Abstract
Objectives:
To evaluate the association between aspirin use during first pregnancy and later maternal cardiovascular risk.
Study Design:
In this secondary analysis of a prospective cohort, we included participants who carried their first pregnancy to 20+ weeks, had data regarding aspirin use, and attended a study visit 2-7 years following delivery. The exposure was aspirin use during the first pregnancy. We calculated aspirin use propensity scores from logistic regression models including baseline variables associated with aspirin use in pregnancy and cardiovascular risk. Outcomes of interest were incident cardiovascular-related diagnoses 2-7 years following delivery. Robust Poisson regression calculated the risk of outcomes by aspirin exposure, adjusting for the aspirin use propensity score.
Main Outcome Measures:
The primary outcome was a composite of incident cardiovascular diagnoses at the time of the study visit: cardiovascular events, chronic hypertension, metabolic syndrome, prediabetes or type 2 diabetes, dyslipidemia, and chronic kidney disease.
Results:
Of 4,480 women included, 84 (1.9%) reported taking aspirin during their first pregnancy. 52.6% of participants in the aspirin-exposed group and 43.0% in the unexposed group had the primary outcome. After adjusting for the aspirin use propensity scores, aspirin use during the first pregnancy was not associated with any of the outcomes.
Conclusion:
We did not detect an association between aspirin use during the first pregnancy and cardiovascular-related diagnoses 2-7 years later. Our study was only powered to detect a large difference in relative risk, so we cannot rule out a smaller difference that may be clinically meaningful.
Keywords: cardiovascular prevention, maternal health, pregnancy as a window to future health, pregnancy complications, adverse pregnancy outcomes
Introduction
Women with adverse pregnancy outcomes, including hypertensive disorders of pregnancy, stillbirth, preterm birth, and delivery of a small-for-gestational age neonate, have higher risk for the subsequent development of cardiovascular disease compared to women with normal pregnancy outcomes [1-7]. While the pathophysiologic mechanisms underlying this association remain poorly understood, low-dose aspirin has been investigated as a preventive therapy for both placenta-mediated pregnancy complications (mostly hypertensive disorders of pregnancy) [8, 9] and cardiovascular disease outside of pregnancy [10]. Even at low doses, aspirin achieves irreversible inhibition of cyclooxygenase enzymes, reducing platelet activation, production of thromboxane A2, and subsequent platelet aggregation [11, 12]. These processes have been implicated in the pathogenesis of both abnormal placentation [13] and cardiovascular events such as myocardial infarction and stroke [14].
Large multinational randomized trials of low-dose aspirin during pregnancy have demonstrated meaningful reductions in the risk for preeclampsia among women assigned to aspirin therapy [8, 9]. In addition, low-dose aspirin has been associated with a decreased risk for perinatal death, preterm birth, and delivery of a small-for-gestational age neonate [15-18]. The United States Preventive Services Task Force (USPSTF) now recommends daily low-dose (81 mg) aspirin for the prevention of preeclampsia among women with risk factors [19]. Numerous studies have assessed the effect of aspirin on pregnancy outcomes, and other studies have assessed the association between pregnancy outcomes and later cardiovascular health. However, the relationship between aspirin use in pregnancy and subsequent cardiovascular risk trajectory is unknown.
We hypothesize that aspirin use during the first pregnancy confers cardiovascular protection beyond the first pregnancy. We aimed to determine whether women who took aspirin during their first pregnancy have more favorable cardiovascular risk profiles several years after delivery compared to women who did not take aspirin, adjusting for propensity for aspirin use.
Methods
This is a secondary analysis of the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers-to-be (nuMoM2b) Heart Health Study (HHS). The nuMoM2b-HHS is a prospective observational cohort that followed participants for 2-7 years following their first pregnancy to investigate relationships between adverse pregnancy outcomes and cardiovascular health [20]. Participants were enrolled in nuMoM2b during the first trimester of their first pregnancy and completed an in-person nuMoM2b-HHS study visit 2-7 years after delivery. The institutional review boards at each study site approved the study protocol, and all participants provided written informed consent before enrollment. We included all 4,484 women who carried their first (index) pregnancy to at least 20 weeks of gestation and participated in a nuMoM2b-HHS in-person study visit. We excluded women who were missing data regarding aspirin exposure (n=4).
The exposure of interest is any aspirin use during the first pregnancy, ascertained prospectively by self-report with verification via chart abstraction. The data collection form was administered via in-person interview by trained research staff. Research staff were instructed to list medications participants were taking other than multivitamins, mineral supplements, prenatal vitamins, or folic acid that were prescribed or recommended by a health care provider. The form did not include information regarding dose or duration of use. Forms were completed at each study visit: visit 1 at 6 0/7 weeks-13 6/7 weeks’ gestation, visit 2 at 16 0/7 weeks-21 6/7 weeks’ gestation, and visit 3 at 22 0/7 weeks-29 6/7 weeks’ gestation. Women were defined as exposed to aspirin if they completed a medication form and reported taking aspirin at any point during the pregnancy. Combination aspirin/acetaminophen/caffeine medications did not qualify as aspirin exposure, because we intended to capture women taking daily low-dose aspirin and these combination medications are taken only as needed for headaches. Women were defined as unexposed if they completed at least one medication form and did not report taking aspirin.
The primary outcome is a composite of incident cardiovascular events and diagnoses at the time of the nuMoM2b-HHS visit, 2-7 years following delivery of the index pregnancy. Components of the composite outcome include incident cardiovascular events (including myocardial infarction, stroke, or transient ischemic attack), chronic hypertension, metabolic syndrome, prediabetes or type 2 diabetes, dyslipidemia, and chronic kidney disease diagnosed during the study period. Secondary outcomes include the individual components of this composite. For each outcome of interest, we excluded women who already had the condition at baseline. This ensured that we were capturing incident outcomes occurring after the first pregnancy. For example, women with chronic hypertension at baseline were excluded from the analysis of incident hypertension but included in the analysis of incident cardiovascular events.
A history of cardiovascular events was defined as self-reported history of myocardial infarction, stroke, or transient ischemic attack. Chronic hypertension was defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, or taking antihypertensive medication [21]. Metabolic syndrome was defined as at least 3 of the following 5 criteria: elevated waist circumference (≥88 cm if non-Asian or ≥80 cm if Asian); elevated triglycerides (≥150 mg/dL or 1.7 mmol/L) or taking triglyceride-lowering medication; elevated fasting glucose (≥100 mg/dL or 5.55 mmol/L) or taking glucose-lowering medication; elevated blood pressure (systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg) or taking antihypertensive medications; and reduced HDL cholesterol (<50 mg/dL or 1.3 mmol/L) or taking lipid-modifying medication. Prediabetes or type 2 diabetes was defined as fasting blood sugar ≥100 mg/dL (5.55 mmol/L) or taking glucose-lowering medication. Dyslipidemia was defined as any of the following: total cholesterol ≥240 mg/dL (6.2 mmol/L), LDL ≥160 mg/dL (4.1 mmol/L), HDL <35 mg/dL (0.9 mmol/L), triglycerides ≥200 mg/dL (2.3 mmol/L), or taking lipid-modifying medication. Chronic kidney disease was defined as a self-reported diagnosis of kidney disease (including nephritis, glomerulonephritis, dialysis, renal transplant, or other condition denoting chronic kidney disease per review by LHT) or albumin-to-creatinine ratio >30 mg/g.
To compare outcomes by aspirin exposure among women with similar baseline risk profiles, we used inverse probability of treatment weighting using propensity scores. Propensity scores were calculated from logistic regression models of aspirin use with explanatory baseline variables related to aspirin exposure during pregnancy [19] and to cardiovascular risk. These variables included age, race, education, insurance, substance use, tobacco use, chronic hypertension, prediabetes or pre-gestational diabetes, chronic kidney disease, autoimmune disease, obesity, and family history of preeclampsia. Study subjects with chronic hypertension, prediabetes or gestational diabetes, and chronic kidney disease at baseline were excluded from the calculation of propensity scores for those outcomes, respectively. The propensity scores ranged from zero to one and estimate the probability of aspirin use based on characteristics included in the logistic regression model. In total, four propensity scores were calculated: 1) for outcomes including the primary composite, metabolic syndrome, and dyslipidemia; 2) for the outcome of chronic hypertension; 3) for the outcome of prediabetes or type 2 diabetes; and 4) for the outcome of chronic kidney disease (Supplement – Generation of Propensity Scores).
Robust Poisson regression was used to calculate the risk of the cardiovascular-related outcomes of interest at 2-7 years after delivery of the first pregnancy, with model covariates including aspirin exposure during the first pregnancy and the aspirin use propensity score. Adverse pregnancy outcomes, including hypertensive disorders of pregnancy, stillbirth, preterm birth, and small-for-gestational age neonate, may be on the causal pathway between aspirin exposure and cardiovascular risk. As such, we stratified our results by 2 categories: history of any of these adverse pregnancy outcomes vs none of these adverse pregnancy outcomes.
Statistical analyses were performed using SAS version 9.4 (Cary, NC). Two-sided p-values <0.05 were considered statistically significant. Based on published results from the nuMoM2b-HHS demonstrating a 20% incidence of stage 2 chronic hypertension among the overall cohort [22] we anticipate that 25% of women in the unexposed/no aspirin group will have the primary outcome composite compared to 15% of women in the exposed/aspirin group. With an overall sample size of 4,484 women, 2.5% of whom are in the exposed/aspirin group (n=112), a two-sided test achieves 80% power at α = 0.050 to detect a relative risk of 0.56.
Results
After excluding 4 participants with missing data regarding aspirin exposure, we included 4,480 women who had completed medication forms during their first pregnancy and subsequently attended an in-person nuMoM2b-HHS study visit (Figure). Eighty-four women (1.9% of the study population) reported taking aspirin during their first pregnancy. Compared with women who did not take aspirin during their first pregnancy, women who reported taking aspirin were older, had higher education levels, private insurance, and were more likely to be white, married, and to have autoimmune disease (Table 1). After excluding women with the outcomes of interest at baseline, we included 3,776 women in the analysis of the primary composite outcome (71 with aspirin and 3,705 with no aspirin), 3,811 women in the analysis of the chronic hypertension outcome (71 with aspirin and 3,740 with no aspirin), 3,879 women in the analysis of the prediabetes or type 2 diabetes outcome (76 with aspirin and 3,803 with no aspirin), and 3,809 women in the analysis of the chronic kidney disease outcome (71 with aspirin and 3,738 with no aspirin). The propensity scores for aspirin exposure in each group are listed in Table 1.
Figure.
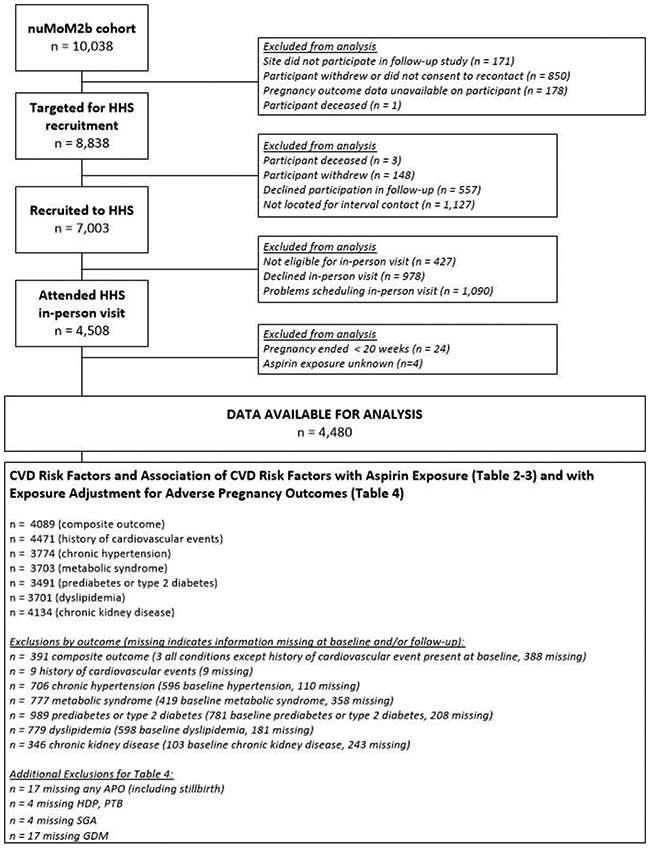
Flow diagram of study population.
Table 1.
Demographic characteristics, baseline cardiovascular risk factors, and propensity scores during index pregnancy according to aspirin exposure.
| Characteristics During nuMoM2b Index Pregnancy | All participants (N=4480) |
nuMoM2b Index Pregnancy Aspirin Exposure |
|
|---|---|---|---|
| Aspirin (N=84) |
No Aspirin (N=4396) |
||
| Maternal age, years, mean (SD) | 27.00 (5.6) | 30.61 (4.7)# | 26.93 (5.6) |
| Category: n (%) | |||
| 13-21 | 905 (20.2) | 2 (2.4)# | 903 (20.5) |
| 22-35 | 3268 (72.9) | 70 (83.3)# | 3198 (72.7) |
| > 35 | 307 (6.9) | 12 (14.3)# | 295 (6.7) |
| Maternal race: n (%) | |||
| White Non-Hispanic | 2783 (62.1) | 71 (84.5)# | 2712 (61.7) |
| Black Non-Hispanic | 618 (13.8) | 4 (4.8)# | 614 (14.0) |
| Hispanic | 734 (16.4) | 4 (4.8)# | 730 (16.6) |
| Asian | 135 (3.0) | 2 (2.4)# | 133 (3.0) |
| Other | 210 (4.7) | 3 (3.6)# | 207 (4.7) |
| Marital status: n (%) | |||
| Single, never married | 1669 (37.3) | 8 (9.5)# | 1661 (37.8) |
| Married | 2756 (61.5) | 76 (90.5)# | 2680 (61.0) |
| Previously partnered | 49 (1.1) | 0 (0.0)# | 49 (1.1) |
| Refused | 6 (0.1) | 0 (0.0)# | 6 (0.1) |
| Education level: n / N (%) | |||
| Less than high school graduate | 327/4478 (7.3) | 1/84 (1.2)# | 326/4394 (7.4) |
| High school graduate or GED completed | 507/4478 (11.3) | 2/84 (2.4)# | 505/4394 (11.5) |
| Some college credit, no degree | 889/4478 (19.9) | 10/84 (11.9)# | 879/4394 (20.0) |
| Associate/technical degree | 501/4478 (11.2) | 7/84 (8.3)# | 494/4394 (11.2) |
| Bachelor's degree | 1260/4478 (28.1) | 35/84 (41.7)# | 1225/4394 (27.9) |
| Degree beyond bachelor's | 994/4478 (22.2) | 29/84 (34.5)# | 965/4394 (22.0) |
| Type of health insurance: n / N (%) | |||
| Commercial/military | 3104/4454 (69.7) | 76/83 (91.6)# | 3028/4371 (69.3) |
| Government | 1199/4454 (26.9) | 4/83 (4.8)# | 1195/4371 (27.3) |
| Self-pay/other | 151/4454 (3.4) | 3/83 (3.6)# | 148/4371 (3.4) |
| Drank during 3 months prior to pregnancy: n / N (%) | 2788/4474 (62.3) | 53/84 (63.1) | 2735/4390 (62.3) |
| Any prior illicit drug use: n / N (%) | 1459/4475 (32.6) | 30/84 (35.7) | 1429/4391 (32.5) |
| Smoked during 3 months prior to pregnancy: n / N (%) | 717/4474 (16.0) | 8/84 (9.5) | 709/4390 (16.2) |
| Chronic hypertension: n / N (%) | 596/4379 (13.6) | 12/83 (14.5) | 584/4296 (13.6) |
| Metabolic syndrome (MetS): n / N (%) | 419/4214 (9.9) | 12/78 (15.4) | 407/4136 (9.8) |
| Prediabetes or pre-gestational diabetes: n / N (%) | 781/4358 (17.9) | 19/78 (24.4) | 762/4280 (17.8) |
| Dyslipidemia: n / N (%) | 598/4362 (13.7) | 17/79 (21.5)# | 581/4283 (13.6) |
| Chronic kidney disease: n / N (%) | 103/4293 (2.4) | 2/83 (2.4) | 101/4210 (2.4) |
| Autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, or antiphospholipid syndrome): n / N (%) | 83/4341 (1.9) | 8/84 (9.5)# | 75/4257 (1.8) |
| BMI, kg/m2, mean (SD) | 26.60 (6.5) | 27.06 (6.4) | 26.59 (6.5) |
| Category: n / N (%) | |||
| < 25 | 2283/4404 (51.8) | 42/83 (50.6) | 2241/4321 (51.9) |
| 25 to < 30 | 1079/4404 (24.5) | 20/83 (24.1) | 1059/4321 (24.5) |
| ≥ 30 | 1042/4404 (23.7) | 21/83 (25.3) | 1021/4321 (23.6) |
| Family history of preeclampsia (participant’s mother or sister): n / N (%) | 401/4120 (9.7) | 10/77 (13.0) | 391/4043 (9.7) |
| Family history of cardiovascular disease (any first-degree relative): n / N (%) | 736/4098 (18.0) | 12/75 (16.0) | 724/4023 (18.0) |
| Any Adverse Pregnancy Outcome (APO): n / N (%) | 1023/4463 (22.9) | 26/83 (31.3) | 997/4380 (22.8) |
| Hypertensive disorders of pregnancy | 612/4476 (13.7) | 19/84 (22.6)# | 593/4392 (13.5) |
| Preterm birth | 387/4476 (8.6) | 8/84 (9.5) | 379/4392 (8.6) |
| Small for gestational age | 187/4463 (4.2) | 4/83 (4.8) | 183/4380 (4.2) |
| Stillbirth | 16/4480 (0.4) | 0 (0.0) | 16/4396 (0.4) |
| Gestational diabetes: n / N (%) | 191/4418 (4.3) | 7/82 (8.5) | 184/4336 (4.2) |
| Propensity scores for aspirin exposure1/ | |||
| Subset to participants included in composite outcome2/, mean (SD) | 0.02 (0.02) | 0.04 (0.05)# | 0.02 (0.02) |
| N with result | 3776 | 71 | 3705 |
| Subset to participants included in chronic hypertension outcome, mean (SD) | 0.02 (0.02) | 0.04 (0.05)# | 0.02 (0.02) |
| N with result | 3811 | 71 | 3740 |
| Subset to participants included in prediabetes or type 2 diabetes outcome, mean (SD) | 0.02 (0.02) | 0.04 (0.05)# | 0.02 (0.02) |
| N with result | 3879 | 76 | 3803 |
| Subset to participants included in chronic kidney disease outcome, mean (SD) | 0.02 (0.02) | 0.04 (0.05)# | 0.02 (0.02) |
| N with result | 3809 | 71 | 3738 |
Abbreviations: GED=General Educational Development; SD = standard deviation; n = number in category; N = sample size; BMI = body mass index.
Statistically significant association at p<0.05 between characteristic and aspirin exposure as calculated by chi-square when possible or Fisher’s Exact when cell counts are low.
Propensity scores calculated from logistic regression models of aspirin exposure with explanatory variables related to aspirin exposure and cardiovascular risk: age, race, education, insurance, substance use, tobacco use, chronic hypertension (excluded from propensity score for chronic hypertension at 2-7 year visit), prediabetes or type 2 diabetes (excluded from propensity score for prediabetes or type 2 diabetes at 2-7 year visit), chronic kidney disease (excluded from propensity score for chronic kidney disease at 2-7 year visit), autoimmune disease, obesity, and family history of preeclampsia. Scores range from zero to one.
Composite outcome: history of cardiovascular events (myocardial infarction or stroke), chronic hypertension (stage 1 or 2 hypertension per the 2017 American College of Cardiology and American Heart Association Guidelines), metabolic syndrome, prediabetes or type 2 diabetes, dyslipidemia, and/or chronic kidney disease diagnoses.
At the time of the nuMoM2b-HHS study visit, 1,766 women experienced the composite cardiovascular-related outcome, with 20 cases of incident cardiovascular events, 679 of incident chronic hypertension, 499 of incident metabolic syndrome, 709 of incident prediabetes or type 2 diabetes, 446 of incident dyslipidemia, and 243 of incident chronic kidney disease.
Among the entire study population, aspirin use during the first pregnancy was not associated with the primary composite outcome at the time of the nuMoM2b-HHS study visit (Table 2). Aspirin use was also not associated with any of the other secondary outcomes at the time of the nuMoM2b-HHS visit.
Table 2.
Association of aspirin exposure during nuMoM2b index pregnancy with cardiovascular-related conditions 2-7 years later, unadjusted and adjusted for propensity of aspirin exposure2,3, among nuMoM2b-HHS participants.
| Cardiovascular-related condition |
All participants n / N (%) |
N with condition among exposed n / N (%) |
N with condition among unexposed n / N (%) |
RR (95% CI) |
|---|---|---|---|---|
| Composite outcome1 | ||||
| Unadjusted | 1766/4089 (43.2) | 41/78 (52.6) | 1725/4011 (43.0) | 1.22 (0.99, 1.51) |
| Adjusted | 1512/3652 (41.4) | 34/70 (48.6) | 1478/3582 (41.3) | 1.18 (0.92, 1.52) |
| Chronic hypertension | ||||
| Unadjusted | 679/3774 (18.0) | 12/71 (16.9) | 667/3703 (18.0) | 0.94 (0.56, 1.58) |
| Adjusted | 567/3253 (17.4) | 9/60 (15.0) | 558/3193 (17.5) | 0.82 (0.44, 1.50) |
| Metabolic syndrome | ||||
| Unadjusted | 499/3703 (13.5) | 4/64 (6.3) | 495/3639 (13.6) | 0.46 (0.18, 1.19) |
| Adjusted | 447/3294 (13.6) | 4/58 (6.9) | 443/3236 (13.7) | 0.56 (0.22, 1.43) |
| Prediabetes or type 2 diabetes | ||||
| Unadjusted | 709/3491 (20.3) | 12/58 (20.7) | 697/3433 (20.3) | 1.02 (0.61, 1.69) |
| Adjusted | 611/3043 (20.1) | 12/52 (23.1) | 599/2991 (20.0) | 1.17 (0.71, 1.92) |
| Dyslipidemia | ||||
| Unadjusted | 446/3701 (12.1) | 8/61 (13.1) | 438/3640 (12.0) | 1.09 (0.57, 2.09) |
| Adjusted | 383/3199 (12.0) | 7/55 (12.7) | 376/3144 (12.0) | 0.96 (0.49, 1.89) |
| Chronic kidney disease | ||||
| Unadjusted | 243/4134 (5.9) | 10/81 (12.3)# | 233/4053 (5.7) | 2.15 (1.19, 3.89) |
| Adjusted | 213/3647 (5.8) | 7/69 (10.1) | 206/3578 (5.8) | 1.71 (0.80, 3.63) |
Abbreviations: n = number in category; N = sample size; RR = relative risk; CI = confidence interval.
Statistically significant association at p<0.05 between characteristic and aspirin exposure as calculated by chi-square when possible or Fisher’s Exact when cell counts are low.
Composite outcome: cardiovascular events (myocardial infarction or stroke), chronic hypertension, metabolic syndrome, prediabetes or type 2 diabetes, dyslipidemia, and/or chronic kidney disease diagnoses
Robust Poisson regression model with sandwich variance estimator was used to calculate RR and 95% CI for association between aspirin exposure and the cardiovascular-related conditions. All models included aspirin exposure during the index pregnancy and the aspirin use propensity score as covariates.
Propensity scores calculated from logistic regression models of aspirin exposure with explanatory variables related to aspirin exposure and cardiovascular risk: age, race, education, insurance, substance use, tobacco use, chronic hypertension (excluded from propensity score for chronic hypertension at 2-7 year visit), prediabetes or type 2 diabetes (excluded from propensity score for prediabetes or type 2 diabetes at 2-7 year visit), chronic kidney disease (excluded from propensity score for chronic kidney disease at 2-7 year visit), autoimmune disease, obesity, and family history of preeclampsia. Scores range from zero to one.
Similarly, stratified analyses by any adverse pregnancy outcome showed no significant associations between aspirin exposure during the first pregnancy and the cardiovascular-related outcomes of interest (Table 3). Only 23 of 1,023 women with an adverse pregnancy outcome took aspirin, so small sample sizes precluded stratifying analyses by individual adverse pregnancy outcomes: 19 of the 612 women with hypertensive disorders of pregnancy took aspirin; 8 of the 387 women with a preterm birth took aspirin; 4 of the 187 women who delivered a small-for-gestational age neonate took aspirin; and none of the 16 women who had a stillbirth took aspirin.
Table 3.
Association of aspirin exposure during nuMoM2b index pregnancy with cardiovascular-related conditions 2-7 years later, unadjusted and adjusted for propensity of aspirin exposure3, 4 and stratified by adverse pregnancy outcome.
| Cardiovascular-related condition | Any Adverse Pregnancy Outcome2 |
|
|---|---|---|
| Yes RR (95% CI) |
No RR (95% CI) |
|
| Composite outcome1 | ||
| Unadjusted | 1.27 (0.95, 1.69) | 1.15 (0.86, 1.54) |
| Adjusted for propensity score | 1.19 (0.82, 1.74) | 1.15 (0.84, 1.59) |
| Chronic hypertension | ||
| Unadjusted | 1.24 (0.68, 2.29) | 0.65 (0.28, 1.50) |
| Adjusted for propensity score | 0.90 (0.38, 2.12) | 0.74 (0.32, 1.70) |
| Metabolic syndrome | ||
| Unadjusted | 0.28 (0.04, 1.88) | 0.55 (0.18, 1.65) |
| Adjusted for propensity score | 0.41 (0.06, 2.82) | 0.63 (0.21, 1.90) |
| Prediabetes or type 2 diabetes | ||
| Unadjusted | 0.78 (0.28, 2.16) | 1.12 (0.62, 2.00) |
| Adjusted for propensity score | 1.02 (0.38, 2.75) | 1.22 (0.69, 2.19) |
| Dyslipidemia | ||
| Unadjusted | 1.57 (0.67, 3.70) | 0.81 (0.32, 2.09) |
| Adjusted for propensity score | 1.57 (0.69, 3.58) | 0.62 (0.21, 1.84) |
| Chronic kidney disease | ||
| Unadjusted | 2.35 (0.93, 5.96) | 2.00 (0.93, 4.31) |
| Adjusted for propensity score | 1.43 (0.35, 5.92) | 1.84 (0.78, 4.34) |
Abbreviations: RR = relative risk; CI = confidence interval.
Statistically significant association at p<0.05 between characteristic and aspirin exposure as calculated by chi-square when possible or Fisher’s Exact when cell counts are low.
Composite outcome: cardiovascular events (myocardial infarction or stroke), chronic hypertension, metabolic syndrome, prediabetes or type 2 diabetes, dyslipidemia, and/or chronic kidney disease diagnoses.
Any adverse pregnancy outcome defined as: hypertensive disorder of pregnancy, preterm birth, small-for-gestational age neonate, or stillbirth
Robust Poisson regression model with sandwich variance estimator was used to calculate RR and 95% CI for association between aspirin exposure and the cardiovascular-related conditions. All models included aspirin exposure during the index pregnancy and the aspirin use propensity score as covariates.
Propensity scores calculated from logistic regression models of aspirin exposure with explanatory variables related to aspirin exposure and cardiovascular risk: age, race, education, insurance, substance use, tobacco use, chronic hypertension (excluded from propensity score for chronic hypertension at 2-7 year visit), prediabetes or type 2 diabetes (excluded from propensity score for prediabetes or type 2 diabetes at 2-7 year visit), chronic kidney disease (excluded from propensity score for chronic kidney disease at 2-7 year visit), autoimmune disease, obesity, and family history of preeclampsia. Scores range from zero to one.
Discussion
In this secondary analysis of the nuMoM2b-HHS prospective cohort, exposure to aspirin during the first pregnancy was not associated with cardiovascular events or diagnoses 2-7 years after delivery. Although not statistically significant, it is worth noting that the point estimate for the association between aspirin exposure and incident chronic kidney disease 2-7 years later was in the positive direction (toward aspirin exposure being associated with chronic kidney disease). This may reflect residual confounding that was not resolved with our propensity score methodology.
Only 1.9% of the women in our study population reported taking aspirin during their first pregnancy. This is likely because the USPSTF guideline recommending low-dose aspirin during pregnancy for women at risk for preeclampsia was published in 2014, while nuMoM2b enrolled pregnant women between 2010-2013. Many of the trials on which the USPSTF based its 2014 guideline, however, were published well before 2010 when nuMoM2b started enrollment [23-29]. Thus, it is noteworthy that white, highly educated, privately insured women were more likely to report aspirin use during their first pregnancy. Because all participants were enrolled early in pregnancy after presenting for prenatal care, this may reflect racial and socioeconomic disparities apart from access to care. It is also possible that this finding reflects differences in practice patterns between study sites with different patient populations, or that participants taking aspirin had other indications such as a history of infertility or recurrent pregnancy loss.
Strengths of our approach include use of prospectively collected data with well-adjudicated cardiovascular and metabolic outcomes. The prospective nature of data collection minimizes the potential for recall bias with self-reported aspirin exposure. Because such a small number of women reported taking aspirin per their provider’s recommendation during their nuMoM2b pregnancy, our study was only powered to detect a relative risk of 0.56 or larger in magnitude (i.e., approximately halving of risk) when comparing exposed and unexposed groups. As such, our findings do not rule out a smaller yet clinically meaningful difference in outcomes between groups. Another limitation of our study is the lack of data regarding aspirin dose, but we presume that healthcare providers would have prescribed or recommended only low-dose aspirin during pregnancy due to fetal renal and cardiovascular effects of high-dose aspirin. We also lack data regarding timing of initiation and duration of aspirin use.
Additional follow-up of the participants in nuMoM2b-HHS is planned, and we anticipate that the incidence of these cardiovascular outcomes of interest will increase as the participants age. Thus, this cohort may yet offer information regarding cardiovascular health following aspirin use during pregnancy. In order to definitively overcome the limitations of this secondary analysis and answer the question of long-term maternal health effects of prophylactic low-dose aspirin during pregnancy, cardiovascular outcomes could be assessed among participants in large multicenter trials of aspirin use to prevent preeclampsia (such as ASPRE and ASPIRIN) in the years following their participation [8, 9].
Supplementary Material
Highlights.
First pregnancy aspirin use was not associated with later cardiovascular outcomes
Propensity scores for aspirin use during first pregnancy were included as covariates
Few women in this cohort were exposed to aspirin, so power is limited
FUNDING SOURCES
This work was supported by cooperative agreement funding from the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HL119991; U10-HL119989; U10-HL120034; U10-HL119990; U10-HL120006; U10-HL119992; U10-HL120019; U10-HL119993; U10-HL120018, and U01HL145358. The study was also supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10 HD063036; U10 HD063072; U10 HD063047; U10 HD063037; U10 HD063041; U10 HD063020; U10 HD063046; U10 HD063048; and U10 HD063053. Support was also provided by the National Institutes of Health: Office of Research on Women's Health through U10-HL119991; Office of Behavioral and Social Sciences Research through U10-HL119991 and U10-HL119992; and the National Center for Advancing Translational Sciences through UL-1-TR000124, UL-1-TR000153, UL-1-TR000439, and UL-1-TR001108; and the Barbra Streisand Women’s Cardiovascular Research and Education Program, and the Erika J. Glazer Women’s Heart Research Initiative, Cedars-Sinai Medical Center, Los Angeles.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the U. S. Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canoy D, Cairns BJ, Balkwill A, et al. Hypertension in pregnancy and risk of coronary heart disease and stroke: A prospective study in a large UK cohort. Int J Cardiol 2016; 222:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gastrich MD, Zinonos S, Bachmann G, et al. Preeclamptic women are at significantly higher risk of future cardiovascular outcomes over a 15-year period. J Womens Health (Larchmt) 2020; 29(1):74–83. [DOI] [PubMed] [Google Scholar]
- 3.Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation 2019; 139(8):1069–79. [DOI] [PubMed] [Google Scholar]
- 4.Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: a population-based study. Am J Obstet Gynecol 2015; 213(4):518 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol 2014; 210(4):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017; 10(2). [DOI] [PubMed] [Google Scholar]
- 7.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. The Lancet 2005; 366(9499):1797–803. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. The Lancet 2020; 395(10220):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377(7):613–22. [DOI] [PubMed] [Google Scholar]
- 10.Thobani A, Dhindsa DS, DeMoss BD, et al. Usefulness of aspirin for primary prevention of atherosclerotic cardiovascular disease. Am J Cardiol 2019; 124(11):1785–9. [DOI] [PubMed] [Google Scholar]
- 11.Patrono C The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J Am Coll Cardiol 2015; 66(1):74–85. [DOI] [PubMed] [Google Scholar]
- 12.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007; 357(24):2482–94. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Shanmugalingam R, Chau K, Pears S, Hennessy A, Makris A. The effect of acetyl salicylic acid (aspirin) on trophoblast-endothelial interaction in vitro. J Reprod Immunol 2017; 124:54–61. [DOI] [PubMed] [Google Scholar]
- 14.Lettino M, Leonardi S, De Maria E, Halvorsen S. Antiplatelet and antithrombotic treatment for secondary prevention in ischaemic heart disease. Eur J Prev Cardiol 2017; 24(3_suppl):61–70. [DOI] [PubMed] [Google Scholar]
- 15.Tan MY, Poon LC, Rolnik DL, et al. Prediction and prevention of small-for-gestational-age neonates: evidence from SPREE and ASPRE. Ultrasound Obstet Gynecol 2018; 52(1):52–9. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet EOG, Askie LA, Mol BWJ, Oudijk MA. Antiplatelet agents and the prevention of spontaneous preterm birth: a systematic review and meta-analysis. Obstet Gynecol 2017; 129(2):327–36. [DOI] [PubMed] [Google Scholar]
- 17.Andrikopoulou M, Purisch SE, Handal-Orefice R, Gyamfi-Bannerman C. Low-dose aspirin is associated with reduced spontaneous preterm birth in nulliparous women. Am J Obstet Gynecol 2018; 219(4):399 e1–e6. [DOI] [PubMed] [Google Scholar]
- 18.Uzan S, Beaufils M, Breart G, et al. Prevention of fetal growth retardation with low-dose aspirin: findings of the EPREDA trial. The Lancet 1991; 337(8755):1427–31. [DOI] [PubMed] [Google Scholar]
- 19.LeFevre ML, Force USPST. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–26. [DOI] [PubMed] [Google Scholar]
- 20.Haas DM, Ehrenthal DB, Koch MA, et al. Pregnancy as a window to future cardiovascular health: design and implementation of the nuMoM2b Heart Health Study. Am J Epidemiol 2016; 183(6):519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2018; 138(17):e426–e83. [DOI] [PubMed] [Google Scholar]
- 22.Haas DM, Parker CB, Marsh DJ, et al. Association of Adverse Pregnancy Outcomes With Hypertension 2 to 7 Years Postpartum. J Am Heart Assoc 2019; 8(19):e013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspi E, Raziel A, Sherman D, Arieli S, Bukovski I, Weinraub Z. Prevention of pregnancy-induced hypertension in twins by early administration of low-dose aspirin: a preliminary report. Am J Reprod Immunol 1994; 31(1):19–24. [DOI] [PubMed] [Google Scholar]
- 24.CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of preeclampsia among 9364 pregnant women. Lancet 1994; 343(8898):619–29. [PubMed] [Google Scholar]
- 25.Hauth JC, Goldenberg RL, Parker CR Jr., , et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol 1993; 168(4):1083–91; discussion 91-3. [DOI] [PubMed] [Google Scholar]
- 26.Hermida RC, Ayala DE, Iglesias M, et al. Time-dependent effects of low-dose aspirin administration on blood pressure in pregnant women. Hypertension 1997; 30(3 Pt 2):589–95. [DOI] [PubMed] [Google Scholar]
- 27.Schiff E, Peleg E, Goldenberg M, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med 1989; 321(6):351–6. [DOI] [PubMed] [Google Scholar]
- 28.Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1993; 329(17):1213–8. [DOI] [PubMed] [Google Scholar]
- 29.Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet 1986; 1(8471):1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


