Abstract
Anaerobic fermentation processes for the production of a succinate-rich animal feed supplement from raw whey were investigated with batch, continuous, and variable-volume fed-batch cultures with Anaerobiospirillum succiniciproducens. The highest succinate yield, 90%, was obtained in a variable-volume fed-batch process in comparison to 80% yield in a batch cultivation mode. In continuous culture, succinate productivity was 3 g/liter/h, and the yield was 60%. Under conditions of excess CO2, more than 90% of the whey-lactose was consumed, with an end product ratio of 4 succinate to 1 acetate. Under conditions of limited CO2, lactose was only partially consumed and lactate was the major end product, with lower levels of ethanol, succinate, and acetate. When the succinic acid in this fermentation product was added to rumen fluid, it was completely consumed by a mixed rumen population and was 90% decarboxylated to propionate on a molar basis. The whey fermentation product formed under excess CO2, which contained mainly organic acids and cells, could potentially be used as an animal feed supplement.
Succinate is the direct in vivo precursor that accounts for up to 73% of the propionate formed in the rumen. Although succinic acid is a product of rumen microbes such as Fibrobacter, Succinivibrio, and Succinomonas, its concentration in the rumen is very low (0.47 ppm) (6, 17, 18). Previous studies demonstrated that high levels of succinate were decarboxylated to propionate when added to lactating cows or mixed-rumen populations (15). The turnover rate of succinate in the rumen is very high (4, 12).
Anaerobiospirillum succiniciproducens is an anaerobe that was previously reported to ferment various saccharides into mixed acids and ethanol (8). We have shown that growth and succinate-versus-lactate production from glucose by A. succiniciproducens were regulated by the level of available carbon dioxide and culture pH (30). The fermentation of lactose by this species has not been previously reported.
Whey, a by-product generated during cheese making, contains about 6 to 7% solids, of which 70 to 80% is lactose and 10 to 15% is milk proteins, lactate, and salts. It is used directly in animal feed mixes (5), and fermentation processes have been developed for upgrading whey to a mixture of single-cell protein, propionate, and ammonium lactate (1, 24, 29).
Feed additives are widely used for enhancing the efficiency of farm animal production. Ionophores, through their selective modification of the ruminal microflora (3, 10), increase propionate production and decrease both amino acid fermentation and the formation of acetate and methane. Increased levels of propionic acid coupled with decreased levels of methane (3, 27, 32) are related to improved animal performance, decreased ruminal protein and amino acid degradation, and stimulation of body protein synthesis (16, 23, 25). The recent trend toward the use of natural food and feed ingredients has stimulated the search for more acceptable animal feed additives that have the potential to stimulate propionate formation in the rumen and to enhance production efficiency (22). One of such additives could be succinate, which can be directly decarboxylated to propionate. Succinate may also probably act as a buffering agent in the pH range of 5.1 to 6.1, because it is a weak acid (pKa2 = 5.64) and thus could reduce the development of subacute acidosis. Also succinate is converted to propionate presumably by a biotin-dependent decarboxylation which results in proton consumption (H+ ion) (11). The fact that whey is a low-cost substrate can be used to produce succinate-based fermentation products for use by the cattle industry.
In preliminary studies, we screened several anaerobic bacterial species for their ability to ferment whey lactose to succinate and selected A. succiniciproducens as a model organism for further studies. We report here that A. succiniciproducens can ferment whey directly into a succinate-rich product that can potentially be used as an animal feed supplement.
All chemicals were of reagent grade and were obtained from Sigma Chemical Co., St. Louis, Mo. Whey was provided by the Michigan State University Dairy Pilot Plant (East Lansing, Mich.). Gases were supplied by Michigan Welding (East Lansing, Mich.) and were scrubbed free of oxygen by passage over heated (370°C) copper filings.
A. succiniciproducens (ATCC 29305) was grown in a seed culture medium prepared in whey diluted approximately twofold to 20 g of lactose per liter. The following ingredients were added (grams per liter): corn steep liquor (50% solids; Sigma), 10; K2HPO4, 3; NaCl, 1; (NH4)2SO4, 1; and MgCO3, 15. The medium was heat sterilized under N2 gas (20 min, 121°C) in vials sealed with black butyl rubber stoppers. To 100 ml of sterile medium in 158-ml serum vials, 0.75 ml of 10 M H2SO4 was added to adjust the pH to 6.8 ± 0.1. Anaerobic culture conditions were established as previously described (34). The headspace nitrogen was replaced by carbon dioxide, and Na2S (0.025%) was added as a reductant. After 15 min, the reduced medium was inoculated with a 1% (vol/vol) addition of an early-stationary-phase culture, and the vials were incubated in a rotary, temperature-controlled (39°C) shaker (New Brunswick Scientific, Edison, N.J.).
Fermentation media were made with sterilized raw whey, and the fermentors were inoculated with 5% samples from seed cultures grown overnight. The final whey lactose concentration ranged between 44.7 and 48.7 g/liter. The concentration of whey lactic acid was 5.5 ± 0.5 g/liter, and that of whey protein was 4.3 ± 0.5 g/liter. The following sterilized ingredients were added to fermentation media in pH-controlled reactors (grams per liter of raw whey): corn steep liquor (50% solids), 20; K2HPO4, 1.5; MgCl2 · 6H2O, 0.2; and tryptophan, 0.02 (7).
One-liter batch and variable-volume fed-batch fermentations were performed with 1.4-liter Multigen New Brunswick vessels. Continuous cultures were run in a 330-ml Bioflo (New Brunswick Scientific Co.) model C30 fermentor. The CO2 needed for growth and product formation was supplied through O2-free CO2 sparging or by the addition of dissolved (NaHCO3 and Na2CO3) and undissolved (MgCO3) carbonates as indicated. The initial pH was adjusted to 6.7 ± 0.1 with H2SO4. Throughout the fermentation, the temperature was controlled at 38 ± 1°C, and the pH was kept at 6.2 ± 0.1 by the addition of sterile solutions of NaOH or Na2CO3 as indicated.
Lactose and fermentation products were analyzed by high-performance liquid chromatography in acidified (1% concentrated HCl) samples (26). They were eluted with 0.012 N H2SO4 from a 300 by 7.8-mm (inside diameter) Bio-Rad HPX-87 column and were detected by a differential refractometer, recorded, and quantified by using a Waters 840 integrator. Standard curves were obtained by using various concentrations of lactose and volatile fatty acids. The concentration was computed from the area under the curve. Succinic acid was determined by an enzymatic assay using a commercial kit from Boehringer Mannheim Biochemicals.
Fermentation product yield, carbon balances, and the recovery of available hydrogen (electron balance) were computed as previously described (21). Protein was determined by the Lowry method (19).
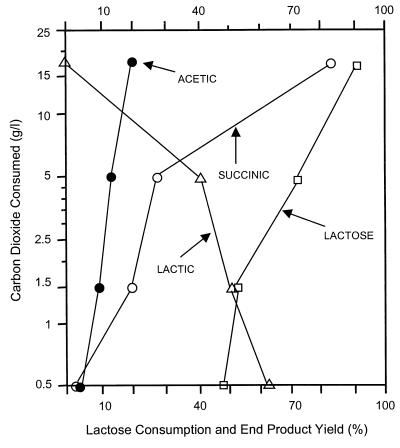
Succinate production dramatically increased when CO2 was added to the fermentation medium. Figure 1 shows the effect of carbon dioxide levels on the yield of major fermentation products formed by A. succiniciproducens. Here, carbon dioxide was supplied as MgCO3, which dissolved during the fermentation. At the highest CO2 levels (i.e., 35 g of MgCO3/liter), vigorous growth and succinate production started after a lag phase of 2 to 3 h, whereas, at the lowest CO2 levels (i.e., 1 g of MgCO3/liter), a 16- to 20-h lag phase was observed. At the highest CO2 levels, more than 90% of the lactose was consumed, the yield of succinic acid was 84%, lactate was not produced, and the ratio of succinate to acetate was 4.3 (g/g). At the lowest CO2 levels tested, 48% of the lactose was used, the main product was lactic acid, the succinate yield was lower than 4%, and the succinate/acetate ratio was 0.65 (g/g). The highest levels of CO2 consumption were correlated with the highest levels of lactose consumption and succinate production.
FIG. 1.
Influence of carbon dioxide levels on lactose consumption and the yield of whey fermentation products in A. succiniciproducens. The experiments were carried out with 158-ml sealed serum vials, and the initial lactose concentration was 45.3 g/liter. Carbon dioxide was supplied with magnesium carbonate, which dissolved and generated CO2 during fermentation.
Studies to understand the kinetics of whey lactose consumption and succinate, acetate, and formate production were conducted with 10-liter batch-mode fermentations. Carbon dioxide was supplied at high levels with 3 M NaHCO3 (45 ml/liter) through the pH control system and with CO2 gas (20 lb/in2) in the headspace. The concentrations of succinate and lactose in the final batch fermentation broth were 34.3 and 2.4 g/liter, respectively. The overall succinate yield (corrected for addition of base) was 84%. Under these CO2 nonlimiting fermentation conditions, there was a direct correlation between lactose consumption and the production of succinate, acetate, and formate. Table 1 summarizes the carbon and electron balance of whey lactose fermentation under high CO2 levels under batch conditions. Under these conditions, 80% of carbon supplied as CO2 was recovered in succinic, acetic, and formic acids.
TABLE 1.
Carbon recovery and electron balance of A. succiniciproducens raw-whey batch fermentation in the presence of high CO2 levelsa
| Substrate or product | Substrate concn (mol/100 mol) | Carbon concn (mol) | Available hydrogen concn
|
|
|---|---|---|---|---|
| mol of H/mol | mol of H/100 mol of lactose | |||
| Substrates | ||||
| Lactose | 100 | 1,200 | 48 | 4,800 |
| Lactic acid | 3 | 9 | 11 | 33 |
| CO2 | 346 | 346 | 0 | 0 |
| Products | ||||
| Succinic acid | 243 | 972 | 14 | 3,402 |
| Acetic acid | 111 | 222 | 8 | 888 |
| Formic acid | 47 | 47 | 2 | 94 |
Carbon and electron balance were determined as described by Zeikus et al. (34), except the carbon and electron balance in cells was not included. CO2 was supplied as magnesium carbonate (35 g/liter). Note that 80% of the carbon (moles) and 92% of the available hydrogen (moles of hydrogen per 100 moles of lactose) were recovered.
Table 2 compares the effect of dilution rate on steady-state parameters for whey lactose conversion to succinic acid under excess CO2 in a continuous culture. Steady-state concentrations of substrate and products were established in the fermentor after six to eight residence times. The growth rate influenced substrate product conversion, and lactose consumption and succinate yield decreased at elevated specific growth rates.
TABLE 2.
A. succiniciproducens whey succinate steady-state fermentation parameters in continuous culture at high CO2 levelsa
| Dilution rate (D, h−1) | Residual lactose concn (S, g/liter) | Succinic acid production (P, g/liter) | Productivity (PD, g/liter/h) | Base dilution factor (%)b | Overall succinate yield (%) |
|---|---|---|---|---|---|
| 0.085 | 9.0 | 24.0 | 2.1 | 8.0 | 72.0 |
| 0.15 | 11.0 | 19.8 | 3.0 | 11.0 | 64.0 |
The concentrations of lactose and NaCO3 in the feed stream were 45 and 5 g/liter, respectively. The culture vessel was continuously gassed with carbon dioxide (0.15 [vol/vol/min]). The results represent the means of 20 resident times after reaching steady-state conditions.
Relative volume of 2 N NaOH through the pH 6.2 control system.
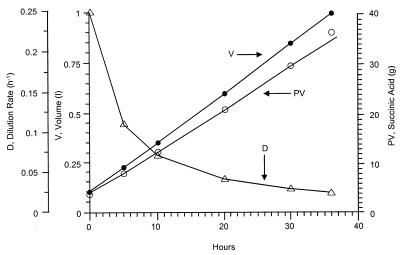
Kinetic parameters for succinate production in a variable-volume fed-batch culture are demonstrated in Fig. 2. The mass balance for lactose is
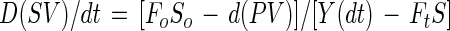 |
where V, S, and P are the time (t)-dependent volume and the concentrations of lactose and succinic acid, respectively. The concentration of lactose in the feed is So, and Y is the yield of succinate on lactose. Fo and Ft denote the constant rates of feed introduction and culture broth withdrawal. Under balanced fermentation conditions, the concentration of lactose is time invariable, and culture broth is not removed until the end of the process (ds/dt = Ft = 0), and the mass balance is FoSoY = Dt (PV)t. In other words, at a constant feed rate and yield, the time-dependent dilution rate, (D)t, and the total amount of succinate produced, (PV)t, are inversely correlated. A 1-liter variable-volume fed-batch fermentation is summarized in Table 3. The process was initiated with 0.1 liter (30 g of succinate per liter) at a constant feed rate (Fo) of 25 ml/h, in which the concentration of lactose (So) was 45 g/liter. The required carbon dioxide was supplied with Na2CO3 through the pH control system and with CO2 gas in the headspace. Following each of the five consecutive runs, 90% of the volume was removed, and the process was repeated. The inhibitory effect at an elevated specific growth rate, observed in continuous culture, is minimized with fed-batch cultivation, due to the fact that the specific growth rate decreases following a short growth period (Fig. 2).
FIG. 2.
Kinetics of succinate production from whey-lactose in variable-volume fed-batch culture with A. succiniciproducens. The constant feed rate (Fo) was 25 ml/h, and the lactose concentration (So) was 45 g/liter. The mass balance is described by Fo So Y = D(PV)t. See the text for a more detailed explanation. The Na2CO3 concentration was 5 g/liter.
TABLE 3.
Succinate production from whey lactose in variable-volume fed-batch culture with A. succiniciproducens
| Process variable | Result |
|---|---|
| Residual lactose (g/liter)a | 1.5 (± 0.5) |
| Succinic acid (g/liter)a | 34.7 (± 2.2) |
| Fermentation cycle (h)a | 34.0 (± 3.0) |
| Medium (45 g/liter of lactose) added (liters)b | 4.35 |
| Base (3 M of Na2CO3) added (liters)b | 0.55 |
| Total lactose consumed (g)b | 188.3 |
| Total succinate produced (g)b | 170.5 |
| Succinate yield (%)a | 90.6 |
Means of five consecutive runs. See Fig. 2 and the text for more experimental details.
During five consecutive runs.
A dried whey succinate fermentation product was produced in a 12-liter fermentor under batch conditions with Na2CO3 (supplied at the beginning and through the pH control system) and CO2 gas in the headspace. The product (35% succinate, 18% protein, 8.7% acetate, salts, and about 1% each lactose, lactate, and formate) was applied to in vitro studies with rumen fluid from a fistulated cow (20). The added succinate, in up to 1% of the fermentation dry solids (3.5 g of succinate per liter) was completely consumed by the mixed-rumen bacterial population, and on a molar basis, more than 90% of the succinate was decarboxylated to propionate (data not shown). The increase in propionate concentration was directly related to the added fermentation product.
The present data indicate that raw whey can be fermented with A. succiniciproducens to a succinate-rich product by using batch, continuous, and fed-batch cultivation methods. High-yield whey lactose conversion to succinic acid depends upon the available carbon dioxide. Under high-CO2 conditions, more than 90% of the lactose is consumed, the succinate yield from lactose is higher than 80%, and the succinate/acetate ratio is 3.6 to 4.3. Under CO2 limitation, lactose is only partially consumed, and the main fermentation product is lactic acid (Fig. 1). In A. succiniciproducens, high levels of CO2 stimulated phosphoenolpyruvate-carboxykinase levels, while the levels of lactate and alcohol dehydrogenases were significantly decreased (30). The highest yield of succinate from whey lactose (90%) was obtained in variable-volume fed-batch fermentation (Table 3). A fed-batch process has the advantages of both batch and continuous processes. As in batch operation, there is no limiting nutrient, and in continuous culture, toxic materials that might accumulate are continuously diluted and removed following each cycle. Fed-batch whey fermentation is widely applied for large-scale processes, and it has been used for the production of yeast single-cell protein accompanied by a reduction in lactose concentration (28).
Whey has been a preferred raw material in the food and feed industries for many years (33), since it contains large amounts of potentially recyclable nutrients. It is uneconomical to transport and expensive to dispose of (because of its high biochemical oxygen demand), and its drying is capital and energy intensive. In the final fermentation product, the concentration of protein is almost doubled, with essential amino acids coming from bacterial protein, and the concentration of whey lactose, which causes bloating in ruminants if directly fed, is markedly reduced.
In whey batch fermentations with A. succiniciproducens, 80% of the lactose carbon source was recovered in end products (Table 1). Succinic acid may be an ideal natural animal feed supplement, since it is the direct precursor of rumen propionate. In experiments with rumen fluid, succinate that had added up to a concentration of 3.26 g/liter was completely consumed, and the concentration of propionate in the ruminal fluid increased linearly with respect to the added succinate. This indicates that manipulation of rumen fermentation toward more propionate production can be achieved by supplying its direct precursor (succinate), alone or together with synthetic feed additives (ionophores) that are currently in use. It can be calculated from the concentration of succinate in the rumen (0.47 ppm) and its turnover rate (10 min−1) that an animal with a rumen capacity of 100 kg has the potential to stimulate the decarboxylation of more than 600 g of succinate to propionate per day. Thus, the potential of the rumen population to decarboxylate succinic acid is much higher than that anticipated from its ruminal concentration or from early studies (12). The turnover rate of rumen succinate is more than 300 times higher than that of the widely used lactic acid (18). In other words, much higher levels of lactate will be required to achieve the same level of propionate formation. However, increased lactate in the rumen can cause lactic acidosis and ruminal dysfunction (31). Succinic acid can be metabolized by rumen microflora to amino acids (2, 13) and sugar (18). Its metabolism in the animal (through the tricarboxylic acid cycle) increases oxygen uptake, inhibits ketosis, and serves as a buffering agent, and its derivatives prevent bloating in cattle (9). The metabolism of succinate by the animal (to acetyl coenzyme A) generates two additional ATPs in comparison to the currently used fumaric acid (14).
Our studies demonstrate that it is possible to produce succinate from whey in high concentrations and yield. The fermentation product can be used potentially as an animal feed supplement directly, or it can be supplemented with essential salts, electrolytes, and ammonia. However, the extent of fermentation product augmentation that will provide sufficient propionate to enhance feedlot performance and its optimal composition will require data that can be achieved only from in vivo experiments.
Acknowledgments
We thank Keith Strevette for technical assistance and Sue Ann Walker and Judy Pennington for preparing the manuscript.
This research was supported by U.S. Department of Energy grant DE-F602-93ER20108.
REFERENCES
- 1.Ahren, W. P., F. A. Dale, and E. S. Lawrence. May 1988. Fermentation of whey to propionic acids. U.S. patent 4,743,453.
- 2.Allison M J, Robinson I M, Baetz A L. Synthesis of α-ketoglutarate by reductive carboxylation of succinate in Veillonella, Selenomonas, and Bacteroides species. J Bacteriol. 1979;140:980–986. doi: 10.1128/jb.140.3.980-986.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergen W G, Bates D B. Ionophores: their effect on production efficiency and mode of action. J Anim Sci. 1984;58:1465–1483. doi: 10.2527/jas1984.5861465x. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn T H, Hungate R E. Succinic acid turnover and propionate production in the bovine rumen. Appl Microbiol. 1963;11:132–135. doi: 10.1128/am.11.2.132-135.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnell T W, Cromwell G L, Stahly T S. Effects of dried whey and copper sulfate on the growth responses to organic acid in diets for weanling pigs. J Anim Sci. 1988;66:1100–1108. doi: 10.2527/jas1988.6651100x. [DOI] [PubMed] [Google Scholar]
- 6.Caspari D, Macy J M. The role of carbon dioxide in glucose metabolism of Bacteroides fragilis. Arch Microbiol. 1983;135:16–24. doi: 10.1007/BF00419476. [DOI] [PubMed] [Google Scholar]
- 7.Datta, R., D. A. Glassner, M. K. Jain, and J. R. Vick Roy. December 1992. Fermentation and purification process for succinic acid. U.S. patent 5,168,055.
- 8.Davis C P, Cleven D, Brown J, Balish E. Anaerobiospirillum, a new genus of spiral-shaped bacteria. Int J Syst Bacteriol. 1976;26:498–504. [Google Scholar]
- 9.Davis J O, Essing H W. Comparison of three blot-preventing compounds for cattle grazing clover. Can J Anim Sci. 1972;52:329–335. [Google Scholar]
- 10.Dimius D M, Simpson M E, March P B. Effect of monensin fed with forage on digestion and the ruminal ecosystem of steers. J Anim Sci. 1976;42:229–234. doi: 10.2527/jas1976.421229x. [DOI] [PubMed] [Google Scholar]
- 11.Dimroth P. Biotin-dependent decarboxylases as energy transducing systems. Ann N Y Acad Sci. 1985;447:72–85. doi: 10.1111/j.1749-6632.1985.tb18426.x. [DOI] [PubMed] [Google Scholar]
- 12.Doetsch R N, Robinson R Q, Brown R E, Storv J C. Catabolic reactions of mixed suspensions of bovine rumen bacteria. J Dairy Sci. 1953;36:825–831. [Google Scholar]
- 13.Emmanual B, Milligan L P. Enzymes of the conversion of succinate to glutamate in extracts of rumen microorganisms. Can J Biochem. 1972;51:1–8. doi: 10.1139/o72-001. [DOI] [PubMed] [Google Scholar]
- 14.Falkowski J F, Aherne F X. Fumaric and citric acid as feed additive in starter pig nutrition. J Anim Sci. 1984;58:935–938. [Google Scholar]
- 15.Fisher L J, Erfle J D, Saur F D. Preliminary evaluation of the addition of glucogenic materials to the ration of lactating cows. Can J Anim Sci. 1971;51:721–727. [Google Scholar]
- 16.Goodrich R D, Garrett J E, Gast D R, Kirck M A, Larson D A, Meijik J C. Influence of monensin on the performance of cattle. J Anim Sci. 1984;58:1484–1498. doi: 10.2527/jas1984.5861484x. [DOI] [PubMed] [Google Scholar]
- 17.Howlett M R, Mountfort D O, Turner K W, Robertson A M. Metabolism and growth yields in Bacteroides ruminocola strain B14. Appl Environ Microbiol. 1976;32:274–283. doi: 10.1128/aem.32.2.274-283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hungate R E. The rumen and its microbes. New York, N.Y: Academic Press; 1966. [Google Scholar]
- 19.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Lumanta, I. G., M. G. Beconi-Baker, N. S. Samuelov, M. K. Jain, and M. T. Yokoyama. Unpublished data.
- 21.Lynd L, Kerby R, Zeikus J G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982;149:255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons T P, editor. Biotechnology in the feed industry. Nicholsville, Ky: Alltech Technical Publications; 1988. pp. 1–16. [Google Scholar]
- 23.Potter E L, Pusser D B, Cline J H. Effects of various energy sources upon plasma-free amino acids in sheep. J Nutr. 1968;95:655–663. doi: 10.1093/jn/95.4.655. [DOI] [PubMed] [Google Scholar]
- 24.Reddy C A, Henderson H E, Erdman M D. Bacterial fermentation of cheese whey for production of a ruminant feed supplement rich in crude protein. Appl Environ Microbiol. 1976;32:769–776. doi: 10.1128/aem.32.6.769-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly P E B, Ford E J H. The effects of different dietary contents of protein on amino acid and glucose production and on the contribution of amino acids to gluconeogenesis in sheep. Br J Nutr. 1971;26:249–263. doi: 10.1079/bjn19710032. [DOI] [PubMed] [Google Scholar]
- 26.Ross L F, Chapital D C. Simultaneous determination of carbohydrates and products of carbohydrate metabolism in fermentation mixtures by HPLC. J Chromatogr Sci. 1987;25:112–117. doi: 10.1093/chromsci/25.3.112. [DOI] [PubMed] [Google Scholar]
- 27.Rumpler W V, Johnson D E, Bates D G. The effect of high cation concentration on methanogenesis by steers fed diets with and without ionophores. J Anim Sci. 1986;62:1737–1741. doi: 10.2527/jas1986.6261737x. [DOI] [PubMed] [Google Scholar]
- 28.Samuelov N S. Upgrading of whey solids in fed-batch culture. Isr J Med Sci. 1982;18:17–23. [Google Scholar]
- 29.Samuelov N S. Single-cell protein production: review of alternatives. In: Mizrahi A, Van Werzel A L, editors. Advances in biotechnological processes. I. Alan R. New York, N.Y: Liss, Inc.; 1983. pp. 293–333. [Google Scholar]
- 30.Samuelov N S, Lamed R, Lowe S, Zeikus J G. Influence of CO2-HCO3− levels and pH on growth, succinate production, and enzyme activities of Anaerobiospirillum succiniciproducens. Appl Environ Microbiol. 1991;57:3013–3019. doi: 10.1128/aem.57.10.3013-3019.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Styler L L. Influence of acidosis on rumen function. J Anim Sci. 1976;43:910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Watanabe J, Kiramitsu S, Maruyama H B. Comparison of the activity of ionophores with other antimicrobial agents against anaerobes. Antimicrob Agents Chemother. 1981;19:519–525. doi: 10.1128/aac.19.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb B H. Proceedings of the whey utilization conference, ARS-73-69. U.S. Philadelphia, Pa: Department of Agriculture; 1970. Utilization of whey in food and feeds; pp. 102–111. [Google Scholar]
- 34.Zeikus J G, Fuchs G, Kenealy W, Thauer R K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977;132:604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]