Abstract
Objective:
To test the hypothesis that forced air warming of critically ill afebrile sepsis patients improves immune function compared to standard temperature management.
Design:
Single-center, prospective, open-label, randomized controlled trial.
Setting:
1200-bed academic medical center.
Patients:
Eligible patients were mechanically ventilated septic adults with: 1) a diagnosis of sepsis within 48 hours of enrollment; 2) anticipated need for mechanical ventilation of > 48 hours; and 3) a maximum temperature < 38.3°C within the 24 hours prior to enrollment. Primary exclusion criteria included: immunologic diseases, immune-suppressing medications, and any existing condition sensitive to therapeutic hyperthermia (e.g., brain injury). The primary outcome was monocyte HLA-DR expression, with secondary outcomes of CD3/CD28-induced IFN-γ production, mortality, and 28-day hospital-free days.
Interventions:
External warming using a forced air warming blanket for 48 hours, with a goal temperature 1.5°C above the lowest temperature documented in the previous 24 hours.
Measurements and Main Results:
We enrolled 56 participants in the study. No differences were observed between the groups in HLA-DR expression (692 vs. 2002, p=0.396) or IFN-γ production (31 vs. 69, p=0.678). Participants allocated to external warming had lower 28-day mortality (18% vs. 43%, absolute risk reduction 25%, 95% CI 2–48%) and more 28-day hospital-free days (difference 2.6 days, 95% CI 0–11.6).
Conclusions:
Participants randomized to external forced air warming did not have a difference in HLA-DR expression or IFN-γ production. In this pilot study, however, 28-day mortality was lower in the intervention group. Future research should seek to better elucidate the impact of temperature modulation on immune and non-immune organ failure pathways in sepsis.
Keywords: sepsis, fever, clinical trial, hyperthermia, immunosuppression
Introduction
Fever is a key feature of infection, yet fewer than half of critically ill patients with sepsis have a fever at the time of diagnosis (1–4). Afebrile sepsis patients have up to twice the mortality and are more likely to develop secondary infections than patients with fever, but the reason for these observations is unknown (2–9). Fever may be an adaptive response to infection that is critical for survival. Animal and in vitro studies have shown elevated temperature to have beneficial effects on adaptive and innate immunity, including increased antibody production, T cell activation, macrophage function, and heat shock protein response (10–14).
Sepsis is associated with pro- and anti-inflammatory mechanisms that can lead to prolonged periods of immunosuppression (15–22). Several biomarkers have been implicated in sepsis-induced immunosuppression, including reduced expression of human leukocyte antigen (HLA)-DR, decreased lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α) production, decreased anti-CD3/anti-CD28-stimulated interferon gamma (IFN-γ) production, and persistent lymphopenia (23). Patients exhibiting persistently depressed levels of these immune markers suffer from higher mortality and increased secondary infections compared to patients with intact immunity (24–32). While fever has been associated with immunocompetence, the role of temperature itself has not been rigorously evaluated.
Therapeutic hyperthermia, artificially raising body temperature through external warming, has been used for immunomodulation to treat several types of cancer (33, 34). This strategy is thought to improve function of natural killer cells, dendritic cells, and T cells (33). In addition, perioperative warming has consistently been shown to decrease postoperative infections (35–37). As a whole, these data provide compelling biologic rationale that therapeutic hyperthermia may be an effective treatment for sepsis.
The objective of this trial was to determine whether forced air warming of critically ill afebrile sepsis patients improves immune function compared to standard temperature management. We hypothesized that warmed patients would exhibit higher levels of HLA-DR expression and CD3/CD28-stimulated IFN-γ production and a reduced incidence of persistent lymphopenia. We also aimed to assess the feasibility and safety of raising body temperature in critically ill sepsis patients and to evaluate the impact of warming on clinical outcomes.
Materials and Methods
Study Design
This study was a single-center, prospective, open-label, randomized controlled trial comparing external warming of afebrile critically ill adult sepsis patients to usual care. The trial reviewed by and approved by the Washington University Human Research Protection Office (IRB#201512121) and was registered on ClinicalTrials.gov (NCT02706275). Written informed consent was obtained from legally authorized representatives, and this study is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement extension for pilot/feasibility trials (38). The study protocol is included in Appendix A.
Participants
This study was conducted in the medical and surgical intensive care units (ICUs) of a 1200-bed tertiary care academic medical center between March 2016 and April 2019. Adult patients admitted to the ICU with a diagnosis of severe sepsis were screened daily by a study coordinator for inclusion. Severe sepsis was defined according to the 2001 Sepsis Taskforce consensus statement (39), and a post hoc review verified that all included patients met Sepsis-3 criteria (40). Enrollment criteria also required participants to be enrolled within 48 hours of sepsis diagnosis (defined as the date/time of the first order for antibiotics), mechanically ventilated with an expected duration of mechanical ventilation > 48 hours, receiving continuous pharmacologic sedation with a Richmond Agitation Sedation Scale (RASS) < 0, and having a maximum temperature < 38.3°C within the 24 hours prior to enrollment. Exclusion criteria included: history of immunological disease; treatment with immunosuppressive medications within the previous 6 months; treatment with corticosteroids at a dose of > 300 mg/day hydrocortisone or equivalent at the time of enrollment; history of chronic infection with hepatitis B or C virus; contraindications to hyperthermia (e.g., sudden cardiac arrest, acute ischemic stroke, acute coronary syndrome, traumatic brain injury or spinal cord injury, sickle cell disease, or multiple sclerosis); pregnancy; comfort care treatment status; contraindications to forced air warming devices per manufacture instructions (i.e. ischemic limbs); or enrollment in another clinical trial. These inclusion/exclusion criteria were selected to maximize participant safety and tolerance of the intervention and to exclude participants in whom immune biomarkers were unreliable or who might be harmed by artificially elevated body temperature. Patients admitted to the ICUs were screened daily and eligible patients were consecutively approached for enrollment.
Randomization
Participants were randomized to usual care or to the study intervention in a 1:1 ratio by computer-generated, block randomization with variable block size (using random blocks of 2, 4, and 6) to ensure allocation concealment. An allocation table based on this randomization scheme was uploaded to REDCap, a secure, web-based application for research data management, by a statistician. A study coordinator obtained the randomization code for each patient from the REDCap database after informed consent had been obtained. Block randomization was used to ensure similar numbers of participants in each study group given the overall small sample size, which was calculated a priori.
Study Treatments
The study intervention consisted of 48 hours of external warming with a forced air warming system (Bair Hugger, 3M, St. Paul, MN) to a goal core temperature 1.5°C greater than the lowest recorded temperature in the 24 hours prior to enrollment or to at least 37.5°C (whichever was higher). This goal temperature was chosen based on evidence from preliminary studies in our lab showing improved monocyte HLA-DR expression in patients with a 1.5°C increase in minimum body temperature during the first 48 hours after sepsis diagnosis (41). All participants enrolled in the study had temperature monitoring using a core temperature monitor (esophageal, bladder, or rectal). Immediately following randomization to the intervention group, a forced air warming blanket on the highest setting (43°C) was applied to participant with the aim of attaining the target temperature within 6 hours of warming onset. After 6 hours, if the goal temperature was not achieved, warm blankets were added on top of the forced air blanket and the room temperature was increased. Once the goal temperature was achieved, the forced air blanket was used to maintain the temperature above the goal for the remainder of the intervention period.
The warming intervention was terminated early if any of the following criteria were met: vasopressor increase > 50% for 6 hours and > 0.1 mcg/kg/min norepinephrine-equivalent dose, heart rate (HR) increase by > 25 bpm and HR > 90, evidence of thermal skin injury, or extubation. These termination criteria were chosen to avoid negative adverse effects of hyperthermia and to ensure participant comfort.
Participants randomized to the control group received usual care temperature management in accordance with our current ICU protocols during the 48-hour intervention period, at the discretion of the clinical team.
Data Collection
Demographics, comorbidities, microbiological culture results and sources of sepsis were extracted from the electronic medical record. Acute Physiology and Chronic Health II (APACHE-II) scores were calculated based on data from the first 24 hours in the ICU. Sequential Organ Failure Assessment (SOFA) scores (excluding the Glasgow Coma Score component) were calculated prior to and following the intervention period. Throughout the 48-hour intervention period, core body temperatures were recorded hourly. Mean arterial blood pressure, vasopressor dose (in norepinephrine-equivalents (42)), and HR were documented every six hours. If warming was terminated early in the participants randomized to the study intervention, the reason for termination was noted. Blood samples were collected at randomization (days 1–2 after sepsis diagnosis) and following the 48-hour intervention period (days 3–4 after sepsis diagnosis) to evaluate markers of immune function. Absolute lymphocyte counts from complete blood cell counts (CBCs) ordered by the treating clinical team were recorded from the electronic health record for the first seven days following sepsis diagnosis.
Outcomes
The primary outcome was monocyte HLA-DR expression following the 48-hour intervention period. This outcome was chosen because (1) HLA-DR expression is one of the most widely accepted measures of sepsis-induced immunosuppression; (2) low levels are associated with mortality and acquisition of nosocomial infection infections in septic patients; and (3) our preliminary data (41) suggested HLA-DR expression is higher in patients who experience fever during the first 48 hours of sepsis. Secondary immune outcomes included CD3/CD28-induced IFN-γ production following the intervention period and incidence of persistent lymphopenia (defined as an absolute lymphocyte count < 1.2 cells/μL x 103, which is the lower limit of normal at our institution, persisting beyond 72 hours after sepsis diagnosis (31)). Clinical outcomes included the delta-SOFA score (change in SOFA score based on laboratory studies and vital signs from the 24-hour period prior to the start of the intervention compared to score based on data from the 24-hour period after completion of the intervention), 28-day hospital free days, 28-day ventilator free days, acquisition of secondary infections, and 28-day mortality. Secondary infections were defined as new positive cultures or new antibiotics started after 48 hours and documentation of a new confirmed infection by the treating clinical team.
The safety outcomes of interest were vasopressor dose (expressed as norepinephrine-equivalent dose), heart rate, and respiratory rate.
Immunological Testing
Immunological testing was performed by laboratory technicians blinded to participant group allocation. Quantification of monocyte HLA-DR was performed according to the Demaret method (43), as previously described (28). Whole blood was incubated with BD Quantibrite Anti-HLA-DR/Anti-Monocyte Stain (Becton-Dickinson, San Jose, CA), lysed using RBC Lysis Buffer (BioLegend, San Diego, CA), and fixed in 2% paraformaldehyde. Samples were acquired on a FACScan (Becton-Dickinson, San Jose, CA) with a 5-color upgrade (CyTech, Fremont, CA). Flow files were acquired and analyzed in CellQuest Pro (Becton-Dickinson, San Jose, CA). Antibodies bound per cell (AB/c) were calculated by standardizing HLA-DR geomean fluorescent intensity (GMFI) of monocytes to BD Quantibrite-phycoerythrin (PE) beads (Becton-Dickinson, San Jose, CA).
CD3/CD28-induced IFN-γ production was determined using an Enzyme-Linked Immunospot (ELISpot) assay, as previously described (44). Peripheral blood mononuclear cells (PMBCs) harvested from whole blood plated at a standardized density, 5 × 105 cells per well, using the Vi-Cell counter (Beckman Coulter, Brea, CA) in wells coated with biotinylated capture antibody specific to IFN-γ. Cells were incubated overnight with RPMI 1640 media (Sigma-Aldrich, St. Louis, MO) containing anti-CD3/anti-CD28 (BioLegend, San Diego, CA). IFN-γ was detected using a colorimetric reagent kit (Strep-AP and BCIP-NBT, R&D Systems, Minneapolis, MN). Images from the ELISpot plates (made by Merk Millipore and acquired through Fisher Scientific, Hampton, NH) were analyzed on Cellular Technologies Ltd (Cleveland, OH) ImmunoSpot 7.0 plate reader and software. The number of spots, representing cells producing IFN-γ, and mean spot size were recorded. The means of two identically treated plates run in duplicate were calculated.
Sample Size Determination
To detect a difference in HLA-DR expression of 12,000 AB/c with a power of 80% using a two-sided α = .05, a total of 44 sepsis participants (22 patients per arm) were required. The detectable difference in HLA-DR expression was the magnitude of the difference in patients with who did and did not experience an increase in body temperature of 1.5°C within 24 hours of sepsis diagnosis in our preliminary data (41). Anticipating control group mortality of 20% prior to acquisition of the post-intervention blood sample, enrollment of 56 participants was planned.
Statistical Analysis
Data were analyzed on an intention-to-treat basis regardless of whether temperature goals were met in the intervention group. Descriptive statistics are presented as counts, means, and medians, as appropriate. Temperature and care-associated variables were compared using t-tests, repeated measures ANOVA, Mann-Whitney U tests, or chi-squared tests as appropriate. The primary HLA-DR and secondary serum IFN-γ analyses were performed among participants who had non-missing values for both the baseline and follow-up immune outcomes using repeated measures ANOVA. Hospital-free days and ventilator-free days were compared using non-parametric tests. To ensure that any 28-day mortality difference observed was not attributable to confounding by illness severity, we conducted a post-hoc multivariable logistic regression analysis, including predictors of both treatment group assignment and enrollment SOFA score. All analyses were conducted in Stata v.16.1 (StataCorp LLC, College Station, TX).
Results
We enrolled 56 participants (28 in each arm), with none lost to follow-up (Supplemental Figure 1). The mean time from first antibiotic administration to enrollment was 29.1 h (SD 15.7). The participants were well balanced on SOFA score, comorbidities, and temperature covariates at randomization. The most common source of infection was pneumonia (n=29), and the most common reason for exclusion was concurrent immune suppression (Table 1).
Table 1. Baseline characteristics.
y, years; SD, standard deviation; BMI, body mass index; ICU, intensive care unit; C, Celsius.
| Variable | Control (n=28) | Warming (n=28) |
|---|---|---|
| Male, n (%) | 16 (57) | 11 (39) |
| Age, y (SD) | 58.7 (16.8) | 58.7 (13.3) |
| BMI, mean (SD) | 33.6 (11.0) | 29.5 (9.1) |
| Body surface area (m2), mean (SD) | 2.07 (0.36) | 1.97 (0.30) |
| ICU, n (%) | ||
| Medical | 21 (75) | 18 (64) |
| Surgical | 7 (25) | 10 (36) |
| Surgery within 1 week, n (%) | 6 (21) | 4 (14) |
| Source, n (%) | ||
| Pneumonia | 15 (54) | 19 (68) |
| Abdominal | 4 (14) | 3 (11) |
| Soft Tissue/Bone | 3 (11) | 1 (4) |
| Urinary | 2 (7) | 1 (4) |
| Other or unknown | 4 (14) | 4 (14) |
| Positive Culture, n (%) | 13 (46) | 20 (71) |
| Culture results susceptible to initial antibiotics | 11 (85) | 20 (100) |
| Organism, n (%) | ||
| Gram negative | 7 (54) | 10 (50) |
| Gram positive | 2 (15) | 5 (25) |
| Mixed | 1 (8) | 1 (5) |
| Viral | 3 (23) | 4 (20) |
| Lactate, mean (SD) | 4.8 (3.1) | 3.5 (2.7) |
| Leukocyte count, mean (SD) | 17.3 (18.1) | 13.6 (7.7) |
| APACHE-II, mean (SD) | 22.1 (5.2) | 20.1 (6.5) |
| SOFA, mean (SD) | 8.8 (3.9) | 8.0 (3.9) |
| Oxygenation score, mean (SD) | 2.4 (1.2) | 2.5 (1.2) |
| Calculated temperature 1.5 degrees C above baseline, mean (SD) | 37.9 (0.6) | 38.0 (0.5) |
| Time from antibiotics to randomization, mean (SD) | 29.7 (16.2) | 28.6 (15.6) |
| Comorbidities, n (%) | ||
| Coronary artery disease | 7 (25) | 6 (21) |
| Congestive heart failure | 5 (18) | 4 (14) |
| Stroke | 2 (7) | 2 (7) |
| Chronic obstructive pulmonary disease | 8 (29) | 8 (29) |
| Diabetes mellitus | 10 (36) | 8 (29) |
| Solid tumor | 4 (14) | 3 (11) |
| Liver disease | 9 (32) | 5 (18) |
| End-stage renal disease | 2 (7) | 2 (7) |
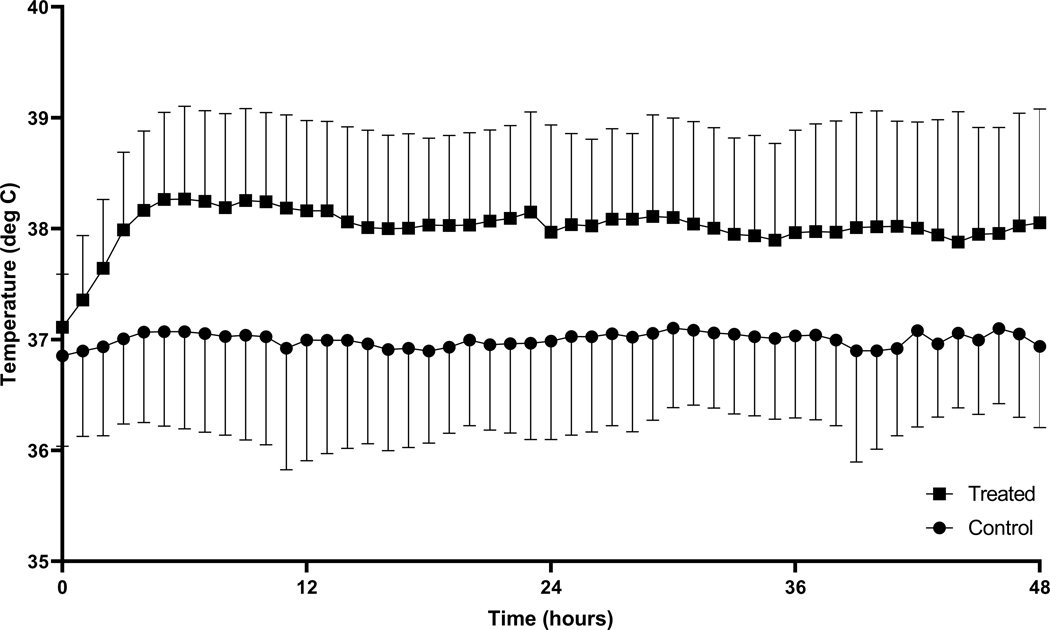
The temperature in participants randomized to therapeutic warming during the study period was higher than those allocated to usual care (repeated measures ANOVA p<0.001) (Figure 1). Twenty-six (93%) participants in the warming group achieved target temperature, with eighteen (69%) reaching the target within 6 hours. The median time to target temperature was 3 hours (IQR 2–4) in the warming group (Supplemental Table 1). Ten participants (36%) had warming terminated prior to 48 hours, with the most common reason being extubation during the intervention period (n=5) (Supplemental Table 2). Two participants had missing immune biomarker values, one due to death prior to sample being drawn and one due to misplacement of the sample (these cases were excluded from immune biomarker analysis). Corticosteroid therapy was administered during the ICU stay (after randomization) for 9 control participants (32%) and 8 intervention participants (29%).
Figure 1. Temperature in the treatment and control groups.
The curves shown demonstrate the separation of temperatures between the therapeutic hyperthermia (squares) group and the usual care (circles) group. The points represent the group mean temperature at each time point, and the error bars represent standard deviation. deg C, degrees Celsius.
Immunological Outcomes
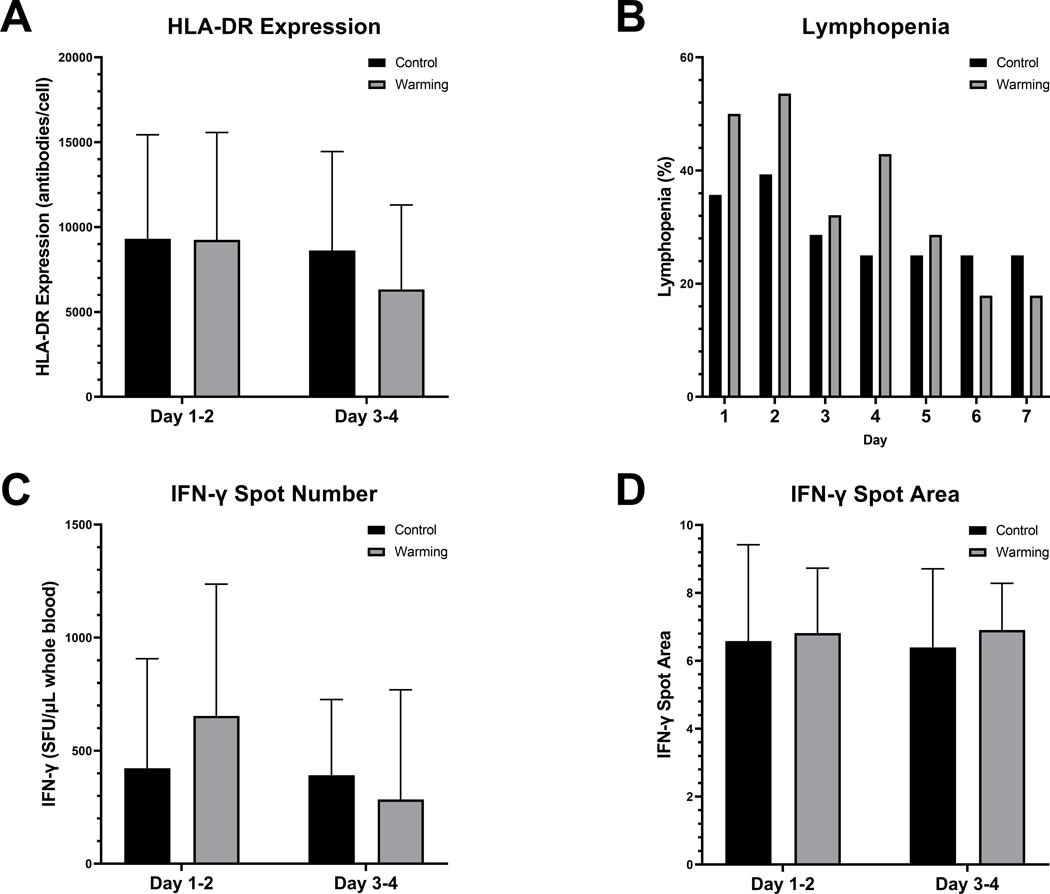
HLA-DR expression at enrollment was similar between the two groups (difference 63.8 AB/c, 95% CI [−3336.8] – 3464.4). For our primary outcome, there was no difference between treatment and control groups in monocyte HLA-DR expression following the 48-hour intervention period (difference −1310.4, 95% CI [−4537.5] – 1916.8, Figure 2). Overall, patients who died had lower HLA-DR expression at enrollment (mean 6522.4 vs. 10438.5, p = 0.03).
Figure 2. Immune Outcomes.
This figure details the differences in immune parameters between the therapeutic hyperthermia (Warming) and usual care (Control) groups. All bars show the mean value for each group, and the error bars represent the standard deviation. A. HLA-DR expression. B. Lymphopenia observed on each day of observation (among participants who had an absolute lymphocyte count recorded on any given day. C. IFN-γ spot number (representing number of cells producing IFN-γ). D. IFN-γ spot area. HLA, human leukocyte antigen; IFN, interferon; SFU, spot-forming unit.
CD3/CD28-induced IFN-γ production was similar between groups at baseline (difference −231.1, 95% CI [−528.5] – 66.2), and no difference was observed at 3–4 days (difference −38.0, 95% CI [−220.4] – 144.4). Persistent lymphopenia was similar between the groups (50% vs. 43%, difference −7% [95% CI (−33) – 19], Supplemental Table 3).
Clinical Outcomes
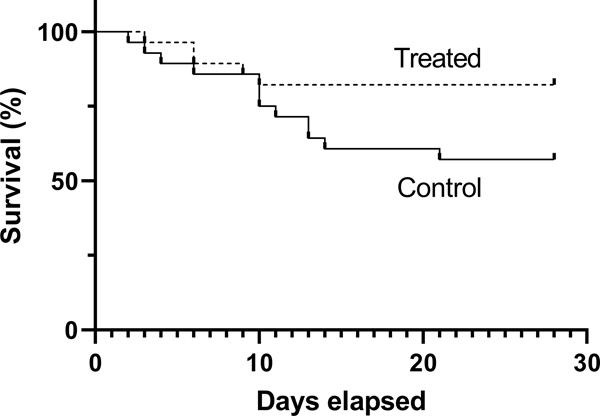
Participants randomized to therapeutic warming had lower 28-day mortality than those in the control group (18% vs. 43%, absolute risk reduction 25%, 95% CI ([−48] – [−2], Figure 3). Post-hoc adjustment of the 28-day mortality outcome for enrollment SOFA score revealed similar effect of treatment group allocation (aOR 0.151, 95% CI 0.029–0.789). They also had more 28-day hospital-free days (2.6 days, 95% CI 0–11.6). There were no differences between 28-day ventilator-free days or the 48-hour delta-SOFA score. Secondary infections were common (30%), but they were not different between the two groups (Table 2).
Figure 3. Survival curve.
This Kaplan-Meier plot shows the proportion of participants in each group alive at each time point through 28 days. The a priori defined secondary outcome was the dichotomous variable 28-day mortality (18% vs. 43%, absolute risk reduction 25%, 95% CI 2–48%).
Table 2.
Clinical Outcomes
| Variable | Control (n=28) | Warming (n=28) | Difference (95% CI) |
|---|---|---|---|
| 28-day mortality, n (%) | 12 (43) | 5 (18) | −25 ([−48]- [−2]) |
| Delta-SOFA, mean (SD) | −1.6 (3.0) | −1.8 (2.4) | −0.2 ([−1.6] – 1.3) |
| 28-day hospital-free days, median (IQR) | 0 (0–11.3) | 13.1 (0–16.8) | 2.6 (0–11.6) |
| 28-day ventilator-free days, median (IQR) | 17.6 (0–24.2) | 21.6 (2.6–24.1) | 0.1 (−6.1–1.0) |
| Secondary infection within 30 days, n (%) | 9 (32) | 8 (29) | −4 ([−28] – 20) |
| Abdominal | 1 (11) | 3 (38) | |
| Bone/soft tissue | 1 (11) | 0 (0) | |
| Catheter | 1 (11) | 1 (13) | |
| Pneumonia | 4 (44) | 4 (50) | |
| Urinary | 1 (11) | 0 (0) | |
| Wound | 1 (11) | 0 (0) |
Safety Outcomes
Participants randomized to therapeutic warming had similar vasopressor doses and vital signs during their intervention period compared to control participants (Supplemental Figure 2). A predetermined safety stopping point for an increase in vasopressor dose was reached in 2 participants (7%) leading to termination of warming, but the same vasopressor criterion was met in 4 control participants (14%). No participants met the predetermined stopping rules for heart rate or respiratory rate.
Discussion
In this pilot randomized controlled trial, we demonstrated that therapeutic hyperthermia in afebrile critically ill patients with sepsis was feasible, but we did not observe a difference in monocyte HLA-DR expression, induced IFN-γ production, or lymphopenia. We also did not observe more adverse events in the therapeutic hyperthermia group. We did, however, observe decreased mortality and increased 28-day hospital-free days in the group that underwent therapeutic hyperthermia. These are important observations because they suggest there may be a clinical benefit of this treatment, although the biological mechanism and replicability in a larger trial remains unknown.
The risk of hypothermia and the survival benefit of fever in patients with sepsis are well described (2–8, 45). A meta-analysis of observational studies cited a pooled estimate of a 47% mortality in hypothermic patients with sepsis versus 22% mortality in those with fever (6). While the control group mortality in our trial was high, it tracks closely with the mortality observed in prior cohorts of afebrile critically ill sepsis patients (2–5). Perhaps more compelling, participants allocated to therapeutic hyperthermia experienced a mortality that was more closely aligned with expected mortality in febrile sepsis patients (2–6). Studies of antipyretic therapy in sepsis have demonstrated little benefit (46, 47). A single trial of external cooling in febrile sepsis patients showed reduced vasopressor doses and lower early mortality in febrile patients cooled to normothermia (48), but these findings were not replicated in a subsequent randomized trial (49). The inflammatory cascade, cytokine expression, and transcription factors in febrile sepsis patients is likely different from afebrile sepsis patients, and having experienced fever may confer benefit to immunocompetent sepsis patients that cannot be undone with cooling.
The mechanism through which therapeutic hyperthermia may have affected clinical outcomes in this study is unclear. Previous data have demonstrated that, compared to afebrile patients with sepsis, febrile patients had a greater increase in monocyte HLA-DR early after sepsis diagnosis (9), suggesting a lower incidence of sepsis-induced immunosuppression in patients with fever. Fever and therapeutic hyperthermia, though, are fundamentally different conditions, and the relationship between fever and HLA-DR expression may be mediated via the inflammatory cascade involved in fever production rather than by heat itself. Notably, the survival curves in our study diverged around day 10, which indicates the effect of warming may be late. Given the breadth of immune changes caused by hyperthermia in animal and in vitro studies, immune-related mechanisms may have played in a role in the clinical outcomes seen in this study, despite our negative immune findings. Alternatively, therapeutic hyperthermia may benefit septic patients through non-immune mechanisms, such as direct effects on microbial clearance, augmentation of antibiotic activity, or temperature-dependent changes in blood flow. Regardless, this is the first paper that has supported the hypothesis that temperature plays a causal role in survival from life-threatening infection.
Another interesting observation in this trial was that forced air warming alone could effectively warm patients in a short time, even above 37°C. This finding suggests that the absence of fever was not an adaptive process associated with a change in set point. Rather, insufficient cytokine response or mitochondrial dysfunction may have contributed to hypothermia, and when adequate heat was applied, substantive temperature elevations could be realized.
Our observation should be confirmed in a multicenter clinical trial powered for clinical outcomes. That trial should better elucidate the most effective temperature target, duration of treatment, and mechanism of effect to fully understand how temperature management might be used in clinical practice. Better understanding the impact of temperature on sepsis physiology and the heterogeneity of treatment effects will be critical to defining the clinical application of sepsis warming.
This trial has several limitations. First, temperature augmentation was modest, with over 25% of our warmed participants not achieving febrile-range hyperthermia. Second, we used a warming technique that was limited in its ability to achieve very high temperatures. Future trials targeting higher temperatures may need to use technology able to transfer more heat. Third, our control group mortality was very high. Although prior studies of hypothermia in sepsis have observed similarly high mortality, our inclusion criteria intentionally selected a group of participants with high predicted mortality. Fourth, our primary outcome could not be measured in one participant who died or in any patients were immune suppressed. These eligibility criteria may limit generalizability if hyperthermia were ultimately found to be effective. Finally, our small sample size also makes Type I error more likely, so future larger multicenter trials should be conducted to ensure balance on important covariates.
In conclusion, induced hyperthermia did not improve early markers of immune function, but in our pilot randomized trial warming was associated with lower mortality. The pathway responsible for our findings is unclear. Future work should seek to confirm our clinical findings in an adequately powered multicenter clinical trial, and to better understand mechanistic changes in sepsis physiology associated with temperature modulation.
Supplementary Material
Acknowledgments
Funding: Dr. Drewry was supported by the Washington University Institute of Clinical and Translational Sciences (UL1TR000448, KL2TR000450) and the National Institutes of Health (K23GM129660). Dr. Mohr received support from the Agency for Healthcare Research and Quality (K08HS025753). Dr. Ablordeppey was supported by the Department of Anesthesiology, Division of Clinical and Translational Research (DoCTR) at Washington University and the K12 Mentored Training in Implementation Science award (K12HL137942). Dr. Hotchkiss is supported by funding from the National Institutes of Health (R35GM126928). These contents are solely the responsibility of the authors and do not necessarily reflect the views of the National Institutes of Health, the Agency for Healthcare Research and Quality, or the U.S. Department of Health and Human Services.
Copyright Form Disclosure: Drs. Drewry and Hotchkiss’s institutions received funding from the National Institutes of Health (NIH). Drs. Drewry and Mohr’s institutions received funding from the Agency for Healthcare Research and Quality. Dr. Drewry’s institution received funding from Washington University in St. Louis. Drs. Drewry, Mohr, Dalton, and Hotchkiss received support for article research from the NIH. Dr. Hotchkiss’s institution received funding from the National Institute of General Medical Sciences; he disclosed that he holds a patent for the ELISpot assay, which was used for immune phenotyping in this paper. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Institution Where the Work was Performed: Washington University in St. Louis, St. Louis, Missouri.
Reprints will not be available.
Conflicts of Interest: Dr. Hotchkiss holds a patent on the ELISpot assay, which was used in the conduct of this research. None of the other authors have conflicts of interest to declare.
References
- 1.Liu VX, Bhimarao M, Greene JD, Manickam RN, et al. : The Presentation, Pace, and Profile of Infection and Sepsis Patients Hospitalized Through the Emergency Department: An Exploratory Analysis. Crit Care Explor 2021; 3(3):e0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushimoto S, Gando S, Saitoh D, Mayumi T, et al. : The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care 2013; 17(6):R271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young PJ, Saxena M, Beasley R, Bellomo R, et al. : Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 2012 [DOI] [PubMed] [Google Scholar]
- 4.Sunden-Cullberg J, Rylance R, Svefors J, Norrby-Teglund A, et al. : Fever in the Emergency Department Predicts Survival of Patients With Severe Sepsis and Septic Shock Admitted to the ICU. Crit Care Med 2017; 45(4):591–599 [DOI] [PubMed] [Google Scholar]
- 5.Inghammar M, Sunden-Cullberg J: Prognostic significance of body temperature in the emergency department vs the ICU in Patients with severe sepsis or septic shock: A nationwide cohort study. PLoS One 2020; 15(12):e0243990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rumbus Z, Matics R, Hegyi P, Zsiboras C, et al. : Fever Is Associated with Reduced, Hypothermia with Increased Mortality in Septic Patients: A Meta-Analysis of Clinical Trials. PLoS One 2017; 12(1):e0170152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arons MM, Wheeler AP, Bernard GR, Christman BW, et al. : Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med 1999; 27(4):699–707 [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Zaloga GP: Hypothermia and cytokines in septic shock. Norasept II Study Investigators. North American study of the safety and efficacy of murine monoclonal antibody to tumor necrosis factor for the treatment of septic shock. Intensive Care Med 2000; 26(6):716–721 [DOI] [PubMed] [Google Scholar]
- 9.Drewry AM, Ablordeppey EA, Murray ET, Dalton CM, et al. : Monocyte Function and Clinical Outcomes in Febrile and Afebrile Patients With Severe Sepsis. Shock 2018; 50(4):381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Oss CJ, Absolom DR, Moore LL, Park BH, et al. : Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc 1980; 27(6):561–565 [PubMed] [Google Scholar]
- 11.Jampel HD, Duff GW, Gershon RK, Atkins E, et al. : Fever and immunoregulation. III. Hyperthermia augments the primary in vitro humoral immune response. J Exp Med 1983; 157(4):1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q, Cross AS, Singh IS, Chen TT, et al. : Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 2000; 68(3):1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozveri ES, Bekraki A, Cingi A, Yuksel M, et al. : The effect of hyperthermic preconditioning on the immune system in rat peritonitis. Intensive Care Med 1999; 25(10):1155–1159 [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, et al. : Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med 1994; 22(6):914–921 [PubMed] [Google Scholar]
- 15.Wolk K, Docke WD, von Baehr V, Volk HD, et al. : Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood 2000; 96(1):218–223 [PubMed] [Google Scholar]
- 16.Huang X, Venet F, Wang YL, Lepape A, et al. : PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009; 106(15):6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venet F, Chung CS, Monneret G, Huang X, et al. : Regulatory T cell populations in sepsis and trauma. J Leukoc Biol 2008; 83(3):523–535 [DOI] [PubMed] [Google Scholar]
- 18.Rigato O, Salomao R: Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock 2003; 19(2):113–116 [DOI] [PubMed] [Google Scholar]
- 19.Ertel W, Kremer JP, Kenney J, Steckholzer U, et al. : Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 1995; 85(5):1341–1347 [PubMed] [Google Scholar]
- 20.Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, et al. : Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defects of monocyte cytokine production. Surgery 2000; 127(3):309–315 [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, et al. : Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999; 27(7):1230–1251 [DOI] [PubMed] [Google Scholar]
- 22.Boomer JS, To K, Chang KC, Takasu O, et al. : Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306(23):2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruse N, Leijte GP, Pickkers P, Kox M: New frontiers in precision medicine for sepsis-induced immunoparalysis. Expert Rev Clin Immunol 2019; 15(3):251–263 [DOI] [PubMed] [Google Scholar]
- 24.Monneret G, Lepape A, Voirin N, Bohe J, et al. : Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 2006; 32(8):1175–1183 [DOI] [PubMed] [Google Scholar]
- 25.Wu JF, Ma J, Chen J, Ou-Yang B, et al. : Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit Care 2011; 15(5):R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, et al. : Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med 2009; 37(10):2746–2752 [DOI] [PubMed] [Google Scholar]
- 27.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, et al. : Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 2013; 41(1):224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drewry AM, Ablordeppey EA, Murray ET, Beiter ER, et al. : Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit Care 2016; 20(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajan G, Sleigh JW: Lymphocyte counts and the development of nosocomial sepsis. Intensive Care Med 1997; 23(11):1187. [DOI] [PubMed] [Google Scholar]
- 30.Chung KP, Chang HT, Lo SC, Chang LY, et al. : Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock 2015; 43(6):569–575 [DOI] [PubMed] [Google Scholar]
- 31.Drewry AM, Samra N, Skrupky LP, Fuller BM, et al. : Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014; 42(5):383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM: A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care 2012; 16(3):R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagawa Y, Tanigawa K, Kobayashi Y, Yamamoto M: Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J Cancer Metastasis Treat 2017; 3:218–230 [Google Scholar]
- 34.van der Zee J: Heating the patient: a promising approach? Ann Oncol 2002; 13(8):1173–1184 [DOI] [PubMed] [Google Scholar]
- 35.Kurz A, Sessler DI, Lenhardt R: Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334(19):1209–1215 [DOI] [PubMed] [Google Scholar]
- 36.Melling AC, Ali B, Scott EM, Leaper DJ: Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001; 358(9285):876–880 [DOI] [PubMed] [Google Scholar]
- 37.Wong PF, Kumar S, Bohra A, Whetter D, et al. : Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg 2007; 94(4):421–426 [DOI] [PubMed] [Google Scholar]
- 38.Eldridge SM, Chan CL, Campbell MJ, Bond CM, et al. : CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; 355:i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellinger RP, Levy MM, Rhodes A, Annane D, et al. : Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41(2):580–637 [DOI] [PubMed] [Google Scholar]
- 40.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315(8):801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drewry AM: Impaired Immunity in Afebrile Critically Ill Patients with Severe Sepsis [oral presentation]. In: American Society of AnesthesiologistsChicago, Illinois, 2016 [Google Scholar]
- 42.Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, et al. : Management of Refractory Vasodilatory Shock. Chest 2018; 154(2):416–426 [DOI] [PubMed] [Google Scholar]
- 43.Demaret J, Walencik A, Jacob MC, Timsit JF, et al. : Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom 2013; 84(1):59–62 [DOI] [PubMed] [Google Scholar]
- 44.Thampy LK, Remy KE, Walton AH, Hong Z, et al. : Restoration of T Cell function in multi-drug resistant bacterial sepsis after interleukin-7, anti-PD-L1, and OX-40 administration. PLoS One 2018; 13(6):e0199497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhavani SV, Carey KA, Gilbert ER, Afshar M, et al. : Identifying Novel Sepsis Subphenotypes Using Temperature Trajectories. Am J Respir Crit Care Med 2019; 200(3):327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young PJ, Bellomo R, Bernard GR, Niven DJ, et al. : Fever control in critically ill adults. An individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 2019; 45(4):468–476 [DOI] [PubMed] [Google Scholar]
- 47.Drewry AM, Ablordeppey EA, Murray ET, Stoll CRT, et al. : Antipyretic Therapy in Critically Ill Septic Patients: A Systematic Review and Meta-Analysis. Crit Care Med 2017; 45(5):806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schortgen F, Clabault K, Katsahian S, Devaquet J, et al. : Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 2012; 185(10):1088–1095 [DOI] [PubMed] [Google Scholar]
- 49.Young PJ, Bailey MJ, Bass F, Beasley RW, et al. : Randomised evaluation of active control of temperature versus ordinary temperature management (REACTOR) trial. Intensive Care Med 2019; 45(10):1382–1391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.