Abstract
Background.
The aim of this study was to determine individual, partner-level and sexual networking factors associated with vaccine- and non-vaccine-type human papillomavirus (HPV) in young women, by vaccination status.
Methods.
Sexually experienced women 13-26 years (N=784) completed a survey and were tested for 36 HPV genotypes. We determined factors associated with 4-valent vaccine-type HPV (HPV6, 11, 16, 18) and non-vaccine-type HPV among vaccinated and unvaccinated women, using univariable and multivariable logistic regression models.
Results.
Participants’ mean age was 19.2 years, 77.7% had received ≥1 vaccine dose, and 7.7% were positive for vaccine-type (HPV6, 11, 16, and/or 18). Factors associated with vaccine-type HPV in vaccinated women included gonorrhea history (adjusted odds ratio [AOR]=2.71), new female sex partner(s) (AOR=4.79), age at vaccination (≥15 vs. <15 years: AOR=2.47), and age discordance with most recent partner (don’t know vs. discordant: AOR=9.17). Factors associated with non-vaccine-type HPV in vaccinated women included history of sexually transmitted infection (AOR=2.69), male most recent partner (AOR=2.85), age of first sex (AOR=1.15), and partner concurrency (don’t know vs. 1 other partner, AOR=2.03). Factors associated with vaccine-type HPV in unvaccinated women included new female sex partner(s) (AOR=7.45) and partner concurrency (don’t know vs. no, AOR=2.95). Factors associated with non-vaccine-type HPV in unvaccinated women included race (White vs. multiracial, AOR=4.10) and partner concurrency (don’t know vs. 0, AOR=4.65).
Conclusions.
Novel findings of this study, including associations between female sex partners and HPV, and between not knowing about partner concurrency and HPV, have implications for sexual education, clinical counseling, and public health interventions.
Keywords: papillomavirus infections, vaccines, sexual behavior, sex partners
Short Summary
Factors associated with HPV infection in young women include partner-level and sexual networking variables, differ depending upon HPV type and vaccination status, and may be different in the post-HPV-vaccination era.
INTRODUCTION
Human papillomavirus (HPV) is a common sexually transmitted infection (STI) that may cause anogenital warts as well as oropharyngeal and anogenital cancers in men and women.1 Safe and effective vaccines that prevent HPV were licensed in the U.S. for young women in 2006 and young men in 2011,2 but vaccination coverage remains suboptimal,3 leaving many women and men at risk for HPV and its sequelae. For example, in surveillance studies we conducted during the 11 years after HPV vaccine introduction among sexually experienced young women 13 to 26 years of age recruited from clinical settings, we demonstrated that although vaccine introduction led to a greater than 80% decline in vaccine-type HPV detection among vaccinated women, the decline in vaccine-type HPV detection among unvaccinated women was only 40%.4 In addition, non-vaccine-type HPV prevalence did not decline overall and increased among unvaccinated women.5 These findings are consistent with those of published meta-analyses examining trends in vaccine-type6 and non-vaccine-type HPV7 globally after vaccine introduction.
Identifying risk factors for HPV in the post-HPV-vaccination era will provide essential information for office, school, and community-based educational initiatives, public health interventions, and vaccination strategies to prevent HPV-associated cancers. Individual-level risk factors for HPV among women have been well-described and include first sexual intercourse at an early age, number of sex partners, and frequency of sexual intercourse.8 9 Vaccination after sexual initiation may also be a risk for HPV among vaccinated individuals, given that the vaccines are preventive. However, little is known about partner-level or sexual networking risk factors for HPV. These include discordance, defined as differences between sex partners in age, race, ethnicity, or number of partners, and concurrency, defined as having more than one sexual partnership at the same time. Discordance may be a marker for engaging in riskier sexual behaviors; e.g., if it leads to a limited ability to negotiate condom use, and concurrency increases risk for STI transmission. Discordance and concurrency have been shown to be associated with bacterial STIs and HIV,10–12 but few studies have explored associations with HPV, and those studies were largely conducted in adult populations.13–17 Furthermore, data are needed regarding whether risk factors for HPV are different after HPV vaccines were introduced, whether factors associated with HPV differ for vaccine-type and non-vaccine-type HPV, and whether any associations vary by vaccination status.
The aim of this study was to determine individual, partner-level, and sexual networking factors associated with vaccine-type and non-vaccine-type HPV in vaccinated and unvaccinated young women. Based on the results of a recent study we conducted among adolescent and young adult men,16 we hypothesized that partner-level and sexual networking factors would be associated with both vaccine-type and non-vaccine-type HPV in vaccinated and unvaccinated young women.
MATERIALS AND METHODS
Participants, setting, and design
The study population for these analyses was comprised of adolescent and young adult women (N=784) who were recruited for two cross-surveillance studies (2013-2014 and 2016-2017). Participants were recruited sequentially from the Teen Health Center at Cincinnati Children’s Hospital Medical Center and the Cincinnati Health Department. Young women 13 to 26 years of age who reported previous sexual contact – defined as genital-oral or genital-genital contact with male or female partners – were eligible to participate, and those who had participated in any previous surveillance study were ineligible to participate again, to ensure that study samples were independent. Participants completed a self-administered survey instrument in English or Spanish assessing vaccination status, sociodemographic characteristics, HPV vaccination history, reproductive health history, substance use, and sexual behaviors. Previous surveillance studies were conducted in 2007-2008 and 2010-2011, but starting with the 2013-2014 surveillance study, partner-level and sexual networking items were added to the survey instrument; therefore, only data from participants enrolled in the 2013-2014 and 2016-2017 surveillance studies were analyzed in this study. Participants also provided a cervicovaginal swab, collected by the participant or clinician using a standard procedure.8 Participants provided written informed consent to participate in the study as described, and the Institutional Review Boards of the hospital and the health department approved each study.
Measures
Self-reported vaccination status was verified via electronic medical records and a statewide vaccine registry.18 Vaccinated participants were defined as having received at least one dose of the 4-valent or 9-valent HPV vaccine. Anogenital samples were analyzed for HPV genotypes using the Roche Linear Array Assay, a polymerase chain reaction amplification technique that uses an L1 consensus primer system and reverse-line blot detection strip to identify 36 different genotypes.19
The two primary outcome or dependent variables were 1) prevalence of ≥1 4-valent vaccine-type HPV (HPV6, 11, 16 and/or 18) and 2) prevalence of a non-4-valent vaccine-type HPV. Independent variables included individual, partner-level and sexual networking measures. Individual measures included participant demographic characteristics, age at HPV vaccination, whether HPV vaccination was received before or after sexual initiation, sexual behaviors, and substance use. Partner-level measures included the most recent partners’ demographic characteristics and sexual behaviors, including discordance (defined as differences between sex partners in age, race, ethnicity, or number of partners) and concurrency (defined as having more than one sexual partnership at the same time).20 Sexual networking measures were assessed for the three most recent sex partners in the last 12 months, and included discordance and concurrency. Participant and partner discordance was assessed as follows: 1) partner-level: discordance considering only the most recent sex partner in three categories (concordant, discordant, and don’t know) and 2) sexual networking: discordance considering the three most recent partners in three categories (100% concordant, discordant for any partner, and do not know for any partner). Race or ethnicity discordance was defined as a reported difference in race or ethnicity between the participant and partner, age discordance was defined as a greater than 3-year difference in age between the participant and partner as in previous studies,21 and discordance in number of sex partners was defined as any difference in the number of reported sex partners (categorized as 0, 1, and 2 or more) between the participant and partners in the past three months and the past twelve months. Similarly, participant and partner concurrency (had sex with any other person between the first and last time they had sex with the partner and participant, respectively) was assessed as follows: 1) partner-level: concurrency considering only the most recent sex partner in three categories (concurrency, no concurrency, and do not know, as well as number of sex partners other than the participant in the past 12 months) and 2) sexual networking: concurrency considering the three most recent partners in three categories (100% no concurrency, concurrency with any partner, and do not know for any partner). Participant lifetime concurrency (had practiced concurrency with any partner in their lifetime) was categorized as no, yes, and don’t remember.
Statistical analyses
Associations between each of the independent variables and each of the two outcomes were assessed using univariable logistic regression models. Independent variables associated with outcome variables at p ≤ .10 in univariable analysis were eligible for inclusion in the multivariable logistic regression models. These independent variables were evaluated for collinearity, and if found one variable was selected, taking into account the degree of statistical significance of the variables and consistency in the variables chosen between multivariable models. Stepwise variable selection was used for multivariable modeling. Only variables associated with the outcome at p ≤ .05 were retained in the final models. All analyses were stratified by participant vaccination status and run separately for each of the two outcomes. Almost all variables had minimal missing data; complete case analysis was used in univariable and multivariable analysis. All analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC).
Because the primary aims of the surveillance studies were to determine long-term trends in vaccine-type and non-vaccine type HPV to assess vaccine effectiveness and herd protection, the study was not statistically powered for the analysis presented in this manuscript. Given the small sample size and the large number of independent variables analyzed, we combined multiple study waves to increase sample size, and used a step-wise approach for dimension reduction and multivariable regression. Strategies used in the step-wise approach were as follows: independent variables were grouped into individual-level, partner-level, and network-level variables, only independent variables associated with outcomes at p ≤ 0.1 were considered in multivariable modeling, independent variables considered for multivariable modeling were examined for multicollinearity (conceptually and statistically), and multivariable regression models were built using candidate independent variables at the individual-level first, followed by adding partner-level variables, then network-level variables.
RESULTS
Individual, partner-level and sexual networking characteristics, proportion vaccinated, and HPV prevalence
The median age of participants was 19 years (Table 1). Most (67.5%) of participants self-reported their race as Black, 7.8% as Multiracial or other, 24.7% as White, and 6.1% of Hispanic ethnicity. Most participants (68.9%) reported they held public health insurance and 10.8% held private insurance. Approximately half of participants reported a lifetime history of an STI (only 2 reported HIV infection), 19.4% reported lifetime cigarette smoking, and 52.9% lifetime marijuana use. Median age of first sexual intercourse was 15 years, median number of lifetime male sex partners was 3, 80.7% reported more than one lifetime male sex partner, 16.1% reported at least one lifetime female sex partner, and 54.7% reported no condom use during the last sexual encounter with their main partner. Discordance by age with one of the three most recent sex partners was reported by 24.7% while 6.4% did not know, 16.3% reported discordance in the number of sexual partnerships while 51.2% did not know, and 16.2% reported that at least one of the three most recent partners practiced concurrency while 40.7% did not know.
Table 1.
Participant characteristics (N=784)1
| Characteristic | N (%) | Median (range) |
|---|---|---|
| HPV vaccination status | ||
|
| ||
| Received ≥1 HPV vaccine dose | 609 (77.7) | |
| Number of HPV vaccine doses received | ||
| 0 | 175 (22.4) | |
| 1 | 53 (6.8) | |
| 2 | 63 (8.1) | |
| 3+ | 491 (62.8) | |
| Vaccine type received | ||
| ≥1 dose of the 4-valent vaccine | 289 (87.8) | |
| ≥1 dose of the 9-valent vaccine | 40 (12.2) | |
| Age at vaccination | ||
| < 15 years | 429 (71.0) | |
| ≥ 15 years | 175 (29.0) | |
| Sexual initiation status at vaccination | ||
| Initiated sex after vaccination | 406 (67.2) | |
| Initiated sex before vaccination | 198 (32.8) | |
|
| ||
| Recruitment site | ||
|
| ||
| Teen Health Clinic | 543 (69.3) | |
| Health Department | 241 (30.7) | |
|
| ||
| Sociodemographic characteristics | ||
|
| ||
| Age (years) | 19 (13, 26) | |
| 13-17 | 222 (28.3) | |
| 18-21 | 437 (55.7) | |
| ≥22 | 125 (15.9) | |
| Race | ||
| White, Asian, or Pacific Islander | 194 (24.7) | |
| Black | 529 (67.5) | |
| Multiracial, other | 61 (7.8) | |
| Ethnicity | ||
| Non-Hispanic White | 158 (20.2) | |
| Non-Hispanic Black | 528 (67.4) | |
| Others | 98 (12.5) | |
| Appalachian descent | 15 (1.9) | |
| Hispanic ethnicity | 48 (6.1) | |
| Marital status | ||
| Never Married | 765 97.6) | |
| Ever Married | 19 (2.4) | |
| Insurance Plan | ||
| Private health insurance | 85 (10.8) | |
| Public health insurance (Medicaid, Medicaid managed care) | 540 (68.9) | |
| Unsure or missing | 159 (20.3) | |
|
| ||
| Sexual health | ||
|
| ||
| History of STI other than warts | 413 (52.7) | |
| History of chlamydia | 327 (41.7) | |
| History of gonorrhea | 155 (19.8) | |
| History of trichomonas | 196 (25.0) | |
| History of herpes | 44 (5.6) | |
| History of pregnancy | 278 (35.5) | |
|
| ||
| Substance use | ||
|
| ||
| Lifetime smoking | 152 (19.4) | |
| Frequency of smoking, past 30 days | ||
| 0 days | 611 (77.9) | |
| >1 day | 173 (22.1) | |
| Lifetime marijuana use | 415 (52.9) | |
| Marijuana use, past 30 days | ||
| 0 days | 504 (64.6) | |
| >1 day | 276 (35.4) | |
|
| ||
| Sexual behaviors | ||
|
| ||
| Age of first sexual intercourse (anal or vaginal), years | 15 (5, 26) | |
| ≤13 | 104 (13.3) | |
| 14-17 | 599 (76.4) | |
| ≥18 | 81 (10.3) | |
| Number of male sexual partners, lifetime (anal or vaginal) | 3 (0, 200) | |
| ≤1 | 151 (19.3) | |
| 2-5 | 409 (52.4) | |
| 6+ | 221 (28.3) | |
| Number of male sexual partners, past 3 months (anal or vaginal) | 1 (0, 10) | |
| 0 | 74 (9.4) | |
| 1 | 565 (72.1) | |
| 2+ | 145 (18.5) | |
| Number of new male sexual partners, past 3 months (anal or vaginal) | 0 (0, 9) | |
| 0 | 537 (68.5) | |
| 1+ | 247 (31.5) | |
| Number of male sexual partners, past 12 months (anal or vaginal) | 1 (0, 70) | |
| ≤1 | 462 (58.9) | |
| 2+ | 322 (41.1) | |
| Number of new male partners, past 12 months (anal or vaginal) | 1 (0, 40) | |
| ≤1 | 651 (83.0) | |
| 2+ | 133 (17.0) | |
| Number of female sexual partners, lifetime | 0 (0, 50) | |
| 0 | 658 (83.9) | |
| 1+ | 126 (16.1) | |
| Number of female sexual partners, past 3 months | 0 (0, 6) | |
| 0 | 734 (93.6) | |
| 1+ | 50 (6.4) | |
| Number of female sexual partners, past 12 months | 1 (0, 9) | |
| 0 | 760 (96.9) | |
| 1+ | 24 (3.1) | |
| Number of new female sexual partners, past 3 months | 0 (0, 6) | |
| 0 | 712 (90.8) | |
| 1+ | 72 (9.2) | |
| Number of new female sexual partners, past 12 months | 0 (0, 7) | |
| 0 | 737 (94.0) | |
| 1+ | 47 (6.0) | |
| History of anal sex | 164 (20.9) | |
| Number of anal sex partners, past 3 months | 0 (0, 10) | |
| 0 | 704 (89.8) | |
| 1+ | 80 (10.2) | |
| Number of anal sex partners, past 12 months | 1 (0, 12) | |
| 0 | 674 (86.0) | |
| 1+ | 110 (14.0) | |
| History of receiving oral sex, past 3 months | ||
| No | 280 (35.7) | |
| Yes | 504 (64.3) | |
| History of giving oral sex, past 3 months | ||
| No | 377 (48.2) | |
| Yes | 406 (51.9) | |
| Frequency of oral sex (given or received) with any partner | 2 (0, 201) | |
| 0 | 234 (29.9) | |
| 1-5 | 362 (46.3) | |
| 6+ | 186 (23.8) | |
| Is your main sexual partner male? | ||
| No | 27 (3.4) | |
| Yes | 671 (85.6) | |
| Don’t have a main partner | 86 (11.0) | |
| Frequency of condom use with main partner past 3 months | ||
| Less than every time | 550 (70.2) | |
| Every time | 105 (13.4) | |
| Missing, not applicable | 129 (16.5) | |
| Condom use, last sexual encounter with main partner | ||
| No | 429 (54.7) | |
| Yes | 242 (30.9) | |
| Missing, not applicable | 113 (14.4) | |
| Are any of your other sexual partners male? | ||
| No | 65 (8.3) | |
| Yes | 289 (36.9) | |
| Don’t have any other male partner | 429 (54.8) | |
| Frequency of condom use with non-main partners, past 3 months | ||
| Less than every time | 150 (19.1) | |
| Every time | 75 (9.6) | |
| Missing or not applicable | 559 (71.3) | |
| Condom use, last sexual encounter with non-main partner | ||
| No | 101 (12.9) | |
| Yes | 188 (24.0) | |
| Missing or not applicable | 495 (63.1) | |
| Sexual orientation | ||
| Attracted to opposite sex | 652 (83.2) | |
| Attracted to same sex or undecided | 132 (16.8) | |
|
| ||
| Discordance and concurrency | ||
|
| ||
| Discordant by race | ||
| 100% concordant | 614 (78.3) | |
| Any discordant | 157 (20.0) | |
| Do not know for ≥1 of the 3 most recent partners | 13 (1.7) | |
| Discordant by ethnicity | ||
| 100% concordant | 741 (94.5) | |
| Any discordant | 30 (3.8) | |
| Do not know for ≥1 of the 3 most recent partners | 13 (1.7) | |
| Discordant by age | ||
| 100% concordant | 540 (68.9) | |
| Any discordant | 194 (24.7) | |
| Do not know for ≥1 of the 3 most recent partners | 50 (6.4) | |
| Discordant by sexual partnerships, 3 months | ||
| 100% concordant | 268 (34.2) | |
| Any discordant | 126 (16.1) | |
| Do not know for ≥1 of the 3 most recent partners | 390 (49.7) | |
| Discordant by sexual partnerships, 12 months | ||
| 100% concordant | 255 (32.5) | |
| Any discordant | 128 (16.3) | |
| Do not know for ≥1 of the 3 most recent partners | 401 (51.2) | |
| Participant’s three most recent partners’ concurrency practice | ||
| 100% no concurrency | 338 (43.1) | |
| Any concurrency | 127 (16.2) | |
| Do not know for ≥1 of the 3 most recent partners | 319 (40.7) | |
| Participant concurrency practice with the 3 most recent partners | ||
| 100% no concurrency | 551 (70.3) | |
| Any concurrency | 208 (26.5) | |
| Do not know for ≥1 of the 3 most recent partners | 25 (3.2) | |
| Participant lifetime concurrency | ||
| No | 618 (78.8) | |
| Yes or don’t remember | 166 (21.2) | |
There were few missing data so missing data are not reported separately in the table: 5 variables had 1 missing value, 3 had 2 missing values, 1 had 3 missing values, and 1 had 4 missing values.
Among all participants, 77.7% had received one or more HPV vaccine doses: 6.8% had received 1 dose, 8.1% had received 2 doses, and 62.8% had received 3 doses. Most had received the 4-valent HPV vaccine (87.8%), but 12.2% had received the 9-valent HPV vaccine as it was introduced toward the end of the recruitment period. Seventy-one percent had received the first vaccine dose before 15 years of age and 67.2% initiated sex after vaccination. Among all participants, 63.4% were infected with at least one HPV type, 45.5% with at least one high-risk (cancer-associated) type, 7.7% with at least one 4-valent vaccine type (HPV 6, 11, 16 and/or 18), 6.3% with HPV16 and/or 18, and 61.7% with at least one non-vaccine-type HPV. Infection rates were higher in unvaccinated vs. vaccinated women for each of these variables (Figure 1). Multivariable results are as follows, and effect sizes and 95% confidence intervals including any overlap are shown in Figures 2, 3, 4, and 5.
Figure 1.
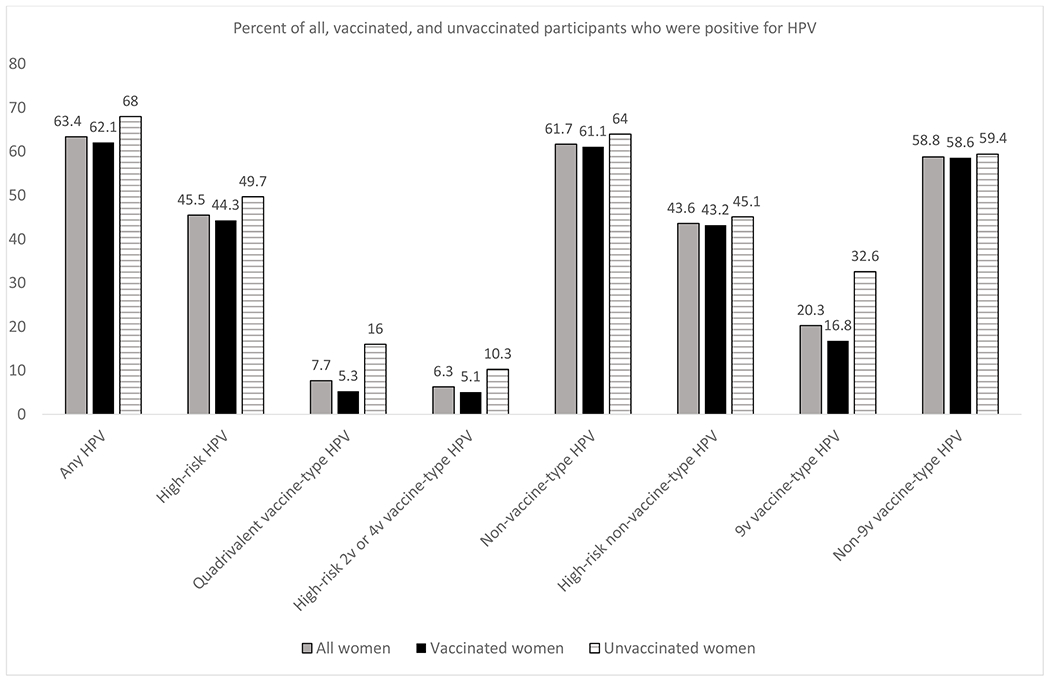
HPV prevalence (% positive for HPV) among all participants (N=784), unvaccinated participants (N=175), and vaccinated participants (N=609)
High-risk HPV: HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 67, 68, 70, 73, and/or 82
Quadrivalent vaccine-type HPV: HPV6, 11, 16, and/or 18
High-risk 2v or 4v vaccine-type HPV: HPV16 and/or 18
Non-vaccine-type HPV: HPV26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55 (now considered a subtype of 44), 56, 58, 59, 61, 62, 64 (now considered a subtype of 34), 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and/or 89
High-risk non-vaccine-type HPV: HPV26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 67, 68, 70, 73, and/or 82
9v vaccine-type HPV: HPV6, 11, 16, 18, 31, 33, 45, 52, and/or 58
Non-9v vaccine-type HPV: HPV26, 35, 39, 40, 42, 51, 53, 54, 55 (now considered a subtype of 44), 56, 59, 61, 62, 64 (now considered a subtype of 34), 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and/or 89
Figure 2.

Independent variables associated with HPV6, 11, 16 and/or HPV18 infection in vaccinated women: results of unadjusted and adjusted logistic regression models (n=609). Unadjusted and adjusted odds ratios (dots, size proportional to the odds ratio) and 95% confidence intervals (horizontal error bars) are graphed on a base 10 logarithmic scale and indicated numerically. The vertical dotted line indicates no effect. Names of variables are shown on the left.
Figure 3.

Independent variables associated with non-vaccine-type HPV infection in vaccinated women: results of unadjusted and adjusted logistic regression models (n=604). Unadjusted and adjusted odds ratios (dots, size proportional to the odds ratio) and 95% confidence intervals (horizontal error bars) are graphed on a base 10 logarithmic scale and indicated numerically. The vertical dotted line indicates no effect. Names of variables are shown on the left.
Figure 4.
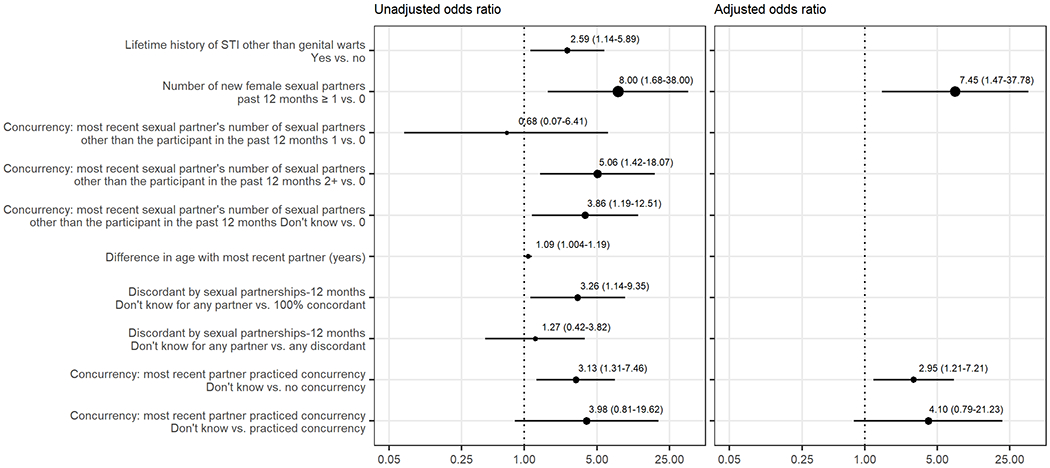
Independent variables associated with vaccine-type HPV infection in unvaccinated women: results of unadjusted and adjusted logistic regression models (n=175). Unadjusted and adjusted odds ratios (dots, size proportional to the odds ratio) and 95% confidence intervals (horizontal error bars) are graphed on a base 10 logarithmic scale and indicated numerically. The vertical dotted line indicates no effect. Names of variables are shown on the left.
Figure 5.

Independent variables associated with non-vaccine-type HPV infection in unvaccinated women: results of unadjusted and adjusted logistic regression models (n=175). Unadjusted and adjusted odds ratios (dots, size proportional to the odds ratio) and 95% confidence intervals (horizontal error bars) are graphed on a base 10 logarithmic scale and indicated numerically. The vertical dotted line indicates no effect. Names of variables are shown on the left.
Variables associated with vaccine-type HPV infection in vaccinated women
The following variables were associated with higher odds of vaccine-type HPV infection in vaccinated women in a multivariable logistic regression model (Figure 2): history of gonorrhea (adjusted odds ratio [AOR] = 2.71; 95% confidence interval [CI] = 1.25-5.89), new female partners in the past 3 months (AOR = 4.79; 95% CI = 1.42-16.15), and age at vaccination (≥15 years vs. <15 years: AOR = 2.47; 95% CI = 1.17-5.20), and age discordance with most recent sex partner (don’t know vs. discordant: AOR = 9.17; 95% CI = 1.58-52.63).
Variables associated with non-vaccine-type HPV infection in vaccinated women
The following variables were associated with higher odds of non-vaccine-type HPV infection in vaccinated women in a multivariable logistic regression model (Figure 3): lifetime history of sexually transmitted infection other than genital warts (AOR = 2.69; 95% CI = 1.88-3.84), most recent sex partner is male (AOR = 2.85; 95% CI = 1.06-7.64), partner concurrency (most recent sex partner’s number of sex partners other than participant, past 12 months: don’t know vs. 0 partners, AOR = 2.34; 95% CI = 1.52-3.59; don’t know vs. 1 partner, AOR = 2.03; 95% CI = 1.17-3.54), and older age of first sex (age defined continuously) with most recent partner (AOR = 1.15; 95% CI = 1.05-1.25).
Variables associated with vaccine-type HPV infection in unvaccinated women
The following variables were associated with higher odds of vaccine-type HPV infection in unvaccinated women in a multivariable logistic regression model (Figure 4): having a new female sex partner in the past 12 months (AOR = 7.45; 95% CI = 1.47-37.78) and concurrency of most recent partner (don’t know vs. no concurrency, AOR = 2.95; 95% CI = 1.21-7.21).
Variables associated with non-vaccine-type HPV infection in unvaccinated women
The following variables were associated with higher odds of non-vaccine-type HPV infection in unvaccinated women in a multivariable logistic regression model (Figure 5): race (White vs. multiracial, AOR = 4.10; 95% CI = 1.21-13.95) and concurrency (most recent partner’s number of sex partners other than participant, past 12 months: don’t know vs. 0, AOR = 4.65; 95% CI = 1.96-11.03; ≥2 vs. 0, AOR = 2.60; 95% CI = 1.02-6.66).
DISCUSSION
This is the first study, to our knowledge, to determine individual, partner-level and sexual networking variables associated with vaccine-type and non-vaccine-type HPV in young women after vaccine introduction, and whether associations were different according to vaccination status. In this study sample, we identified several individual factors associated with HPV including White race, history of STI, later age of first sexual intercourse, and older age at vaccination. The finding that older age at vaccination was independently associated with vaccine-type HPV in vaccinated women underscores the importance of vaccinating both boys and girls at the recommended age of 11-12 years, prior to sexual initiation, given that HPV vaccines are preventative.
In contrast to individual-level factors, which differed across models, partner-level and sexual networking variables were more consistently associated with HPV outcomes across models. As hypothesized, we found that these variables were associated with both vaccine-type and non-vaccine type HPV and in both vaccinated and unvaccinated young women. We noted two patterns in the multivariable models. The first was gender of recent sex partners: among both vaccinated and unvaccinated women, having a new female sex partner was associated with a higher risk of vaccine-type HPV. Conversely, among vaccinated women, male gender of the most recent sex partner was associated with a higher risk of non-vaccine-type HPV. The few studies that have examined HPV infection among sexual minority women have demonstrated disparities by sexual orientation. Solazzo and colleagues found that women reporting that they were heterosexual but had same-sex partners, were mostly heterosexual, or were bisexual had higher odds of HPV infection compared with completely heterosexual women, while lesbian women had lower odds of HPV infection.22 Reiter et al. similarly reported that bisexual women and women who reported partners of both sexes had higher odds of HPV infection in univariable analyses, while lesbian women and women who reported only same-sex partners had lower odds of HPV infection in multivariable analyses.23 Our finding that having a new female sex partner was associated with a higher risk of vaccine-type HPV in both vaccinated and unvaccinated women raises concern about the risk for HPV-associated cancers among sexual minority women. Compared to heterosexual women, sexual minority women may be less aware of their risk for HPV, less likely to practice safer sexual behaviors, less likely to discuss safer sex practices with partners, and less likely to obtain a Pap test.22 24 Additional research is needed to determine what drives these differences, though previous work suggests they may be influenced by misconceptions regarding STI transmission in female same-sex partnerships and sexual orientation disparities in sex education and in medical care.25 Taken together, these findings suggest that clinicians should routinely assess sexual orientation and sexual practices among women, and should educate sexual minority women about practices and behaviors to prevent STIs, encourage HPV vaccination, and advocate for patients to participate in cervical cancer screening.
The second pattern we noted was that partner-level and sexual networking variables were consistently associated with a higher risk of HPV. Age discordance with the most recent partner was associated with vaccine-type HPV in vaccinated women, and partner’s practice of concurrency was associated with both vaccine-type HPV in unvaccinated women and with non-vaccine-type HPV among vaccinated and unvaccinated women. Our findings are consistent with a small number of previous studies that have examined associations between concurrency and HPV, primarily conducted in adults. In a study of unvaccinated adult women in the United Kingdom, partner concurrency was associated with high-risk HPV infection.13 In a sample of unvaccinated adult women in the U.S., those who reported a concurrent partnership had an increased risk for a high-risk HPV infection.14 In a study of adult Taiwanese men who have sex with men, concurrency was associated with penile HPV detection.15 Finally, in a study of 13-26 year-old young men in the U.S., partner concurrency was associated with HPV16/18 detection in unvaccinated and vaccinated men.16 Our findings imply that young women whose partners practice concurrency are at elevated risk for HPV in the post-HPV-vaccination era and should be targeted for education about cervical cancer prevention through safer sexual behaviors, vaccination (if unvaccinated), and cervical cancer screening when indicated. However, partner-level and sexual networking behaviors are not routinely assessed in the sexual history, and should be considered by clinicians as a component of routine care given their consistent association with HPV and other STIs. Finally, investigators designing interventions to reduce HPV and other STIs should consider addressing concurrency behaviors as a component of the intervention. Wingood et al. reported that an intervention to reduce HIV infection that aimed to decrease concurrent partnerships also decreased HPV infection.26
An unexpected finding was that in all multivariable models – predicting vaccine-type and non-vaccine-type HPV among both vaccinated and unvaccinated women – the “don’t know” category for sexual networking variables was associated with a higher risk of HPV. Not knowing one’s partner’s age (even in comparison to report of age discordance) and whether one’s partner was practicing concurrency (even in comparison to report of one’s partner having a sex partner in addition to the participant) were associated with HPV infection. Drumright et al. similarly demonstrated in a sample of young adults that lack of awareness of a partner’s concurrency was associated with STI risk.27 They note that individuals who are unaware of their partner’s concurrency may practice less safe sexual behaviors because they assume the partner is monogamous, whereas those who are aware of a partner’s concurrency practice safer behaviors because they perceive higher risk for STI acquisition from the partner. In addition, lack of knowledge may be a proxy for poor communication with one’s sex partners. Communication with sex partners has been linked to safer sexual behaviors,28 and safer-sex communication skills training has been shown to improve communication and behaviors such as condom use which reduce the risk of STIs.29 Lack of knowledge regarding age discordance or concurrency of one’s sex partner may also be reflective of a greater proportion of casual or short-term sexual partnerships. The finding that a lack of knowledge about one’s partner’s age or sexual practices is associated with higher risk for HPV warrants further research and has implications for counseling in clinical care settings and sexual education initiatives. Clinicians should consider assessing knowledge about a patient’s partner and providing targeted counseling to those who lack knowledge about their partners. Sexual education and public health interventions should both incorporate messaging centering on the importance of communicating about risks for STIs with potential sex partners and address communication barriers.
This study has several limitations. First, data were cross-sectional, so causal inferences cannot be assumed. Second, behaviors were self-reported, and socially-desirable responses (including responses of “don’t know” if participants did not want to admit to a partner’s concurrency) or recall bias may have limited the validity of these data. Similarly, partner-level and sexual networking variables were assessed based on participants’ responses, so were not validated based on partner responses. In addition, we did not ask participants about their partners’ vaccination status. Study participants in our study were recruited from clinical sites providing care to a patients who were predominantly racial minorities and low-income, limiting generalizability. Finally, although we used a reliable and accurate method of HPV DNA testing, there are limitations to all HPV tests in terms of accuracy.30
In conclusion, the results of this study demonstrate that those factors associated with HPV infection in young women may be different in the post-HPV-vaccination era, and differ by HPV type (vaccine-type and non-vaccine-type) and vaccination status. These findings have implications for sexual education initiatives, office-based clinical counseling, and public health interventions.
ACKNOWLEDGMENTS
The authors thank the providers and staff of the Teen Health Center at Cincinnati Children’s Hospital and the Cincinnati Health Department for their collaboration. The data presented in this article were presented at the Society for Adolescent Health and Medicine 2018 Annual Meeting.
Conflicts of Interest and Source of Funding.
The authors have no relevant conflicts of interest to report. This work was funded by the National Institute of Allergy and Infectious Diseases, grants R01 AI073713 and R01 AI104709, the National Center for Advancing Translations Sciences of the National Institutes of Health under Award Number UL1 TR001425, and the NIH Medical Student Summer Research Program Training Grant T35 DK 060444.
REFERENCES
- 1.Dunne EF, Park IU. HPV and HPV-associated diseases. Infect Dis Clin North Am 2013;27(4):765–78. [DOI] [PubMed] [Google Scholar]
- 2.Meites E, Szilagyi PG, Chesson HW, et al. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68(32):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elam-Evans LD, Yankey D, Singleton JA, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69(33):1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinner C, Ding L, Bernstein DI, et al. Human Papillomavirus Vaccine Effectiveness and Herd Protection in Young Women. Pediatrics 2019;143(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saccucci M, Franco EL, Ding L, et al. Non-Vaccine-Type Human Papillomavirus Prevalence After Vaccine Introduction: No Evidence for Type Replacement but Evidence for Cross-Protection. Sex Transm Dis 2018;45(4):260–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drolet M, Bénard É, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394(10197):497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesher D, Soldan K, Lehtinen M, et al. Population-Level Effects of Human Papillomavirus Vaccination Programs on Infections with Nonvaccine Genotypes. Emerg Infect Dis 2016;22(10):1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shikary T, Bernstein DI, Jin Y, et al. Epidemiology and risk factors for human papillomavirus infection in a diverse sample of low-income young women. J Clin Virol 2009;46(2):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn JA, Burk RD, Squires KE, et al. Prevalence and Risk Factors for HPV in HIV-Positive Young Women Receiving Their First HPV Vaccination. J Acquir Immune Defic Syndr 2012;61(3):390–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sex Transm Dis 2005;32(1):7–12. [DOI] [PubMed] [Google Scholar]
- 11.Maulsby C, Jain K, Sifakis F, et al. Individual-Level and Partner-Level Predictors of Newly Diagnosed HIV Infection Among Black and White Men Who Have Sex with Men in Baltimore, MD. AIDS Behav 2015;19(5):909–17. [DOI] [PubMed] [Google Scholar]
- 12.Kraut-Becher JR, Aral SO. Patterns of age mixing and sexually transmitted infections. Int J STD AIDS 2006;17(6):378–83. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AM, Mercer CH, Beddows S, et al. Epidemiology of, and behavioural risk factors for, sexually transmitted human papillomavirus infection in men and women in Britain. Sex Transm Infect 2012;88(3):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javanbakht M, Gorbach PM, Amani B, et al. Concurrency, sex partner risk, and high-risk human papillomavirus infection among African American, Asian, and Hispanic women. Sex Transm Dis 2010;37(2):68–74. [DOI] [PubMed] [Google Scholar]
- 15.Strong C, Yu YF, Zou H, et al. Sexual network and detection of anogenital human papillomavirus in a community cohort of men who have sex with men in Taiwan. PloS One 2019;14(5):e0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen BL, Gorbach P, Ding L, et al. Sexual Network Patterns and Their Association With Genital and Anal Human Papillomavirus Infection in Adolescent and Young Men. J Adolesc Health 2021;68(4):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burchell AN, Rodrigues A, Moravan V, et al. Determinants of Prevalent Human Papillomavirus in Recently Formed Heterosexual Partnerships: A Dyadic-Level Analysis. J Infect Dis 2014;210(6):846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R, Higgins L, Ding L, et al. Factors Associated With HPV Vaccine Initiation, Vaccine Completion, and Accuracy of Self-Reported Vaccination Status Among 13- to 26-Year-Old Men. AJMH 2018;12(4):819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol 2000;38:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty IA, Padian NS, Marlow C, et al. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis 2005;191 Suppl 1:S42–54. [DOI] [PubMed] [Google Scholar]
- 21.Koon-Magnin S, Kreager DA, Ruback RB. Partner age differences, educational contexts and adolescent female sexual activity. Perspect Sex Reprod Health 2010;42(3):206–13. [DOI] [PubMed] [Google Scholar]
- 22.Solazzo AL, Agénor M, Austin SB, et al. Sexual Orientation Differences in Cervical Cancer Prevention among a Cohort of U.S. Women. Women’s Health Issues 2020;30(4):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter PL, McRee AL. HPV infection among a population-based sample of sexual minority women from USA. Sex Transm Infect 2017;93(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto VM, Tancredi MV, Tancredi Neto A, et al. Sexually transmitted disease/HIV risk behaviour among women who have sex with women. AIDS 2005;19 Suppl 4:S64–9. [DOI] [PubMed] [Google Scholar]
- 25.Solazzo AL, Tabaac AR, Agénor M, et al. Sexual orientation inequalities during provider-patient interactions in provider encouragement of sexual and reproductive health care. Prev Med 2019;126:105787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wingood GM, Diclemente RJ, Robinson-Simpson L, et al. Efficacy of an HIV intervention in reducing high-risk human papillomavirus, nonviral sexually transmitted infections, and concurrency among African American women: a randomized-controlled trial. J Acquir Immune Defic Syndr 2013;63 Suppl 1(01):S36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drumright LN, Gorbach PM, Holmes KK. Do People Really Know Their Sex Partners?: Concurrency, Knowledge of Partner Behavior, and Sexually Transmitted Infections Within Partnerships. Sex Transm Dis 2004;31(7):437–42. [DOI] [PubMed] [Google Scholar]
- 28.Noar SM, Carlyle K, Cole C. Why communication is crucial: meta-analysis of the relationship between safer sexual communication and condom use. J Health Commun 2006;11(4):365–90. [DOI] [PubMed] [Google Scholar]
- 29.Gause NK, Brown JL, Welge J, et al. Meta-analyses of HIV prevention interventions targeting improved partner communication: effects on partner communication and condom use frequency outcomes. J Behav Med 2018;41(4):423–40. [DOI] [PubMed] [Google Scholar]
- 30.Tsakogiannis D, Gartzonika C, Levidiotou-Stefanou S, et al. Molecular approaches for HPV genotyping and HPV-DNA physical status. Exp Rev Mol Med 2017;19:e1. [DOI] [PubMed] [Google Scholar]


