction in proptosis and CAS was significantly greater in the worse affected orbit, improving symmetry. In the placebo arm, while the mean CAS in the study eye reduced over time, proptosis and diplopia did not change in either orbit.
Conclusion
The findings in this study suggest the differential impact of teprotumumab on orbits that are clinically more affected by TED, suggesting that teprotumumab reduces asymmetry.
Keywords: eye (globe), eye lids, immunology, orbit, pathology
Introduction
Thyroid eye disease (TED) is a rare and complex autoimmune condition with an incidence of 16 per 100 0001 in females and 3 per 100 000 in males.2 Patients develop permanent disfiguring facial changes, and may experience proptosis, diplopia and vision changes or loss.3 The burden of morbidity is considerable, with a marked impact on quality of life4 and mental health,5 which may lead to increased rates of suicide.6
Over 90% of patients with TED also have hyperthyroidism,3 however, patients may be hypothyroid or euthyroid.7 The manifestations of TED typically involve expansion of soft tissues within the bony orbital cavity, with associated ocular inflammation.8–10
Most patients present with varying degrees of bilateral disease; however, some patients present asymmetrically. In a 2020 report on 269 TED patients, 83 (30.9%) were considered to have asymmetric disease, with a mean difference of 2.5 mm exophthalmos between the eyes. Higher disease burden among patients with asymmetric vs symmetric disease was also observed.9 Asymmetry has been identified as a significant contributor to psychosocial distress and impaired quality of life in patients with TED.10 11 Asymmetry also appears to be a marker for more acute, severe and possibly recalcitrant disease.9
Orbital changes in TED occur due to orbital fibroblast (OF) deposition of glycosaminoglycans such as hyaluronan in the muscle, and expansion of fat within the orbit.12 Recent research has led to a growing body of evidence highlighting the central role of the insulin-like growth factor 1 receptor (IGF-1R) pathway in the pathogenesis of the disease. Over activation of the IGF-1R increases the production of interleukin 2 (IL-2),13 transforming growth factor-α,14 and IL-8,15 by T cells and monocytes, in vitro. It also enhances T cell-independent humoral immune responses in B cells.16 The IGF-1R pathway also likely plays a significant role in modifying the extracellular matrix (ECM), suggesting a regulatory role in the expression of collagen,17 fibronectin, vitronectin, laminins and integrins.18
Further, OFs from patients with TED demonstrate a threefold increase in surface expression of the IGF-1R.19 When OFs from patients with TED were treated with IGF-1 or IgG derived from patients with Graves’ disease, they were found to produce increased levels of hyaluronan.20 These OFs also produced increased amounts of the powerful T cell chemoattractants; IL-16 and chemokine (C-C motif) ligand 5.21 Autoantibodies directed against the IGF-1R can be detected more frequently in patients with GD, when compared with controls. The role of these antibodies remains unclear, but the IGF-1R pathway appears clearly central to the development of TED and its sequelae.22
A novel human monoclonal IGF-1R inhibitor, teprotumumab, binds to the IGF-1R abrogating signalling and inducing internalisation and degradation of the antibody-receptor complex.20 Phases 2 and 3 randomised placebo-controlled trials (RCTs) of teprotumumab (NCT01868997,23 NCT03298867,24 respectively) revealed marked improvement in the Clinical Activity Score (CAS), diplopia and proptosis of patients with moderate to severe TED following 8 infusions of teprotumumab over a 24-week period.23 25 In addition, patients treated with teprotumumab demonstrated reduction of extraocular muscle and orbital fat volume.24
Given the overexpression of IGF-1Rs on OFs in TED,19 we hypothesise that the effect of teprotumumab is specific to orbital tissue which has undergone expansion during TED pathogenesis. Therefore, we expect that patients with asymmetric TED would have a differential reduction in proptosis and CAS, thereby achieving improved symmetry. There is a paucity of data in the published literature on pharmacological treatment of asymmetrical TED. To examine this, we conducted a post hoc subgroup analysis of patients who had asymmetric TED at baseline in the pooled data of the phase 2 (NCT01868997)23 and phase 3 (NCT03298867)24 trials. Examining these two placebo-controlled trials allows us a unique opportunity to examine whether patients with asymmetry become more symmetrical because of treatment with teprotumumab as opposed to improvement associated with the natural history of the disease. Further, it also provides unique insight into the effects of teprotumumab on the non-study eye, which have not been reported previously.
Methods
All patients provided informed consent.
Patients
Patients with moderate to severe, active TED who were randomised to receive teprotumumab or placebo treatment during the phase 2 (NCT01868997)23 and phase 3 (NCT03298867)24 trials were screened for study eligibility in this subanalysis. In the clinical trials, patients received 8 infusions of teprotumumab (10 mg/kg for the first infusion and 20 mg/kg for subsequent infusions) every 3 weeks over 24 weeks. For each patient, the clinically more severely proptotic eye was designated the study eye and the contralateral fellow eye was designated the non-study eye in accordance with the original clinical trial protocols. As there is no currently agreed to formal definition of asymmetry, patients were defined by asymmetry in anterior globe position of 3 mm or greater at baseline.
Measurement of clinical outcomes
The primary key outcomes of the trials, proptosis, diplopia and CAS response, were evaluated in both eyes to examine differential response from baseline to week 24. Observed data on both placebo and teprotumumab treated patients who met the criteria for asymmetry are reported and compared here for patients with data at both time points.
Proptosis measurements were made using a Hertel exophthalmometer. Changes in diplopia grade were assessed using the Gorman subjective diplopia score26 (range 0–3). A score of 0 indicates no diplopia; (1) intermittent diplopia; (2) inconstant diplopia and 3, constant diplopia. An improvement ≥1 grade is considered as clinically significant. The 7-point CAS27 was used to measure inflammation.25 The presence of each of the following symptoms/signs is scored: retrobulbar eye pain, pain on eye movement, eyelid erythema, eyelid swelling, conjunctival redness, chemosis, inflammation of the caruncle or plica. Higher scores indicate more inflammation.28
Statistical analysis
Statistical analysis was performed using SPSS V.22.0 (SPSS). The difference between pre and post treatment exophthalmometry measurements was calculated using a dependent t-test, while the difference between the proptosis in the placebo and treatment group was calculated using an independent t-test. The pre-CAS and post-CAS and diplopia scores were analysed using the Mann-Whitney U test. Statistical significance was defined as p<0.05.
Results
Patients
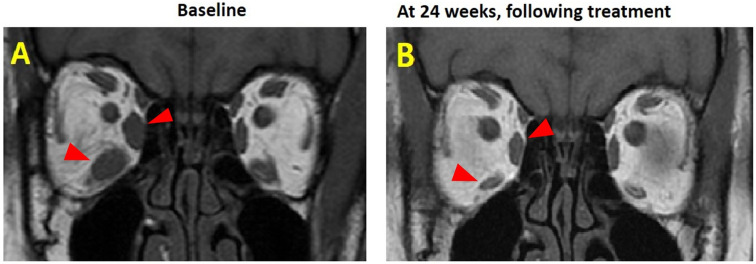
From a pooled group of 84 patients randomised to receive teprotumumab and 87 randomised to placebo, 11 (13%) and 12 (14%), respectively, met the inclusion criteria for asymmetry at baseline and were included in this analysis. Only patients who had assessments at baseline and week 24 were included. One of the 11 teprotumumab patients did not have a week 24 visit assessment and therefore excluded, leaving 10 patients (12%) for this analysis. Demographic details are provided in table 1, while clinical details are provided in table 2. An MRI scan of a patient treated with teprotumumab is provided in figure 1.
Table 1.
Patient demographics
| Patient demographics at baseline | ||
| Teprotumumab group, N = 10 | Placebo, N = 12 | |
| Age (years), mean (SD) | 50 (13) | 59 (7) |
| Gender, (N) | ||
| Male | 4 | 2 |
| Female | 6 | 10 |
| Race, (N) | ||
| Caucasian | 10 | 10 |
| Black | 0 | 0 |
| Asian | 0 | 2 |
| Smokers, (N) | 2 | 4 |
| Months since diagnosis of Graves’ disease, mean (SD) | 16.4 (20) | 24.8 (41.3) |
| Months since diagnosis of TED, mean (SD) | 5.8 (2.2) | 6.0 (2.3) |
TED, thyroid eye disease.
Table 2.
Proptosis measurements for patients
| Study phase | Case | Baseline study eye | Week 24 study eye | Baseline fellow eye | Week 24 fellow eye | Baseline asymmetry | Week 24 asymmetry |
| A: Teprotumumab group | |||||||
| 3 | 1 | 27 | 20 | 17 | 17 | 10 | 3 |
| 3 | 2 | 23 | 18 | 18 | 17 | 5 | 1 |
| 3 | 3 | 26 | 20 | 23 | 18.5 | 3 | 1.5 |
| 2 | 4 | 26 | 21 | 22 | 21 | 4 | 0 |
| 2 | 5 | 24 | 20 | 20 | 19 | 4 | 1 |
| 2 | 6 | 26 | 20 | 23 | 21 | 3 | 1 |
| 2 | 7 | 25 | 21 | 22 | 19 | 3 | 2 |
| 2 | 8 | 21 | 16 | 15 | 12 | 6 | 4 |
| 2 | 9 | 23 | 19 | 18 | 18 | 5 | 1 |
| 2 | 10 | 22 | 21 | 19 | 16 | 3 | 5 |
| Mean | 24.2 | 19.6 | 19.55 | 17.85 | 4.64 | 1.75 | |
| SD | 1.9 | 21.58 | 2.66 | 2.63 | 2.06 | 1.81 | |
| B: Placebo group | |||||||
| 3 | 1 | 28 | 28 | 24 | 24 | 4 | 4 |
| 3 | 2 | 21 | 19 | 18 | 18 | 3 | 1 |
| 3 | 3 | 20 | 20 | 17 | 18 | 3 | 2 |
| 3 | 4 | 25 | 26 | 22 | 23 | 3 | 3 |
| 3 | 5 | 21 | 21 | 17 | 18 | 4 | 3 |
| 3 | 6 | 24 | 24 | 19 | 20 | 5 | 4 |
| 2 | 7 | 24 | 21 | 21 | 19 | 3 | 2 |
| 2 | 8 | 16 | 17 | 12 | 16 | 4 | 1 |
| 2 | 9 | 19 | 21 | 16 | 17 | 3 | 4 |
| 2 | 10 | 28 | 30 | 25 | 25 | 3 | 5 |
| 2 | 11 | 25 | 25 | 22 | 23 | 3 | 2 |
| 2 | 12 | 22 | 23 | 18 | 17 | 4 | 6 |
| Mean | 22.8 | 23 | 19.3 | 19.8 | 3.5 | 3.1 | |
| SD | 3.6 | 3.9 | 3.7 | 3.1 | 0.7 | 1.6 | |
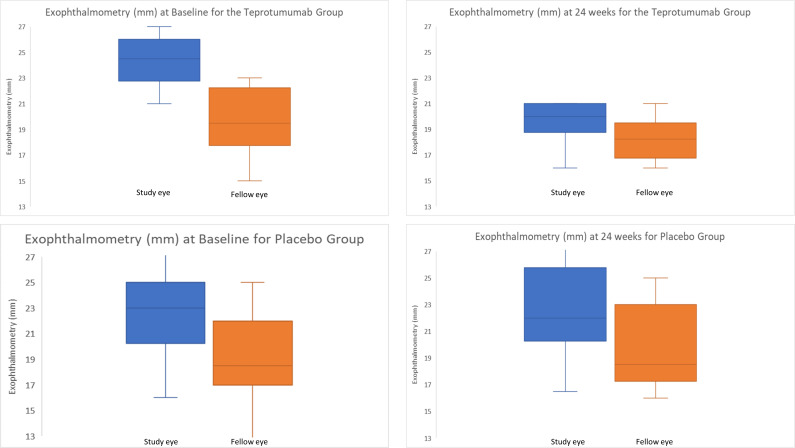
Figure 1.
Change in proptosis from baseline to 24 weeks, in the treatment group and placebo group.
Proptosis
In the group treated with teprotumumab, the baseline exophthalmometry measurement (mean (SD)) was 24.3 mm (2.0) for the study eye and 19.7 mm (2.8) for the fellow eye. The mean difference or asymmetry between the study eye and fellow eye at baseline was 4.6 mm (2.2). In the placebo group, the mean (SD) baseline exophthalmometry for the study eye was 22.8 mm (3.6) and 19.3 mm (3.7) for the fellow eye. The mean asymmetry in the placebo group was 3.5 mm (0.7) at baseline. There were no significant baseline differences in exophthalmometry between the teprotumumab group and placebo group (p=0.18 for study eye and p=0.6 for fellow eye).
Following treatment with teprotumumab, there was a larger mean (SD) reduction from baseline in proptosis in the study eye (4.7 mm (1.6)) than the fellow eye (1.9 mm (1.5)) (figure 2, p<0.01 for both). The mean (SD) asymmetry between the study eye and fellow eye at week 24 was 1.8 mm (1.8). Seven of the 10 patients (70%) no longer demonstrated asymmetry (as defined by a difference of <3 mm) at week 24. In contrast, 5 of the 12 patients (42%) in the placebo group did not have asymmetry at 24 weeks. The mean (SD) change from baseline in this group was 0.1 mm (1.5) for the study eye and 0.6 mm (1.4) for the fellow eye. After 24 weeks, there was a significant reduction in asymmetry in the teprotumumab group (p<0.01), while there was no significant reduction in asymmetry in the placebo group (p=0.3). Further, there was a significant difference in asymmetry after 24 weeks between the placebo and treated group (p<0.05).
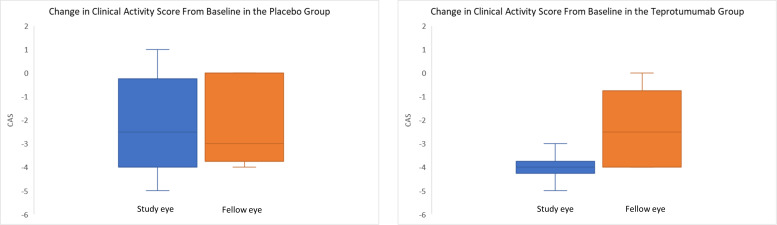
Figure 2.
Change in clinical activity score from baseline to 24 weeks, in the treatment and placebo groups.
Clinical Activity Score
At baseline, in the teprotumumab group, all study eyes and 5 (50%) fellow eyes had a CAS greater than 3. Following treatment, one patient had a CAS greater than three in both eyes and the remaining nine patients all had a CAS of 0 or 1 in both eyes. The change in CAS from baseline to 24 weeks in each eye, following completion of a course of teprotumumab therapy, is shown in figure 3. In the placebo group, all study eyes and 10 fellow eyes had a CAS greater than 3 at baseline. After 24 weeks, five study eyes and four fellow eyes continued to have a CAS greater than 3 (table 3).
Figure 3.
(A) MRI scan of the same patient at baseline and (B) at 24 weeks, following therapy with teprotumumab (red arrows indicate extraocular muscles)
Table 3.
Clinical activity score for the teprotumumab and placebo groups
| Study | Case | Baseline study eye |
Week 24 study eye |
Baseline fellow eye |
Week 24 fellow eye |
| A: Clinical activity score in patients who received teprotumumab | |||||
| 3 | 1 | 5 | 1 | 0 | 0 |
| 3 | 2 | 5 | 0 | 1 | 0 |
| 3 | 3 | 4 | 0 | 4 | 0 |
| 2 | 4 | 5 | 1 | 4 | 1 |
| 2 | 5 | 4 | 0 | 4 | 0 |
| 2 | 6 | 7 | 4 | 7 | 4 |
| 2 | 7 | 5 | 0 | 4 | 0 |
| 2 | 8 | 4 | 1 | 1 | 0 |
| 2 | 9 | 4 | 0 | 0 | 0 |
| 2 | 10 | 5 | 1 | 2 | 0 |
| Mean | 5 | 0.8 | 2.7 | 0.5 | |
| SD | 0.9 | 1.2 | 2.3 | 1.3 | |
| B: Clinical Activity Score in patients who received placebo | |||||
| Phase 3 | 1 | 7 | 3 | 7 | 3 |
| Phase 3 | 2 | 5 | 0 | 4 | 0 |
| Phase 3 | 3 | 5 | 2 | 5 | 2 |
| Phase 3 | 4 | 7 | 2 | 5 | 2 |
| Phase 3 | 5 | 5 | 5 | 4 | 4 |
| Phase 3 | 6 | 6 | 5 | 4 | 4 |
| Phase 2 | 7 | 5 | 1 | 5 | 1 |
| Phase 2 | 8 | 4 | 4 | 4 | 4 |
| Phase 2 | 9 | 4 | 5 | 3 | 0 |
| Phase 2 | 10 | 6 | 3 | 4 | 1 |
| Phase 2 | 11 | 6 | 5 | 6 | 5 |
| Phase 2 | 12 | 5 | 3 | 2 | 2 |
| Mean | 5.4 | 3.2 | 4.4 | 2.3 | |
| SD | 1 | 1.7 | 1.3 | 1.7 | |
Diplopia
In the teprotumumab group, at baseline, all patients had diplopia. Following treatment, eight (80%) had an improvement in one or more grades, with a mean (SD) reduction of 1.8 (1.4) on the diplopia score. Five patients had complete resolution of diplopia at week 24 (grade 0). The difference between pretherapy and post-therapy diplopia scores was significant (p<0.05). In the placebo group, eight patients had diplopia at baseline and nine patients had diplopia at week 24 (one more patient who did not have diplopia at baseline, developed diplopia during the 24 weeks), with the mean (SD) diplopia score increasing from 1.4 (1.3) to 2.0 (1.1) after 24 weeks (table 4).
Table 4.
Gorman Diplopia Score for the teprotumumab and placebo groups
| Study | Case | Baseline | Week 24 |
| A: Gorman Diplopia Score in patients who received teprotumumab | |||
| 3 | 1 | 3 | 0 |
| 3 | 2 | 3 | 0 |
| 3 | 3 | 1 | 1 |
| 2 | 4 | 3 | 1 |
| 2 | 5 | 3 | 1 |
| 2 | 6 | 3 | 2 |
| 2 | 7 | 3 | 0 |
| 2 | 8 | 2 | 0 |
| 2 | 9 | 3 | 0 |
| 2 | 10 | 1 | 2 |
| Mean | 2.3 | 0.7 | |
| SD | 1.1 | 0.8 | |
| B: Gorman diplopia score in patients who received Placebo | |||
| Phase 3 | 1 | 0 | 2 |
| Phase 3 | 2 | 2 | 2 |
| Phase 3 | 3 | 3 | 3 |
| Phase 3 | 4 | 0 | 0 |
| Phase 3 | 5 | 3 | 3 |
| Phase 3 | 6 | 3 | 2 |
| Phase 2 | 7 | 1 | 3 |
| Phase 2 | 8 | 3 | 3 |
| Phase 2 | 9 | 0 | 2 |
| Phase 2 | 10 | 0 | 0 |
| Phase 2 | 11 | 1 | 1 |
| Phase 2 | 12 | 1 | 3 |
| Mean | 1.4 | 2 | |
| SD | 1.3 | 1.1 | |
Discussion
The prevalence of asymmetric TED has been found to be as high as 30%–40% in some studies9 29 and is associated with a significant detrimental impact on patient quality of life,11 30 often requiring surgical correction. This study examined the differential impact of teprotumumab on asymmetric TED and to our knowledge, is the first report of placebo-controlled data to provide an analysis of the impact of a therapy for TED, on the fellow eye. The results of this study indicate that teprotumumab may have greater efficacy on the orbit that is more affected by TED, thereby potentially improving symmetry by reducing proptosis where it is needed most. In the present study, in the treated group, baseline asymmetry was 4.6 mm (2.2), reducing to 1.8 mm (1.8) after 24 weeks. The reduction of asymmetry to below 2 mm may be significant, since in a previous study it was found that only asymmetry (due to proptosis) greater than 2 mm, was noticeable by observers.31 Further, this study indicates that this balancing of symmetry does not occur during the natural short-term course of the disease.
The teprotumumab-treated asymmetric patients demonstrated significant reductions in proptosis, CAS and diplopia in both orbits of each patient and this was not seen with placebo. Furthermore, the magnitude of the reduction in proptosis and CAS was significantly greater in the orbit that was most affected by TED, achieving improved symmetry. In the placebo arm, while the mean CAS in the study eye reduced over time, proptosis and diplopia did not change in either the study or fellow eye.
Asymmetric TED remains an enigma. It is uncommon for an autoimmune condition to present disproportionately on one side of the body. This study provides a rare insight into the characteristics and treatment of this phenomenon. In the placebo arm, approximately 40% of the patients had worsening proptosis in the fellow eye over the course of the 6-month period, suggesting that the disease may eventually affect this eye as well. Although at this stage it is unclear why teprotumumab has a greater effect on tissue more affected by TED, a brief insight into the pathogenesis of TED may provide some clues. Over the past decade, there has been a growing interest in the role of CD34+ fibrocytes. These bone marrow derived cells are frequently found at sites of fibrosis.32 They have more recently been found to enter the orbit in TED, in some cases virtually replacing the native population of OFs,33 and their migration appears to be biased toward the more affected orbit.33 They have the propensity toward adipogenesis32 or a myofibroblast phenotype34 based on the local inflammatory milieu and have a significant impact on the constituents of the ECM by increasing the expression of collagen and fibronectin.32 Given the central role played by fibrocytes and the IGF-1R pathway in the modification of the ECM and orbital inflammation, it is likely that the differential effects of teprotumumab in patients with asymmetric TED indicate its specificity for the IGF-1R.
Since the data analysed in this study has been pooled from two previous RCTs,23 24 the advantages of this study relate to those of the aforementioned RCTs. The limitations of this study include the relatively small number of patients in the treatment group, however, it was made more robust by comparing to a similar group of patients in a placebo arm. Further, the study reviewed patients for 6 months and there is a possibility that given a longer period of time proptosis in some patients may have reduced in the placebo group.
The findings in this study underscore the differential impact of teprotumumab on orbits that are clinically more affected by TED. They also suggest that teprotumumab reduces asymmetry and therefore might circumvent the requirement for surgical correction.
Footnotes
Correction notice: This paper has been corrected since it was published online. In table 2, the data in section B was duplicated in section A. Also, the figure and tables were incorrectly labelled and these have now been updated.
Contributors: SU: Data collection, analysis of the data, preparation of manuscript. YW and TM: Data collection, examination of patients, coordination of clinical trial, review of paper. GK and RD: Design of study, collection of data, analysis of data, review of manuscript. Horizon Therapeutics agreed to pay for the open access publication cost.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RD: Consultant Horizon Therapeutics, Immunovant. GK: The JGU Medical Centre has received research-associated funding from Horizon Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The two trials from which this analysis was constructed adhered to the tenets of the Declaration of Helsinki, was performed in accordance with the Health Insurance Portability and Accountability Act (HIPAA) and was approved by the sites’ institutional review boards.
References
- 1. Perros P, Crombie AL, Matthews JN, et al. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol 1993;38:367–72. 10.1111/j.1365-2265.1993.tb00516.x [DOI] [PubMed] [Google Scholar]
- 2. Phelps PO, Williams K. Thyroid eye disease for the primary care physician. Dis Mon 2014;60:292–8. 10.1016/j.disamonth.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 3. Bahn RS. Graves' ophthalmopathy. N Engl J Med 2010;362:726–38. 10.1056/NEJMra0905750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park JJ, Sullivan TJ, Mortimer RH, et al. Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol 2004;88:75–8. 10.1136/bjo.88.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wickwar S, McBain HB, Ezra DG, et al. What are the psychosocial outcomes of treatment for thyroid eye disease? A systematic review. Thyroid 2014;24:1407–18. 10.1089/thy.2014.0037 [DOI] [PubMed] [Google Scholar]
- 6. Ferløv-Schwensen C, Brix TH, Hegedüs L. Death by suicide in Graves' disease and Graves' orbitopathy: a nationwide Danish register study. Thyroid 2017;27:1475–80. 10.1089/thy.2017.0365 [DOI] [PubMed] [Google Scholar]
- 7. Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996;121:284–90. 10.1016/S0002-9394(14)70276-4 [DOI] [PubMed] [Google Scholar]
- 8. Ugradar S, Rootman DB. Orbital fat expansion in thyroid eye disease is related to age. Eur J Ophthalmol 2020;30:1004–7. 10.1177/1120672119852322 [DOI] [PubMed] [Google Scholar]
- 9. Perros P, Žarković MP, Panagiotou GC. Asymmetry indicates more severe and active disease in Graves’ orbitopathy: results from a prospective cross-sectional multicentre study. J Endocrinol Invest 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villagelin D, Romaldini J, Andrade J, et al. Evaluation of quality of life in the Brazilian Graves' disease population: focus on mild and moderate Graves' orbitopathy patients. Front Endocrinol 2019;10:192. 10.3389/fendo.2019.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wickwar S, McBain HB, Ezra DG, et al. Which factors are associated with quality of life in patients with Graves' orbitopathy presenting for orbital decompression surgery? Eye 2015;29:951–7. 10.1038/eye.2015.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naik VM, Naik MN, Goldberg RA, et al. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol 2010;55:215–26. 10.1016/j.survophthal.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kooijman R, Rijkers GT, Zegers BJ. IGF-I potentiates interleukin-2 production in human peripheral T cells. J Endocrinol 1996;149:351–6. 10.1677/joe.0.1490351 [DOI] [PubMed] [Google Scholar]
- 14. Renier G, Clément I, Desfaits AC, et al. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production. Endocrinology 1996;137:4611–8. 10.1210/endo.137.11.8895324 [DOI] [PubMed] [Google Scholar]
- 15. Kooijman R, Coppens A, Hooghe-Peters E. IGF-I stimulates IL-8 production in the promyelocytic cell line HL-60 through activation of extracellular signal-regulated protein kinase. Cell Signal 2003;15:1091–8. 10.1016/S0898-6568(03)00069-X [DOI] [PubMed] [Google Scholar]
- 16. Baudler S, Baumgartl J, Hampel B, et al. Insulin-Like growth factor-1 controls type 2 T cell-independent B cell response. J Immunol 2005;174:5516–25. 10.4049/jimmunol.174.9.5516 [DOI] [PubMed] [Google Scholar]
- 17. Reiser K, Summers P, Medrano JF, et al. Effects of elevated circulating IGF-1 on the extracellular matrix in "high-growth" C57BL/6J mice. Am J Physiol 1996;271:R696–703. 10.1152/ajpregu.1996.271.3.R696 [DOI] [PubMed] [Google Scholar]
- 18. Beattie J, McIntosh L, van der Walle CF. Cross-Talk between the insulin-like growth factor (IGF) axis and membrane integrins to regulate cell physiology. J Cell Physiol 2010;224:605–11. 10.1002/jcp.22183 [DOI] [PubMed] [Google Scholar]
- 19. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol 2008;181:4397–405. 10.4049/jimmunol.181.6.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krieger CC, Neumann S, Place RF, et al. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins. J Clin Endocrinol Metab 2015;100:1071–7. 10.1210/jc.2014-3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 2003;170:6348–54. 10.4049/jimmunol.170.12.6348 [DOI] [PubMed] [Google Scholar]
- 22. Marinò M, Rotondo Dottore G, Ionni I, et al. Serum antibodies against the insulin-like growth factor-1 receptor (IGF-1R) in Graves' disease and Graves' orbitopathy. J Endocrinol Invest 2019;42:471–80. 10.1007/s40618-018-0943-8 [DOI] [PubMed] [Google Scholar]
- 23. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017;376:1748–61. 10.1056/NEJMoa1614949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020;382:341–52. 10.1056/NEJMoa1910434 [DOI] [PubMed] [Google Scholar]
- 25. Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol 1989;73:639–44. 10.1136/bjo.73.8.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Go. Eur J Endocrinol 2008;158:273–85. 10.1530/EJE-07-0666 [DOI] [PubMed] [Google Scholar]
- 27. European Group on Graves' Orbitopathy (EUGOGO), Wiersinga WM, Perros P, et al. Clinical assessment of patients with Graves' orbitopathy: the European group on Graves' orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol 2006;155:387–9. 10.1530/eje.1.02230 [DOI] [PubMed] [Google Scholar]
- 28. Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European thyroid Association/European group on Graves' orbitopathy guidelines for the management of Graves' orbitopathy. Eur Thyroid J 2016;5:9–26. 10.1159/000443828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kavoussi SC, Giacometti JN, Javier Servat J. The relationship between sex and symmetry in thyroid eye disease. Clin Ophthalmol 2014;8:1295–300. 10.2147/OPTH.S61041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponto KA, Binder H, Diana T, et al. Prevalence, phenotype, and psychosocial well-being in Euthyroid/Hypothyroid thyroid-associated orbitopathy. Thyroid 2015;25:942–8. 10.1089/thy.2015.0031 [DOI] [PubMed] [Google Scholar]
- 31. Lin LK, Andreoli CM, Hatton MP, et al. Recognizing the protruding eye. Orbit 2008;27:350–5. 10.1080/01676830802336645 [DOI] [PubMed] [Google Scholar]
- 32. Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011;11:427–35. 10.1038/nri2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010;95:430–8. 10.1210/jc.2009-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong KM, Belperio JA, Keane MP, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem 2007;282:22910–20. 10.1074/jbc.M703597200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.