Abstract
Pulmonary arterial hypertension (PAH) remains a deadly disease, and there currently is no cure for this life-threating medical problem. The average lifespan is about 5–7 years after diagnosis of PAH. Therefore, a conceptual breakthrough to develop new therapeutic strategies for PAH is urgently needed. Growing evidence shows that stem cells are emerging as a novel effective treatment, but the understanding of its underlying mechanisms is still limited. This review highlights the mechanisms through which stem cells successfully reverse pulmonary vascular endothelial dysfunction, pulmonary artery smooth muscle cell (PASMC) over-proliferation, and mitochondrial dysfunction in PAH patients and common rodent models used in PAH research. They can modulate common underlying pathways involved in PAH, including the nitric oxide synthase (eNOS), mitochondrial regulators, microRNAs and STAT3-BMPR signaling. Genetic modifications further enhance the therapeutic effects of stem cells on PAH. Clinical trials showed promising therapeutic potential of mesenchymal stem cells and endothelial progenitor cells for PAH. Potential limitations and challenges are also discussed. The current findings support the need for further investigation and validation of stem cell therapy for PAH.
Keywords: Stem cell, endothelial progenitor cell, pulmonary arterial hypertension, microRNA, extracellular vesicles
Tweet
Stem cell therapy holds a great promise for pulmonary vascular dysfunction, pulmonary arterial hypertension and right ventricular failure.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of vascular resistance in which the pulmonary blood vessels are narrowed and the pulmonary arterial pressure is elevated (mPAP>= 20 mmHg). Symptoms of PAH include fatigue and dyspnea, often the result from lack of oxygen. While a relatively rare disease, the prognosis for those who are diagnosed is poor as the disease leads to right heart failure (1). PAH primarily affects two types of cells in pulmonary arteries: endothelial cells and smooth muscle cells(2). Damaged endothelial cells result in a loss of normal endothelial function while pulmonary artery smooth muscle cells (PASMCs) exhibit excessive proliferation. Additionally, these cells demonstrate impaired mitochondrial function and disrupted metabolic balance. These abnormal phenotypes precede vascular remodeling, or the structural alterations in the vessels. The fact that both cell types are abnormally affected in different ways explains the complexity of PAH. Current treatments do not improve long term outcomes or address the molecular mechanisms to reverse vascular remodeling. An effective treatment must be capable of restoring normal endothelial function, as well as prevent the abnormal proliferation and buildup of PASMCs.
One of the rising areas in PAH research involves the use of adult stem cell therapy for treatment. Adult stem cells are primarily multipotent with some instances of pluripotency (including umbilical cord blood).(3) Government supported research investigating the ability for adult stem cells to be used therapeutically is ever increasing. There are also a number of preclinical and clinical studies using stem cells for PAH (4). Two fields that have gained much momentum in PAH research involve the use of mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs). These therapies have yielded promising results, but it is important to examine whether they can target the known underlying mechanisms, pathways, and gene expressions that lead to PAH. This review will focus on the usage of MSCs and EPCs, and their ability to address endothelial dysfunction, PASMC proliferation, epigenetic mechanisms, mitochondrial dysfunction, and the potential usage of stem cell-derived materials for vascular remodeling. Potential limitations and challenges are also discussed in this review.
Types of Stem Cells
Mesenchymal Stem Cells (MSCs)
Stem cell therapy using MSCs has attracted significant attention because MSCs are unique in possessing a potential to differentiate into other cell types and have the ability to secrete paracrine factors leading to the improvement of injured tissue (5). In addition, MSCs are easy to isolate and expand ex vivo, can easily be genetically engineered and are “home” to sites of tissue injury or inflammation (6, 7). Differing routes of MSC administration have been investigated including intravenous, intratracheal and direct implantation into the organ (8–10). The MSC-based clinical trials have already been initiated for testing for the treatment of diseases that do not have many therapeutic options including congenital diseases and cancers (11). MSCs harboring the prostacyclin synthase (PCS) gene were shown to prevent monocrotaline (MCT)-induced pulmonary arteriolar remodeling and attenuate RV hypertrophy (7). Most importantly, this study showed that a single injection of PCS-MSCs was able to improve the prognosis and survival of PAH rats up to seven weeks after a single delivery of PCS-MSCs.
Endothelial Progenitor Cells (EPCs)
One of the more recent and interesting discoveries in the course of PAH research is the potential therapeutic application for EPCs. EPCs are angiogenic cells that mobilize from the bone marrow in response to injury. The regenerative properties of EPCs were first discovered in vitro when isolated EPCs differentiated into mature endothelial cells (12). However, research examining the therapeutic potential for EPCs has been extensively debated since their introduction to the field. JunHui et al. first demonstrated that EPC numbers and functional capacity (migratory and adhesive capacity) were reduced in human PAH patients, which implicated a role for EPCs in the maintenance of normal endothelial function.(13) Additionally, several investigators have examined their potential for regenerating new vessels in a variety of PAH animal models. In the MCT model, experiments showed transplantation of EPCs attenuated PAH.(10, 14) Takahashsi et al. reported that transplanted autologous EPCs improved mean pulmonary artery pressure, cardiac output and pulmonary vascular resistance in a dog PAH model.(10) Furthermore, cell-based gene therapy of EPCs with eNOS or angiogenic factors (VEGF and angiopoietin-1) reduced monocrotaline-induced pulmonary hypertension.(15) In pilot clinical trials in PAH patients, infusion of autologous EPCs significantly improved exercise capacity and pulmonary hemodynamics compared with patients who received conventional therapy (16).
Restoration of PA endothelial dysfunction by stem cells
A disbalance between vasodilators and vasoconstrictors is a major characteristic of endothelial dysfunction in PAH (17). One important mediating factor in endothelial dysfunction is the vasodilator nitric oxide (NO)(18). Patients with PAH present with low levels of nitric oxide synthase (eNOS), the enzyme that produces NO (17). While inhaled NO is used as a treatment for PAH patients, there are limitations with this treatment, namely withdrawal effects from discontinuing therapy that accentuate PAH severity (19). Such treatments rely on exogenous NO and do not address the underlying mechanisms behind PAH. There is promising evidence that stem cell therapy can restore this disbalance. Stem cells induce vasodilation by increasing endogenous levels of NO (Fig. 1) (20). One of the early reports of genetically engineered MSCs involved the use of the eNOS gene. In this study by Kanki-Horimoto et al., MSCs overexpressing the eNOS gene markedly lowered the RV/body weight ratio and elevated endogenous levels of NO in the MCT PAH rat model (9). RV impairment and RV systolic pressure improved significantly as well (9). Importantly, these improvements were more pronounced in rats receiving MSCs overexpressing eNOS than rats receiving MSCs alone (9).
Figure 1. A diagram illustrating the mechanistic effects of stem cell delivery on pulmonary arterial endothelial cell (PAEC) and pulmonary arterial smooth muscle cell (PASMC) dysfunctions in PAH.
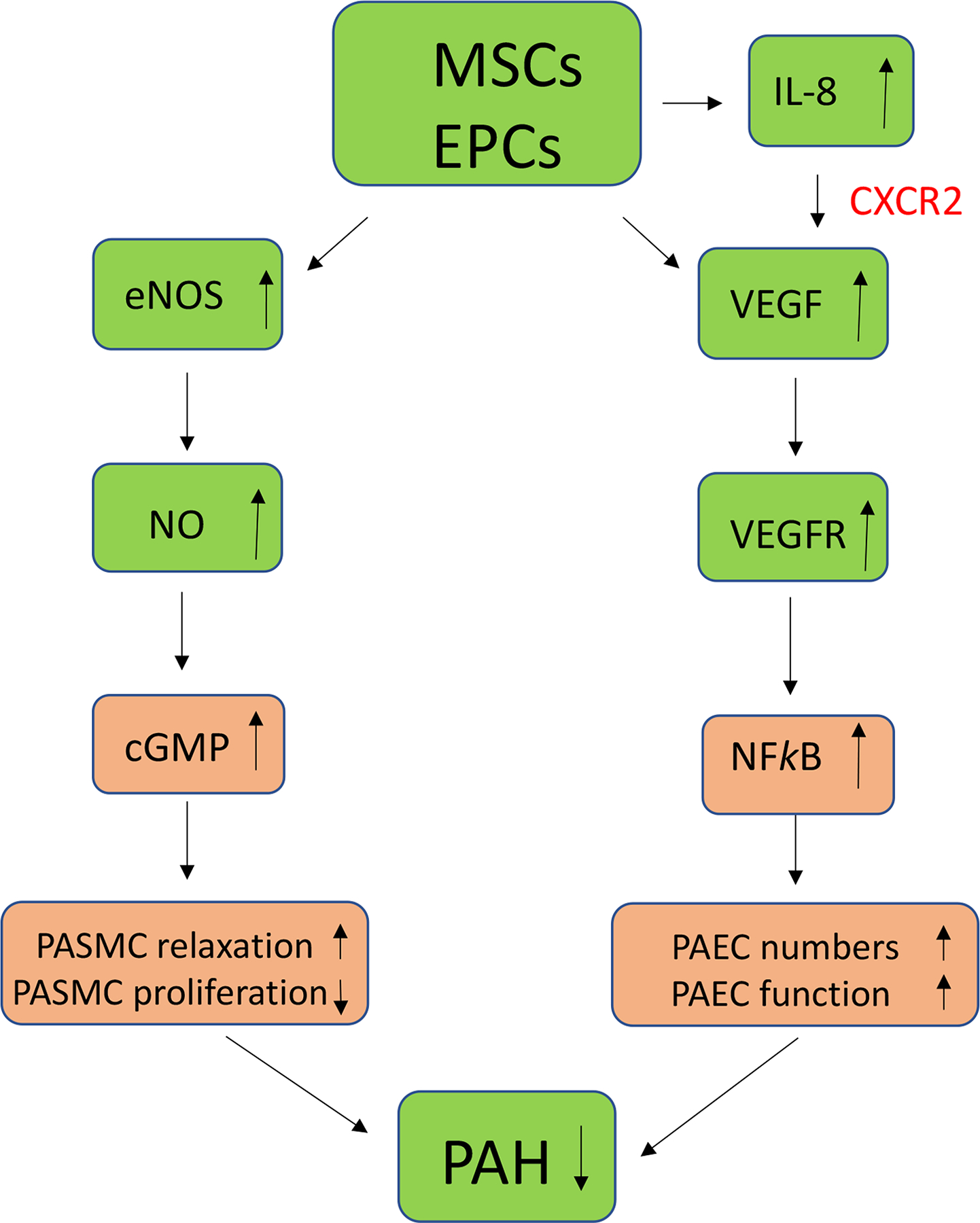
Endothelial progenitor cells (EPCs) secrete vascular endothelial growth factor (VEGF) which promotes PAEC proliferation and function in PAH via the VEGF/VEGFR-NFkB pathway. Mesenchymal stem cells (MSCs) enhance nitric oxide (NO) levels which promote PASMC relaxation and inhibit PASMC proliferation via the NO-cGMP pathway.
Additionally, endothelial cell damage, failure of repair, and compromise of barrier integrity are important components of the endothelial dysfunction in PAH patients (21). A recent study by Varshney et al showed that transfecting MSCs with the secreted Klotho (SKL) gene have protective effects on the pulmonary vascular endothelium (22). MSCs overexpressing SKL (MSC-SKL) upregulated the SIRT1 pathway and restored eNOS levels, with therapeutic effects more pronounced than MCT rats receiving MSCs only (22). Klotho is an anti-aging protein with protective effects on multiple systems(23–26), including endothelial function(27), smooth muscle cell function(28, 29), cardiac function(30–32), and the SIRT1 pathway is a major mediating factor in its protective role (33, 34). Thus, Klotho can enhance the therapeutic potential of stem cells in restoring the endothelial function. These results demonstrate the possibility of safe and efficient cell-based gene therapies utilizing MSCs to deliver specific paracrine factors to a target organ using the homing ability of the MSCs.
EPCs could also be an effective therapeutic to restore endothelial function in PAH patients. These cells have the capacity to circulate, proliferate, and differentiate into mature endothelial cells but have neither acquired mature endothelial markers nor formed a lumen (16). EPCs are suspected to have proliferative potential and play a vital role in vascular regeneration by replacing or restoring damaged endothelial cells (35). EPCs can secrete IL-8 and vascular endothelial growth factor (VEGF) that enhance the regeneration of endothelial cells (Fig. 1) (36). IL8 stimulates vascular VEGF expression and the autocrine activation of VEGF receptor 2 (VEGFR2) in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex (Fig. 1). Specifically, the elevated levels of IL-8 activate VEGF through the chemokine CXCR2 receptor (Fig. 1)(37). Conversely, hypoxia induces elevated levels of VEGF that also recruit stem cells for vasculogenesis (38). In the MCT rat model, EPCs were capable of incorporating into the endothelial layer of the pulmonary vessels (39). EPC injection led to return of normal levels of pulmonary arterial blood pressure, RV/LV ratio, perfusion, and muscularization (39). Interestingly, these results were more pronounced when EPCs were transduced with eNOS, increasing survival rates beyond rats which only received EPCs (39). Thus, the transplanted stem cells can also be successfully engrafted within the host’s tissue as means of treating endothelial cell damage in PAH.
Targeting PASMC proliferation and PA remodeling with stem cells
Stem cell treatment can reverse the extracellular matrix modifications caused by vascular remodeling in PAH. It was reported that MSCs injected intravenously reduced vascular muscularity in a hypoxia-induced mouse PAH model (40). The MSCs also restored normal levels of collagen and the receptor for advanced glycation end product (RAGE) protein (40). RAGE expression levels are increased in PAH patients by as much as six times, and its downstream signaling effects include activating the STAT3-BMPR pathway implicated in cell proliferation (Fig. 1) (41). STAT-3 is activated by inflammatory cytokine IL-6 and plays a significant role in the pathogenesis of multiple characteristics of PAH, including downregulation of eNOS expression (42). Bone morphogenetic proteins (BMP), a member of the transforming growth factor beta (TGF-β) family, prohibits PASMC proliferation via activation of BMPR2 signaling (Fig. 2) (43). PAH patients generally present with decreased expression of BMPR2 (17). Recent evidence also shows that inhibiting the RAGE signaling can reduce PASMC proliferation (44). Although collagen may not directly cause PAH, it has become an established biomarker of PAH severity in patients (45). Normalization of collagen levels is thus important for reversing pulmonary vascular remodeling. Human MSC therapy improved pulmonary vascular remodeling and reversed the lowered pulmonary arterial acceleration time/right ventricle ejection time (PAAT/RVET) ratio in PAH mice, suggesting a restoration of pulmonary cardiovascular performance (40).
Figure 2. A diagram illustrating the action of stem cell delivery of peptides on proliferation and apoptosis of pulmonary arterial smooth muscle cells (PASMCs).
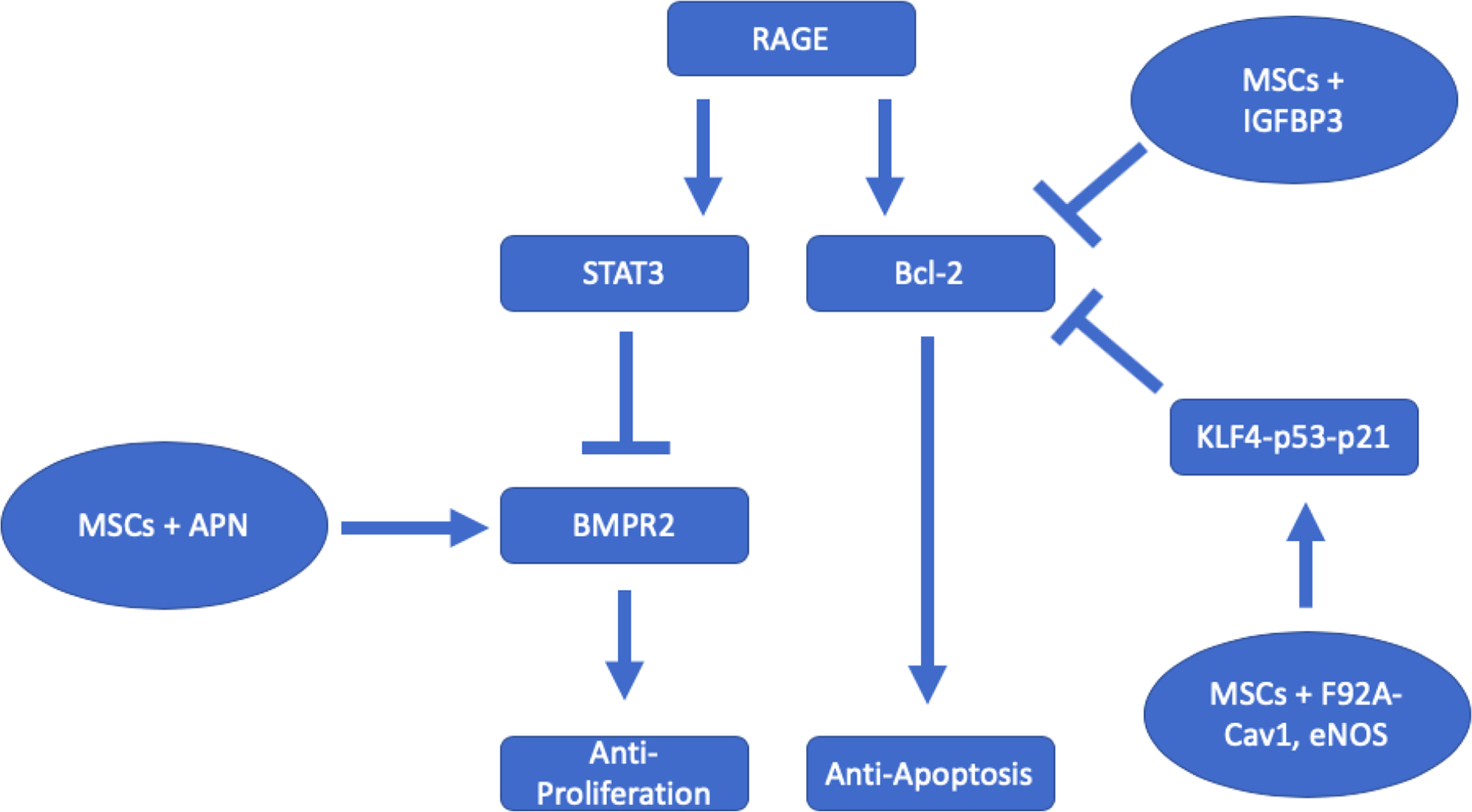
Stem cells transfected with peptides act on the proliferative and apoptotic pathways in PASMCs. Adiponectin (APN) stimulates BMPR2, a receptor that plays a major role in suppressing proliferation but is usually downregulated in PAH patients. Insulin-like growth factor binding protein (IGBFP3) and mutant Caveolin-1 (Cav1) suppress Bcl-2 expression. Bcl-2 prevents cell apoptosis and is upregulated in PAH patients.
A study by Mendonca et al. found that adipose-derived MSCs attenuated vascular remodeling, collagen content, and the endothelial-mesenchymal transition (EMT) in the MCT-induced PAH model (46). The EMT process allows the cell to eventually develop into a smooth muscle cell, so attenuating this process is another important strategy to alleviate PASMC proliferation. Upregulation of inflammatory factors (e.g., IL-6, TNFα, macrophages) is involved in PASMC hyper-proliferation and pulmonary arterial remodeling (47, 48). Pro-inflammatory factors (IL-6), macrophages (CD68+) and anti-apoptosis biomarkers (B-cell lymphoma (Bcl-2) and survivin) were all decreased while expression of the pro-apoptosis factor procaspase-3 was increased as a result of treatment with MSCs (Fig. 2) (46). Bcl-2 expression occurs downstream of RAGE activation (Fig. 1) (49). This finding suggests that RAGE is also involved in cell survival and that MSCs can act on multiple steps of the same pathway involved in the abnormal behavior of PASMCs. Thus, stem cells can modulate underlying factors of both pro-proliferative and anti-apoptotic phenotypes in PASMCs to reverse arterial remodeling.
Transcription factors play important roles in PASMC over-proliferation and PAH. Nuclear factor-κB (NF-κB) and nuclear factor of activated T cells-1 (NFAT-1) participate in inflammation and tissue remodeling. NFAT-1 and NF-κB signaling pathways are upregulated in MCT-induced PAH (50). Downregulation of NAFT-1 and NF-κB due to pharmacological inhibition of inflammation may contribute to attenuation of MCT-induced PAH and PA remodeling (50). Furthermore, RNAi silencing of NFATc2, NFATc3 and NFATc4 attenuates the excessive proliferation and migration and apoptosis resistance in PASMCs isolated from MCT-treated rats (51). RUNX family transcription factor 2 (RUNX2) transcriptionally regulates potential target genes that may promote cell proliferation (52). Hypoxia-inducible factor-1α plays an important role in PASMC proliferation and PA remodeling in PAH (53, 54). Activation of the NOTCH signaling also participates in PASMC proliferation (55, 56).
Another promising target for genetically modified stem cells is adiponectin (APN), a hormone also known to be implicated in inhibiting cell proliferation (57). Adipose-derived stem cells genetically modified with the APN gene effectively reduced SMC proliferation in PAH rats (Fig. 1) (58). APN attenuated PASMC proliferation by acting on the BMPR2 receptor (Fig. 1). The effect of APN on proliferation was decreased by a BMPR2 inhibitor (58). However, the APN’s primary function is to regulate metabolism by acting on the AMPK pathway. This finding is important because it signifies that the AMPK pathway is involved in cell growth, metabolism, survival, and division (59).
Some studies have attempted to modify the stem cell in order to deliver a peptide of interest, and many have successfully reversed vascular remodeling and PAH by inhibiting anti-apoptotic factors in cells. Some of these peptides used in stem cell treatments include the insulin growth factor binding protein-3 (IGFBP-3) and caveolin-1 with an alanine for phenylalanine substitution (F92A-Cav1) (Fig. 1) (60, 61). Although each peptide may act on a different pathway, they all eventually downregulate the bcl-2 protein that is critical to anti-apoptosis (Fig. 1). Cheng et al found that the pro-apoptotic effects of IGFBP-3 in MSCs are due to the downregulation of Bcl-2 (60). In another study, MSCs modified with eNOS and F92A-Cav1 activated the KLF4-p53-p21 pathway (61). Modulating KLF4 directly regulates Bcl-2 expression, suggesting a link between Cav1 and Bcl-2 (62). Many of these peptides themselves have vasodilatory, anti-inflammatory, or other cytoprotective functions, but their similar downstream effects may explain why they all help reverse remodeling in PAH. Thus, modifying stem cells to target such known pathways to prevent anti-apoptosis in cells is a strategic method to aid in treating vascular remodeling. This evidence suggests that peptides that target Bcl-2 or a similarly effective anti-apoptotic downstream protein will help treat vascular remodeling (Fig. 1).
Epigenetic modification of stem cells for the treatment of PAH
The role of epigenetics in PAH is a new area of investigation but has already produced insightful findings that can help develop future therapeutic targets. DNA methylation, histone acetylation, and microRNA dysregulation are all involved in PAH. Histone acetylation plays an important role in pulmonary arterial hypertension (63). It was reported that high altitude long-term hypoxia induces PASMC proliferation and PA remodeling via reduced histone acetylation (64). BRD4 has been identified as a critical epigenetic driver for pulmonary hypertension (65–68). Recent evidence shows that transfecting stem cells with microRNAs can modulate the underlying pathways in PAH by binding to mRNA and altering gene expression. Currently, it is believed that dysregulation of microRNAs miR-17~92, −21, −124, −145, −204 and −210 in PASMCs are involved in PAH and are hypothesized to contribute to cell proliferation (69). Zhu et al. also summarizes a host of microRNAs whose abnormal expression levels act on familiar pathways (70). For example, miR-17~92 propagate the STAT3-BMPR pathway, and miR-145 inhibits BMPR function (70). Another microRNA of interest is let-7a due to its association with cancer and other cell proliferation diseases. Let-7a expression levels are lower in PH patients and negatively correlates with PAH severity (71). MSCs modified with let-7a significantly reduced PASMC resistance to apoptosis, arterial wall thickness, and cell proliferation in the MCT rat model (Fig. 3) (72). Cheng et al. demonstrated that MSC-let-7a treatment lowered STAT-3 expression levels, which led to an increase in bone morphogenic protein receptor (BMPR) receptor levels (Fig. 3) (72). Thus, epigenetic modulation using microRNA in stem cells is another attractive strategy for treating PAH.
Figure 3. A diagram illustrating an epigenetic mechanism by which stem cells regulate pulmonary arterial smooth muscle cell (PASMCs) proliferation in PAH.
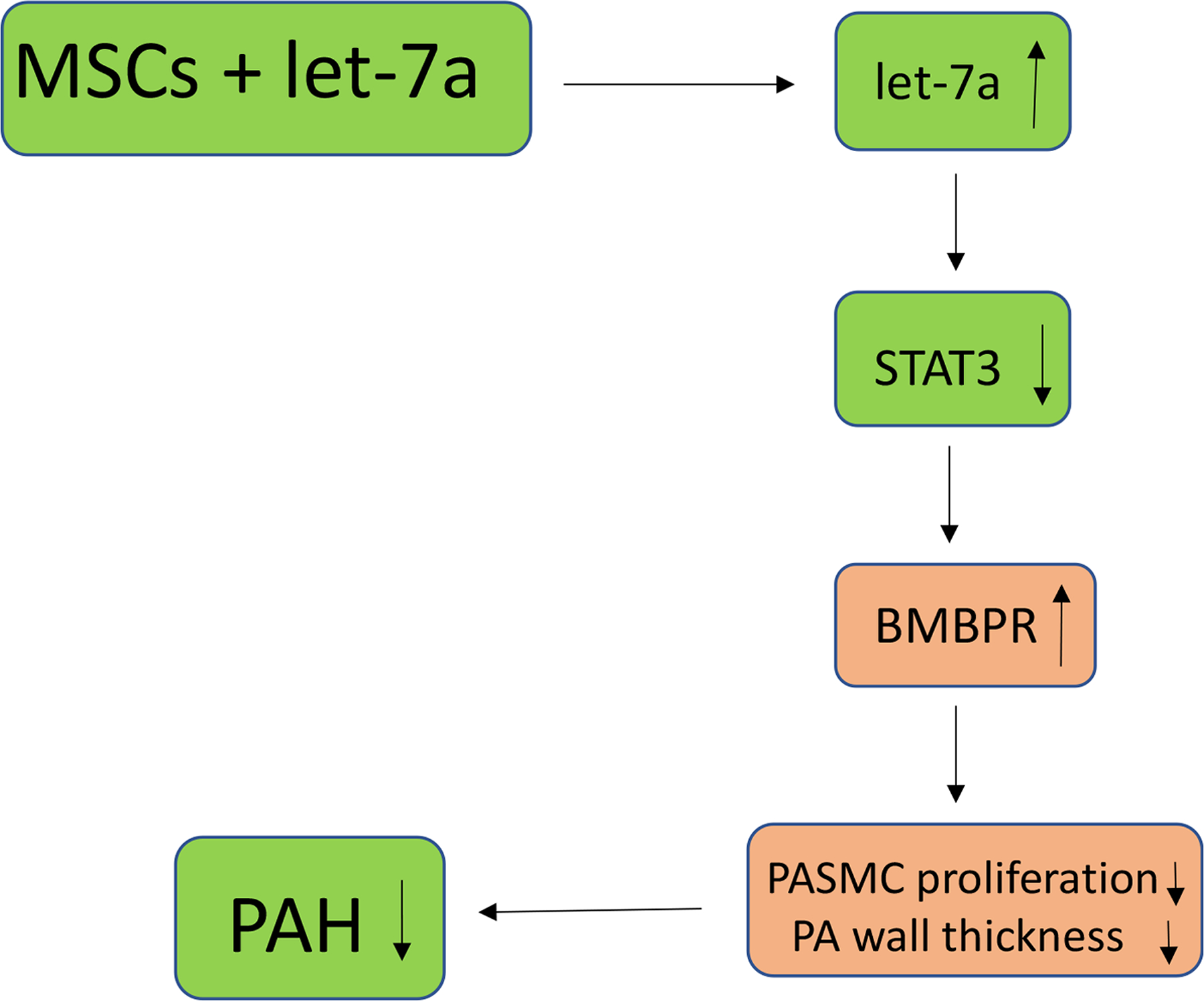
In vivo delivery of mesenchymal stem cells overexpressing microRNA let-7a increases let-7a levels which inhibits PASMC proliferation and attenuates PA wall thickness and PAH via the let-7a-STAT3-BMBPR pathway.
Unfortunately, many of the other microRNAs mentioned still have unknown mechanisms. It is also unknown how microRNAs might interact with or influence genes with distinct DNA methylation and histone acetylation patterns. One particular gene of interest is SOD2, a mitochondrial gene that produces proteins that decompose byproducts of oxidative phosphorylation (73). In PAH patients, hypermethylation of the SOD2 gene silences its expression, resulting in an apoptotic-resistant and enhanced proliferative cell (74). A DNA methyltransferase inhibitor reversed these effects. Overexpression of histone deacetylase (HDAC) genes are associated with PA remodeling (75). Epigenetic mechanisms cannot be ignored when considering stem cell therapy for PAH, and fully understanding how modulation of microRNAs can reverse PAH will be important for further exploring its therapeutic potential.
Regulation of apoptosis by stem cell-derived exosomes: targeting on mitochondrial function in PAH
The mitochondria are often overlooked, but recently, they have emerged as a very potent therapeutic target for reversing pulmonary vascular remodeling. Although PAH is often classified as a cardiovascular disease, it should be also considered a metabolic disease due to its significant disruptions in normal mitochondrial function, adding another dimension to the complexity of the disease that makes current treatments insufficient. Indeed, metabolic function is impaired in PAH. The hypoxia inducible factor (HIF) induces the expression of pyruvate dehydrogenase kinase (PDK) gene (76). This kinase inhibits the pyruvate dehydrogenase complex, preventing metabolites from entering the citric acid cycle and effectively suppressing aerobic metabolism. HIF expression may also account for lower mitochondrial count in endothelial cells (77), suggesting its role in endothelial dysfunction. The altered mitochondrial function, coupled with a reduced supply of oxygen, also results in a decrease in aerobic metabolism to rely on glycolysis and fermentation for energy, which is characterized as the Warburg effect. A recent study demonstrated that MSC-derived exosomes significantly improved mitochondrial function in a rat and mouse model (Fig. 2) (78). The exosome treatment reduced SIRT4 expression, a gene known to inhibit pyruvate dehydrogenase (PDH) and glutamate dehydrogenase, resulting in a return to normal levels of citric acid cycle metabolites and oxygen consumption (Fig. 4) (78). Additionally, the stem cell-derived exosomes decreased PASMC proliferation in an in vitro assay (78).
Figure 4. A diagram showing that stem cell treatment restores normal mitochondrial function via epigenetic mechanisms.
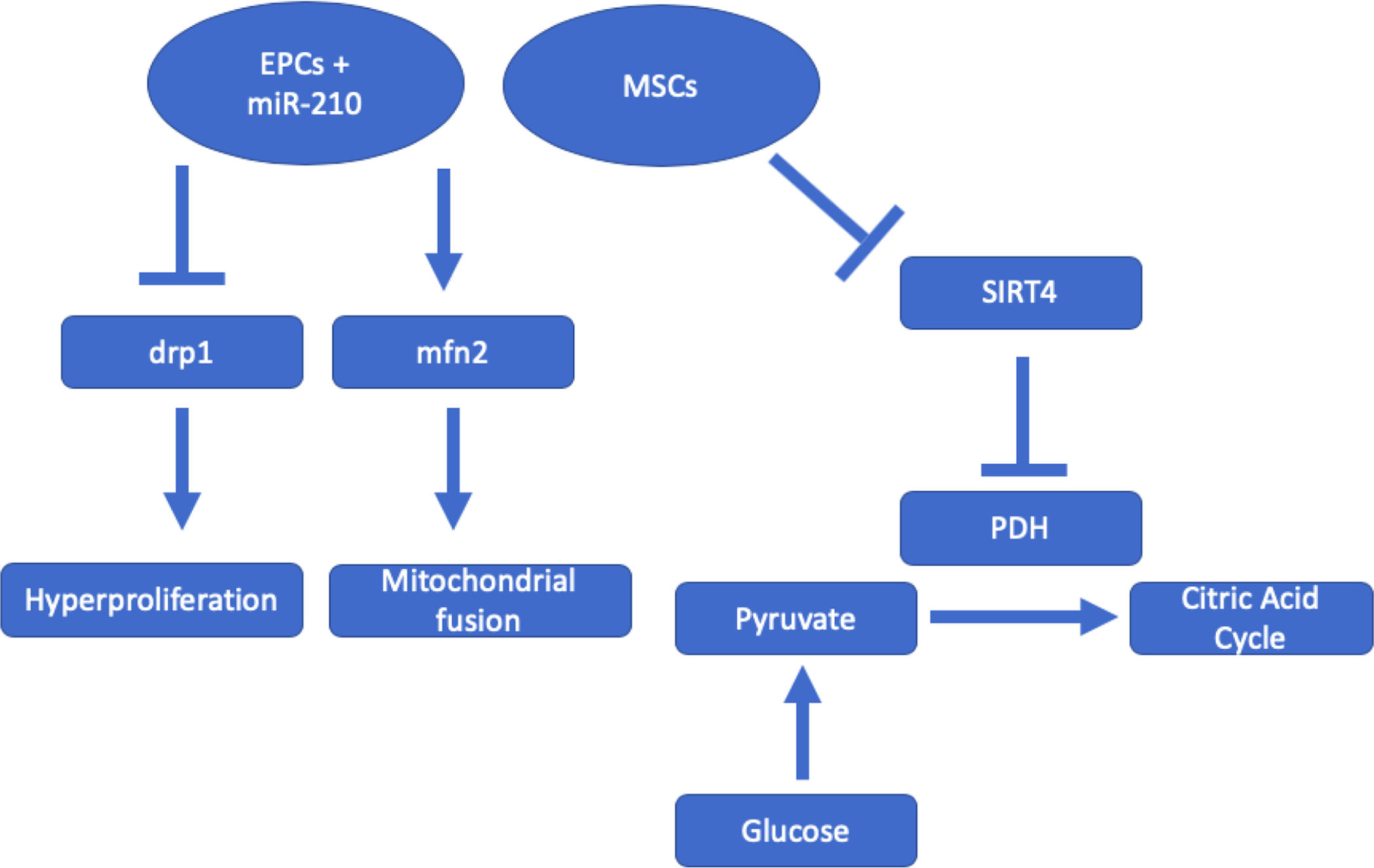
MSCs downregulating the SIRT4 pathway allows the cell to return to aerobic metabolism due to disinhibition of pyruvate dehydrogenase kinase (PDH). SIRT4 is a histone demethylase that regulates PDH expression. EPCs transfected with microRNA-210 attenuate drp1 and increase mfn2, mitochondrial proteins important for hyperproliferation and mitochondrial fusion, respectively.
It is hypothesized that alteration of mitochondrial function may contribute to the apoptosis-resistant characteristic of PASMCs. Apoptosis is usually initiated in the mitochondria. In PAH patients, mitochondria are hyperpolarized which suppresses the mitochondrial function leading to apoptotic resistance (79). EPC-derived exosomes loaded with miR-210 reduced angiogenic dysfunction and elevated ATP levels and protected mitochondrial function (80). MiR-210 EPC-exosomes augmented expression of mitochondrial proteins mitofusin-2 (mfn2) and attenuated expression of dynamin-related protein (drp1) (Fig. 4)(80). Drp1 plays a direct role in mitochondrial division, and its peak expression levels concur with mitosis (81, 82). Drp1 mediates PASMC hyperproliferation in human PAH patients and MCT rats (83). Mfn2 is vital for mitochondrial fusion, and its deficiency in MCT rats leads to mitochondrial fragmentation and PASMC hyperproliferation, whereas mfn2 overexpression reverses these effects (84, 85). Hogan et al. reported that mesenchymal stromal cell-derived exosomes improve mitochondrial health and decreased PASMC proliferation in pulmonary arterial hypertension (78). These results suggest that the stem cell therapies help the cell mitochondria regain its apoptotic function.
The detoxifying DNA enzyme NUDT1 (nudrix hydrolase 1) expression levels were increased in cells and tissues from PAH patients and animal models. Upregulation of NUDT1 may hijack persistent oxidative stress which prevents incorporation of oxidized nucleotides into DNA, thus allowing the cell to escape apoptosis and proliferate in PAH-PASMCs (86). Pharmacological inhibition of NUDT1 attenuated pulmonary vascular remodeling and improved pulmonary hemodynamics (86). Under metabolic stress conditions, HSP90 accumulates in PAH-PASMC mitochondria to ensure cell survival by maintaining mitochondrial DNA integrity and bioenergetic capabilities (87–89). Selective inhibition of mitochondrial HSP90 decreased mitochondrial DNA content and repair capacity and bioenergetic functions which suppress PAH-PASMC proliferation and resistance to apoptosis, and thus attenuating PAH and PA remodeling (87). It would be interesting to explore whether stem cell-derived exosomes loaded with NUDT1 and HSP90 siRNAs could improve PAH.
Therapeutic potential of stem cell-derived extracellular vesicles and materials for PAH
One of the major concerns against using stem cell therapy is the potential for graft VS host complications after transplants. However, recent studies demonstrated, stem cells can still offer therapeutic benefits via paracrine signaling without the risk of graft VS host. The works of Hogan et al. and Ma et al. demonstrate the potential uses of stem cell-derived exosomes in treating PAH (78),(80). Exosomes and other materials are packed in stem cells by the invagination of the cell membrane to form a micro vesicle. The vesicle is transported out and fuses with the membrane of the target cell, allowing the vesicle’s contents to enter the cell.
Growing evidence supports that the extracellular vesicles (EVs) derived from stem cells can treat hypertrophy, vascular remodeling, inflammation, and hypertension associated with PAH (90). Stem cell-derived EVs often act on pathways commonly associated with PAH. MSC-derived EVs can effectively upregulate the AMPK pathway and increase eNOS expression via the SIRT1 pathway (91). MSC-derived EVs reverse Sugen/hypoxia pulmonary hypertension (92). Lee et al. found that the MSC-derived exosomes can decrease levels of IL-6, which effectively suppressed the STAT3 signaling (93). Treatment with MSC-derived exosomes blunts hypoxia-associated inflammation and alters the hyperoxic lung transcriptome which alleviates pulmonary vascular remodeling and hypertension (94). The mechanistic action of MSC-exosomes may be associated with modulation of lung macrophage phenotype (94). The MSC exosomes were more effective than the fibroblast vesicles. Additionally, these exosomes suppressed miR-17 expression, another microRNA believed to be involved in cell proliferation (93, 95). The use of stem cell exosomes loaded with microRNAs maximizes the benefits of stem cell therapy by selectively modulating gene expression. Aliotta et al. found that stem cell exosome microRNA cargo can determine its therapeutic effects (96). Making this potential a reality will require a precise understanding of all microRNAs implicated in the pathogenesis of PAH. The idea of using stem cell exosomes for therapy via paracrine signaling itself is also a relatively new area of study, but one that is already yielding promising results. There is a need for additional studies involving stem cell exosomes transfected with microRNAs.
Stem cells also secreted some paracrine factors that can regulate pulmonary vascular function. Delivery of MSCs overexpressing secreted Klotho (SKL) gene abolished MCT-induced pulmonary vascular endothelial dysfunction and PA remodeling (22). This beneficial effect is mediated by secreted Klotho released from MSCs (22).
Impact of stem cell therapies on RV failure
Stem cell therapies attenuate right ventricular (RV) hypertrophy and ameliorates RV failure which may be partially due to attenuation of PAH. Recent studies showed that stem cell therapies may also have direct beneficial effect in the RV myocardium in PAH (97). RV angiogenesis is impaired in PAH leading to decompensated RV failure (98–101). Therefore, targeting RV angiogenesis may be a potential therapeutic approach for RV failure in PAH. Smits et al demonstrated that transplantation of highly proliferative endothelial colony forming cells (ECFC) restored myocardial vascular density in PAH rats (98), suggesting improved angiogenesis. It was reported that transplantation of autologous umbilical cord blood mononuclear cells preserves RV function in a rodent model of chronic RV volume overload (102). Intramyocardial delivery of human umbilical cord blood mononuclear cells improved murine RV structure and function, increased angiogenesis, and attenuated RV fibrosis in chronic RV pressure overload (103). Mesenchymal stromal cells can improve RV hypertrophy, RV ejection fraction, and RV systolic pressure (5, 104). Administration of EPCs reduces RV systolic pressure and RV hypertrophy in PAH(105, 106) and more effectively attenuates PAH progression when combined with conventional pharmacological therapies for PAH (107). Adipose-derived stem cells (ADSCs) can promote angiogenesis via differentiation into endothelial cells in the newly formed vascular walls and via secretion of paracrine factors (97). In addition, delivery of ADSCs decreased RV hypertrophy and RV function in PAH (108, 109). Transplantation of induced pluripotent stem cells (iPS) can generate cardiomyocytes which integrate into host myocardium and formed sarcomeric structures and effectively improve LV function and angiogenesis and attenuate RV hypertrophy (110).
Beneficial effects of stem cell therapies on systemic vascular dysfunction in PAH
Recent evidence indicates that PAH patients also showed systemic vascular dysfunction as manifested by impaired brachial artery flow-mediated dilation, abnormal cerebral blood flow, skeletal myopathy, and coronary arterial dysfunction (111–114). Capillary rarefaction within the skeletal muscle may contribute to skeletal muscle dysfunction and poor exercise tolerance through impaired muscle oxygen supply in PAH (112, 113, 115). Although some of the systemic vascular anomalies in PAH are partially due to right ventricular and pulmonary insufficiency, recent data support the concept that systemic vascular dysfunction and PAH may share some common genetic and molecular mechanisms (111). While the improvement in skeletal muscle and coronary function by stem cell therapy may be partly secondary to its therapeutic effects on PAH and RV failure, stem cells may also directly ameliorate skeletal and coronary vascular function. It should be mentioned that stem cell therapy for PAH and systemic vascular diseases were investigated separately in most studies. However, there is no doubt that systemic delivery of stem cells could directly benefit both systemic and pulmonary circulation. For example, EPCs could improve PA dysfunction and PAH (16) but EPCs also can ameliorate systemic vascular dysfunction and contribute to restoring blood supply and tissue regeneration after ischemic injury (116–118). EPCs tend to mobilize to the injured vascular sites for vascular repair and angiogenesis (116–118) without noticeable selectivity to pulmonary or systemic vessels. Further studies are needed to delineate effects of stem cell therapy on pulmonary and systemic vascular dysfunctions simultaneously in PAH.
Clinical studies on stem cell therapy for PAH
The promising preclinical data on the impressive therapeutic effects of EPCs on experimental PAH motivated several clinical trials. Wang et al performed a prospective, randomized trial to determine the effects of transplantation of autologous EPCs on idiopathic pulmonary arterial hypertension on IPAH (16). They reported that the 6-minute walking distance increased by 48.2 m in IPAH patients at 12 weeks after EPC transplantation. In contrast, the walking distance of the conventional treatment group only increased by 5.7 m. EPC transplantation significantly improved mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac output (16). No obvious adverse effects were found following EPC transplantation. This clinical trial suggests that intravenous infusion of autologous EPCs is feasible and safe and has beneficial effects on exercise capacity and pulmonary hemodynamics in IPAH patients (16).
In the pulmonary hypertension and angiogenic cell therapy (PHACeT) trial, Granton et al investigated the effects of culture-derived EPCs transfected with endothelial nitric oxide synthase (eNOS) gene in PAH patients who are refractory to conventional PAH-specific treatments (119). They reported that infusion of the EPCs carrying eNOS gene was not associated with obvious adverse hemodynamic effects. This trial demonstrated that EPCs tended to improve the total pulmonary resistance in PAH patients during the 3-day delivery period. The 6-minute walking distance was largely increased at 1, 3, and 6 months after EPC delivery. Unfortunately, no sustained hemodynamic improvements were observed at 3 months. This clinical study suggests that delivery of EPCs overexpressing eNOS was well tolerated in PAH patients. The short-term hemodynamic improvement was followed by long-term benefits in physical performance. Nevertheless, additional clinical trials are needed to further validate the efficacy and safety of delivery of EPCs overexpressing eNOS in PAH.
In an open label study, Zhu et al tested the effects of transplantation of autologous EPCs on exercise capacity and pulmonary hemodynamics in children with IPAH (120). This study also showed that transplantation of autologous EPCS is feasible and safe. This study suggested that intravenous infusion of autologous EPCs significantly improved the exercise tolerance and pulmonary hemodynamics in children with IPAH. However, this is a small pilot trial with only a limited number of participants. Additional well-controlled and randomized clinical trials are needed to validate these findings in children with IPAH.
Potential limitations and challenges
While initial studies supporting EPC therapy in PAH patients demonstrated much promise, in-depth research has accumulated evidence for caution. The regeneration capacity of EPCs is reduced in prehypertensive or hypertensive patients (121). In this study, the repair capacity of EPCs was determined by EPCs isolated from subjects. They found that EPCs from prehypertensive and hypertensive subjects produced less nitric oxide (NO) and shortened telomere length, indicating increased EPC senescence (121). This finding could suggest that stem cell therapy may be less effective for certain patients. Additionally, the variation in potency of different donors’ stem cells could create the need to screen potential donors for such complications that compromise stem cell repair capacity. More studies elucidating these limitations of stem cell capacity are needed before its use as a treatment for PAH in patients.
Upon further examination, EPCs were reported to be of two distinct populations: 1) endothelial-like culture modified monocytes (E-CMMs) with no proliferative potential and 2) late-outgrowth EPCs (L-EPCs) known to display a strong endothelial phenotype and proliferative potential (122). In fact, early studies demonstrating the therapeutic potential of EPCs used cells conforming to the description of E-CMMs and suggested that only a small subset of these cells were late EPCs and acted as true progenitors (122). Additional evidence against the clinical use of EPC therapy includes the reported development of PAH as a complication to allogeneic bone marrow-derived stem cell transplantation (123). More recently, EPCs were demonstrated to inhibit apoptosis of pulmonary endothelial cells via VEGF A/B, stromal derived factor-1, and nitric oxide, which could partially mediate the therapeutic benefits of EPC therapy in PAH patients (124).
Despite our current limited understanding of EPCs and their biology, the fact remains that there is great potential for developing EPCs into effective therapeutics. Many questions remain before therapy can be applied to PAH patients. As our understanding of EPC biology and mechanisms of action grow, so will the potential for applying the therapy to not only PAH patients but also other disorders where vascular dysfunction plays a role. While most therapies involving bone marrow-derived stem cells target endothelial function, more recent cell-based therapy with genes engineered to reduce SMC proliferation and growth would be worth exploring. When working with adult stem cells however, universal challenges remain that need to be addressed prior to its use in patients including how to isolate pure cell populations in sufficient quantities and the self-renewal abilities of the isolated cells. Nevertheless, these challenges do not take away from its promising value.
Stem cell-derived EVs have shown promising therapeutic potential for PAH. It can also avoid complications associated with stem cell therapy. Engineering EVs could further enhance their therapeutic potential. However, EVs may be decomposed quickly by target cells resulting in a short half-life. Thus, a limitation of EV therapy is that continuous doses of EVs are required for achieving stable therapeutic effects. EVs do not home specifically to the injury site as stem cells do.
Translational Perspectives
Our understanding of the underlying mechanisms of PAH has grown rapidly over the last several years, which have allowed for improvements in the clinical management of this disease. Despite these current treatments, there is a continual need to develop even more effective therapies. This review demonstrates that stem cells hold promising potential to modulate molecular pathways, restore normal mitochondrial function, improve pulmonary vascular endothelial dysfunction, mitigate cell over-proliferation, and reverse pulmonary arterial remodeling and PAH. However, these recent findings should be treated with cautious optimism. The area of stem cell-derived exosomes could prevent graft VS host complications, but evidence on its usage and efficacy is still relatively limited. Patient-dependent responses may lead to varied therapeutic effects of stem cells. More rigorous and well-controlled human clinical trials are needed to replicate many of the exciting findings from animal models. A pilot randomized controlled trial demonsrated that transplantation of autologous endothelial progenitor cells showed remarkable ability to decrease vascular resistance in patients with IPAH (16). Stem cells engineered with therapeutic genes hold promise for the treatment of PAH patients with dysregulation of specific genes. The use of autologous stem cells would minimize the rejection responses upon transplantation. In some cases, a combination of both stem cell therapy and other forms of therapy may help achieve synergistic therapeutic effects on PAH (125). Overall, a wide body of findings warrant further development of stem cell therapy for PAH.
Source of Funding
This work was supported by NIH R01 HL154147, HL116863, AG062375, and AG049780.
Abbreviations;
- PAH
Pulmonary Arterial Hypertension
- PASMC
Pulmonary Artery Smooth Muscle Cells
- MSCs
Mesenchymal Stem Cells
- EPCs
Endothelial Progenitor Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None of the authors have any competing interests in the manuscript.
Ethics approval and consent to participate
The experiment was approved by the University of Oklahoma Health Sciences Center.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Naeije R, Manes A: The right ventricle in pulmonary arterial hypertension. European Respiratory Review 2014;23:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crosswhite P, Sun Z: Molecular mechanisms of pulmonary arterial remodeling. Molecular Medicine 2014;20:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. : Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–9. [DOI] [PubMed] [Google Scholar]
- 4.van der Laarse A, Cobbaert CM, Umar S: Stem and progenitor cell therapy for pulmonary arterial hypertension: effects on the right ventricle (2013 Grover Conference Series). Pulm Circ 2015;5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umar S, de Visser YP, Steendijk P, et al. : Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;297:H1606–16. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Morse JH, Slager SL, et al. : Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T: Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic research in cardiology 2010;105:409–17. [DOI] [PubMed] [Google Scholar]
- 8.Archer S, Rich S: Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 2000;102:2781–91. [DOI] [PubMed] [Google Scholar]
- 9.Kanki-Horimoto S, Horimoto H, Mieno S, et al. : Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 2006;114:I181–5. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y: Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng 2004;10:771–9. [DOI] [PubMed] [Google Scholar]
- 11.Baksh D, Song L, Tuan RS: Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 2004;8:301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asahara T, Murohara T, Sullivan A, et al. : Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7. [DOI] [PubMed] [Google Scholar]
- 13.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C: Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008;102:1073–9. [DOI] [PubMed] [Google Scholar]
- 14.Nagaya N, Kangawa K, Kanda M, et al. : Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003;108:889–95. [DOI] [PubMed] [Google Scholar]
- 15.Dewachter L, Dewachter C, Naeije R: New therapies for pulmonary arterial hypertension: an update on current bench to bedside translation. Expert Opin Investig Drugs 2010;19:469–88. [DOI] [PubMed] [Google Scholar]
- 16.Wang XX, Zhang FR, Shang YP, et al. : Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–71. [DOI] [PubMed] [Google Scholar]
- 17.Crosswhite P, Sun Z: Molecular mechanisms of pulmonary arterial remodeling. Mol Med 2014;20:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosswhite P, Sun Z: Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. Journal of Hypertension 2010;28:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavoie A, Hall JB, Olson DM, Wylam ME: Life-threatening effects of discontinuing inhaled nitric oxide in severe respiratory failure. Am J Respir Crit Care Med 1996;153:1985–7. [DOI] [PubMed] [Google Scholar]
- 20.You D, Waeckel L, Ebrahimian TG, et al. : Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation 2006;114:328–38. [DOI] [PubMed] [Google Scholar]
- 21.Toshner M, Voswinckel R, Southwood M, et al. : Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. American journal of respiratory and critical care medicine 2009;180:780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varshney R, Ali Q, Wu C, Sun Z: Monocrotaline-Induced Pulmonary Hypertension Involves Downregulation of Antiaging Protein Klotho and eNOS Activity. Hypertension 2016;68:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Sun Z: Molecular basis of Klotho: from gene to function in aging. Endocr Rev 2015;36:174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z: Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ Res 2021;128:492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Fan J, Wang S, Sun Z: Secreted Klotho Attenuates Inflammation-Associated Aortic Valve Fibrosis in Senescence-Accelerated Mice P1. Hypertension 2018;71:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao D, Wang S, Lin Y, Sun Z: In vivo AAV delivery of glutathione reductase gene attenuates anti-aging gene klotho deficiency-induced kidney damage. Redox Biol 2020;37:101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Chen J, Sun Z: Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension 2016;67:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Sun Z: Klotho deficiency-induced arterial calcification involves osteoblastic transition of VSMCs and activation of BMP signaling. J Cell Physiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Wang S, Sun Z: Kidney-Specific Klotho Gene Deletion Causes Aortic Aneurysm via Hyperphosphatemia. Hypertension 2021;78:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K, Sun Z: Estrogen inhibits renal Na-Pi Co-transporters and improves klotho deficiency-induced acute heart failure. Redox Biol 2021;47:102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Wang S, Sun Z: In Vivo Cardiac-specific Expression of Adenylyl Cyclase 4 Gene Protects against Klotho Deficiency-induced Heart Failure. Transl Res 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Zhang B, Sun Z: MicroRNA 379 Regulates Klotho Deficiency-Induced Cardiomyocyte Apoptosis Via Repression of Smurf1. Hypertension 2021;78:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Sun Z: Current Understanding of Klotho. Ageing research reviews 2008;8:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao D, Zuo Z, Tian J, et al. : Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension (Dallas, Tex : 1979) 2016;68:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barst R: Is it possible to reverse the endothelial dysfunction in pulmonary arterial hypertension? Journal of the American College of Cardiology 2007;49:1572–4. [DOI] [PubMed] [Google Scholar]
- 36.Yoon CH, Hur J, Park KW, et al. : Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005;112:1618–27. [DOI] [PubMed] [Google Scholar]
- 37.Martin D, Galisteo R, Gutkind JS: CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 2009;284:6038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Sharpe EE, Maupin AB, et al. : VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. Faseb j 2006;20:1495–7. [DOI] [PubMed] [Google Scholar]
- 39.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ: Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–50. [DOI] [PubMed] [Google Scholar]
- 40.Alencar AKN, Pimentel-Coelho PM, Montes GC, et al. : Human Mesenchymal Stem Cell Therapy Reverses Su5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Mice. Front Pharmacol 2018;9:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meloche J, Courchesne A, Barrier M, et al. : Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2013;2:e005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulin R, Meloche J, Bonnet S: STAT3 signaling in pulmonary arterial hypertension. JAKSTAT 2012;1:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Fantozzi I, Tigno DD, et al. : Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L740–54. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Sakaguchi M, Matsubara H, et al. : Crucial role of RAGE in inappropriate increase of smooth muscle cells from patients with pulmonary arterial hypertension. PLoS One 2018;13:e0203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safdar Z, Tamez E, Chan W, et al. : Circulating collagen biomarkers as indicators of disease severity in pulmonary arterial hypertension. JACC Heart Fail 2014;2:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Mendonça L, Felix NS, Blanco NG, et al. : Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res Ther 2017;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crosswhite P, Chen K, Sun Z: AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension 2014;64:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crosswhite P, Sun Z: Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 2010;55:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H: Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 2000;275:40096–105. [DOI] [PubMed] [Google Scholar]
- 50.Bai Y, Li ZX, Wang HL, Lian GC, Wang Y: The protective effects of PCPA against monocrotaline-induced pulmonary arterial hypertension are mediated through the downregulation of NFAT-1 and NF-κB. Int J Mol Med 2017;40:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He RL, Wu ZJ, Liu XR, Gui LX, Wang RX, Lin MJ: Calcineurin/NFAT Signaling Modulates Pulmonary Artery Smooth Muscle Cell Proliferation, Migration and Apoptosis in Monocrotaline-Induced Pulmonary Arterial Hypertension Rats. Cell Physiol Biochem 2018;49:172–89. [DOI] [PubMed] [Google Scholar]
- 52.Yang DP, Huang WY, Chen G, et al. : Clinical significance of transcription factor RUNX2 in lung adenocarcinoma and its latent transcriptional regulating mechanism. Comput Biol Chem 2020;89:107383. [DOI] [PubMed] [Google Scholar]
- 53.Han XJ, Zhang WF, Wang Q, et al. : HIF-1α promotes the proliferation and migration of pulmonary arterial smooth muscle cells via activation of Cx43. J Cell Mol Med 2021;25:10663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, He H, Cui Y, et al. : Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell Signal 2021;87:110140. [DOI] [PubMed] [Google Scholar]
- 55.Smith KA, Voiriot G, Tang H, et al. : Notch Activation of Ca(2+) Signaling in the Development of Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension. Am J Respir Cell Mol Biol 2015;53:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao J, Fang X, Zhang C, et al. : Astragaloside IV attenuates hypoxia- induced pulmonary vascular remodeling via the Notch signaling pathway. Mol Med Rep 2021;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Lam KS, Xu JY, et al. : Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 2005;280:18341–7. [DOI] [PubMed] [Google Scholar]
- 58.Luo L, Zheng W, Lian G, et al. : Combination treatment of adipose-derived stem cells and adiponectin attenuates pulmonary arterial hypertension in rats by inhibiting pulmonary arterial smooth muscle cell proliferation and regulating the AMPK/BMP/Smad pathway. Int J Mol Med 2018;41:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mihaylova MM, Shaw RJ: The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng GS, Zhang YS, Zhang TT, He L, Wang XY: Bone marrow-derived mesenchymal stem cells modified with IGFBP-3 inhibit the proliferation of pulmonary artery smooth muscle cells. Int J Mol Med 2017;39:223–30. [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Yang H, Yue H, et al. : Mesenchymal Stem Cells Expressing eNOS and a Cav1 Mutant Inhibit Vascular Smooth Muscle Cell Proliferation in a Rat Model of Pulmonary Hypertension. Heart, Lung and Circulation 2017;26:509–18. [DOI] [PubMed] [Google Scholar]
- 62.Mohan N, Ai W, Chakrabarti M, Banik NL, Ray SK: KLF4 overexpression and apigenin treatment down regulated anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK-N-DZ and IMR-32 cells. Molecular Oncology 2013;7:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chelladurai P, Boucherat O, Stenmark K, et al. : Targeting histone acetylation in pulmonary hypertension and right ventricular hypertrophy. Br J Pharmacol 2021;178:54–71. [DOI] [PubMed] [Google Scholar]
- 64.Yang Q, Lu Z, Ramchandran R, Longo LD, Raj JU: Pulmonary artery smooth muscle cell proliferation and migration in fetal lambs acclimatized to high-altitude long-term hypoxia: role of histone acetylation. Am J Physiol Lung Cell Mol Physiol 2012;303:L1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dutta P, Gomez D, Gladwin MT: Do BRD(4)S of a Feather Flock Together? How an Inflammation-Driven Epigenetic Regulator May Link Pulmonary Hypertension and Coronary Artery Disease. Arterioscler Thromb Vasc Biol 2017;37:1428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin S, Du L: The therapeutic potential of BRD4 in cardiovascular disease. Hypertens Res 2020;43:1006–14. [DOI] [PubMed] [Google Scholar]
- 67.Meloche J, Potus F, Vaillancourt M, et al. : Bromodomain-Containing Protein 4: The Epigenetic Origin of Pulmonary Arterial Hypertension. Circ Res 2015;117:525–35. [DOI] [PubMed] [Google Scholar]
- 68.Van der Feen DE, Kurakula K, Tremblay E, et al. : Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2019;200:910–20. [DOI] [PubMed] [Google Scholar]
- 69.Zhou G, Chen T, Raj JU: MicroRNAs in pulmonary arterial hypertension. American journal of respiratory cell and molecular biology 2015;52:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Z, Fang Z, Hu X, Zhou S: MicroRNAs and mesenchymal stem cells: hope for pulmonary hypertension. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular 2015;30:380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izumiya Y, Jinnn M, Kimura Y, et al. : Expression of Let-7 family microRNAs in skin correlates negatively with severity of pulmonary hypertension in patients with systemic scleroderma. Int J Cardiol Heart Vasc 2015;8:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng G, Wang X, Li Y, He L: Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling. Stem Cell Res Ther 2017;8:34–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flynn JM, Melov S: SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radical Biology and Medicine 2013;62:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Archer SL, Marsboom G, Kim GH, et al. : Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 2010;121:2661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng X, Wang Y, Du L: Epigenetic Modulation in the Initiation and Progression of Pulmonary Hypertension. Hypertension 2019;74:733–9. [DOI] [PubMed] [Google Scholar]
- 76.Archer SL, Weir EK, Wilkins MR: Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fijalkowska I, Xu W, Comhair SAA, et al. : Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 2010;176:1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hogan SE, Rodriguez Salazar MP, Cheadle J, et al. : Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2019;316:L723–l37. [DOI] [PubMed] [Google Scholar]
- 79.Paulin R, Michelakis ED: The metabolic theory of pulmonary arterial hypertension. Circ Res 2014;115:148–64. [DOI] [PubMed] [Google Scholar]
- 80.Ma X, Wang J, Li J, et al. : Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function. Cell Physiol Biochem 2018;46:664–75. [DOI] [PubMed] [Google Scholar]
- 81.Peña-Blanco A, Haschka MD, Jenner A, et al. : Drp1 modulates mitochondrial stress responses to mitotic arrest. Cell Death & Differentiation 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smirnova E, Griparic L, Shurland DL, van der Bliek AM: Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001;12:2245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marsboom G, Toth PT, Ryan JJ, et al. : Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 2012;110:1484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan JJ, Marsboom G, Fang Y-H, et al. : PGC1α-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. American journal of respiratory and critical care medicine 2013;187:865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC: Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 2003;160:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vitry G, Paulin R, Grobs Y, et al. : Oxidized DNA Precursors Cleanup by NUDT1 Contributes to Vascular Remodeling in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2021;203:614–27. [DOI] [PubMed] [Google Scholar]
- 87.Boucherat O, Peterlini T, Bourgeois A, et al. : Mitochondrial HSP90 Accumulation Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2018;198:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu L, Zhao R, Liu Q, Li Q: New Insights Into Heat Shock Protein 90 in the Pathogenesis of Pulmonary Arterial Hypertension. Frontiers in physiology 2020;11:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malikova E, Kmecova Z, Doka G, et al. : Pioglitazone restores phosphorylation of downregulated caveolin-1 in right ventricle of monocrotaline-induced pulmonary hypertension. Clin Exp Hypertens 2022;44:101–12. [DOI] [PubMed] [Google Scholar]
- 90.Worthington EN, Hagood JS: Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. International journal of molecular sciences 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z: Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. Journal of Extracellular Vesicles 2020;9:1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klinger JR, Pereira M, Del Tatto M, et al. : Mesenchymal Stem Cell Extracellular Vesicles Reverse Sugen/Hypoxia Pulmonary Hypertension in Rats. Am J Respir Cell Mol Biol 2020;62:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee C, Mitsialis SA, Aslam M, et al. : Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012;126:2601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willis GR, Fernandez-Gonzalez A, Anastas J, et al. : Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med 2018;197:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ventura A, Young AG, Winslow MM, et al. : Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008;132:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aliotta JM, Pereira M, Wen S, et al. : Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res 2016;110:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loisel F, Provost B, Haddad F, et al. : Stem cell therapy targeting the right ventricle in pulmonary arterial hypertension: is it a potential avenue of therapy? Pulm Circ 2018;8:2045893218755979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smits J, Tasev D, Andersen S, et al. : Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez-Arroyo J, Sandoval J, Simon MA, Dominguez-Cano E, Voelkel NF, Bogaard HJ: Treatment for pulmonary arterial hypertension-associated right ventricular dysfunction. Ann Am Thorac Soc 2014;11:1101–15. [DOI] [PubMed] [Google Scholar]
- 100.Potus F, Hindmarch CCT, Dunham-Snary KJ, Stafford J, Archer SL: Transcriptomic Signature of Right Ventricular Failure in Experimental Pulmonary Arterial Hypertension: Deep Sequencing Demonstrates Mitochondrial, Fibrotic, Inflammatory and Angiogenic Abnormalities. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Potus F, Ruffenach G, Dahou A, et al. : Downregulation of MicroRNA-126 Contributes to the Failing Right Ventricle in Pulmonary Arterial Hypertension. Circulation 2015;132:932–43. [DOI] [PubMed] [Google Scholar]
- 102.Yerebakan C, Sandica E, Prietz S, et al. : Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transplant 2009;18:855–68. [DOI] [PubMed] [Google Scholar]
- 103.Oommen S, Yamada S, Cantero Peral S, et al. : Human umbilical cord blood-derived mononuclear cells improve murine ventricular function upon intramyocardial delivery in right ventricular chronic pressure overload. Stem Cell Res Ther 2015;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luan Y, Zhang X, Kong F, Cheng GH, Qi TG, Zhang ZH: Mesenchymal stem cell prevention of vascular remodeling in high flow-induced pulmonary hypertension through a paracrine mechanism. Int Immunopharmacol 2012;14:432–7. [DOI] [PubMed] [Google Scholar]
- 105.Yip HK, Chang LT, Sun CK, et al. : Autologous transplantation of bone marrow-derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit Care Med 2008;36:873–80. [DOI] [PubMed] [Google Scholar]
- 106.Ormiston ML, Deng Y, Stewart DJ, Courtman DW: Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol 2010;43:546–54. [DOI] [PubMed] [Google Scholar]
- 107.Yen CH, Tsai TH, Leu S, et al. : Sildenafil improves long-term effect of endothelial progenitor cell-based treatment for monocrotaline-induced rat pulmonary arterial hypertension. Cytotherapy 2013;15:209–23. [DOI] [PubMed] [Google Scholar]
- 108.Luo L, Lin T, Zheng S, et al. : Adipose-derived stem cells attenuate pulmonary arterial hypertension and ameliorate pulmonary arterial remodeling in monocrotaline-induced pulmonary hypertensive rats. Clin Exp Hypertens 2015;37:241–8. [DOI] [PubMed] [Google Scholar]
- 109.Somanna NK, Wörner PM, Murthy SN, et al. : Intratracheal administration of cyclooxygenase-1-transduced adipose tissue-derived stem cells ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 2014;307:H1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang WC, Ke MW, Cheng CC, et al. : Therapeutic Benefits of Induced Pluripotent Stem Cells in Monocrotaline-Induced Pulmonary Arterial Hypertension. PLoS One 2016;11:e0142476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nickel NP, Yuan K, Dorfmuller P, et al. : Beyond the Lungs: Systemic Manifestations of Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2020;201:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malenfant S, Potus F, Fournier F, et al. : Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J Mol Med (Berl) 2015;93:573–84. [DOI] [PubMed] [Google Scholar]
- 113.Malenfant S, Potus F, Mainguy V, et al. : Impaired Skeletal Muscle Oxygenation and Exercise Tolerance in Pulmonary Hypertension. Med Sci Sports Exerc 2015;47:2273–82. [DOI] [PubMed] [Google Scholar]
- 114.Meloche J, Lampron MC, Nadeau V, et al. : Implication of Inflammation and Epigenetic Readers in Coronary Artery Remodeling in Patients With Pulmonary Arterial Hypertension. Arterioscler Thromb Vasc Biol 2017;37:1513–23. [DOI] [PubMed] [Google Scholar]
- 115.Potus F, Malenfant S, Graydon C, et al. : Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med 2014;190:318–28. [DOI] [PubMed] [Google Scholar]
- 116.Li JH, Li Y, Huang D, Yao M: Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration. Tissue Eng Regen Med 2021;18:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu H, Wang B, Jiang C, Li R, Zhao J: Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21–5p to modulate Thrombospondin-1 expression. Clin Sci (Lond) 2019;133:1629–44. [DOI] [PubMed] [Google Scholar]
- 118.Real C, Caiado F, Dias S: Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc Hematol Disord Drug Targets 2008;8:185–93. [DOI] [PubMed] [Google Scholar]
- 119.Granton J, Langleben D, Kutryk MB, et al. : Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension: The PHACeT Trial. Circ Res 2015;117:645–54. [DOI] [PubMed] [Google Scholar]
- 120.Zhu JH, Wang XX, Zhang FR, et al. : Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant 2008;12:650–5. [DOI] [PubMed] [Google Scholar]
- 121.Giannotti G, Doerries C, Mocharla PS, et al. : Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension 2010;55:1389–97. [DOI] [PubMed] [Google Scholar]
- 122.Ormiston ML, Deng Y, Stewart DJ, Courtman DW: Innate Immunity in the Therapeutic Actions of Endothelial Progenitor Cells in Pulmonary Hypertension. American journal of respiratory cell and molecular biology 2009. [DOI] [PubMed] [Google Scholar]
- 123.Steward CG, Pellier I, Mahajan A, et al. : Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol 2004;124:63–71. [DOI] [PubMed] [Google Scholar]
- 124.Urbich C, Aicher A, Heeschen C, et al. : Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of molecular and cellular cardiology 2005;39:733–42. [DOI] [PubMed] [Google Scholar]
- 125.Patel NM, Burger CD: Two cases of stem cell therapy for pulmonary hypertension: A clinical report. Respiratory Medicine CME 2011;4:70–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


