Abstract
In some countries of sub-Saharan Africa, the prevalence of HIV exceeds 20%; in South Africa, 20.4% of people are living with HIV. We examined the impact of HIV infection on the overall survival (OS) of women with non-metastatic breast cancer (BC) enrolled in the South African Breast Cancer and HIV Outcomes (SABCHO) study. We recruited women with newly diagnosed BC at six public hospitals from July 1, 2015, to June 30, 2019. Among women with stages I-III BC, we compared those with and without HIV infection on socio-demographic, clinical, and treatment factors. We analyzed the impact of HIV on OS using multivariable Cox proportional hazard models. Of 2367 women with stages I-III BC, 499 (21.1%) had HIV and 1868 (78.9%) did not. With a median follow-up of 29 months, 2-year OS was poorer among women living with HIV (WLWH) than among HIV-uninfected women (72.4% vs. 80.1%, p<0.001; adjusted hazard ratio (aHR) 1.49, 95% confidence interval (CI) = 1.22–1.83). This finding was consistent across age groups ≥45 years and <45 years, stage I-II BC and stage III BC, and ER/PR status (all p<0.03). Both WLWH with <50 viral load copies/mL and WLWH with ≥50 viral load copies/mL had poorer survival than HIV-uninfected BC patients (aHR: 1.35 (1.09–1.66) and 1.54 (1.20–2.00), respectively), as did WLWH who had ≥200 CD4+ cells/mL at diagnosis (aHR: 1.39 (1.15–1.67)). Because receipt of antiretroviral therapy has become widespread, WLWH are surviving long enough to develop BC; more research is needed on the causes of their poor survival.
Keywords: Breast cancer, HIV, Overall survival, South Africa
Graphical Abstract
INTRODUCTION
Of the estimated 36.7 million people living with HIV worldwide, 7 million (19%) live in South Africa 1. The rollout of effective antiretroviral therapy (ART) has dramatically increased their life expectancy, but as they age, the burden of breast cancer (BC) among them has risen 2, 3.
Several retrospective studies from the United States have shown that HIV infection adversely affects BC survival 4–6. In 2019, Coghill et al. showed that women living with HIV (WLWH) who were diagnosed with stages I-III BC had worse mortality than HIV-uninfected women; their absolute mortality rates were 41.7% vs. 15.8% (hazard ratio (HR) 1.85 (95% confidence interval (CI) 1.68–2.04) 4.
Although BC survival data from Africa are sparse, the African Breast Cancer-Disparities in Outcomes (ABC-DO) study estimated the 3-year overall survival (OS) of 2,156 women with BC from five countries in sub-Saharan Africa (SSA) to be 50% (95% CI 48–53), while BC 5-year survival is >80% in high-income countries (HICs) 7, 8. In a meta-analysis of BC outcomes in SSA, Brandão et al showed that WLWH were diagnosed with BC at a more advanced stage and had worse OS (HR: 1.43; 95%CI: 1.06–1.92) than HIV-uninfected women 9. We have found no data on receipt of BC treatment and ART, viral loads, or CD4 counts among WLWH with BC in SSA.
Since July 2015, the South African Breast Cancer and HIV Outcomes (SABCHO) Study has been prospectively enrolling women newly diagnosed with BC at six public academic hospitals located within Gauteng and KwaZulu-Natal provinces in South Africa 10. The primary aims of the SABCHO study are to examine the impact of HIV on BC survival and to investigate possible reasons for survival disparities. We have found that WLWH are diagnosed with BC at a younger age and are less likely to achieve a pathological complete response after neoadjuvant chemotherapy than others 10–12, but we have not found associations of HIV status with BC subtype or the quality of treatment received. We previously found that HIV status was not associated with survival among women with stage IV BC 13. In this paper, we report our findings regarding patients diagnosed with non-metastatic BC.
METHODS
Context and settings
South Africa is an upper-middle-income country, but despite socioeconomic improvements since 1994 (the post-apartheid era), high levels of inequality, unemployment, and poverty persist, adversely affecting the 80% majority black population 14. South Africa has dual healthcare systems. The wealthiest fifth of the population is privately insured; the remaining 80% are dependent on the resource-constrained public health care system 15. In the SABCHO study, we enrolled subjects from the breast units of six public tertiary referral hospitals: Chris Hani Baragwanath Academic Hospital (CHBAH), Soweto, Johannesburg; Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), Johannesburg; Inkosi Albert Luthuli Central Hospital, Durban; Addington Hospital, Durban; Grey’s Hospital, Pietermaritzburg; and Ngwelezana Hospital, Empangeni. The two hospitals in Durban share facilities and staff and are analyzed here as a single unit.
In 2015, when our study began, national guidelines recommended antiretroviral therapy (ART) initiation for all people living with HIV (PLWH) whose CD4 counts were <350 cells/mL. All PLWH with a new cancer diagnosis were also initiated on ART irrespective of their CD4 count. In September 2016, South Africa adopted the universal-test-and-treat policy.
Cancer surgery is available in the district and tertiary provincial hospitals. Chemotherapy, radiation therapy, and endocrine therapy are available at tertiary referral hospitals; treatment costs to patients are low or waived according to income. Both breast conserving surgery and total mastectomy are offered at all study sites. The most common BC chemotherapy regimen in our hospitals is a combination of anthracycline and cyclophosphamide, with or without 5-fluorouracil, and usually followed by a taxane. Human epidermal growth factor receptor-2 (HER2)-targeted agents were not available during our study period.
Participants
Between July 1, 2015, and June 30, 2019, we recruited as SABCHO participants all women >18 years of age, recently diagnosed with histologically-confirmed invasive BC, residing in South Africa for ≥5 years, free of a self-reported prior cancer diagnosis (excluding in-situ cervical cancer and non-melanoma skin cancer), and providing written informed consent. For this analysis, we included only patients with known HIV status, American Joint Committee on Cancer (AJCC) 7th edition stage I-III disease, and known tumour receptor (oestrogen, progesterone, and HER2) status. We categorized all cases by tumour receptor expression as: oestrogen receptor (ER)+/progesterone receptor (PR)+/HER2−, ER+/PR+/HER2+, ER−/PR−/HER2+, and ER−/PR−/HER2−. For the purpose of our analysis, we classified the 105 women (4.4%) who had equivocal HER2 testing (i.e., HER2 2+ by immunohistochemistry with missing confirmatory HER2 fluorescence in situ hybridization testing) as HER2 negative. These women were enrolled before HER2-targeted treatment became available in our hospitals. We excluded patients with bilateral BC because we could not differentiate them from patients with de-novo metastatic disease.
Data collection and processing
Data on socio-demographics (i.e., age, self-reported ethnicity (black, Asian, white, and mixed race), marital status, the highest level of education, employment status), height, weight, comorbidities, clinical tumour size, nodal status, tumour grade, ER/PR, and HER2 status were collected at diagnosis. We derived a wealth index from the principal component analysis of a survey of household possessions and facilities, as previously described 16, grouping patients into quintiles based on their wealth index ranking. For all patients, BC staging work-up at diagnosis included: full blood count; electrolytes, urea, and creatinine; liver function tests; chest X-ray and abdominal ultrasound. Patients who presented with symptoms and signs suggestive of metastatic disease underwent computerized tomography scans and bone scans; patients with confirmed stage IV disease at diagnosis were excluded from this analysis. Treatment data were collected directly from the medical record.
Participants were grouped and analyzed based on their HIV status. Those who did not report that they were living with HIV were tested for HIV at BC diagnosis, after providing consent. HIV testing was performed using the enzyme-linked immunosorbent assay through the National Health Laboratory Services. Approximately 4.7% of women in our cohort had unknown HIV status and were excluded from the analysis 17. Repeat negative tests were not mandatory for the HIV-uninfected cohort at any time during the follow-up period. Repeat testing was ordered at the discretion of the managing physician with the consent of the patient.
Outcome variables
Our primary outcome was OS defined as the time from the date of BC diagnosis to the date of death, the date on which the participant was last known to be alive, or our administrative censoring date (September 30, 2020) 18. Patients were contacted every 3 months after enrolment to determine vital status. If we were unable to reach the patient, her next of kin, or other persons whom she named as close contacts for two consecutive follow-up calls, we searched Verify ID (a publicly available administrative database) to determine the patient’s vital status (Death certification is mandatory for everyone dying in South Africa regardless of cause or place of death). In a quality control analysis, we found 100% agreement between the publicly available administrative data and our own data for patients whose date of death we had documented.
Patients were censored at the last date when they were known to be alive. Sources of the date of death information were 70.7% from next of kin, 7.0% from hospital records, and 22.3% from publicly available administrative data. There were no differences in the source of death data by HIV status.
Statistical analysis
We compared the distribution of the categorical and continuous variables by HIV status, using Pearson’s chi-squared test or the Wilcoxon Rank Sum test as appropriate. We constructed Kaplan–Meier survival curves stratified by HIV status for the overall cohort and subgrouped by age, stage at diagnosis and ER/PR status. We dichotomized the age variable using the median age of WLWH in our cohort and compared women ≤45 years and >45years. Survival comparisons were performed using the log-rank test.
We tested the association of the variables with OS in a univariate proportional hazards model. We then constructed a multivariable Cox proportional hazards model to investigate the effect of HIV status on OS, while adjusting for the effects of other demographic and clinical characteristics. In that model, we included covariates known a priori to impact BC survival (age, ECOG performance status at diagnosis19, clinical stage (I & II vs. III) 7, receptor subtype (ER+/PR+/HER2− and ER+/PR+/HER2+ vs. ER−/PR−/HER2+ and ER−/PR−/HER2−) 20, Ki67 score (<20% vs. ≥20%) 21 and treatment received (surgery and chemotherapy vs. surgery/no chemotherapy vs. chemotherapy/no surgery vs. no surgery or chemotherapy) 22, 23). Additional variables showing an association with OS in the univariate analysis with a significance of p<0.1 and not part of the a priori set of covariates were also included in the multivariable model; these included ethnicity, highest level of education achieved, and body mass index (BMI). We examined hazard ratios for HIV before and after adjustment for these variables to identify both confounding effects (e.g., age, stage) and mediating pathways (e.g., BC receptor subtypes). We excluded tumour grade due to a high number of missing values; wealth index because of collinearity with ethnicity and educational status; diabetic and cerebrovascular disease because of their low prevalence in WLWH; and radiation therapy because the indications varied based on both indication and scheduled dose. Among WLWH, we compared OS within subgroups based on the use of ART (yes/no), HIV viral load (<50 vs. ≥50 copies/mL), and CD4 cell count (<200 vs. ≥ 200 cells/mL) at BC diagnosis. All statistical analyses were performed using Stata version 16 (StataCorp Ltd, College Station, TX).
RESULTS
Between July 1, 2015, and June 30, 2019, we enrolled 2995 women into the SABCHO study. Of these, we excluded 26 (<1%) women with missing clinical stage, 523 (17.5%) diagnosed with stage IV BC, 23 (<1%) with unknown HIV status, 8 (<1%) with unknown hormone receptor status, and 48 (<1%) with bilateral BC. Table 1 shows the socio-demographic and comorbidity characteristics of the remaining 2,367 women. The 499 (21.1%) WLWH were younger than the 1868 (78.9%) without HIV (Median age (interquartile range): 45.0 (39.6–52.4) vs. 58.8 (48.0–68.3), p<0.001). Compared to HIV-uninfected women, WLWH were less wealthy and more likely to be of black African descent, educated beyond the primary level, of normal body size (BMI <25kg/m2), (p<0.001 for all comparisons). They were less likely to report having diabetes, hypertension, or cerebrovascular disease (p<0.001 for all comparisons) (Table 1); these differences persisted when we restricted the analysis to women ≥45 years of age (Data not shown).
Table 1:
Socio-demographic and pre-morbid characteristics of women with stages I-III breast cancer in the SABCHO cohort by HIV status
| HIV negative | HIV positive | Total | P-value | |
|---|---|---|---|---|
| HIV status (row %) | 1868 (78.9%) | 499 (21.1%) | 2367 (100.0%) | |
| Age at diagnosis in years | ||||
| <40 | 198 (10.6) | 128 (25.7) | 326 (13.8) | <0.001 |
| 40–49 | 359 (19.2) | 209 (41.9) | 568 (24.0) | |
| 50–59 | 441 (23.6) | 116 (23.2) | 557 (23.5) | |
| 60–69 | 463 (24.8) | 36 (7.2) | 499 (20.1) | |
| ≥70 | 407 (21.8) | 10 (2.0) | 417 (17.6) | |
| a Age at diagnosis in years, Median (IQR) | 58.8 (48.0–68.3) | 45.0 (39.6–52.4) | 55.1 (44.8–65.8) | <0.001 |
| Menopausal status | ||||
| Premenopausal | 543 (29.1) | 308 (61.7) | 851 (36.0) | <0.001 |
| Menopausal | 1325 (70.9) | 191 (38.3) | 1516 (64.0) | |
| Ethnicity | ||||
| Black | 1358 (72.7) | 487 (97.6) | 1845 (77.9) | <0.001 |
| Asian | 245 (13.1) | 2 (0.4) | 247 (10.4) | |
| White | 172 (9.2) | 0 (0.0) | 172 (7.3) | |
| Mixed race | 93 (5.0) | 10 (2.0) | 103 (4.4) | |
| Highest level of education | ||||
| Primary education and below | 558 (30.2) | 101 (20.8) | 659 (28.2) | <0.001 |
| Secondary education and above | 1292 (69.8) | 393 (79.2) | 1685 (71.8) | |
| Wealth index | ||||
| 1 | 313 (16.8) | 158 (31.7) | 471 (19.9) | <0.001 |
| 2 | 347 (18.6) | 130 (26.1) | 477 (20.2) | |
| 3 | 381 (20.4) | 93 (18.6) | 474 (20.0) | |
| 4 | 413 (21.1) | 64 (12.8) | 477 (20.2) | |
| 5 (Wealthiest) | 414 (22.2) | 54 (10.8) | 468 (19.8) | |
| Alcohol | ||||
| Yes | 342 (18.4) | 120 (24.1) | 462 (19.6) | 0.004 |
| No | 1521 (81.6) | 377 (75.9) | 1898 980.4) | |
| Smoking | ||||
| Yes | 265 (14.2) | 41 (8.3) | 306 (13.0) | <0.001 |
| No | 1598 (85.8) | 456 (91.7) | 2054 (87.0) | |
| Body mass index (BMI) | ||||
| <25 | 280 (15.8) | 131 (27.4) | 411 (18.2) | <0.001 |
| 25–29.9 | 457 (25.7) | 138 (28.9) | 595 (26.4) | |
| ≥30 | 1038 (58.5) | 209 (43.7) | 1247 (55.3) | |
| Diabetes | ||||
| Yes | 300 (16.1) | 21 (4.2) | 321 (13.6) | <0.001 |
| No | 1563 (83.9) | 476 (95.8) | 2039 (86.4) | |
| Hypertension | ||||
| Yes | 871 (46.8) | 106 (21.3) | 977 (41.4) | <0.001 |
| No | 992 (53.2) | 391 (78.7) | 1383 (58.6) | |
| Cerebrovascular disease | ||||
| Yes | 137 (7.4) | 10 (2.0) | 147 (6.2) | <0.001 |
| No | 1726 (92.6) | 487 (98.0) | 2213 (93.8) | |
| b ECOG PS | ||||
| 0 & 1 | 1738 (93.3) | 484 (97.2) | 2222 (94.1) | 0.001 |
| 2–4 | 125 (6.7) | 14 (2.8) | 139 (5.9) | |
| Hospitals | ||||
| c CHBAH | 699 (37.4) | 225 (45.1) | 924 (39.0) | <0.001 |
| d CMJAH | 503 (26.9) | 101 (20.2) | 604 (25.5) | |
| Durban | 339 (18.1) | 60 (12.0) | 399 (16.9) | |
| Greys | 398 (16.0) | 93 (18.7) | 391 (16.5) | |
| Ngwelezana | 29 (1.6) | 20 (4.0) | 49 (2.1) |
Abbreviations:
IQR (Interquartile range),
ECOG (Eastern Cooperative Oncology Group) PS (Performance status) at baseline,
CHBAH (Chris Hani Baragwanath Academic Hospital),
CMJAH (Charlotte Maxeke Johannesburg Academic Hospital). Missing data: Highest level of education (n=23), Alcohol (n=7), Smoking (n=7), BMI (n=114), Diabetes (n=7), hypertension (n=7), cerebrovascular disease (n=7), ECOG (n=6)
Overall, nearly half the women presented with stage III BC, but WLWH were more likely than others to have stage III disease (p=0.017). WLWH were also more likely to receive chemotherapy (82.4%) than HIV-uninfected women (72.1%, p<0.001) overall (Table 2), among those with stage I-II BC (76.0% vs. 64.5%, p=0.001), and among those with stage III BC (87.8% vs. 80.4%, p=0.004) (Supplementary table 1).
Table 2:
Clinical characteristics at diagnosis and treatment of women with stages I-III breast cancer in the SABCHO cohort by HIV status
| HIV negative | HIV positive | Total | P-value | |
|---|---|---|---|---|
| HIV status (row %) | 1868 (78.9%) | 499 (21.1%) | 2367 (100.0%) | |
| Tumour stage | ||||
| T0 | 4 (0.2) | 1 (0.2) | 5 (0.2) | 0.401* |
| T1 | 201 (10.8) | 41 (8.2) | 242 (10.2) | |
| T2 | 820 (43.9) | 212 (42.5) | 1032 (43.6) | |
| T3 | 300 (16.1) | 88 (17.6) | 388 (16.4) | |
| T4 | 543 (29.1) | 157 (31.5) | 700 (29.6) | |
| Nodal stage | ||||
| 0 | 649 (34.7) | 132 (26.5) | 781 (33.0) | 0.003 |
| 1 | 772 (41.3) | 226 (45.3) | 998 (42.2) | |
| 2 | 362 (19.4) | 109 (21.8) | 471 (19.9) | |
| 3 | 85 (4.6) | 32 (6.4) | 117 (4.9) | |
| Stage | ||||
| Stage I | 142 (7.6) | 23 (4.6) | 165 (7.0) | 0.017 |
| Stage II | 819 (43.8) | 206 (41.3) | 1025 (43.3) | |
| Stage III | 907 (48.6) | 270 (54.1) | 1177 (49.7) | |
| Histological diagnosis | ||||
| Invasive ductal | 1782 (95.4) | 483 (96.8) | 2265 (95.7) | 0.172 |
| Other histological type | 86 (4.6) | 16 (3.2) | 102 (4.3) | |
| Grade | ||||
| Grade 1 | 123 (7.7) | 30 (7.0) | 153 (7.5) | 0.883 |
| Grade 2 | 880 (55.0) | 239 (55.7) | 1119 (55.1) | |
| Grade 3 | 598 (37.3) | 160 (36.3) | 758 (37.3) | |
| Breast cancer subtype | ||||
| a ER+ or PR+/HER2− | 1163 (62.3) | 271 (54.3) | 1434 (60.6) | 0.003 |
| ER+/PR+/HER2+ | 299 (16.0) | 111 (22.2) | 410 (17.3) | |
| ER−/PR−/HER2+ | 117 (6.3) | 31 (6.2) | 148 (6.3) | |
| ER−/PR−/HER2− | 289 (15.5) | 86 (17.2) | 375 (15.8) | |
| KI67 | ||||
| <20 | 581 (32.3) | 130 (27.3) | 711 (31.2) | 0.036 |
| ≥20 | 1220 (67.7) | 347 (72.7) | 1567 (68.8) | |
| Surgical treatment | ||||
| No | 434 (23.2) | 137 (27.5) | 571 (24.1) | 0.050 |
| Yes | 1434 (76.8) | 362 (72.5) | 1796 (75.9) | |
| Chemotherapy treatment | ||||
| No | 522 (27.9) | 88 (17.6) | 610 (25.8) | <0.001 |
| Yes | 1346 (72.1) | 411 (82.4) | 1757 (74.2) | |
| Radiation therapy | ||||
| No | 893 (47.8) | 248 (49.7) | 1141 (48.2) | 0.452 |
| Yes | 975 (52.2) | 251 (50.3) | 1226 (51.8) | |
| Treatment received | ||||
| Surgery, no chemotherapy | 303 (16.2) | 40 (8.1) | 343 (14.5) | <0.001 |
| Surgery + chemotherapy | 1131 (60.5) | 322 (65.5) | 1453 (61.4) | |
| Chemotherapy, no surgery | 215 (11.5) | 89 (17.8) | 304 (12.8) | |
| No surgery or chemotherapy | 219 (11.7) | 48 (9.6) | 267 (11.3) | |
| Endocrine therapy (ER+/PR+ patients only, N=1844) | ||||
| No | 252 (17.2) | 81 (21.2) | 333 (18.1) | 0.073 |
| Yes | 1210 (82.8) | 301 (78.8) | 1511 (81.9) | |
| b Median follow-up time in months (IQR) | 29.0 (19.0–42.0) | 26.0 (17.0–38.0) | 29.0 (19.0–41.0) | <0.001 |
Abbreviations:
ER/PR (Oestrogen receptor/Progesterone receptor), HER2 (Human Epidermal Growth Factor Receptor 2),
IQR (Interquartile range). Missing data: Grade (n=337), Ki67 (n=89).
Fisher’s exact test
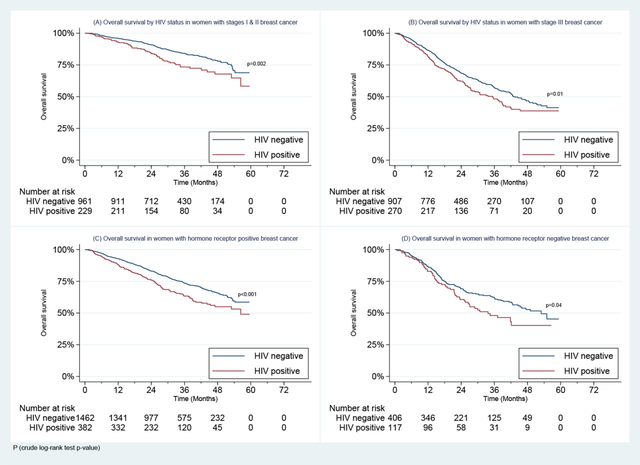
By the median follow-up time of 29.0 months (IQR 19.0–41.0), 728 (30.8%) women had died. WLWH had poorer OS at 2 years than others (72.4% vs. 80.1%, p<0.001) overall, and in younger (age <45 years: 70.4% vs.81.0%, p=0.004) and older age groups (≥45 years: 74.3% vs. 79.9%, p=0.001) (Figure 1).
Figure 1:
Overall Survival in Women with Stages I-III Breast Cancer Enrolled in the SABCHO cohort by HIV status and by age group.
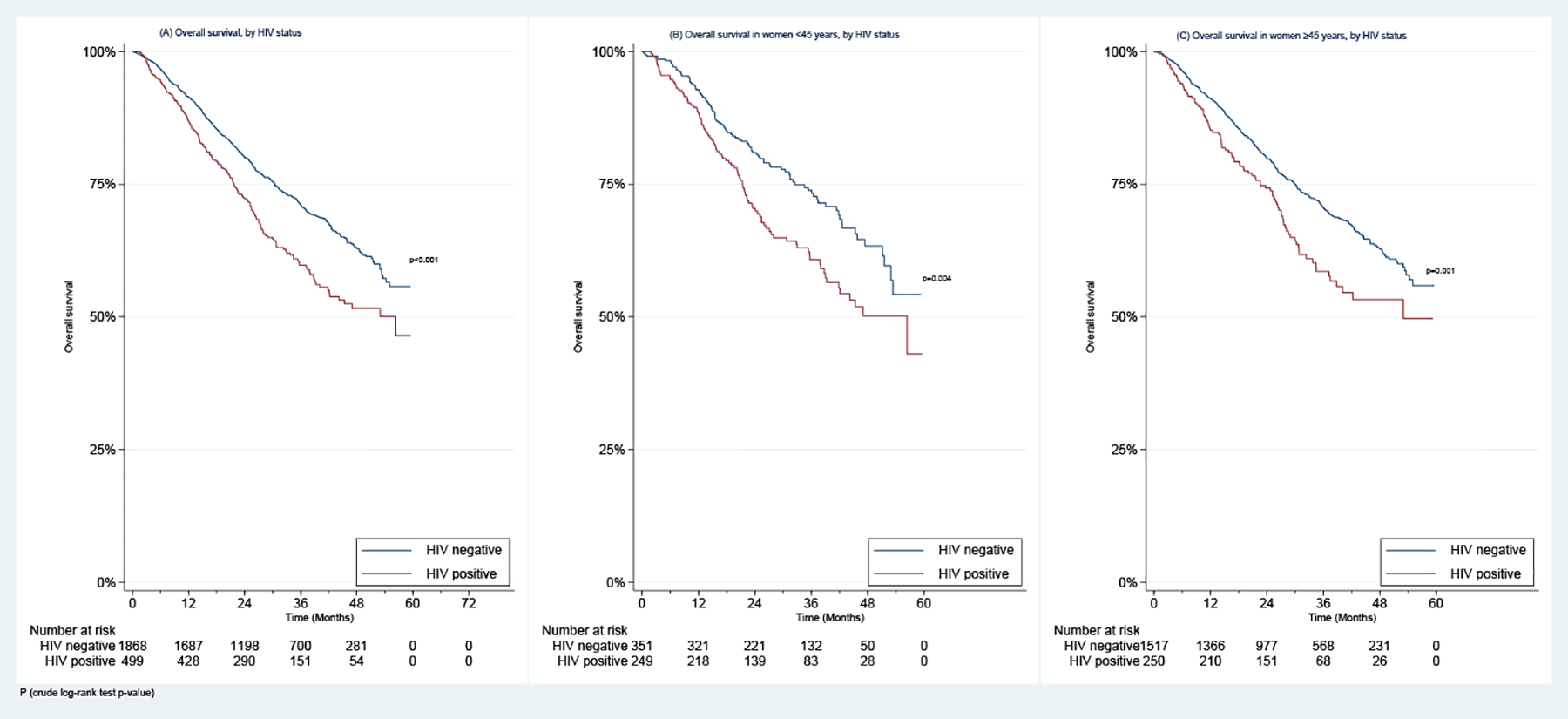
In addition, WLWH had poorer 2-year OS than HIV-uninfected women whether they had stage I-II disease (84.3% vs. 90.8%, p=0.002) or stage III disease (62.2% vs. 68.6%, p=0.011) (Figure 2). WLWH also had poorer 2-year OS in both the ER+/PR+ (76.1% vs. 83.1%, p<0.001) and ER−/PR− negative (60.6% vs. 69.1%, p=0.037) subgroups (Figure 2).
Figure 2:
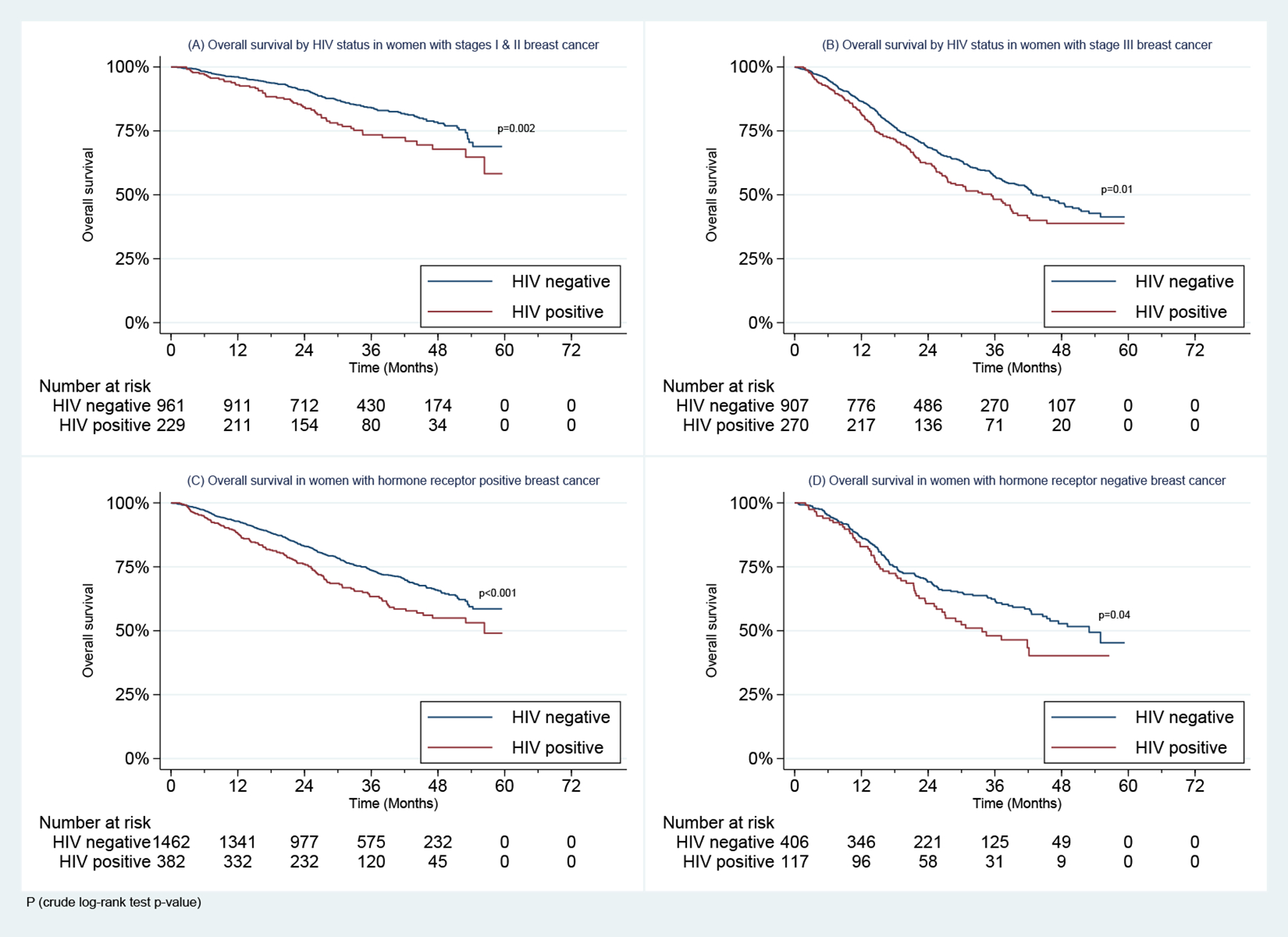
Overall Survival in Women with Stages I-III Breast Cancer Enrolled in the SABCHO cohort by (A) & (B) HIV status and stage, and (C) & (D) HIV status and Breast Cancer Hormone receptor status
In our univariate and multivariate model, all-cause mortality remained higher among WLWH than among HIV-uninfected women (Crude HR: 1.45, 95%CI: 1.23–1.72 and adjusted HR (aHR): 1.49, 95%CI: 1.22–1.83) (Supplementary table 2 and Table 3). Other predictors of survival were performance status, ECOG 2–4 vs. 0–1 (aHR: 1.73 (1.29–2.32); stage III vs. stages I & II BC (aHR: 2.13 (1.77–2.56); ER−/PR−/HER2+ (aHR: 1.45 (1.06–1.97) and ER−/PR−/HER2− (aHR: 1.78 (1.44–2.19) vs. ER+/PR+/HER2− BC subtype; and Ki67 ≥20 vs. <20 (aHR: 1.45 (1.20–1.75). Survival was worse among women who had chemotherapy with no surgery (aHR: 3.75 (3.07–4.58)) and women who had no surgery or chemotherapy (aHR: 3.83 (2.98–4.92)) than among women who had surgery with chemotherapy (Table 3). In a sensitivity analysis restricted to those who received surgery and at least some form of systemic therapy, the factors influencing OS were similar to those that did so in the full cohort (Supplementary table 3). In a sub-group analysis of WLWH only, significant predictors of survival were stage, hormone receptor status, and Ki67 score (aHR for stage III vs. stages I & II: 2.24 (1.53–3.28); aHR for ER−/PR−/HER2− vs. ER+/PR+/HER2− BC subtype: 2.14 (1.37–3.36); and aHR for Ki67 score ≥20 vs. <20: 1.57 (1.03–2.38) (Supplementary table 4).
Table 3:
Multivariate Cox Proportional Hazard Ratio Model of Risk Factors for Mortality in Women with Stages I-III Breast Cancer Enrolled in the SABCHO cohort
| Died, N=728 (row %) | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|
| HIV | |||
| Negative | 538 (28.8) | 1.00 (Reference) | |
| Positive | 190 (38.1) | 1.49 (1.22–1.83) | <0.001 |
| Age at diagnosis in years | |||
| <40 | 109 (33.4) | 1.00 (Reference) | |
| 40–49 | 160 (28.2) | 0.84 (0.64–1.08) | 0.173 |
| 50–59 | 155 (27.8) | 0.84 (0.64–1.10) | 0.207 |
| 60–69 | 137 (27.4) | 0.86 (0.64–1.16) | 0.330 |
| ≥70 | 167 (40.1) | 0.95 (0.69–1.31) | 0.771 |
| Ethnicity | |||
| Others | 137 (26.2) | 1.00 (Reference) | |
| Black | 591 (32.0) | 1.01 (0.81–1.24) | 0.954 |
| Highest level of education | |||
| Primary education and below | 247 (37.5) | 1.00 (Reference) | |
| Secondary education and above | 466 (27.7) | 0.73 (0.61–0.88) | 0.001 |
| Body mass index (BMI) | |||
| <25 | 154 (37.5) | 1.00 (Reference) | |
| 25–29.9 | 155 (26.1) | 0.76 (0.60–0.96) | 0.020 |
| ≥30 | 376 (30.2) | 0.95 (0.78–1.18) | 0.663 |
| a ECOG PS | |||
| 0 & 1 | 643 (28.9) | 1.00 (Reference) | |
| 2–4 | 82 (59.0) | 1.73 (1.29–2.32) | <0.001 |
| Stage at diagnosis | |||
| I & II | 214 (18.0) | 1.00 (Reference) | |
| III | 514 (43.7) | 2.13 (1.77–2.56) | <0.001 |
| Breast cancer subtype | |||
| b ER+/PR+/HER2− | 385 (26.8) | 1.00 (Reference) | |
| ER+/PR+/HER2+ | 133 (32.4) | 1.19 (0.96–1.47) | 0.106 |
| ER−/PR−/HER2+ | 57 (38.5) | 1.45 (1.06–1.97) | 0.019 |
| ER−/PR−/HER2− | 153 (40.8) | 1.78 (1.44–2.19) | <0.001 |
| KI67 | |||
| <20 | 174 (24.5) | 1.00 (Reference) | |
| ≥20 | 520 (33.2) | 1.45 (1.20–1.75) | <0.001 |
| Treatment received | |||
| Surgery + chemotherapy | 334 (23.0) | 1.00 (Reference) | |
| Surgery, no chemotherapy | 61 (17.8) | 1.19 (0.87–1.64) | 0.280 |
| Chemotherapy, no surgery | 189 (62.2) | 3.75 (3.07–4.58) | <0.001 |
| No surgery or chemotherapy | 144 (53.9) | 3.83 (2.98–4.92) | <0.001 |
Abbreviations:
ECOG (Eastern Cooperative Oncology Group) PS (Performance status),
ER/PR (Oestrogen receptor/Progesterone receptor), HER2 (Human Epidermal Growth Factor Receptor 2). We tested for interactions between HIV and each of the above covariates, and none was statistically significant. Missing data: Highest level of education (n=23), BMI (n=114), ECOG (n=6), Ki67 (n=89).
In exploratory analyses, both WLWH with <50 HIV copies/mL and WLWH with ≥50 copies/mL had greater mortality than HIV-uninfected BC patients (aHR: 1.35 (1.09–1.66), p=0.005 and 1.54 (1.20–2.00), p<0.001, respectively). In addition, WLWH with ≥200 CD4+ cells/mL and those with <200 cells/mL both had worse OS than HIV-uninfected women (aHR: 1.39 (1.15–1.67), p<0.001 and 1.55 (0.96–2.48), p=0.07, respectively) (Table 4 & Figure 3).
Table 4:
HIV-related factors at diagnosis and Hazard Ratio for mortality in Women with Stages I-III Breast Cancer Enrolled in the SABCHO cohort
| Characteristic | Died, *N=728 (row %) | HR (95% CI) | P-value | HR (95% CI)a | P-value a |
|---|---|---|---|---|---|
| ART use | |||||
| HIV− | 538 (28.8) | 1.00 (Ref) | 1.00 (Ref) | ||
| HIV+, not on ART | 141 (36.1) | 1.76 (1.31–2.37) | 0.001 | 1.68 (1.24–2.27) | 0.001 |
| HIV+, on ART | 47 (46.1) | 1.37 (1.14–1.65) | <0.001 | 1.37 (1.12–1.66) | 0.002 |
| HIV viral load (copies/mL) | |||||
| HIV− | 538 (28.8) | 1.00 (Ref) | |||
| HIV+, VL < 50 | 112 (35.7) | 1.33 (1.09–1.64) | 0.006 | 1.35 (1.09–1.66) | 0.005 |
| HIV+, VL ≥ 50 | 70 (42.7) | 1.63 (1.27–2.09) | <0.001 | 1.54 (1.20–2.00) | 0.001 |
| CD4 count (cells/mL) | |||||
| HIV− | 538 (28.8) | 1.00 (Ref) | |||
| HIV+, CD4 ≥ 200 | 163 (37.3) | 1.40 (1.18–1.67) | <0.001 | 1.39 (1.15–1.67) | <0.001 |
| HIV+, CD4 < 200 | 18 (42.9) | 1.66 (1.04–2.66) | 0.034 | 1.55 (0.96–2.48) | 0.070 |
ART (Antiretroviral therapy), VL (Viral load),
Adjusted for age and stage.
Missing data for *N: ART use (n=2), HIV viral load (n=8), CD4 count (n=9).
Figure 3:
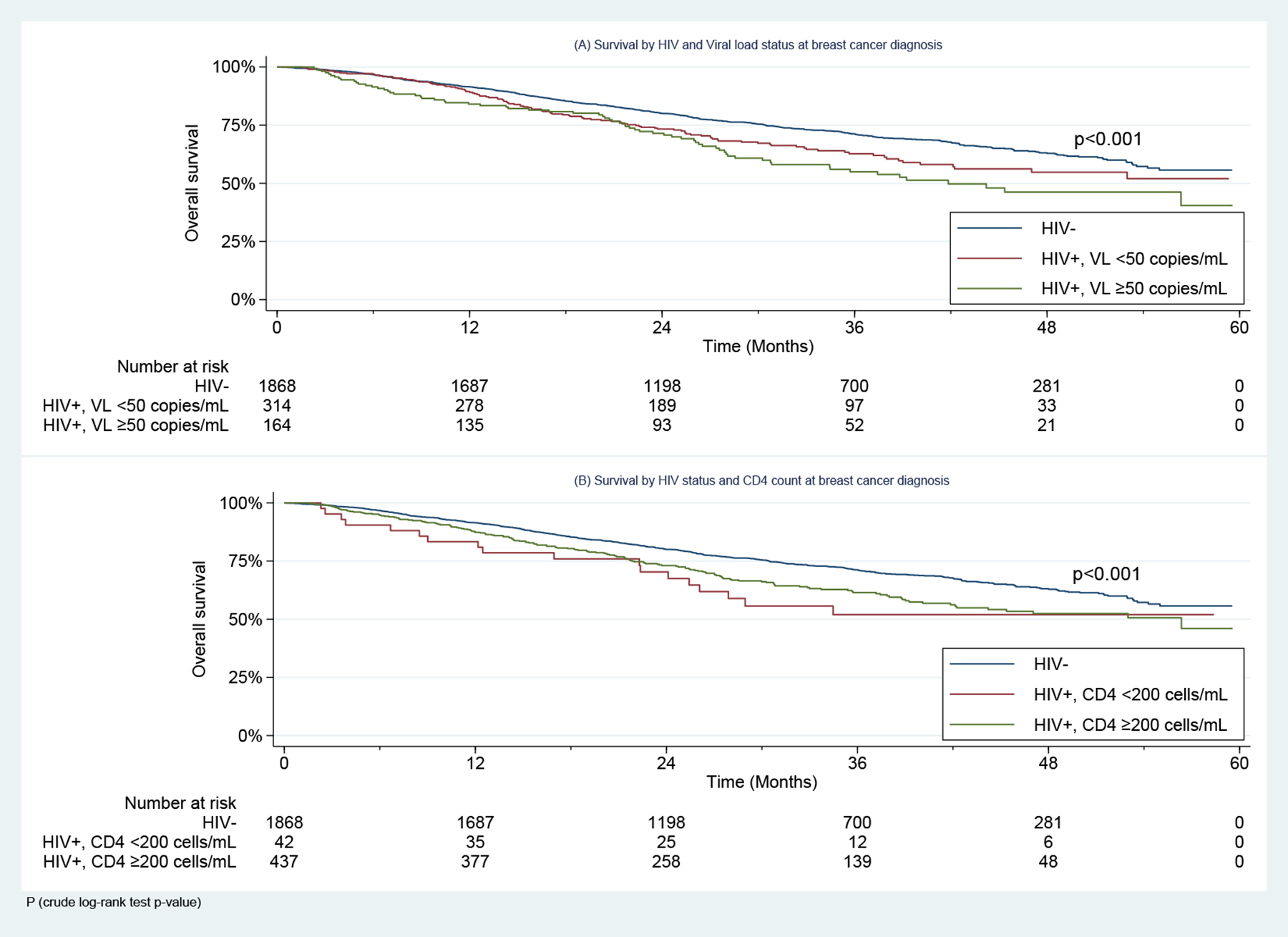
Overall Survival in Women Living with HIV with Stages I-III Breast Cancer Enrolled in the SABCHO cohort, by (A) Viral Load and (B) CD4 count at Breast Cancer diagnosis.
DISCUSSION
In our cohort of women with stages I-III BC at six public hospitals in South Africa, 21.1% were WLWH at BC diagnosis. At a median follow-up of 29.0 months, 2-year OS was 72.4% among WLWH vs. 80.1% among HIV-uninfected women (aHR for all-cause mortality: 1.49, 95%CI 1.22–1.83). WLWH also had poorer OS within strata of age, stage, and BC hormone receptor status.
Our findings are consistent with prior work from HICs demonstrating higher mortality rates among patients with BC and HIV than among HIV-uninfected BC patients 4, 24. In US National Cancer Database data from 2004 to 2014, mortality was higher among the 1,197 BC patients with HIV than among the 1,448,757 HIV‐uninfected BC patients (HR; 1.85, 95%CI 1.68‐2.04). A recent meta-analysis of 18 studies (4 from North America, 14 from SSA) also found that WLWH and BC had poorer overall survival than HIV-uninfected women with BC, in both North America (HR; 2.45; 95%CI 1.11–5.41) and SSA (HR 1.43; 95%CI 1.06–1.92) 9.
The reasons for these survival disparities are likely to be multifactorial. Many WLWH, both in South Africa and elsewhere, come from vulnerable populations at risk for poor cancer outcomes 25–27. However, even controlling for age, ethnicity, and education, our study found poorer survival among BC patients with than without HIV infection. Our models may not have controlled for unknown socio-demographic risk factors that may have affected access to high-quality BC treatment. Even so, our prior work on BC treatment quality in South Africa did not show any differences in receipt of timely adjuvant chemotherapy, endocrine therapy, or radiotherapy based on HIV status 28. WLWH in our cohort were more likely to have stage III disease, but they were also younger and therefore less likely to have slow-growing, ER+/PR+ BC.
The relative youth of the WLWH in our cohort is attributable to the younger age of WLWH in the general population. Breast cancer in younger women has a more aggressive phenotype than that in older women, with high proportions of ER/PR loss and HER2 overexpression 29, 30. Accordingly, younger age at diagnosis, especially of early-stage BC, is associated with poorer survival 31, 32. Even though our multivariable analyses adjusted for age, unknown factors related to age and HIV may have contributed to the survival disparity.
HER2-targeted agents, such as trastuzumab, were not available during our study period; adjuvant trastuzumab was added to the National Essential Medicines List in 2019. The lack of trastuzumab may have been of particular importance to the survival of WLWH, given that they were modestly more likely to have HER2+ BC. However, this discrepancy probably does not account for much of the difference in OS because we controlled for HER2 status in multivariable analysis, and because BC patients living with HIV also have poorer survival in high-income settings despite routine trastuzumab use.
Few studies have evaluated the association of comorbid HIV and malignancy with adherence to treatment of either condition. A nationwide study in Korea found that a cancer diagnosis was a risk factor for low ART adherence, and, among PLWH in SSA, increasing pill burden decreased ART adherence 33, 34. WLWH may also experience greater myelosuppression, and therefore more dose reductions or delays, than others from cytotoxic chemotherapy. Both incomplete adherence to endocrine therapy and reduced chemotherapy dose intensity can worsen BC outcomes 35, 36; we are currently investigating the impact of HIV on both of these aspects of BC treatment quality.
Comorbid HIV also may inhibit the immune response to breast tumours. Intraepithelial and stromal tumour-infiltrating lymphocyte density influences systemic therapy response and BC recurrence rates 37, 38. HIV also indirectly causes persistent immune activation via its effect on the gut, where acute infection damages tight junction proteins and promotes microbial dysbiosis 39. Microbial gut translocation persists after viral suppression, contributing to a chronic pro-inflammatory state which may also increase the risk of BC progression and metastases 40–42.
Finally, HIV increases risks for many competing causes of death unrelated to BC. The life expectancy of PLWH in South Africa has improved drastically in the past two decades, but even patients who initiate ART early have a 20% lower life expectancy than adults without HIV 43, 44. Cause of death data on our cohort members was not reliable enough to enable us to compute the exact proportions of excess deaths attributable to BC and of those due to complications of HIV.
However, our exploratory findings that even WLWH with HIV viral loads <50 copies/mL and CD4+ T-cell count ≥200 cells/mL had worse survival suggests that HIV-related complications are not wholly responsible for the excess mortality observed in WLWH. Future work should include close monitoring of HIV indicators in patients undergoing BC treatment to determine if those cancer therapies lead to loss of HIV control and increase the risk of acute HIV-related events.
Most women in our cohort were diagnosed with stage III disease, but the proportion was higher among WLWH than among HIV-uninfected women (54.1% vs. 48.6%). Several studies have found large proportions of advanced stage disease at diagnosis and overall poor survival among BC patients in SSA 7, 45–47. Overall, our crude 2-year OS was 78.5% but 89.6% for women with stage I & II BC and 67.1% for those with stage III BC. Women with stage III BC in our cohort had a higher estimated 2-year OS than stage III BC patients in the overall ABC-DO cohort, and in the Nigeria and Uganda sub-cohorts (ABC-DO overall cohort: ~60%; Nigeria: ~50%; Uganda: ~56% 7. However, in HICs, 5-year survival probability is ≥ 89% 8, 48 among women diagnosed with early-stage BC. Some reasons for advanced-stage BC diagnosis in our setting include low BC awareness, difficulty in accessing healthcare, lack of population-based screening 49–51; belief in alternative sources of healing and fear of conventional medicine 52, 53; and cumbersome referral pathways within the healthcare system 50, 54. The poor survival by stage of our WLWH (Figure 2) may be due in part to a higher risk of additional causes of death such as HIV‐associated comorbidities and AIDS.
The survival pattern in our cohort shows that HIV has a later effect on survival in the ER−/PR− group than in the ER+/PR+ group. In the women with ER−/PR− BC, there was no significant disparity in survival between WLWH and HIV-uninfected women in the first 12 months of follow-up. In our prior work, we observed that WLWH had poorer responses to neoadjuvant chemotherapy than HIV-uninfected women. This effect was concentrated in the ER+/PR+ group; we found no difference in proportions with pathologic complete response between WLWH and HIV-negative women in the ER−/PR− group. We are currently analysing endocrine therapy adherence among our WLWH and HIV-negative women.
Overall, our women who had chemotherapy with no surgery and those who had no surgery or chemotherapy had poorer survival than women who had both surgery and chemotherapy. This finding is expected. Patients who did not have surgery after chemotherapy probably had irresectable tumours or disease progression, or they abandoned treatment. The South African national policy specifies that low-cost or no-cost BC surgery, chemotherapy, radiotherapy, and endocrine therapy should be available at tertiary public hospitals. However, resource constraints within the national health system mean that timely access to these treatments is inconsistent. We previously found that baseline care for our patients was reasonably concordant with the American Society of Clinical Oncology BC care quality measures for chemotherapy and endocrine therapy but poor for radiotherapy 55. We lacked data to explain whether individual patients failed to receive surgery or chemotherapy because of lapses at the hospital level, incomplete patient adherence, or clinically appropriate decisions following disease progression.
Strengths of our study include the large sample size of our cohort, the prospective multi-center design, and the availability of detailed socio-demographic, clinical, and outcome data, all unprecedented for a BC population from SSA. South Africa’s high HIV prevalence and widespread access to ART make it an important setting for the study of HIV’s impact on BC, and given our study’s setting and multicentre design, our results are probably generalizable to SSA, the region with the world’s largest absolute number of WLWH and comorbid BC.
Some limitations should be noted. We were not able to collect detailed information on HIV treatment or to assess treatment adherence, which may have differed by HIV status. We did not consider our information on disease-free survival and cause of death reliable enough to support analyses of the excess mortality seen in WLWH. Our median follow-up time was only 29 months, but the mortality we observed was higher than in most BC cohort studies in HICs.
CONCLUSIONS
In the largest prospectively collected BC cohort we know of describing survival in WLWH with BC, we found compelling evidence that WLWH had worse OS than HIV-uninfected women. Our study supports the conclusions of smaller studies from SSA and more precisely describes the survival disadvantages of WLWH 4, 5, 24. The reasons why BC survival among WLWH is so poor call for further research focusing on differences in access to care, treatment-related adverse events, a possible biological associations between HIV and tumour behaviour, and cause of death unrelated to BC but known to be associated with HIV, such as trauma, suicide, and specific complications of HIV/AIDs. In the future, we also hope to examine survival in South African BC patients diagnosed after 2019 to see to what extent access to trastuzumab ameliorated the OS disparity between women with and without HIV.
Supplementary Material
Novelty and impact statement.
Breast cancer (BC) patients living with HIV are a growing population globally. In this large cohort of South African women with non-metastatic BC, patients living with HIV at the time of BC diagnosis had a 49% higher risk of death from any cause than women with BC without HIV-infection. This finding persisted after accounting for differences in age, ethnicity, BC stage, subtype, and treatments received. Our work shows that HIV adversely affects the survival of women with non-metastatic BC, including those on antiretroviral therapy.
Funding:
NIH/NCI grant; Grant/award numbers: NIH/R01-CA19262701 and R01-CA250012, Cancer Association of South Africa (CANSA) grant “Down-staging and improving survival of breast cancer in South Africa”, The South African Medical Research Council/University of the Witwatersrand Common Epithelial Cancer Research Center (MRC/ WITS CECRC). This study was in part funded by a 2021 American Society of Clinical Oncology Conquer Cancer – Janssen Oncology Young Investigator Award to Y.S.P.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Wits Health Consortium (PTY) Ltd provided support in the form of payroll administration of salaries from grant funds for authors OA, MJ, WM, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of abbreviations
- ABC-DO
Africa breast Cancer Disparity in Outcomes
- ART
Antiretroviral therapy
- BC
Breast cancer
- BMI
Body mass index
- CHBAH
Chris Hani Baragwanath Academic Hospital
- CMJAH
Charlotte Maxeke Johannesburg Academic Hospital
- CI
Confidence interval
- ER
Oestrogen receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- HICs
High income countries
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- IQR
Interquartile range
- OR
Odds ratio
- OS
Overall survival
- PLWH
People living with HIV
- PR
Progesterone receptor
- SABCHO
South African Breast Cancer and HIV Outcomes Study
- SD
Standard deviation
- SSA
Sub-Saharan Africa
- WLWH
Women living with HIV
Footnotes
Conflict of interest:
AIN: I have consulted for Otsuka, Eisai, GlaxoSmithKline, United biosource Corp, Hospira. I have grant support from Otsuka. I am on the medical advisory board of EHE Intl.
All other authors declare that they have no conflict of interest.
Publisher's Disclaimer: Disclaimer: “Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of these organizations.”
Ethics statement: The SABCHO study was approved by the University of the Witwatersrand Human Research Ethics Committee in Johannesburg, South Africa, and the Institutional Review Board of Columbia University in New York, NY. All participants provided written informed consent
Data Availability Statement:
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.UNAIDS. AIDS Data, 2016. Available at: https://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 2.Grover S, Martei YM, Puri P, Prabhakar P, Mutebi M, Balogun OD, Price AJ, Freeman AH, Narasimhamurthy M, Rodin D, Rayne S, Zetola NM. Breast Cancer and HIV in Sub-Saharan Africa: A Complex Relationship. Journal of Global Oncology 2018: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prager GW, Braga S, Bystricky B, Qvortrup C, Criscitiello C, Esin E, Sonke GS, Martínez GA, Frenel J-S, Karamouzis M. Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO open 2018;3: e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 2019;125: 2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiology and Prevention Biomarkers 2017;26: 1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coghill AE, Suneja G, Rositch AF, Shiels MS, Engels EA. HIV infection, cancer treatment regimens, and cancer outcomes among elderly adults in the United States. JAMA oncology 2019;5: e191742–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C, Anele A, Parham G, Pinder LF, Cubasch H. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. The Lancet Global Health 2020;8: e1203–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 2018;391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandão M, Bruzzone M, Franzoi M-A, De Angelis C, Eiger D, Caparica R, Piccart-Gebhart M, Buisseret L, Ceppi M, Dauby N, Carrilho C, Lunet N, et al. Impact of HIV infection on baseline characteristics and survival of women with breast cancer. AIDS 2021;35: 605–18. [DOI] [PubMed] [Google Scholar]
- 10.Cubasch H, Ruff P, Joffe M, Norris S, Chirwa T, Nietz S, Sharma V, Duarte R, Buccimazza I, Čačala S. South African breast cancer and HIV outcomes study: Methods and baseline assessment. Journal of global oncology 2017;3: 114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phakathi B, Cubasch H, Nietz S, Dickens C, Dix-Peek T, Joffe M, Neugut AI, Jacobson J, Duarte R, Ruff P. Clinico-pathological characteristics among South African women with breast cancer receiving anti-retroviral therapy for HIV. The Breast 2019;43: 123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nietz S, O’Neil DS, Ayeni O, Chen WC, Joffe M, Jacobson JS, Neugut AI, Ruff P, Mapanga W, Buccimazza I. A comparison of complete pathologic response rates following neoadjuvant chemotherapy among South African breast cancer patients with and without concurrent HIV infection. Breast cancer research and treatment 2020;184: 861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pumpalova YS, Ayeni OA, Chen WC, O’Neil DS, Nietz S, Phakathi B, Buccimazza I, Čačala S, Stopforth LW, Farrow HA, Joffe M, Mapanga W, et al. Impact of HIV infection on overall survival among women with stage IV breast cancer in South Africa. Breast Cancer Res Treat 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis D, Webster E. Inequality in South Africa. Development Southern Africa 2019;36: 733–4. [Google Scholar]
- 15.Mayosi BM, Benatar SR. Health and health care in South Africa—20 years after Mandela. New England Journal of Medicine 2014;371: 1344–53. [DOI] [PubMed] [Google Scholar]
- 16.O’Neil DS, Nietz S, Buccimazza I, Singh U, Čačala S, Stopforth LW, Joffe M, Jacobson JS, Neugut AI, Crew KD. Neoadjuvant chemotherapy use for nonmetastatic breast cancer at five public South African hospitals and impact on time to initial cancer therapy. The oncologist 2019;24: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayeni OA, Norris SA, Joffe M, Cubasch H, Nietz S, Buccimazza I, Singh U, Čačala S, Stopforth L, Chen WC. The multimorbidity profile of South African women newly diagnosed with breast cancer. International journal of cancer 2020;147: 361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman J-AW, Sparano JA, Hunsberger S, Enos RA, Gelber RD. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. Journal of Clinical Oncology 2007;25: 2127–32. [DOI] [PubMed] [Google Scholar]
- 19.Karakaya S, Karadağ İ, Duran AO, Oksuzoglu OB, Demirci U. Clinical outcomes and prognostic factors in HER-2 positive breast cancer with brain metastasis: A single-center experience. Journal of Clinical Oncology 2020;38: e13015–e. [DOI] [PubMed] [Google Scholar]
- 20.Johansson ALV, Trewin CB, Hjerkind KV, Ellingjord-Dale M, Johannesen TB, Ursin G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer 2019;144: 1251–61. [DOI] [PubMed] [Google Scholar]
- 21.Mannell A. The role of Ki-67 in breast cancer. South African Journal of Surgery 2016;54: 10–3. [PubMed] [Google Scholar]
- 22.Zarcos-Pedrinaci I, Redondo M, Louro J, Rivas-Ruiz F, Téllez T, Pérez D, Medina Cano F, Machan K, Domingo L, Mar Vernet M, Padilla-Ruiz M, Castells X, et al. Impact of adjuvant chemotherapy on the survival of patients with breast cancer diagnosed by screening. Cancer Med 2019;8: 6662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin M, Verschraegen C, Vincent V-H, Patel SM, George T, Truica CI. Impact of lack of surgery on outcomes in elderly women with nonmetastatic breast cancer—A surveillance, epidemiology, and end results 18 population based study. Medicine 2020;99: e18745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. Journal of Clinical Oncology 2015;33: 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denning P, DiNenno E: Communities in crisis: is there a generalized HIV epidemic in impoverished urban areas of the United States XVIII international AIDS conference 2010; 1. [Google Scholar]
- 26.Fox AM. The HIV-poverty thesis re-examined: poverty, wealth or inequality as a social determinant of HIV infection in sub-Saharan Africa? Journal of biosocial science 2012;44: 459. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan AS, Rosenberg E, Shouse RL, Sullivan PS. Connecting race and place: a county-level analysis of White, Black, and Hispanic HIV prevalence, poverty, and level of urbanization. American journal of public health 2014;104: e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Neil DS, Chen WC, Ayeni O, Nietz S, Buccimazza I, Singh U, Čačala S, Stopforth L, Joffe M, Crew KD. Breast cancer care quality in South Africa’s public health system: an evaluation using American society of clinical oncology/national quality Forum measures. Journal of global oncology 2019;5: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26: 3324–30. [DOI] [PubMed] [Google Scholar]
- 30.Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP, Partridge AH. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 2012;131: 1061–6. [DOI] [PubMed] [Google Scholar]
- 31.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. Journal of the American College of Surgeons 2009;208: 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One 2009;4: e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Lee E, Park B-J, Bang JH, Lee JY. Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: a nationwide study. Scientific reports 2018;8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ global health 2016;1: e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Yu Q, Wu X-C, Hsieh M-C, Loch M, Chen VW, Fontham E, Ferguson T. Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I–III breast cancer: ER+/PR+, HER2-vs. triple-negative. Breast cancer research and treatment 2018;169: 175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, Gelber RD, Goldhirsch A, Smith I, Rabaglio M. Treatment adherence and its impact on disease-free survival in the Breast International Group 1–98 trial of tamoxifen and letrozole, alone and in sequence. Journal of clinical oncology 2016;34: 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, De Azambuja E, Eidtmann H, Ellis CE, Baselga J. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA oncology 2015;1: 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. The lancet oncology 2018;19: 40–50. [DOI] [PubMed] [Google Scholar]
- 39.Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019;11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nature Reviews Endocrinology 2019;15: 139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Molecular and cellular endocrinology 2014;382: 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine & growth factor reviews 2006;17: 325–37. [DOI] [PubMed] [Google Scholar]
- 43.Johnson L, Mossong J, Dorrington R, Schomaker M, Hoffmann C, Keiser O, Fox M, Wood R, Prozesky H, Giddy J. International Epidemiologic Databases to Evaluate AIDS Southern Africa Collaboration Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013;10: e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bor J, Herbst AJ, Newell M-L, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013;339: 961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World journal of surgical oncology 2015;13: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebremariam A, Dereje N, Addissie A, Worku A, Assefa M, Abreha A, Tigeneh W, Pace LE, Kantelhardt EJ, Jemal A. Factors associated with late-stage diagnosis of breast cancer among women in Addis Ababa, Ethiopia. Breast Cancer Research and Treatment 2021;185: 117–24. [DOI] [PubMed] [Google Scholar]
- 47.Islami F, Lortet-Tieulent J, Okello C, Adoubi I, Mbalawa CG, Ward EM, Parkin DM, Jemal A. Tumor size and stage of breast cancer in Côte d’Ivoire and Republic of Congo–Results from population-based cancer registries. The Breast 2015;24: 713–7. [DOI] [PubMed] [Google Scholar]
- 48.Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T. Cancer survival in five continents: a worldwide population-based study (CONCORD). The lancet oncology 2008;9: 730–56. [DOI] [PubMed] [Google Scholar]
- 49.Akuoko CP, Armah E, Sarpong T, Quansah DY, Amankwaa I, Boateng D. Barriers to early presentation and diagnosis of breast cancer among African women living in sub-Saharan Africa. PloS one 2017;12: e0171024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joffe M, Ayeni O, Norris SA, McCormack VA, Ruff P, Das I, Neugut AI, Jacobson JS, Cubasch H. Barriers to early presentation of breast cancer among women in Soweto, South Africa. PloS one 2018;13: e0192071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickens C, Joffe M, Jacobson J, Venter F, Schüz J, Cubasch H, McCormack V. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. International journal of cancer 2014;135: 2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donkor A, Lathlean J, Wiafe S, Vanderpuye V, Fenlon D, Yarney J, Opoku S, Antwi W, Kyei K. Factors contributing to late presentation of breast cancer in Africa: A systematic literature review. Archives of Medicine 2015;8: 1–10. [Google Scholar]
- 53.Espina C, McKenzie F, dos-Santos-Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Annals of epidemiology 2017;27: 659–71. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKenzie F, Zietsman A, Galukande M, Anele A, Adisa C, Parham G, Pinder L, Cubasch H, Joffe M, Kidaaga F. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer–disparities in outcomes (ABC‐DO) study. International journal of cancer 2018;142: 1568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neil DS, Chen WC, Ayeni O, Nietz S, Buccimazza I, Singh U, Čačala S, Stopforth L, Joffe M, Crew KD, Jacobson JS, Neugut AI, et al. Breast Cancer Care Quality in South Africa’s Public Health System: An Evaluation Using American Society of Clinical Oncology/National Quality Forum Measures. Journal of global oncology 2019;5: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.


