Abstract
Background:
Nonviral sexually transmitted infections (STIs) increase risk of sexually-acquired HIV infection. Updated risk estimates carefully scrutinizing temporality bias of studies are needed.
Methods:
We conducted a systematic review (PROSPERO # CRD42018084299) of peer-reviewed studies evaluating variation in risk of HIV infection among high-risk heterosexuals diagnosed with any of: Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, Treponema pallidum, and/or Trichomonas vaginalis. We searched PubMed, Web of Science, and Embase databases through December 2017 and included studies where STIs and HIV were assessed using laboratory tests or medical exams and where STI was diagnosed before HIV. After dual screening, data extraction, and risk of bias assessment, we meta-analytically pooled risk ratios (RR).
Results:
We found 32 eligible studies reporting k=97 effect size estimates of HIV acquisition risk due to infection with one of the above STIs. Most data were based on females engaged in sex work or other high-risk occupations in developing countries. Many studies did not measure or adjust for known confounders including drug injection and condom use and most were at medium or high risk of bias due to potential for undetected HIV infection to have occurred prior to STI infection. HIV acquisition risk increased among females infected with any pathogen; the effect was greatest for females infected with Mycoplasma genitalium (RR=3.10; 95% CI 1.63, 5.92; k=2) and gonorrhea (RR=2.81; 95% CI 2.25, 3.50; k=16) but also statistically significant for females infected with syphilis (RR=1.67; 95% CI 1.23, 2.27; k=17), trichomonas (RR=1.54; 95% CI 1.31, 1.82; k=17) and chlamydia (RR=1.49; 95% CI 1.08, 2.04; k=14). For males, data were space except for syphilis (RR=1.77; 95% CI 1.22, 2.58; k=5).
Conclusion:
Nonviral STI increases risk of heterosexual HIV acquisition, although uncertainty remains due to risk of bias in primary studies.
Keywords: HIV, STI, systematic review, heterosexual
SUMMARY
We examine temporal relationships between heterosexual acquisition of nonviral STIs and HIV, finding increased risk for females with Mycoplasma genitalium, gonorrhea, syphilis, trichomonas, or chlamydia and males with syphilis.
INTRODUCTION
Nonviral sexually transmitted infections (STIs) are among the most common infectious diseases globally, with incidence increasing.1 In 2012, there were an estimated 131 million new cases of chlamydia, 78 million new cases of gonorrhea, 143 million new cases of trichomoniasis, and 6 million new cases of syphilis.1 Longstanding evidence has associated STI infection with increased risk of HIV transmission and acquisition2–9 due to ulceration, localized immune responses involving CD4 cell proliferation, and elevated HIV shedding, among other mechanisms.10,11
Rationale for systematic review
Since 1992, numerous systematic reviews have examined the relationship between STIs and HIV infections2–10 although effect size estimates vary.4,10,12,13 Some change in estimates over time is expected due to advances in diagnostic technology, e.g., nucleic acid amplification that more accurately classifies disease status by detecting infections with greater sensitivity and specificity14,15 and improved antiretroviral treatment that dramatically lowers risk of HIV transmission.16 Review methods also may influence effect estimates through criteria for selecting primary studies: many prior reviews included cross-sectional studies that reported correlation between STI and HIV infection but could not address infection sequence. Other reviews included cohort studies that involved simultaneous STI and HIV diagnosis, similarly obscuring the issue of infection temporality.17–19
Refined, updated estimates of the effect of STI infections on HIV acquisition and transmission risk can improve the epidemiologic modeling that informs HIV prevention strategies. With more accurate estimates, policymakers and public health leaders can better project population-level impacts of budgetary and programmatic investments in STI testing, pre-exposure prophylaxis (PrEP), and other HIV prevention strategies. This systematic review and meta-analysis addresses these issues through an exclusive focus on studies where STI diagnosis was confirmed to precede HIV diagnosis.
METHODS
Full methods for this review are described elsewhere.20 Briefly, we conducted a parent systematic review on the effect of six STI pathogens (Chlamydia trachomatis, Herpes Simplex Virus type 2 (HSV-2), Mycoplasma genitalium, Neisseria gonorrhoeae, Treponema pallidum, and Trichomonas vaginalis) on HIV acquisition and transmission among high-risk populations. This manuscript addresses high-risk heterosexual populations; our database search included studies on men who have sex with men (MSM).
We followed Cochrane Collaboration recommendations.21 We registered our protocol in the PROSPERO database (CRD42018084299).22,23 We used the Population, Exposure, Comparator, Outcomes schema to guide screening and data extraction. We followed Grading of Recommendations Assessment, Development and Evaluation Guideline (GRADE) methods to assess risk of bias at the effect-size level24 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting.25
Study searches and screening
We combined keywords and database-specific syntax to develop search strategies implemented in PubMed in December 2017 and Web of Science and Embase in January 2018. Two authors reviewed records independently. (Appendices A–C).
Study eligibility
We included peer-reviewed studies where participants were confirmed to be HIV-uninfected at baseline and were classified as STI-infected or -uninfected prior to HIV diagnosis or censoring. We included studies on risk of HIV acquisition (comparing STI-infected and uninfected participants who were HIV-uninfected at baseline) as well as transmission to partners (published separately). We included the following high-risk populations: female sex workers and their clients, persons in other high-risk occupations (e.g., bar workers, migrant workers), STI clinic patients, serodiscordant couples, and other high-risk heterosexually-active persons as defined by study authors.
We excluded studies for three reasons: self-reported data on either infection, an interval between STI and HIV assessment of two years or greater, and STI diagnosis not confirmed to precede HIV diagnosis. We included effect sizes with sufficient data to calculate the effect size in the form of risk ratio (RR) and 95% confidence interval (CI).
Data extraction and standardization
We developed standardized data extraction tools in Google Sheets to record essential data including effect size, year and location of data collection, demographics, intervention exposure (including antiretroviral therapy among partners, PrEP, condom use, etc.), diagnosis and treatment of STIs, diagnostic methods and timing, and factors affecting risk of bias. We conducted dual independent data extraction with raters using spreadsheet formulas to identify discrepancies, which they resolved via discussion or supervisor consultation. We contacted authors for missing information.
Risk of bias assessment
We adapted our risk of bias assessment from Making GRADE the Irresistible Choice (MAGIC).21,24,26,27 We integrated criteria for timing and accuracy of STI and HIV diagnosis into the MAGIC domains for exposure, outcome, and prognostic indicator assessment. (Appendix D). For example, shorter intervals between STI diagnosis and HIV outcome assessment and/or the use of an RNA test for HIV resulted in lower-risk ratings. We rated each domain on the following four-point scale: “very low,” “low,” “medium”, and “high” risk of bias.
Data analysis and synthesis
We used Stata v14.228 for data analysis. We converted all effect sizes to RR; for studies reporting odds ratios (OR), we used the Zhang and Yu29 method for conversion. For each STI pathogen, we meta-analytically pooled effect sizes using a random-effects model given methodological and implementation heterogeneity among included studies. We reported heterogeneity using the I2 statistic (percentage)21 and performed sensitivity analyses by recalculating pooled estimates without each effect size.
In sub-group analysis, we assessed the effect of geographic setting, HIV and STI assessment methods, and assessment intervals. We also conducted sub-group meta-analysis that excluded data with the highest potential risk of bias: that from case-control studies, unadjusted effect sizes, and studies with more than 12 months between STI and HIV assessments.
Results
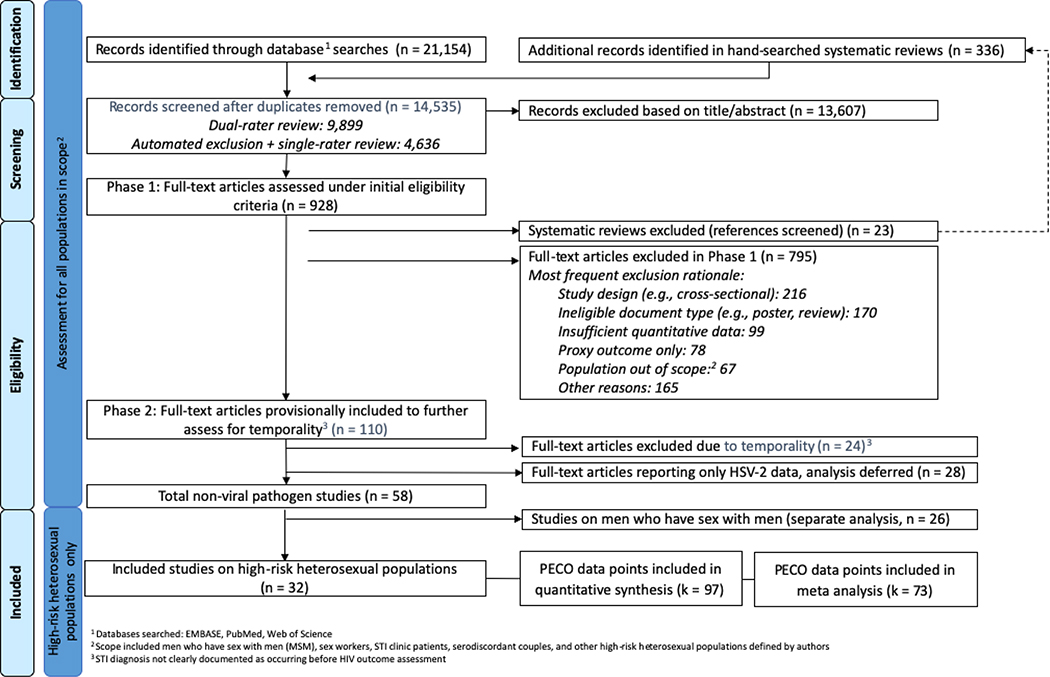
Our searches returned 14,535 unique records on both heterosexual and MSM populations. We excluded 13,607 based on title and/or abstract review (Figure 1) and 842 in full-text review (Appendix E). We also excluded 28 studies on HSV-2 infection because that pathogen was addressed in a recent review.30 Of the 58 eligible studies, 32 addressed risk of HIV among high-risk heterosexual populations (Table 1) and were included in this review.
Figure 1. Identification and screening of bibliographic records for systematic review of the effect of nonviral STI diagnosis on the risk of HIV seroconversion among high-risk heterosexuals (search up to January 2018).
Table 1.
Included studies assessing the effect of nonviral STI on the risk of HIV acquisition among high-risk heterosexuals (n=32)
| Females (n=25) | |||||||||||
|
| |||||||||||
| Author & Year | Risk Group | Country (study location) | Min. Age | Data or Recruitment Source | Sample | Study Period | Study Design | STI Pathogen | STI Assessment | Risk Ratio (calculated) † | Confounders Adjusted For |
|
| |||||||||||
| Auvert 2011331 | FSW | South Africa | 19 | Participants in a Nonoxynol 9 trial (COL-1492), who were recruited from truck stops along a major highway, Kwazulu-Natal Midlands | N=88 FSW Median age: 24 Randomized to intervention or placebo gel |
1996–2000 | Prospective Cohort | CT | ELISA/ NAAT | 0.25 (0.06, 1.04) | HPV genotypes, other STIs, age group, intervention group, condom use, anal sex, duration of sex work, number of clients per week |
| NG | Culture/ NAAT | 2.30 (0.53, 9.98) | |||||||||
| TP | Serology | 0.66 (0.17, 2.56) | |||||||||
| TV | NAAT | 1.40 (0.41, 4.78) | |||||||||
|
| |||||||||||
| Braunstein 2011332 | FSW | Rwanda | 18 | New cohort recruited from community meetings in 3 Kigali districts | N=397 FSW Median age: 24 |
2006–2009 | Prospective Cohort | CT -baseline | NAAT | 1.10 (0.10, 12.10) | NA |
| NG - baseline | NAAT | 2.80 (0.90, 8.71) | NR | ||||||||
| NG - incident | NAAT | 1.70 (0.50, 5.90) | |||||||||
| TP-baseline | Serology | 1.70 (0.40, 7.10) | |||||||||
| TP-incident | Serology | 5.70 (1.30, 24.99) | |||||||||
| TV-baseline | Culture and wet mount | 0.90 (0.30, 3.10) | NA | ||||||||
| TV-incident | Culture and wet mount | 1.00 (0.30, 3.33) | |||||||||
|
| |||||||||||
| Ghys 2001333 | FSW | Cote d'Ivoire | NR | Ministry of Health HIV/ STD Prevention campaign, Programme de Prevention et de Prise en charge des MST/SIDA chez les femmes libres et leurs Partenaires (PPP), Abidjan |
N=542 FSW Median age: 27 |
1992–1998 | Prospective Cohort | NG | Culture | 4.80 (2.10, 10.97) | NR |
| TP | Serology | 1.13 (0.39, 3.27) | NA | ||||||||
| TV | Medical Exam | 2.80 (1.30, 6.03) | NR | ||||||||
|
| |||||||||||
| Hanson 2005334 | Mixed, recruited from STI clinic attendees | United States | 14 | STI clinic in New Orleans | N=10,879 STI clinic patients 75% male Med. Age: 28.0 (males), 23.9 (females) PWID: 4.7% MSM: 4.5% |
1990–1998 | Retrospective Cohort | NG | Culture or NAAT | 0.60 (0.10, 3.60) | NR |
| TP | Serology, exam, and medical records | 7.40 (1.70, 32.21) | |||||||||
|
| |||||||||||
| Hughes 2012335 | Sero-discordant couples | South Africa, Zambia, Kenya, Rwanda, Tanzania, and Uganda | 18 | Partners in Prevention HSV/HIV Transmission Study | N=3,408 Serodiscordant couples where HIV-infected partner was co-infected with HSV-2 HIV-infected partner: 97.4% Female Med. Age: 32 ART: 27.6% Virally suppressed: 0% |
2004–2007 | Prospective Cohort | TV | NAAT | 2.57 (1.42, 4.65) | Viral load, age, HSV-2 status at enrollment, GUD during follow-up, cervicitis or vaginitis during follow-up. |
|
| |||||||||||
| Kapiga 2007336 | Mixed, recruited from women in high-risk occupation | Tanzania | 14 | New cohort recruited from bars and hotels in Moshi | N=845 High-risk women (27.4% FSW) Mean Age: 27.9 |
2002–2005 | Prospective Cohort | CT- baseline | ELISA | 5.20 (1.90, 14.4) | GUD during follow-up, CT at baseline, disturbances in vaginal flora and BV at baseline, male partner who had other partners during follow-up |
| CT-incident | ELISA | 0.90 (0.10, 8.10) | NA | ||||||||
| TP- baseline | Serology | 2.10 (0.30, 15.40) | |||||||||
| TP-incident | Serology | 2.80 (0.90, 8.71) | |||||||||
| TV- baseline | Wet mount | 1.20 (0.40, 4.10) | |||||||||
| TV-incident | Wet mount | 1.40 (0.30, 6.53) | |||||||||
|
| |||||||||||
| Kaul 2004337 | FSW | Kenya | 18 | New cohort recruited from Kibera urban slum area of Nairobi | N=466 FSW Mean age: 28.6 PWID ever: 4.0% |
1998–2002 | Prospective Cohort | CT | NAAT | 3.00 (1.10, 8.18) | NA. Other STIs and number of partners were tested and found not significant |
| NG | NAAT | 4.90 (1.70, 14.12) | |||||||||
| TV | Culture | 0.70 (0.20, 2.45) | |||||||||
|
| |||||||||||
| Laga 1993338 | FSW | Zaire | 15 | New cohort of FSW in Kinshasa | N=431 FSW Mean age: 25.8 |
1988–1991 | Nested case control | CT | Unspecified lab test | 2.23 (1.28, 3.88) | NR |
| NG | Unspecified lab test | 3.49 (2.11, 5.77) | |||||||||
| TP | Serology | 1.91 (0.78, 4.72) | NA | ||||||||
| TV | Smear | 1.58 (0.92, 2.72) | NR | ||||||||
|
| |||||||||||
| Martin 1998339 | FSW | Kenya | 18 | New cohort recruited from STI clinic in Mombasa | N=3,639 FSW Mean age: 26 PWID: 0% |
1993–1997 | Prospective Cohort | CT | EIA | 1.30 (0.50, 3.38) | Workplace, number of sex partners, condom use, parity, vulvitis, GUD, vaginal discharge, BV, candida, and NG |
| NG | Culture | 1.80 (1.00, 3.24) | |||||||||
| TP | Serology | 1.60 (0.60, 4.27) | |||||||||
| TV | Wet mount | 1.20 (0.70, 2.06) | |||||||||
|
| |||||||||||
| Masese 201540 | FSW | Kenya | 18 | Mombasa Cohort | N=1,964 FSW Med. age=25 |
1993–2012 | Prospective Cohort | NG | Culture or NAAT | 2.05 (1.38, 3.05) | Age, workplace, hormonal contraceptive use, number of sexual partners, condomless sex, tobacco use, calendar year, other STIs |
| TV | Wet mount | 1.41 (0.99, 2.01) | |||||||||
|
| |||||||||||
| McClelland 200741 | FSW | Kenya | NR | Municipal clinic in Mombasa | N=1,335 FSW Med. Age=26 |
1993–2004 | Prospective Cohort | TV | Wet mount | 1.52 (1.04, 2.22) | NR |
|
| |||||||||||
| Metha 200642 | STI clinic attendees | United States | 12 | Records from STI clinics in Baltimore | N=10,535 STI clinic patients Male: 59.2% PWID (ever): 5% |
1993–2002 | Prospective Cohort | NG | Culture, stain, or NAAT | 2.78 (1.21, 6.39) | NR |
| TP | Serology and exam | 2.82 (1.08, 7.36) | |||||||||
|
| |||||||||||
| Mlisana 201243 | FSW | South Africa | 16 | CAPRISA 002 Acute HIV Infection Study of high-risk women in Durban | N=245 High-risk women (78.8% FSW) Median age: 34.2 |
2004–2005 | Prospective Cohort | CT | NAAT | 0.90 (0.18, 4.50) | STIs, clinical symptoms, demographic and behavioral factors |
| MG | NAAT | 4.08 (0.83, 20.06) | |||||||||
| NG | NAAT | 4.62 (1.34, 15.93) | |||||||||
| TV | NAAT | 1.74 (0.62, 4.88) | |||||||||
|
| |||||||||||
| Nagot 200544 | FSW | Burkina Faso | NR | New cohort recruited from SW workplaces in Bobo-Dioulasso | N=377 Women who exchanged sex for money or goods |
1998–2002 | Prospective Cohort | CT | DIF | 0.56 (0.21, 1.49) | NA |
| TV | Wet mount | 0.71 (0.22, 2.29) | |||||||||
|
| |||||||||||
| Plourde 199445 | STI clinic attendees | Kenya | 18 | New cohort recruited from Nairobi City Commission Special Treatment Clinic | 134 STI clinic patients (7.4% SW history) Female: 100% Med. age: 33 |
1988–1990 | Prospective Cohort | CT | Culture | 0.81 (0.05, 12.53) | NA |
| NG | Culture | 5.00 (1.00, 25.00) | |||||||||
| TP | Serology and darkfield microscopy | 0.28 (0.02, 4.62) | |||||||||
| TV | Wet mount | 0.61 (0.14, 2.74) | |||||||||
|
| |||||||||||
| Plummer 199146 | FSW | Kenya | NR | New cohort recruited from the local community, Nairobi | N=595 FSW Median age: 30.2 |
1985–1987 | Prospective Cohort | CT | Culture | 1.58 (0.92, 2.71) | Oral contraceptive use, GUD, CT, condom use, number of partners |
| TP | Culture | 1.10 (0.86, 1.41) | NA | ||||||||
|
| |||||||||||
| Priddy 201147 | FSW | Kenya | 18 | New cohort recruited from FSW social empowerment none in Nairobi | N=200 FSW Mean age: 28 Illicit drug use history: 21.5% |
2008 | Prospective Cohort | CT | NAAT | 1.46 (0.08, 26.64) | Age, income, ever/never married, number of dependents, age at first sex, regular paid partners per week, regular casual partners per week, condom use by partner group and sexual act, vaginal washing, lubricant use, alcohol use, other STIs |
| NG | NAAT | 1.33 (0.07, 25.27) | |||||||||
| TP | Serology | 3.15 (0.15, 66.15) | |||||||||
| TV | Culture | 0.87 (0.05, 15.14) | |||||||||
|
| |||||||||||
| Ramjee 200549 | FSW | South Africa | NR | New cohort recruited from 5 truck stops, KwaZulu-Natal | N=196 FSW Mean age: 25 |
NR | Prospective Cohort | NG | Culture | 1.92 (0.84, 4.39) | NR |
|
| |||||||||||
| Riedner 200650 | High-risk occupation | Tanzania | 16 | Mbeya Medical Research Programme recruitment at 14 trading centers and towns in Mbeya Region | N=600 Female bar workers Mean age: 25.5 |
2000–2004 | Prospective Cohort | TP | Serology and NAAT | 2.23 (1.03, 4.83) | NR |
|
| |||||||||||
| Su 201651 | FSW | China | 16 | Kaiyuan longitudinal study of FSW, recruitment from local SW venues in Yunnan | N=1,158 FSW Mean age: 26.7 History of drug use: 16.1% |
2006–2014 | Prospective Cohort | TP at baseline | Serology | 2.69 (1.11, 6.53) | NA |
| TP during follow-up | Serology | 3.23 (1.36, 7.67) | |||||||||
|
| |||||||||||
| Vandepitte 201353* | FSW | Uganda | NR | New cohort of self-reporting FSWs and/or women employed in entertainment facilities in Kampala | N=646 FSW Illicit drug use: 2.3% |
2008–2011 | Prospective Cohort | CT | NAAT | 1.91 (0.83, 4.40) | Age, calendar time, age at first sexual intercourse, number of lifetime sexual partners, use of alcohol in past 3 months, number of paying clients in past 3 months, inconsistent condom use with paying clients in past 3 months, current pregnancy, and NG, TV, MG |
| MG | NAAT | 2.19 (1.11, 4.36) | |||||||||
| NG | NAAT | 5.41 (2.76, 10.60) | |||||||||
| TP | Serology | 1.64 (0.48, 5.60) | |||||||||
| TV | Culture | 2.26 (1.03, 4.96) | |||||||||
|
| |||||||||||
| Vandepitte 201452* | FSW | Uganda | 14 | Same cohort as Vandepitte 2013 | N=646 High-risk women (89.2% FSW) |
2008–2011 | Nested case control | CT | NAAT | 2.93 (0.78, 4.05) | NA |
| MG | NAAT | 2.94 (1.45, 5.96) | Source of income, alcohol use, HSV-2 infection | ||||||||
| NG | NAAT | 2.94 (1.55, 4.03) | NA | ||||||||
| TP | Serology | 1.46 (0.93, 1.92) | |||||||||
| TV | Culture | 1.00 (0.37, 2.08) | |||||||||
|
| |||||||||||
| Wall 201754 | Sero-discordant couples | Zambia | NR | New cohort recruited from couples' VCT, Lusaka | N=2,949 couples Serodiscordant couples Female HIV+: 54.3% ART: 0% |
1994–2012 | Prospective Cohort | TP | Serology | 0.93 (0.56, 1.54) | NA |
|
| |||||||||||
| Wang 201255 | FSW | China | 16 | New cohort recruited from known SW venue in Kaiyuan City | N=2,051 FSW PWID: 9.5% |
2006–2009 | Prospective Cohort | CT | NAAT | 1.20 (0.40, 3.60) | NA |
| NG | NAAT | 2.20 (0.51, 9.49) | |||||||||
| TP | Serology | 2.50 (0.71, 8.80) | |||||||||
| TV | Wet mount | 0.80 (0.11, 5.82) | |||||||||
|
| |||||||||||
| Watson-Jones 200956 | High-risk occupation | Tanzania | 16 | New cohort recruited from bars, guesthouses, and similar facilities in 19 communities | N=821 High-risk women 100% HSV-2 infected |
NR-2008 | Prospective Cohort | CT | NAAT | 2.56 (1.21, 5.42) | Age |
| NG | NAAT | 2.91 (1.23, 6.88) | |||||||||
| TP | NAAT | 0.69 (0.21, 2.27) | |||||||||
| TV | Culture | 1.81 (1.05, 3.12) | |||||||||
| Males (n=5) | |||||||||||
|
| |||||||||||
| Author & Year | Risk Group |
Country (study location) |
Min. Age | Data or Recruitment Source | Sample | Study Period | Study Design | STI Pathogen | STI Assessment | Risk Ratio (calculated) † | Confounders Adjusted For |
|
| |||||||||||
| Hanson 2005334 | Mixed, recruited from STI clinic attendees | United States | 14 | STI clinic in New Orleans | N=10,879 STI clinic patients 75% male Med. Age: 28.0 (males), 23.9 (females) PWID: 4.7% MSM: 4.5% |
1990–1998 | Retrospective Cohort | NG | Medical records | 2.80 (1.50, 5.20) | NR |
| TP | Serology, exam, and medical records | 2.10 (0.70, 6.30) | |||||||||
|
| |||||||||||
| Heffron 201157 | High-risk occupation | Zambia | 18 | New cohort of seasonal farm workers from a town on a major roadway | N=842 Male farm workers 46.9% migrant workers |
2006–2007 | Prospective Cohort | TP | Serology | 2.10 (0.60, 7.35) | Age, widowhood, circumcision, self-report of genital ulcers, HSV-2 at baseline |
|
| |||||||||||
| Rakwar 199948 | High-risk occupation | Kenya | 16 | New cohort of Male trucking-company employees, Mombasa | N=992 Male trucking company employees Med. age: 29 PWID: 0% |
1993–1997 | Prospective Cohort | CT | Stain or EIA | 0.80 (0.30, 1.90) | NA |
| TP | Serology | 2.70 (1.30, 5.61) | |||||||||
|
| |||||||||||
| Telzak 199358 | STI clinic attendees | United States | NR | Clinic records of patients who tested HIV-negative and returned for results, New York | N=1,679 STI clinic patients (heterosexual risk only) Med. Age: 30 |
Approx.1990 | Prospective Cohort | TP | Serology and darkfield microscopy | 3.40 (0.82, 14.12) | NA |
|
| |||||||||||
| Wall 201754 | Sero-discordant couples | Zambia | NR | New cohort recruited from couples' VCT, Lusaka | N=2,949 couples Serodiscordant couples Female HIV+: 54.3% ART: 0% |
1994–2012 | Prospective Cohort | TP | Serology | 1.26 (0.80, 1.98) | NA |
|
Mixed-Sex None Not Included in Meta-Analysis (n=6) Studies reporting sex-specific data are also listed above | |||||||||||
|
| |||||||||||
| Author & Year | Risk Group |
Country (study location) |
Min. Age | Data or Recruitment Source | Sample | Study Period | Study Design | STI Pathogen | STI Assessment | Risk Ratio (calculated) † | Confounders Adjusted For |
|
| |||||||||||
| Deschamps 199659 | Sero-discordant couples | Haiti | NR | New cohort recruited from Group Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes at National Institute for Laboratory Research, Port-au-Prince | N=475 serodiscordant couples Mean Age: 33 ART: 0% PWID: 0% |
1988–1992 | Prospective Cohort | TP-both partners | Serology | 4.47 (1.33, 14.98) | NA |
| TP-HIV-uninfected partner only | Serology | 2.89 (1.36, 6.16) | |||||||||
|
| |||||||||||
| Hughes 2012335 | Sero-discordant couples | South Africa, Zambia, Kenya, Rwanda, Tanzania, and Uganda | 18 | Partners in Prevention HSV/HIV Transmission Study | N=3,408 Serodiscordant couples where HIV-infected partner was co-infected with HSV-2 HIV-infected partner: 97.4% Female Med. Age: 32 ART: 27.6% Virally suppressed: 0% |
2004–2007 | Prospective Cohort | CT | NAAT | 1.67 (0.53, 5.30) | NR |
| TP | Serology | 2.44 (1.22, 4.88) | |||||||||
|
| |||||||||||
| Kassler 199460 | Mixed, recruited from STI clinic attendees | United States | NR | Records from Baltimore City Health Department STI clinics | N=6,175 STI clinic attendees Med. Age: 25 PWID: 17.4% |
1988–1990 | Case control | NG | Stain | 3.12 (1.24, 5.03) | NR |
| TP | Serology | 1.51 (0.14, 2.90) | NA | ||||||||
| TV | Wet mount | 1.69 (0.77, 2.55) | |||||||||
|
| |||||||||||
| Metha 200642 | STI clinic attendees | United States | 12 | Records from STI clinics in Baltimore | N=10,535 STI clinic attendees Male: 59.2% PWID (ever): 5% |
1993–2002 | Prospective Cohort | NG | Culture, stain, or NAAT | 1.67 (0.99, 2.80) | NR |
| TP | Serology and exam | 2.25 (1.08, 4.67) | |||||||||
|
| |||||||||||
| Otten 199461 | STI clinic attendees | United States | NR | Records from 4 public STI clinics in Dade County (Miami), Florida | N=5,164 STI clinic patients Male: 65.8% |
1987–1990 | Retrospective Cohort | TP | Serology | 3.50 (0.10, 6.90) | NA |
|
| |||||||||||
| Ruzagira 201162 | Sero-discordant couples | Uganda | 18 | New cohort of couples referred from various VCT programs in Masaka District | N=495 serodiscordant couples Male: 69% Mean age: 36.2 |
2006–2009 | Prospective Cohort | TP-baseline | Serology | 1.80 (0.50, 5.90) | NA |
| TP-incident | Serology | 3.20 (1.30, 7.70) | |||||||||
Vandepitte 2013 and Vandepitte 2014 report data from the same study. We included multivariate-adjusted data from Vandepitte 2013 in our analysis of CT, NG, TP, and TV. Because both studies reported multivariate-adjusted data on MG, we used data from Vandepitte 2014, which reported on a shorter interval between MG and HIV diagnoses.
Meta-analyzed RR reflect confidence intervals as calculated with Stata v.14.2, the upper limits of which may differ from RR reported as published in primary studies.
ART = Antiretroviral Therapy; BV = Bacterial Vaginosis; CT = Chlamydia; DIF = Direct Immunofluorescence; EIA = Enzyme Immunoassay; ELISA = Enzyme-Linked Immunosorbent Assay; FSW = Female Sex Workers; GUD = Genital Ulcer Disease; HR = Hazard Ratio; HSV= Herpes Simplex Virus; Med. = Median; MG=Mycoplasma genitalium; NA = Not Applicable; NAAT = Nucleic Acid Amplification; NG = Gonorrhea; NR = Not Reported; PWID = People who Inject Drugs; RR = Risk Ratio; STI = Sexually Transmitted Infection; TP = Syphilis; TV=trichomoniasis vaginalis, VCT = Voluntary HIV Counseling and Testing Italic = effect size not included in meta-analysis
Study-level descriptive data
Table 2 summarizes the characteristics of included studies. Studies were published from 1991–2017, with data collection beginning between 1985–2008. The large majority (27, 84.4%) were prospective cohorts. The same number (27, 84.4%) were conducted in low- or middle-income countries that are not members of the Organisation for Economic Co-operation and Development (OECD). Five (15.6%) studies were conducted in the United States (US), the only OECD country represented.
Table. 2.
Characteristics of included studies (n=32) and effect sizes (k=97) assessing the effect of nonviral STI on the risk of HIV seroconversion among high-risk heterosexuals
| Total Studies (n=32) | Total Effect Sizes (k=97*) | |||
|---|---|---|---|---|
| Characteristics of Included Studies | n | % | k | % |
| Study Design | ||||
| Prospective cohort | 27 | 84.4% | 78 | 80.4% |
| Retrospective cohort | 2 | 6.3% | 7 | 7.2% |
| Case control | 1 | 3.1% | 3 | 3.1% |
| Nested case control | 2 | 6.3% | 9 | 9.3% |
| Data Collection Start Year | ||||
| 1985–1994 | 15 | 46.9% | 39 | 40.2% |
| 1995–2004 | 8 | 25.0% | 28 | 28.9% |
| 2004–2008 | 9 | 28.1% | 30 | 30.9% |
| Publication Year | ||||
| 1991–2000 | 9 | 28.1% | 23 | 23.7% |
| 2001–2010 | 10 | 31.3% | 29 | 29.9% |
| 2011–2017 | 13 | 40.6% | 45 | 46.4% |
| Geographical Distribution | ||||
| OECD Countries | ||||
| United States | 5 | 15.6% | 13 | 13.4% |
| Non-OECD Countries | ||||
| Kenya | 8 | 25.0% | 22 | 22.7% |
| South Africa | 3 | 9.4% | 9 | 9.3% |
| Tanzania | 3 | 9.4% | 11 | 11.3% |
| Uganda | 3 | 9.4% | 12 | 12.4% |
| Other | 10 | 31.3% | 30 | 30.9% |
| Sex | ||||
| Females only | 21 | 65.6% | 78† | 80.4% |
| Males only | 3 | 9.4% | 7† | 7.2% |
| Mixed-sex group | 8 | 25.0% | 12 | 12.4% |
| Risk Group (total exceeds 100% due to overlap) | ||||
| High-risk occupation – females | 20 | 62.5% | 68 | 70.1% |
| High-risk occupation – males | 2 | 6.3% | 3 | 3.1% |
| Serodiscordant partnership – females | 4 | 12.5% | 8 | 8.2% |
| Serodiscordant partnership – males | 4 | 12.5% | 7 | 7.2% |
| STI clinic patients – females | 5 | 15.6% | 14 | 14.4% |
| STI clinic patients – males | 5 | 15.6% | 9 | 9.3% |
| Mixed risk none – females | 3 | 9.4% | 11 | 11.3% |
| Mixed risk none – males | 2 | 6.3% | 5 | 5.2% |
| People who inject drugs (PWID) | ||||
| PWID not reported | 23 | 71.9% | 70 | 72.2 |
| Reported 0% PWID | 4 | 12.5% | 9 | 9.3% |
| Reported >0% <10% PWID | 4 | 12.5% | 15 | 15.5% |
| Reported >10% PWID | 1 | 3.1% | 3 | 3.1% |
| Reporting of Intervention Coverage | ||||
| Condom use (coverage range 0–100%, median 46.8%) | 23 | 71.9% | 63 | 64.9% |
| STI Treatment (completion NR) | 25 | 78.1% | 73 | 75.3% |
| Male population circumcised (coverage range 8.0–87.0%) | 6 | 18.8% | 23 | 23.7% |
| HIV-uninfected population on PrEP | 0 | 0% | 0 | 0% |
| Total Effect Sizes (k=97 * ) | ||||
| Characteristics of Included Effect Sizes | k | % | ||
|
| ||||
| Pathogen | ||||
| Syphilis | 34 | 35.1% | ||
| Trichomonas | 21 | 21.6% | ||
| Gonorrhea | 21 | 21.6% | ||
| Chlamydia | 18 | 18.6% | ||
| Mycoplasma genitalium | 3 | 3.1% | ||
| Effect Size Type | Multivariate-Adjusted | Unadjusted | Multivariate-Adjusted | Unadjusted |
|
| ||||
| Hazard ratio | 34 | 20 | 35.1% | 20.6% |
| Odds ratio | 11 | 7 | 11.3% | 7.2% |
| Risk ratio | 4 | 12 | 4.1% | 12.4% |
| Percentage | 0 | 4 | 0.0% | 4.1% |
| Incidence rate ratio | 4 | 0 | 4.1% | 0.0% |
| Incidence rate | 0 | 1 | 0.0% | 1.0% |
| Timing of STI Assessment | ||||
| Baseline only | 42 | 43.3% | ||
| Incident STI only | 14 | 14.4% | ||
| Baseline or incident, or not reported | 41 | 42.3% | ||
| STI Diagnostic Method | ||||
| Culture or stain | 40 | 41.2% | ||
| Serology for syphilis | 34 | 35.1% | ||
| Nucleic acid amplification test (NAAT) | 23 | 23.7% | ||
| Anatomical Site | ||||
| Vaginal | 55 | 56.7% | ||
| Ureteral | 1 | 1.0% | ||
| Unspecified (includes diagnosis via serology) | 41 | 42.3% | ||
| HIV Diagnostic Procedure - Baseline | ||||
| RNA Test | 4 | 4.1% | ||
| Polymerase chain reaction | 10 | 10.3% | ||
| Western Blot (WB) or p24 test | 2 | 2.1% | ||
| 4th-Generation ELISA using venous blood | 6 | 6.2% | ||
| 3rd-Generation ELISA | 28 | 28.9% | ||
| 2nd-Generation ELISA | 2 | 2.1% | ||
| Unspecified or Mixed ELISA | 45 | 46.4% | ||
| HIV Diagnostic Procedure -Outcome | ||||
| RNA Test | 4 | 4.1% | ||
| Polymerase chain reaction | 4 | 4.1% | ||
| 4th-Generation ELISA using venous blood | 6 | 6.2% | ||
| 3rd-Generation ELISA | 31 | 32.0% | ||
| Any ELISA + WB to Confirm Positives | 35 | 36.1% | ||
| Unspecified or Mixed ELISA | 17 | 17.5% | ||
| Follow-Up Intervals (Months) | ||||
| 1 | 12 | 12.4% | ||
| 3 | 27 | 27.8% | ||
| 4 to 4.5 | 3 | 3.1% | ||
| 6 | 22 | 22.7% | ||
| 12 | 3 | 3.1% | ||
| NR | 30 | 30.9% | ||
73 effect sizes were included in meta-analysis
Sex-specific effect sizes were drawn from both studies with mixed-sex and single-sex populations.
Legend: ELISA=Enzyme-linked immunosorbent assay; IRR=Incidence rate ratio; NAAT=Nucleic acid amplification test; NR=Not reported; OECD=Organisation for Economic Co-operation and Development; PrEP= Pre-exposure prophylaxis; PWID=People who inject drugs; RNA = Ribonucleic acid; STI=Sexually transmitted infection; WB = Western Blot
Most (21, 65.6%) studies reported on female participants exclusively. Three (9.4%) reported on male participants exclusively and eight (25.0%) reported on both. The majority of studies (22, 68.8%) reported on people in high-risk occupations, including female sex workers, other female workers in bars/hotels or entertainment venues, and male trucking-company and seasonal farm workers. Four (12.5%) studies reported on serodiscordant couples; the remaining six (18.8%) reported on STI clinic attendees. We classified three (9.4%) studies as “mixed” because they reported on populations with mixed risk behavior despite recruiting from a single source. These included one study of high-risk females recruited from bars and hotels who did not report sexual risk behaviors consistent with sex work (5.5% reported exchanging sex for money/gifts and 82.9% reported no more than one partner in the past year)36s and two studies using data from STI clinics that reported significant participation by people who inject drugs (PWID), MSM, and/or people involved in sex work; one of these reported results for a mixed-sex population and thus was not included in meta-analysis.34s,42s
Confounding factors
Most (23, 71.9%) studies did not report on the proportion of PWID. Four (12.5%) reported no drug injection history in the cohort and four (12.5%) reported less than 10% of participants were currently or previously PWID.
Other factors known to confound risk for HIV were reported with varying frequency. Twenty-three studies (71.9%) reported rates of condom use, although only two stratified this by STI status. While most studies (25, 78.1%) reported that STI-infected participants received or were offered treatment, none reported on treatment completion. Of the 11 studies reporting on either male participants or serodiscordant couples where female participants’ partners were known, six (54.5%) reported male circumcision proportions (range: 8.0–87.0%). No studies reported on the use of PrEP. Except for the serodiscordant-couple studies, the HIV and ART statuses of participants’ partners were not reported.
Effect-size level descriptive data
We calculated 97 effect sizes. Twelve (12.4%) reported on risk among mixed-sex groups for which we did not conduct meta-analysis. Another twelve (12.4%) effect sizes overlapped with others from the same studies and were excluded from meta-analysis.
STI Pathogens
More than a third (34, 35.1%) of effect sizes were on syphilis. Trichomonas and gonorrhea were the next-most reported STIs (each 21, 21.6%), followed by chlamydia (18, 18.6%) and Mycoplasma genitalium (3, 3.1%).
Most (54, 55.7%) effect sizes were reported as hazard ratios. Eighteen (18.6%) were reported as odds ratios, 16 (16.5%) as risk ratios, four (4.1%) as percentages, four (4.1%) as incidence rate ratios, and one (1.0%) as an incidence rate. Forty-two (43.3%) effect sizes reported HIV risk following STI diagnosed at baseline, 14 (14.4%) for incident STI, and 41 (42.3%) reported HIV risk following STI diagnosis that could have occurred either at baseline or a previous follow-up.
Forty (41.2%) effect sizes reported on STI diagnosed via a culture or gram stain. All 34 (35.1%) effect sizes reporting on syphilis diagnosis used serologic tests. Nucleic acid amplification tests (NAAT) were used in the remaining 23 (23.7%) effect sizes. Fifty-six (57.7%) effect sizes were reported in association with STI diagnosis at a genital site (vaginal=55, ureteral=1) and 41(42.3%, including all 34 syphilis effect sizes) did not specify the site of infection. No effect sizes specified STI infection at oral or rectal sites.
HIV infection
HIV diagnostic practices varied. Twenty-two (22.7%) effect sizes were from studies that used best-in-class diagnostic practices at baseline: RNA tests (4, 4.1%), polymerase chain reaction (PCR, 10, 10.3%), Western Blot or p24 test given to all participants (2, 2.1%), or a fourth-generation enzyme-linked immunoassay (ELISA) (6, 6.2%). The largest number (45, 46.4%) of effect sizes came from studies that used ELISA tests of multiple generations or did not report baseline diagnostic methods and thus limited our ability to assess the potential for false-negative HIV results at baseline. At follow-up, 35 (36.1%) effect sizes determined HIV outcomes using ELISA tests with Western Blot confirming positive results. RNA and PCR tests were used for four (4.1%) effect sizes each and fourth-generation ELISA tests were used for six (6.2%).
Factors influencing effect sizes
Precise follow-up interval timing was not reported for 30 (30.9%) effect sizes, although nine of those came from studies with no more than one year of follow-up. Twelve (12.4%) effect sizes were reported for intervals of one month, 30 (30.9%) reported average intervals between three and 4.5 months, and 25 (25.8%) between six to twelve months. When reported, mean follow-up time was 5.5 months. Only seven effect sizes came from studies reporting follow-up intervals under six months and used methods to preclude the possibility of HIV infection at baseline.32s
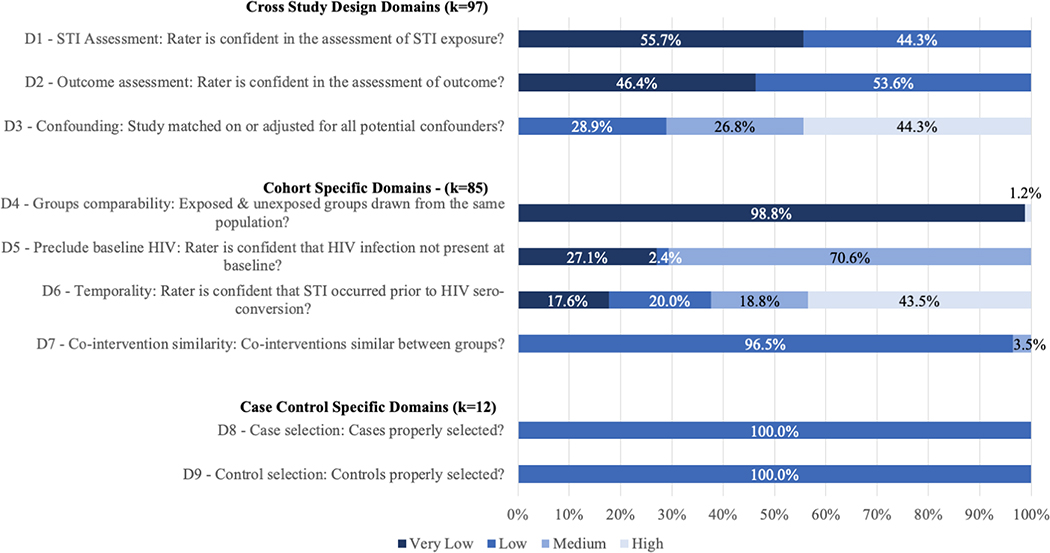
Risk of bias varied by risk domain (Figure 2/Appendix F). All effect sizes were rated as having low or very low risk of bias in STI and in HIV outcome assessments, since all studies reported using laboratory tests. Higher risk of bias was present around accounting for potential confounders (inadequate multivariate adjustment or matching; D3) with 43 (44.3%) effect sizes rated as high risk and 26 (26.8%) as medium risk. Of the 85 effect sizes from cohort studies, all but one were rated as very low risk of bias for recruitment from the same population (D4). Factors related to baseline HIV testing (precluding the possibility of false negative results, D5) had greater risk of bias: 60 (70.5%) effect sizes were rated medium risk, although none were rated high-risk. Temporality (likelihood of STI infection occurring prior to HIV infection, which bears on the strength of potential association between the two infections; D6) was rated as high risk in 37 (43.5%) effect sizes, medium risk in 16 (18.8%), low risk in 17 (20.0%), and very low risk in 15 (17.6%). All 12 effect sizes from case-control studies were rated low risk for both case and control selection (D8 and D9).
Figure 2. Assessment of risk of bias for effect-size-level data (k=97) on the effect of nonviral sexually transmitted infection diagnosis on the risk of HIV acquisition among high-risk heterosexuals.
Effects of STI on risk of HIV acquisition
Effects of STI on risk of HIV acquisition among females, by pathogen
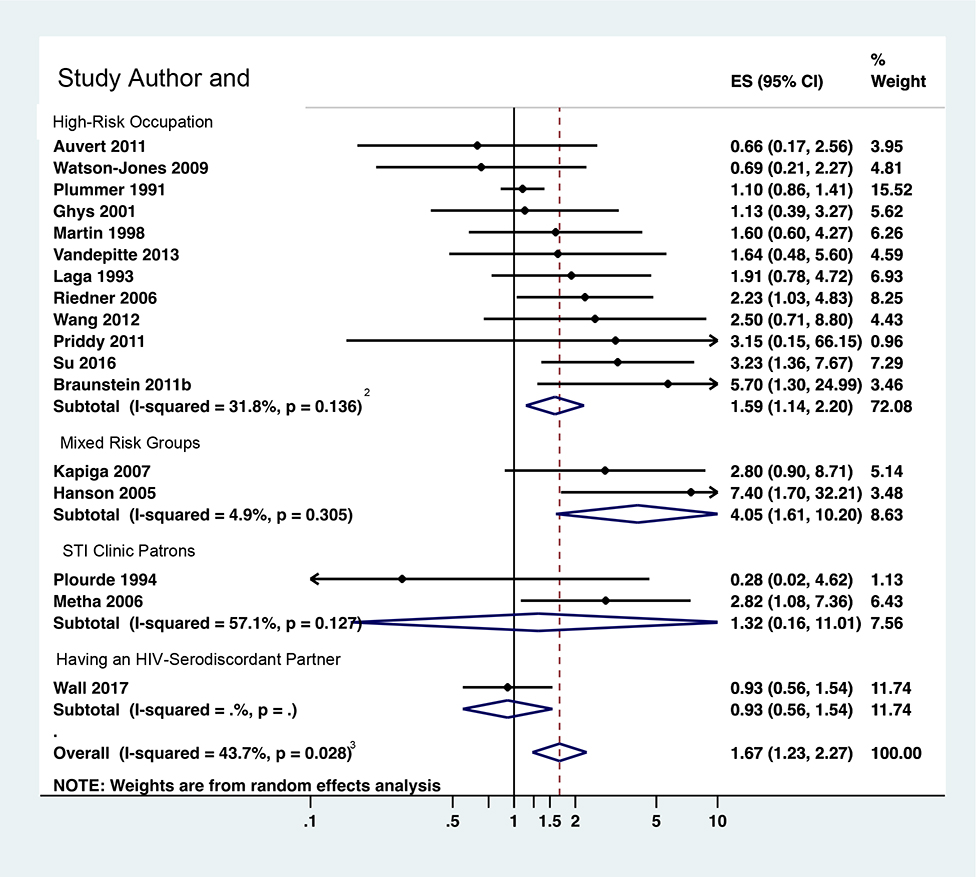
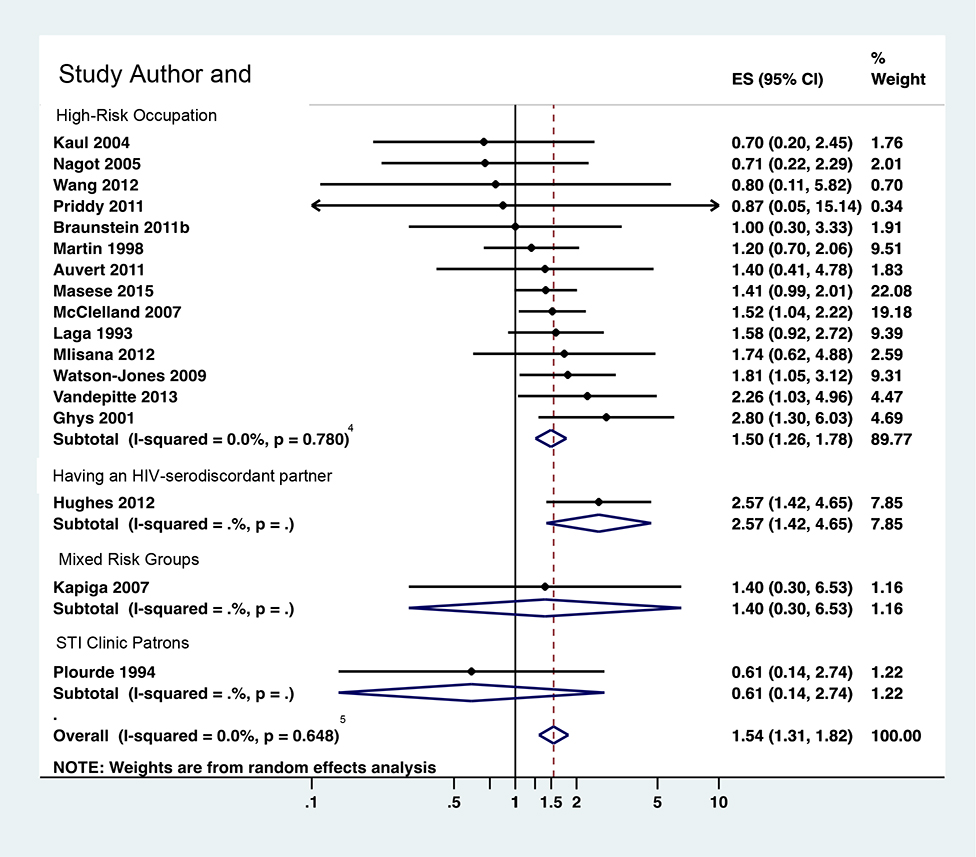
Table 3 reports estimates of increased HIV risk due to infection with each pathogen among female high-risk heterosexuals, overall and by sub-group analysis. Figures 3a–3d illustrate estimates for each pathogen overall and by sub-population and report RRs from each study in meta-analysis.
Table 3.
Comparison of risk of bias groupings on the effect of nonviral STI diagnosis on risk of HIV acquisition among female high-risk heterosexuals (k=66)
| Syphilis | Trichomoniasis | Gonorrhea | Chlamydia | Mycoplasma Genitalium | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Female Populations | ||||||||||
| Pooled RR (95% CI) | 1.67 (1.23, 2.27) | 1.54 (1.31, 1.82) | 2.81 (2.25, 3.50) | 1.49 (1.08, 2.04) | 3.10 (1.63, 5.92) | |||||
| I2, p value | 43.7%, 0.028 | 0.0%, 0.648 | 10.9%, 0.329 | 23.4%, 0.200 | 0.0%, 0.712 | |||||
| SA RR Range | 1.56–1.821 | 1.48–1.58 | 2.58–3.052 | 1.37, 1.693 | 2.94–4.084 | |||||
| k | 17 | 17 | 16 | 14 | 2 | |||||
| By Multivariate Adjustment | ||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Pooled RR (95% CI) | 1.64 (1.01, 2.67) |
1.75 (1.12, 2.72) |
0.82 (0.47, 1.45) |
1.64 (1.38, 1.95) |
3.97 (1.86, 8.46) |
2.74 (2.14, 3.51) |
1.19 (0.65, 2.17) |
1.61 (1.11, 2.35) |
- | 3.10 (1.63, 5.92) |
| I2, p value | 40.8%, 0.119 | 50.0%, 0.035 | 0.0, 0.975 | 0.0%, 0.700 | 0.0%, 0.651 | 20.1%, 0.240 | 11.9%, 0.339 | 30.3%, 0.186 | 0.0%, 0.712 | |
| k | 7 | 10 | 6 | 11 | 3 | 13 | 6 | 8 | 0 | 2 |
| By Risk of Bias in Temporality | ||||||||||
| Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | |
| Pooled RR (95% CI) | 1.56 (0.76, 3.21) |
1.77 (1.23, 2.53) |
2.32 (1.55, 3.48) |
1.42 (1.18, 1.70) |
3.11 (2.00, 4.84) |
2.76 (2.10, 3.62) |
0.51 (0.19, 1.36) |
1.71 (1.31, 2.23) |
3.10 (1.63, 5.92) |
- |
| I2, p value | 62.1%, 0.032 | 38.0%, 0.088 | 0.0%, 0.731 | 0.0%, 0.837 | 0.0%, 0.421 | 21.9%, 0.241 | 0.0%, 0.400) | 0.0%, 0.471 | 0.0%, 0.712 | |
| k | 5 | 12 | 4 | 13 | 6 | 10 | 3 | 11 | 2 | 0 |
| Higher-Quality Data Only | ||||||||||
| Pooled RR (95% CI) | 1.49 (0.98, 2.26) | 1.51 (1.25, 1.84) | 2.64 (1.92, 3.63) | 1.90 (1.40, 2.56) | - | |||||
| I2, p value | 32.9%, 0.177 | 0.0%, 0.874 | 37.0%, 0.146 | 0.0%, 0.848 | ||||||
| SA RR Range | 1.19–1.835 | 1.48–1.576 | 2.33–2.877 | 1.77–2.068 | ||||||
| k | 7 | 7 | 7 | 6 | 0 | |||||
| High-Risk Occupation Only | ||||||||||
| Pooled RR (95% CI) | 1.59 (1.14, 2.20) | 1.50 (1.26, 1.78) | 2.84 (2.25, 3.58) | 1.49 (1.06, 2.10) | 3.10 (1.63, 5.92) | |||||
| I2, p value | 31.8%, 0.136 | 0.0%, 0.780 | 11.3%, 0.332 | 33.3%, 0.124 | 0.0%, 0.712 | |||||
| SA RR Range | 1.40–1.83 9 | 1.44–1.5310 | 2.60–3.1311 | 1.37–1.70 12 | 2.94–4.08 13 | |||||
| k | 12 | 14 | 13 | 12 | 2 | |||||
| By Multivariate Adjustment | ||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Pooled RR (95% CI) | 2.11 (1.29, 3.46) |
1.39 (0.94, 2.04) |
0.79 (0.41, 1.53) |
1.57 (1.31, 1.88) |
3.72 (1.58, 8.77) |
2.81 (2.18, 3.62) |
1.24 (0.55, 2.82) |
1.61 (1.11, 2.35) |
- | 3.10 (1.63, 5.92) |
| I2, p value | 0.0%, 0.499 | 28.1%, 0.204 | 0.0%, 0.975 | 0.0%, 0.847 | 0.0%, 0.3.85 | 18.7%, 0.265 | 45.6%, 0.138 | 30.3%, 0.186 | 0.0%, 0.712 | |
| k | 4 | 8 | 4 | 10 | 2 | 11 | 4 | 8 | 0 | 2 |
| By Risk of Bias in Temporality | ||||||||||
| Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | Higher Risk | Lower Risk | |
| Pooled RR (95% CI) | 0.92 (0.40, 2.13) |
1.75 (1.21, 2.55) |
2.13 (1.23, 3.69) |
1.44 (1.20, 1.73) |
3.80 (2.20, 6.56) |
2.72 (2.04, 3.62) |
0.51 (0.19, 1.38) |
1.73 (1.29, 2.31) |
- | 3.10 (1.63, 5.92) |
| I2, p value | 0.0%. 0.541 | 40.0%, 0.091 | 0.0%, 0.582 | 0.0%, 0.809 | 0.0%, 0.770 | 26.8%, 0.205 | 0.0%, 0.400 | 11.4%, 0.340 | 0.0%, 0.712 | |
| k | 2 | 10 | 3 | 11 | 4 | 9 | 3 | 9 | 0 | 2 |
| Higher-Quality Data Only | ||||||||||
| Pooled RR (95% CI) | 1.49 (0.98, 2.26) | 1.51 (1.25, 1.84) | 2.64 (1.92, 3.63) | 1.90 (1.40, 2.56) | - | |||||
| I2, p value | 32.9%, 0.177 | 0.0%, 0.874 | 37.0%, 0.146 | 0.0%, 0.848 | ||||||
| SA RR Range | 1.19–1.8314 | 1.48–1.5715 | 2.33–2.8716 | 1.30–2.5617 | ||||||
| k | 7 | 7 | 7 | 6 | 0 | |||||
k = Number of effect size estimates included; RR = Risk ratio; SA = Sensitivity analysis; SA RR range = Range when one study removed from analysis
Where studies reported multiple effect sizes for the same population-pathogen pairing, estimates and SA RR ranges above reflect better-quality data (i.e., multivariate-adjusted vs unadjusted and/or shorter duration of follow-up). SA RR ranges for lower-quality data are reported in footnotes.
RR when each study removed from analysis, where RR changed by >0.05: Auvert 2011: 1.74 (1.27, 2.39); Braunstein 2011: 1.58 (1.18, 2.13); Ghys 2001: 1.73 (1.25, 2.39); Hanson 2005: 1.56 (1.17, 2.07); Metha 2006: 1.61 (1.18, 2.20); Plummer 1991: 1.81 (1.29, 2.54); Su 2016: 1.57 (1.16, 2.13); Wall 2017: 1.82 (1.30, 2.54); Watson-Jones 2009: 1.75 (1.28, 2.40). RR when lower-quality effect size was substituted for Braunstein 2011 was 1.58 (1.19, 2.10).
RR when each study removed from analysis: Ghys 2001: 2.69 (2.16, 3.35); Kaul 2004: 2.74 (2.19, 3.43); Laga 1993: 2.71 (2.12, 3.46); Martin 1998: 2.94 (2.36, 3.67); Masese 2015: 3.05 (2.42, 3.84); Ramjee 2005: 2.89 (2.29, 3.65); Vandepitte 2013: 2.58 (2.1, 3.18). RR when lower-quality effect size was substituted from Vandepitte 2013 was 2.62 (2.15, 3.19).
RR when each study removed from analysis: Auvert 2011: 1.68 (1.29, 2.17); Kaul 2004: 1.41 (1.02, 1.95); Laga 1993: 1.37 (0.97, 1.94); Nagot 2005: 1.69 (1.28, 2.22); Plummer 1991: 1.43 (0.98, 2.08); Vandepitte 2013: 1.42 (1.00, 2.02); Watson-Jones 2009: 1.39 (0.99, 1.94). RR when lower-quality effect size was substituted from Kapiga 2007 was 1.60 (1.12, 2.29).
RR when each study removed from analysis: Mlisana 2012: 2.94 (1.45, 5.96), Vandepitte 2013: 4.08 (0.83, 20.06). RR when lower-quality effect size was substituted from Vandepitte 2013 was 2.41 (1.29, 4.50).
RR when each study removed from analysis: Braunstein 2011: 1.19 (0.96, 1.49); Plummer 1991: 1.83 (1.13, 2.98); Riedner 2006: 1.33 (0.87, 2.04); Watson-Jones 2009: 1.65 (1.04, 2.61).
RR when Martin 1998 removed from analysis: 1.57 (1.27, 1.93).
RR when each study removed from analysis: Laga 1993: 2.46 (1.71, 3.53); Martin 1998: 2.86 (2.01, 4.06); Masese 2015: 2.87 (1.96, 4.19); Ramjee 2005: 2.75 (1.92, 3.94); Vandepitte 2013: 2.33 (1.81, 2.98).
RR when each study removed from analysis: Laga 1993: 1.77 (1.24, 2.53); Martin 1998: 1.98 (1.44, 2.71); Plummer 1991: 2.06 (1.43, 2.95); Watson-Jones 2009: 1.79 (1.29, 2.48).
RR when each study removed from analysis: Auvert 2011: 1.67 (1.19, 2.34); Braunstein 2011: 1.45 (1.09, 1.94); Ghys 2001: 1.65 (1.15, 2.37); Plummer 1991: 1.83 (1.32, 2.56); Riedner 2006: 1.52 (1.07, 2.15); Su 2016: 1.40 (1.05, 1.87); Watson-Jones 2009: 1.68 (1.20, 2.36). RR when lower-quality effect size was substituted from Braunstein 2011 was 1.42 (1.09, 1.84); when substituted from Vandepitte 2013 was 1.54 (1.16, 2.06)
RR when lower-quality effect size was substituted from Braunstein 2011 was 1.44 (1.21, 1.72).
RR when each study removed from analysis: Ghys 2001: 2.71 (2.16, 3.41); Kaul 2004: 2.77 (2.19, 3.51); Laga 1993: 2.75 (2.12, 3.56); Martin 1998: 2.97 (2.37, 3.72); Masese 2015: 3.13 (2.45, 4.00); Ramjee 2005: 2.94 (2.30, 3.76); Vandepitte 2013: 2.60 (2.09, 3.23). RR when lower-quality effect size was substituted from Vandepitte 2013 was 2.61 (2.11, 3.24).
RR when each study removed from analysis: Auvert 2011 1.70 (1.31, 2.21); Kaul 2004: 1.40 (0.98, 2.00); Laga 1993: 1.37 (0.94, 2.02); Nagot 2005: 1.69 (1.24, 2.29); Plummer 1991: 1.44 (0.95, 2.17); Vandepitte 2013: 1.43 (0.97, 2.1); Watson-Jones 2009: 1.38 (0.96, 2.00).
RR when each study removed from analysis: Mlisana 2012: 2.94 (1.45, 5.96), Vandepitte 2013: 4.08 (0.83, 20.06). R when lower-quality effect size was substituted from Vandepitte 2013 was 2.41 (1.29, 4.50).
RR when each study removed from analysis: Braunstein 2011: 1.19 (0.96, 1.49); Plummer 1991: 1.83 (1.13, 2.98); Riedner 2006: 1.33 (0.87, 2.04); Watson-Jones 2009: 1.65 (1.04, 2.61).
RR when Martin 1998 removed from analysis: 1.57 (1.27, 1.93).
RR when each study removed from analysis: Laga 1993: 2.46 (1.71, 3.53); Martin 1998: 2.86 (2.01, 4.06); Masese 2015: 2.87 (1.96, 4.19); Ramjee 2005: 2.75 (1.92, 3.94); Vandepitte 2013: 2.33 (1.81, 2.98).
RR when each study removed from analysis: Martin 1998: 1.3 (0.5, 3.38); Priddy 2011: 1.46 (0.08, 26.64); Plummer 1991: 1.58 (0.92, 2.71); Laga 1993: 2.23 (1.28, 3.88); Watson-Jones 2009: 2.56 (1.21, 5.42).
Figures 3a to 3d. Forest plots for risk ratios for nonviral STI diagnosis and risk of HIV acquisition among female high-risk heterosexuals1.
Figure 3a: RR for syphilis diagnosis and risk of HIV acquisition among female high-risk heterosexuals (k=17)
Figure 3b: RR for trichomonas vaginalis diagnosis and risk of HIV acquisition among female high-risk heterosexuals (k-17)
Figure 3c: RR for gonorrhea diagnosis and risk of HIV acquisition among female high-risk heterosexuals (k=16)
Figure 3d: RR for chlamydia diagnosis and risk of HIV acquisition among female high-risk heterosexuals (k=14)
1Where studies reported multiple effect sizes for the same population-pathogen pairing, estimates and sensitivity analysis (SA) risk ratio (RR) ranges above reflect higher-quality data (i.e., multivariate-adjusted vs unadjusted and/or shorter duration of follow-up). SA RR ranges for lower-quality data are reported in footnotes.
2Syphilis-high-risk occupation SA RR range: 1.40–1.83. Removing the following studies changed RR ≥0.05: Auvert 2011: 1.67 (1.19, 2.34); Braunstein 2011: 1.45 (1.09, 1.94); Ghys 2001: 1.65 (1.15, 2.37); Plummer 1991: 1.83 (1.32, 2.56); Riedner 2006: 1.52 (1.07, 2.15); Su 2016: 1.40 (1.05, 1.87); Watson-Jones 2009: 1.68 (1.20, 2.36). RR when lower-quality effect size was substituted from Braunstein 2011 was 1.42 (1.09, 1.84); when substituted from Vandepitte 2013 was 1.54 (1.16, 2.06)
3Syphilis overall SA RR range: 1.56–1.82. Removing the following studies changed RR ≥0.05: Auvert 2011: 1.74 (1.27, 2.39); Braunstein 2011: 1.58 (1.18, 2.13); Ghys 2001: 1.73 (1.25, 2.39); Hanson 2005: 1.56 (1.17, 2.07); Metha 2006: 1.61 (1.18, 2.20); Plummer 1991: 1.81 (1.29, 2.54); Su 2016: 1.57 (1.16, 2.13); Wall 2017: 1.82 (1.30, 2.54); Watson-Jones 2009: 1.75 (1.28, 2.40). RR when lower-quality effect size was substituted for Braunstein 2011 was 1.58 (1.19, 2.10).
4Trichomoniasis high-risk occupation SA RR range: 1.44–1.53. RR when lower-quality effect size was substituted from Braunstein 2011 was 1.44 (1.21, 1.72).
5Trichomoniasis overall SA RR range: 1.48–1.58.
6Gonorrhea high-risk occupation SA RR range: 2.60–3.13.. Removing the following studies changed RR ≥0.05: Ghys 2001: 2.71 (2.16, 3.41); Kaul 2004: 2.77 (2.19, 3.51); Laga 1993: 2.75 (2.12, 3.56); Martin 1998: 2.97 (2.37, 3.72); Masese 2015: 3.13 (2.45, 4.00); Ramjee 2005: 2.94 (2.30, 3.76); Vandepitte 2013: 2.60 (2.09, 3.23). RR when lower-quality effect size was substituted from Vandepitte 2013 was 2.61 (2.11, 3.24).
7Gonorrhea overall SA RR range: 2.58–3.05. Removing the following studies changed RR ≥0.05: Ghys 2001: 2.69 (2.16, 3.35); Kaul 2004: 2.74 (2.19, 3.43); Laga 1993: 2.71 (2.12, 3.46); Martin 1998: 2.94 (2.36, 3.67); Masese 2015: 3.05 (2.42, 3.84); Ramjee 2005: 2.89 (2.29, 3.65); Vandepitte 2013: 2.58 (2.1, 3.18). RR when lower-quality effect size was substituted from Vandepitte 2013 was 2.62 (2.15, 3.19).
8Chlamydia high-risk occupation SA RR range: 1.37–1.70. Removing the following studies changed RR ≥0.05: Auvert 2011 1.70 (1.31, 2.21); Kaul 2004: 1.40 (0.98, 2.00); Laga 1993: 1.37 (0.94, 2.02); Nagot 2005: 1.69 (1.24, 2.29); Plummer 1991: 1.44 (0.95, 2.17); Vandepitte 2013: 1.43 (0.97, 2.1); Watson-Jones 2009: 1.38 (0.96, 2.00).
9Chlamydia overall SA RR range: 1.37, 1.69. Removing the following studies changed RR ≥0.05: Auvert 2011: 1.68 (1.29, 2.17); Kaul 2004: 1.41 (1.02, 1.95); Laga 1993: 1.37 (0.97, 1.94); Nagot 2005: 1.69 (1.28, 2.22); Plummer 1991: 1.43 (0.98, 2.08); Vandepitte 2013: 1.42 (1.00, 2.02); Watson-Jones 2009: 1.39 (0.99, 1.94). RR when lower-quality effect size was substituted from Kapiga 2007 was 1.60 (1.12, 2.29).
Diagnosis of syphilis increased risk of HIV acquisition among females (RR=1.67; 95% CI 1.23, 2.27; I2=43.7%; k=17; Figure 3a). When only multivariate-adjusted RRs were pooled, risk was slightly increased (RR=1.75; 95% CI 1.12, 2.72; I2=50.0%; k=10), as it was when RRs were restricted to low risk of bias in temporality/timing (RR=1.77; 95% CI 1.23, 2.53; I2=38.0%; k=12), or to higher-quality data (RR=1.49; 95% CI 0.98, 2.26; I2=32.9%, k=7). Most (12, 70.6%) effect sizes reflected females in high-risk occupations, the pooled RR for which was similar to the overall estimate (RR=1.59; 95% CI 1.14, 2.20; I2=31.8%). The estimate was greater for the few effect sizes from OECD countries (RR=3.86; 95% CI 1.59, 9.38; I2=13.7%, k=2) than non-OECD countries (RR=1.48; 95% CI 1.11, 1.98; I2=32.5%; k=15; Appendix G); notably both OECD-country studies were conducted among STI clinic patients in the United States.
Trichomoniasis results similarly showed increased risk, with an overall pooled RR=1.54 (95% CI 1.31, 1.82; I2=0%; k=17; Figure 3b) and RR=1.64 (95% CI 1.38, 1.95; I2=0.0%; k=11) when restricted to multivariate-adjusted effect sizes. Pooled RR was slightly lower when analysis included RRs with lower risk of bias in temporality (RR=1.42; 95% CI 1.18, 1.70; I2=0.0%; k=13) and for higher-quality RRs (RR=1.51; 95% CI 1.25, 1.84; I2=0.0%; k=7). By risk group, females in discordant partnerships had the highest risk (RR=2.57, 95% CI 1.42, 4.64), although that estimate reflects only one effect size. Females in high-risk occupations had risk similar to the overall estimate (RR=1.50; 95% CI 1.26, 1.78; I2=0.0%; k=14) and, again, comprised the majority of the effect sizes. Results for STI clinic patients (k=1) and mixed groups (k=2, from the same study) were not significant.
Our analysis showed that prior diagnosis of gonorrhea almost tripled risk of HIV acquisition (RR=2.81; 95% CI 2.25, 3.50; Figure 3c), particularly notable since it combined 16 RRs with low heterogeneity (I2=10.9%). Pooled multivariate-adjusted RRs showed a similar result (RR=2.74; 95% CI 2.14, 3.51; I2=20.1%; k=13), as did RRs with a lower risk of bias in temporality (RR=2.76; 95% CI 2.10, 3.62; I2=21.9%; k=10). Pooled higher-quality RR was 2.64 (95% CI 1.92, 3.63; I2=37.0%; k=7). Most (13, 81.3%) effect sizes reflected females in high-risk occupations whose pooled RR (2.84; 95% CI 2.25, 3.58; I2=11.3%) for HIV acquisition was very close to the overall estimate. We found a higher pooled RR among STI clinic patients (3.15; 95% CI 1.50, 6.59; I2=0.0%; k=2). Pooled RR was lower in OECD countries (1.60; 95% CI 0.38, 6.77; I2=56.8%; k=2, both US) than non-OECD countries (2.86; 95% CI 2.29, 3.57; I2=7.3%; k=14; Appendix G).
Pooled RR for chlamydia (RR=1.49; 95% CI 1.08, 2.04; I2=23.4%; k=14, Figure 3d) was the smallest of the five pathogens, although it increased slightly when restricted to multivariate-adjusted RRs (RR=1.61; 95% CI 1.11, 2.35; I2=30.3%; k=8), lower risk of bias in temporality (RR=1.71; 95% CI 1.31, 2.23; I2=0.0%; k=11), and higher-quality data (RR=1.90; 95% CI 1.40, 2.56; I2=0.0%; k=6). Females in high-risk occupations had nearly the same risk as the overall estimate (RR=1.49; 95% CI 1.06, 2.10; I2=33.3%; k=12). One effect size was reported for each of STI clinic patrons and mixed populations; neither were statistically significant.
Mycoplasma genitalium had the greatest effect size, with a pooled RR=3.10 (95% CI 1.63, 5.92; I2=0.0%), however this reflects just two effect sizes, both from studies of female sex workers in non-OECD countries that used similar methods, so no stratified analysis was possible.
Effects of STI diagnosis among males
The effect of a syphilis diagnosis on risk of HIV acquisition among males was slightly higher (RR=1.77; 95% CI 1.22, 2.58; I2=8.5%; k=5; Table 4/Appendix H) than for females. When pooed, multivariate-adjusted RRs were larger than unadjusted RRs (RR=2.10; 95% CI 0.92, 4.80; I2=0.00; k=2). The one effect size with a low risk of bias in temporality had a higher RR (3.40; 95% CI 0.82, 14.12) than did the pooled estimate for the four other effect sizes (RR=1.71; 95% CI 1.15, 2.54; I=12.4%; k=4). The pooled RR for OECD countries was larger (RR=2.51; 95% CI 1.05, 6.00; I2=0.0%; k=2) than non-OECD countries RR=1.74; 95% CI 1.02, 2.97; I2=8.5%; k=3).
Table 4.
Summary of results on the effect of bacterial nonviral STI diagnosis on risk of HIV Acquisition among male high-risk heterosexuals (k=7)
| Syphilis1 | Gonorrhea2 | Chlamydia3 | ||
|---|---|---|---|---|
| Pooled RR (95% CI) | 1.77 (1.22, 2.58)4 | 2.80 (1.50–5.20) | 0.80 (0.30–1.90) | |
| I2, p value | 8.5%, 0.358 | NA | NA | |
| SA RR Range | 1.51–2.53 | |||
| k | 5 | 1 | 1 | |
| By Multivariate Adjustment | Unadjusted RR | Adjusted RR | Single data point is multivariate adjusted | Single data point is unadjusted |
| Pooled RR (95% CI) | 1.92 (1.02, 3.62) | 2.10 (0.92, 4.80) | ||
| I2, p value | 51.1%, 0.129 | 0.0%, 1.000 | ||
| k | 3 | 2 | ||
| By Risk of Bias in Temporality | Higher Risk | Lower Risk | Single data point is lower-risk | Single data point is higher-risk |
| Pooled RR (95% CI) | 1.71 (1.15, 2.54) | 3.40 (0.82, 14.12) | ||
| I2, p value | I=12.4%, p=0.331 | NA | ||
| k | 4 | 1 | ||
K = Number of effect size estimates included; NA = Not applicable; RR = Risk ratio; SA = Sensitivity analysis; SA RR range = range when one study removed from analysis
Populations reflected: Men in high-risk occupations (trucking company workers, farm workers): k=2, pooled RR 2.53 (1.35–4.76); STI clinic attendees: k = 1; men with serodiscordant partner: k=1; mixed risk groups: k=1.
Mixed risk groups
Men in high-risk occupations (trucking company workers)
RR when each study removed from analysis: Hanson 2005: 1.85 (1.14–3.01), Heffron 2011: 1.86 (1.15–2.99), Rakwar 1999: 1.51 (1.03–2.22), Telzak 1993: 1.71 (1.15–2.54), Wall 2017: 2.53 (1.52–4.21).
Only two effect sizes reported on the effects of diagnosis with other pathogens on risk of HIV acquisition among males: one on gonorrhea (RR=2.80; 95% CI 1.50, 5.20) and one on chlamydia (RR=0.80; 95% CI 0.30, 1.90) (Table 4).
DISCUSSION
Based on the updated body of evidence we identified, high-risk heterosexual persons diagnosed with a nonviral STI are at approximately 1.5 to three times greater risk of acquiring HIV, depending on the pathogen. Analyses restricted to effect sizes with lower risk of bias show similar results, and multivariate-adjusted effect sizes yield higher RRs for every pathogen except gonorrhea.
These estimates incorporate rigorous methodological nuance around infection temporality. Our study accounts for variation in testing protocols, technologies, and intervals by considering whether studies attempted to identify false-negative HIV-test results at enrollment. It presents sub-group analysis that excludes the longest follow-up intervals, which is helpful because longer intervals increase the potential to misclassify risk factors.
As with every systematic review, ours is subject to the limitations of primary studies. Because studies of the effect of STI on HIV must, ethically, use an observational design, some bias may be introduced. Just over half of effect sizes used some multivariate adjustment, however none accounted for all of the following known major confounders: partner HIV status, number of partners, drug injection, other STIs, condom use, and partner type.
Despite our efforts to isolate sources of potential error, STI infection is not optimally measured and reported. Studies compared HIV outcomes for persons who were and were not diagnosed with a specified STI, however persons in either group may have been infected with a different STI, which could have affected risk for HIV. While 20 (62.5%) studies controlled for diagnosis of other STIs, none tested for every possible STI and thus none could entirely control for this variable. Additionally, more than half of effect sizes reflected follow-up intervals longer than three months, meaning that STIs diagnosed may have been cured or resolved prior to HIV acquisition, participants could have acquired new STIs not detected before HIV diagnosis, or participants could have engaged in unmeasured behaviors increasing risk of HIV. In these cases, the elevated risk of HIV acquisition observed among the STI-infected group could reflect added risk due to factors common to both HIV and STIs, such as unprotected sex. Finally, although 25 (78.1%) studies confirmed that STI treatment was provided to participants, no data on treatment adherence/completion were reported, so the effects of treatment are unmeasured.
Most studies did not indicate whether any participants injected drugs. Of those that did, not all distinguished between recent and past practices. The absence of data on drug injection introduces substantial uncertainty in reported estimates.
Most studies of females with nonviral STI were conducted among those engaged in sex work or a similar activity. Thus, our overall effect estimates are similar to those for sex workers. Data on other risk groups were often insufficient for meta-analysis. We found few studies conducted on males with nonviral STI. Sub-group analysis by geography was also limited because the United States was the only OECD country represented.
Few studies obtained data on participants’ partners, including their HIV status, antiretroviral therapy or viral suppression status (if HIV-infected), STI, and circumcision status of male partners. No studies included participants reported to be taking PrEP. These constrain our ability to extrapolate on how STI may shape HIV acquisition risk within the context of daily PrEP use63s or sustained viral suppression,64s both of which effectively prevent HIV transmission.
Heterogeneity was low (<24%) across estimates for trichomoniasis, gonorrhea, mycoplasma genitalium, and chlamydia among females and of syphilis among males, and moderate (44%) across estimates of the effect of syphilis among females. Because there was relatively little variation in population and setting (non-OECD countries) in studies reporting on females, caution is warranted when results are applied to other populations and settings.
This paper presents updated, rigorous evidence of the effects of nonviral STI on HIV acquisition among high-risk heterosexual populations, incorporating uncommon scrutiny around the temporality and timing between STI and HIV diagnoses and variations in diagnostic accuracy. Uncertainty persists due to lack of data on confounding factors and participants’ partners, lengthy follow-up intervals, limited evidence on males and on the effects of mycoplasma genitalium, and limited variety in the study settings and risk groups involved in research of high-risk females. Future research that explores or accounts for these elements could enhance the breadth of evidence.
Supplementary Material
LIST OF SUPPLEMENTAL DIGITAL CONTENT:
Appendix A: Database search strategies and de-duplication process
Appendix B: Filtering and partitioning of likely-irrelevant titles
Appendix C. Citation screening process flowchart2-4
Appendix D. Risk of bias indicator definitions used for data extraction
Appendix E: Studies excluded after full-text review
Appendix F. Risk of Bias Results for Meta-Analyzed Effect Sizes (k=97)
Appendix G. Comparison of Organization for Economic Co-Operation and Development (OECD)- and non-OECD study settings on the effect of nonviral STI diagnosis on risk of HIV acquisition among high-risk heterosexuals
Appendix H. Forest plot for risk ratios of diagnosis of syphilis and risk of HIV acquisition among male high-risk heterosexuals
Appendix References
Acknowledgment
We would like to extend our special gratitude to Wei Chang for her support in screening and extraction of Chinese-language studies, Devon McCabe for project management support, and authors of included studies who provided additional data not reported in their manuscripts.
Funding
The U.S. Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NEEMA, # 5U38PS004649).
Prospero Number: CRD42018084299
Footnotes
Competing Interest: None known.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Newman L, Rowley J, Vander Hoorn S, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 2015; 10(12): e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually transmitted diseases 1992; 19(2): 61–77. [PubMed] [Google Scholar]
- 3.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sexually transmitted diseases 2001; 28(10): 579–97. [DOI] [PubMed] [Google Scholar]
- 4.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sexually transmitted diseases 2005; 32(6): 351–7. [DOI] [PubMed] [Google Scholar]
- 5.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS (London, England) 2006; 20(1): 73–83. [DOI] [PubMed] [Google Scholar]
- 6.Bonell C, Hickson F, Beaumont M, Weatherburn P. Sexually transmitted infections as risk factors for HIV infection among MSMs: systematic review. Sexually transmitted diseases 2008; 35(2): 209. [DOI] [PubMed] [Google Scholar]
- 7.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. The Lancet Infectious diseases 2009; 9(2): 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutua FM, M’Imunya JM, Wiysonge CS. Genital ulcer disease treatment for reducing sexual acquisition of HIV. The Cochrane database of systematic reviews 2012; (8): Cd007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilber AM, Francis SC, Chersich M, et al. Intravaginal practices, vaginal infections and HIV acquisition: systematic review and meta-analysis. PLoS One 2010; 5(2): e9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sexually transmitted diseases 2008; 35(11): 946–59. [DOI] [PubMed] [Google Scholar]
- 11.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Current opinion in HIV and AIDS 2010; 5(4): 305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora P, Nagelkerke NJ, Jha P. A systematic review and meta-analysis of risk factors for sexual transmission of HIV in India. PLoS One 2012; 7(8): e44094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS (London, England) 2012; 26(17): 2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp JR, Schachter J, Gaydos CA, Van Der Pol B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae−−2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports 2014; 63(Rr-02): 1–19. [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. 2015 Sexually Transmitted Diseases Treatment Guidelines. Available: https://www.cdc.gov/std/tg2015/default.htm [accessed 12 June 2017]. CDC; 2015. [Google Scholar]
- 16.Melo MG, Sprinz E, Gorbach PM, et al. HIV-1 heterosexual transmission and association with sexually transmitted infections in the era of treatment as prevention. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2019; 87: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li HM, Peng RR, Li J, et al. HIV incidence among men who have sex with men in China: a meta-analysis of published studies. PLoS One 2011; 6(8): e23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y, Bu K, Li M, Zhang X, Jin S, Wang L. [Meta-analysis of HIV infection incidence and risk factors among men who have sex with men in China]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2015; 36(7): 752–8. [PubMed] [Google Scholar]
- 19.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. The Journal of infectious diseases 2002; 185(1): 45–52. [DOI] [PubMed] [Google Scholar]
- 20.Malekinejad M, Barker EK, Merai R, et al. Risk of HIV acquisition among men who have sex with men infected with bacterial sexually transmitted infections: A systematic review and meta-analysis. Under Review. 2020. [DOI] [PMC free article] [PubMed]
- 21.Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Available from: http://www.cochrane-handbook.org [accessed 18 September 2015]. The Cochrane Collaboration; 2011. [Google Scholar]
- 22.Malekinejad M, al. e. Risk of HIV transmission and acquisition among HIV high-risk populations infected with other sexually transmitted infections. 2018. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=84299 (accessed November 5, 2018 2018).
- 23.PROSPERO International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/prospero/ (accessed November 25, 2018 2018).
- 24.GRADE handbook for grading quality of evidence and strength of recommendations. Available from: http://www.guidelinedevelopment.org/handbook/ [accessed 21 Jan 2018]. GRADE Working Group; 2013. [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tool to Assess Risk of Bias in Cohort Studies. http://help.magicapp.org/knowledgebase/topics/32139-grade-and-methodology-for-development-of-guideline (accessed November 25, 2018.
- 27.How to rate Risk of bias in Observational studies. http://help.magicapp.org/knowledgebase/articles/294933-how-to-rate-risk-of-bias-in-observational-studies (accessed November 25, 2018.
- 28.StataCorp. Stata Statistical Software: Release 14.2. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 29.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama 1998; 280(19): 1690–1. [DOI] [PubMed] [Google Scholar]
- 30.Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. The Lancet Infectious diseases 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LIST OF SUPPLEMENTAL DIGITAL CONTENT:
Appendix A: Database search strategies and de-duplication process
Appendix B: Filtering and partitioning of likely-irrelevant titles
Appendix C. Citation screening process flowchart2-4
Appendix D. Risk of bias indicator definitions used for data extraction
Appendix E: Studies excluded after full-text review
Appendix F. Risk of Bias Results for Meta-Analyzed Effect Sizes (k=97)
Appendix G. Comparison of Organization for Economic Co-Operation and Development (OECD)- and non-OECD study settings on the effect of nonviral STI diagnosis on risk of HIV acquisition among high-risk heterosexuals
Appendix H. Forest plot for risk ratios of diagnosis of syphilis and risk of HIV acquisition among male high-risk heterosexuals
Appendix References