Abstract
Activation of an immune response is energetically costly and excessive immune system activity can result in immunopathology, yet a slow or insufficient immune response carries the risk of pathogen establishment with consequent pathology arising from the infection. Mathematical theory and empirical data demonstrate that hosts balance the costs of immunity against the risk of infection by closely regulating immunological dynamics. An optimal immune system is rapidly and robustly deployed against a true infectious threat and rapidly deactivated once the threat has been controlled. Genetic variation in the sensitivity of an immune system, as well as in the activation and shutdown kinetics of host immune responses, can contribute to the evolution of pathogen virulence and host tolerance of infection. Improved understanding of the adaptive forces that operate on immune regulatory dynamics will clarify fundamental principles governing the evolution and maintenance of innate immune systems.
Keywords: insect immunity, immune regulation, immunopathology, infection kinetics, evolution, adaptation, resistance, tolerance, infection
Intuition suggests that pathogenic infection should be met immediately with an overwhelming immune defense that smothers the infection before it can become established. In fact, one might envision an ever-active immune system poised to suppress any pathogen that breaches epithelial barriers. Yet most immune systems remain fairly inactive under normal circumstances and are only induced to high levels of activity after infection occurs. The ubiquity of inducible immune reactions implies that the fully active state is costly, and that the cost is not worth bearing in the absence of dangerous infection. The costs are real and manifest in terms of energetic demands and risk of autoimmune damage. Yet the alternative strategy of deactivating the immune system in the absence of infection carries a risk of its own, providing the pathogen with time to become established while immune defense is being ramped up. The lag time to full activation of an inducible defense therefore sets up a high-stakes race between the host and pathogen, and the host must balance risk against costs in assessing what threats warrant a response and how strongly to react.
Insects and other non-vertebrates rely on innate immune systems, while vertebrates supplement their innate immunity with highly specific, antibody-mediated acquired immune systems. In innate antimicrobial immune responses, a eukaryotic host recognizes conserved molecular structures characteristic of microbes but absent from eukaryotes, including β-glucans (fungal cell walls), peptidoglycan and lipopolysaccharides (bacterial cell walls and membranes), and components of flagella (bacteria). These stimulatory molecules are sometimes called Microbe-Associated Molecular Patterns, or MAMPs [1]. Recognition of parasitic infection stimulates an induced response, which in insects can be composed of phagocytosis of small pathogens by macrophage-like cells, encapsulation of large pathogens within multilayered sheaths of host cells, and production of cytotoxic oxidative free radicals and antimicrobial peptides to kill pathogens [2,3]. Each of these defense mechanisms may come at considerable cost. Phagocytes are finite in number and cannot always be quickly recycled [3]. Forming a multicellular capsule around a pathogen requires substantial investment into hematopoiesis [4]. Reactive oxygen species are undiscriminating and can do damage to host tissues as well as to pathogens [5–7]. Antimicrobial peptides may be produced at levels as high as 100 μmol in insect hemolymph [8], posing a substantial transcriptional and translational burden on host cells. The costs of an immune response in terms of energetic expenditure and self-damage can be so great that, in some cases, the pathology associated with an infection is due as much to the host response as it is to direct virulence of the pathogen [9].
How, then, should a host optimize the immune response? Rapid activation is critical and small differences in the speed or intensity of immune induction over the first few hours of infection can make a life-or-death difference in infection outcome [10–11, P. Lafont et al. bioRxiv doi: 10.1101/2021.10.19.464998], but the costs of an overactive response could be substantial. The host must react quickly and intensely to a true threat without being hypersensitive to non-threats such as beneficial microbes and benign commensals. Since even non-pathogenic microbes present MAMPs, simple recognition that a microbe is present is too low of a threshold for inducing full immune activation. Instead, a more discriminating strategy is to couple surveillance for microbes with surveillance for indicators of cellular damage such as intracellular molecules in the extracellular space (sometimes called Damage Associated Molecular Patterns; DAMPs) [12,13] or for common pathogen virulence mechanisms such as toxin production or protease secretion [14–16]. That a discriminating immune system should mount a full response only in the joint presence of both DAMPs and MAMPs is the central premise of Maztinger’s Danger Model [17]. Extending Matzinger’s logic and incorporating the costs of immunity, Lazzaro and Rolff subsequently argued that the healthiest host need not completely eradicate a pathogen but need only reduce pathogen burden below a threshold where the cost of damage done by the pathogen is less than would be the cost of an immune reaction to control the infection [18]. The residual pathogen burden would be tolerated by the host. Those authors drew a parallel to the economic injury level as applied in agriculture [19], which is the threshold at which herbicide or insecticide application is warranted because the cost of damage done by an agricultural pest exceeds the cost of implementing control. Similarly, a well-tuned immune system might balance risks and costs by activating a response only when both MAMPs and danger signals are jointly present, and only when the potential damage from the pathogen is greater than the energetic and immunopathological cost of defense.
Of course, it is quite a lot to ask that any system should be able to anticipate future costs of unchecked infection and titrate exactly the correct amount of immunity for control. In practice, infection presents a combination of stimuli, some of which may be only indirectly related to potential virulence or toxicity of the pathogen, and the immune system must respond to those stimuli as they exist in the moment as opposed to what they might represent for the future. While activating immunity certainly comes with costs, failing to adequately suppress a pathogenic infection would cost much more. In an early theoretical model, Frank showed that hosts achieve highest fitness when pathogenic infection is rapidly detected and an intense response is mounted to guarantee control of infection, provided the response is quickly shut down after the infection has been cleared to limit immunopathology [20]. Subsequent work by Urban et al. [21] demonstrated that the optimal host strategy is to err in the direction of excess immune activation to ensure suppression of the pathogen, but again emphasized the importance of rapid shutdown after the infection has been cleared. Interestingly, however, the fitness surface in the Frank [20] model was fairly flat and the Urban et al model [21] yielded a flat shoulder to the fitness surface beyond the point where immune activity was adequate for control of infection. These theoretical findings suggested that natural populations might harbor substantial genetic variability for immune regulatory kinetics as long as activation exceeds some minimum threshold. Consistent with these models, natural insect populations harbor considerable genetic variation for the quantitative degree of immune gene expression in response to infection challenge [e.g., 22–24]. However, more empirical work is necessary to determine the degree to which that variability stems specifically from polymorphism in surveillance sensitivity, intensity of activation, and rate of shutdown, as well as how such variability relates to pathogen control and host fitness.
The theoretical prediction that potential threats should elicit a rapid and overwhelming initial response is well supported by data. In insects, a sterile injury is sufficient to induce a transient prophylactic antimicrobial response even in the absence of microbes or MAMPs [e.g., 25]. However, the presence of microbes is required to sustain the response beyond a few hours. Insect immune reactions to sterile injury or legitimate bacterial infection include several-hundred-fold increase in expression of genes encoding a broad suite of antimicrobial peptides [e.g., 25–29]. Surprisingly, however, most of the antimicrobial peptides produced seem to have no effect in controlling any given infection [30]. Thus, it would appear that instead of attempting to restrict expression to the specific peptide(s) that are appropriate for controlling a given infection, insects abundantly express an entire catalog of peptides so that a few functionally effective peptides will be included among them. This may be due to lack of host ability to rapidly and accurately discriminate among infections, but the consequence could be that an unnecessarily high cost of immune activation is borne in order to ensure that infection is controlled.
The prediction of robust negative regulation is also well supported empirically. A ubiquitous insect defense mechanism is production of cytotoxic reactive oxygen species (ROS) from quinone and semiquinone intermediates via phenoloxidase. This is an extremely rapid response, activated in minutes by protease cascades that are much faster than responses that depend on transcription and translation. However, simultaneously, ROS detoxifiers like superoxide dismutase and catalase are produced to defuse the ROS even as they are being generated [3; Figure 1]. Similarly, the expression of genes encoding antimicrobial peptides is triggered by activation of two signaling pathways, the Toll and Imd pathways, which are almost completely conserved across insects. These two pathways activate expression of proteins that dampen flux through the pathways [1; Figure 1], resulting in a negative feedback loop that shuts down the immune system once the activating stimulus is gone. Furthermore, the Toll and Imd pathways upregulate production of proteins that degrade immunostimulatory MAMPs [31,32; Figure 1], actively removing the stimulus to prevent sustained signaling in response to microbes that have already been killed or in response to benign commensals [33]. Theory predicts [34] that shutdown kinetics should be especially rapid when the cost of immune deployment is high, as in the case of antimicrobial peptide production, or when there is a high risk of immunopathology, as is the case with ROS production. High levels of immunopathology can even shift the fitness balance toward sustaining chronic infections, provided the pathology of the chronic infection is less than the pathology associated with a level of immunity required to eradicate it [34].
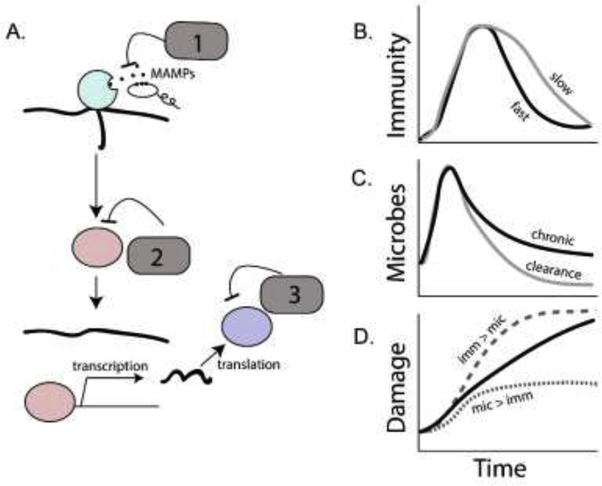
Figure 1.
Regulation of immune signaling during infection. Negative regulators of immune responses (A) can degrade MAMPs and DAMPs to prevent signal transduction from immune receptors (1, e.g. peptidoglycan-recognition proteins with amidase activity), they can inhibit or degrade signaling proteins and transcription factors that propagate those signals (2, e.g. the proteins Cactus and PIRK), or they can modify the activity of the resulting effector response (3, e.g. antioxidants that detoxify immune-generated ROS). The net effect of these regulators is to slow the induction of an immune response and to accelerate its decay. Lower activity of a negative regulator can result in a prolonged immune response (B, gray line) relative to a response that returns more quickly to baseline (black line). While reducing negative regulation on the system could potentially result in faster activation and/or more complete clearance of microbes compared to a dampened reaction that might allow chronic persistence of an infection (C), a host with more aggressive immunity could suffer a net higher damage (D, gray dashed line, where immunopathology exceeds the damaged done by infection) compared to a host whose immune system is under moderate negative regulation (D, black line, where immunopathology is reduced but damage due to chronic infection continues to accumulate) or one whose immune system is strongly regulated so that immunopathological damage is lower than direct damage from the infection (D, dotted gray line).
Genetic variation in sensitivity to immune stimulation and consequent immunopathology could contribute to among-individual variation in tolerance of infection, where tolerance is defined as the ability to sustain health and fitness despite a pathogen burden [35]. Tolerance can be empirically measured as the relationship between health and parasite number, and variation in tolerance can be determined at the population level by measuring both host health and pathogen burden in a sample of individuals or genotypes drawn from the population. Immune sensitivity is determined by several distinct variables, including the number of microbes needed to cross activation thresholds [36], the strength of induction per microbe [23], and the kinetics of negative regulators relative to positive ones [37]. Genetic variation in any of these traits could lead to variability in the capacity to control infection, as well as to variation in immunopathology and tolerance of infection. Insects are especially amenable to studying variation in tolerance because they often can be maintained as genetically homogeneous strains, which can be used for replicated measure of host health over an experimental range of pathogen burdens. Such studies have revealed considerable naturally occurring genetic variation in tolerance of microbial infection [e.g., 38–42]. This observation is qualitatively consistent with the theoretical predictions of Frank [20] and Urban et al [21] that populations may be genetically variable for immune activation and shutdown kinetics, and indeed a pair of recent articles recently showed that negative regulators of the Drosophila immune system promote tolerance of infection [Prakash et al. bioRxiv doi: doi.org/10.1101/2021.09.23.461574, Prakash et al. bioRxiv doi: 10.1101/2021.09.23.461578]. Future research should additionally test the relationship between tolerance and sensitivity to immune activation, while recognizing that a vast number of physiological processes could contribute to tolerance and that the determinants of genetic variation in tolerance are unlikely to lie solely in the immune system [28,40,43].
Optimal immune reactivity may vary demographically. Metcalf et al [44] noted that long-lived hosts might be expected to evolve reduced sensitivity to infection in order to minimize immunopathology accumulated over a lifetime. This is superficially contrary to an intuitive expectation that long-lived hosts should be more immunologically vigilant because of cumulative risk of exposure, but is supported by widespread observation that overactivation of immune systems early in life results in decreased longevity [6,45,46]. Instead, long-lived hosts could be predicted to evolve forms of acquired immunological memory and immune plasticity to deal with rapidly evolving pathogens [47–49]. This hypothesis could be tested by contrasting the immune systems of long-lived and short-lived invertebrates.
When immunopathology is a major determinant of overall pathology (in contrast to when virtually all pathology arises from parasite exploitation of the host), immunopathology can become an indirect driver of pathogen virulence evolution [50]. For the host, the cost of infection is the cumulative combination of pathology arising from both pathogen virulence and the host’s own immune reactions (Figure 1). Direct selection on the pathogen is always for increased transmission, but host immunopathology can become a factor in transmission-virulence tradeoffs for the pathogen. When pathogen virulence mechanisms stimulate increased immunopathology to the point of decreasing transmission (e.g., because of early host death), there can be selective pressure on pathogens to become both less virulent and less immunostimulatory, effectively increasing host tolerance of infection [51,52]. Reduced immunostimulation may be less likely to evolve when transmission and immunopathology are positively correlated (e.g., when host symptoms mediate transmission). Similarly, host evolution of tolerance mechanisms that mitigate pathogen-induced damage without directly attacking the pathogen itself (such as wound repair and neutralization of virulence factors) can facilitate pathogen evolution of escalated virulence if the virulence mechanisms directly promote transmission [53].
While both theoretical and empirical studies have begun to explore the challenge of optimizing immune reactivity to sufficiently control infection while minimizing immunopathology, much work remains to be done. How the host might achieve this optimization given limited and imperfect information about potential virulence in the early stages of an infection is a question ripe for theoretical exploration. Mechanistically disentangling the counterbalancing forces of immune induction versus active signal decay and system shutdown is a top priority, including comparative evaluation of how direct versus indirect negative regulation impacts infection dynamics. So, too, is determining the health and fitness consequences of drifting away from immunological optimality. Because of their experimental tractability, insect systems are ideal for performing this work. Insects are regularly observed to tolerate low-level chronic infections instead of immunologically eradicating pathogens [e.g. 38,41,54,55], and the importance of this for the evolution of pathogen virulence and transmission should be explored theoretically. Achieving better and more complete understanding of the proximate and evolutionary consequences of variation in the regulation of inducible immune responses will clarify fundamental principles driving the evolution and maintenance of innate immune systems.
HIGHLIGHTS.
Energetic demands and immunopathology contribute to costs of immunity.
Hosts must titrate immune responses appropriately to maintain health and fitness in the face of infection.
Optimal immunity activates quickly in response to pathogenic infection, but does not react to non-pathogenic challenge and is quickly shut down after an infection has been eradicated.
Immune sensitivity and immunopathology can contribute to the evolution of pathogen virulence and host tolerance.
Mathematical theory and empirical study jointly enhance understanding of the evolution and maintenance of innate immunity.
ACKNOWLEDGEMENTS
We thank Jess Metcalf and Andrea Graham for valuable discussion on these topics and for comments on the manuscript. ATT is supported by NIH grant R35 GM138007 and BPL is supported by NIH grant R01 AI141385. The funding sources had no involvement in the conception or preparation of this article.
Footnotes
Declaration of Financial Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1]. Zhang W, Tettamanti G, Bassal T, Heryanto C, Eleftherianos I, Mohamed A: Regulators and signalling in insect antimicrobial innate immunity: Functional molecules and cellular pathways. Cell Signal 2021, 83:110003. • This recent review article presents an overview of the regulatory pathways that control immune signaling in insects.
- [2].Wu Q, Patočka J, Kuča K: Insect antimicrobial peptides, a mini review. Toxins 2018, 10:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Eleftherianos I, Heryanto C, Bassal T, Zhang W, Tettamanti G, Mohamed A: Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021. doi: 10.1111/imm.13390. 164:401–432. • This recent review article presents an overview of cellular processes associated with immune defense in insects.
- [4].Bajgar A, Kucerova K, Jonatova L, Tomcala A, Schneedorferova I, Okrouhlik J, Dolezal T: Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol 2015, 13:e1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sadd BM, Siva-Jothy MT: Self-harm caused by an insect’s innate immunity. Proc Biol Sci 2006, 273:2571–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pursall ER, Rolff J: Immune responses accelerate ageing: proof-of-principle in an insect model. PLoS One 2011, 6:e19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Li X, Rommelaere S, Kondo S, Lemaitre B: Renal purge of hemolymphatic lipids prevents the accumulation of ROS-induced inflammatory oxidized lipids and protects Drosophila from tissue damage. Immunity 2020, 52:374–387. •• This work demonstrates a physiological mechanism by which lipids are removed from circulation in the hemolymph upon immune challenge. This removal is essential because lipids are particularly prone oxidation by reactive oxygen species (ROS) and can result in immunopathology associated with ROS-mediated defense. The elimination of lipids from circulation therefore reduces immunopathology.
- [8].Lemaitre B, Hoffmann J: The host defense of Drosophila melanogaster. Annu Rev Immunol 2007, 25:697–743. [DOI] [PubMed] [Google Scholar]
- [9].Graham AL, Allen JE, Read AF: Evolutionary causes and consequences of immunopathology. Annu Rev Ecol Evol Syst 2005, 36:373–397. [Google Scholar]
- [10].Duneau D, Ferdy JB, Revah J, Kondolf H, Ortiz GA, Lazzaro BP, Buchon N: Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. eLife 2017, 6:e28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Ellner SP, Buchon N, Dörr T, Lazzaro BP: Host-pathogen immune feedbacks can explain widely divergent outcomes from similar infections. Proc Biol Sci 2021, 288:20210786. •• Building from reference [10], this paper presents a theoretical model showing that the outcome of infection is determined by a race between pathogen proliferation and host immune activation, and that quantitative variability in feedback dynamics between host and pathogen is sufficient to explain why some hosts survive infection while others succumb. A related theoretical analysis that is available as a preprint is cited inline in the article text.
- [12].Srinivasan N, Gordon O, Ahrens S, Franz A, Deddouche S, Chakravarty P, Phillips D, Yunus AA, Rosen MK, Valente RS, Teixeira L, Thompson B, Dionne MS, Wood W, Reis e Sousa C. Actin is an evolutionarily-conserved damage-associated molecular pattern that signals tissue injury in Drosophila melanogaster. eLife 2016, 5:e19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roh JS, Sohn DH: Damage-Associated Molecular Patterns in inflammatory diseases. Immune Netw 2018, 18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D: Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 2006, 127:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Issa N, Guillaumot N, Lauret E, Matt N, Schaeffer-Reiss C, Van Dorsselaer A, Reichhart JM, Veillard F: The circulating protease Persephone is an immune sensor for microbial proteolytic activities upstream of the Drosophila Toll pathway. Mol Cell 2018, 69:539–550.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Piffer AC, Kuczera D, Rodrigues ML, Nimrichter L: The paradoxical and still obscure properties of fungal extracellular vesicles. Mol Immunol 2021, 135:137–146. • This article describes extracellular vesicles derived from fungi during infection, which can be immunostimulatory.
- [17].Matzinger P: Tolerance, danger, and the extended family. Annu Rev Immunol 1994, 12:991–1045. [DOI] [PubMed] [Google Scholar]
- [18].Lazzaro BP, Rolff J. Immunology: Danger, microbes, and homeostasis. Science 2011, 332:43–4. [DOI] [PubMed] [Google Scholar]
- [19].Stern VM, Smith RF, Van Den Bosch R, Hagen KS: The integrated control concept. Hilgardia 1959, 29:81–101. [Google Scholar]
- [20].Frank SA: Immune response to parasitic attack: evolution of a pulsed character. J Theor Biol 2002, 219:281–90. [DOI] [PubMed] [Google Scholar]
- [21].Urban MC, Bürger R, Bolnick DI: Asymmetric selection and the evolution of extraordinary defences. Nat Commun. 2013. 4:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sackton TB, Lazzaro BP, Clark AG: Genotype and gene expression associations with immune function in Drosophila. PLoS Genet 2010, 6:e1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Jent D, Perry A, Critchlow J, Tate AT: Natural variation in the contribution of microbial density to inducible immune dynamics. Mol Ecol 2019, 28:5360–5372. • This paper demonstrates genetic variation in Tribolium flour beetles for immunological sensitivity and regulatory kinetics of immune response to bacterial infection. The paper links quantitative variation in expression of antimicrobial peptide genes to control of infection and costs to other life history traits.
- [24]. Nardini L, Holm I, Pain A, Bischoff E, Gohl DM, Zongo S, Guelbeogo WM, Sagnon N, Vernick KD, Riehle MM: Influence of genetic polymorphism on transcriptional enhancer activity in the malaria vector Anopheles coluzzii. Sci Rep 2019. 9:15275. • This article documents genetic variation in transcriptional control of immune genes in a mosquito vector of human malaria.
- [25].Lemaitre B, Reichhart JM, Hoffmann JA: Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA 1997, 94:14614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Behrens S, Peuβ R, Milutinović B, Eggert H, Esser D, Rosenstiel P, Schulenburg H, Bornberg-Bauer E, Kurtz J: Infection routes matter in population-specific responses of the red flour beetle to the entomopathogen Bacillus thuringiensis. BMC Genomics 2014. 15:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim IW, Lee JH, Subramaniyam S, Yun EY, Kim I, Park J, Hwang JS: De novo transcriptome analysis and detection of antimicrobial peptides of the American cockroach Periplaneta americana (Linnaeus). PLoS One 2016, 11:e0155304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Troha K, Im JH, Revah J, Lazzaro BP, Buchon N: Comparative transcriptomics reveals CrebA as a novel regulator of infection tolerance in D. melanogaster. PLoS Pathog 2018, 14:e1006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Lee JH, Chung H, Shin YP, Kim MA, Natarajan S, Veerappan K, Kim SH, Park J, Hwang JS: Deciphering novel antimicrobial peptides from the transcriptome of Papilio xuthus. Insects. 2020. 11:776. • This article is one of many published over the past two decades on a variety of insects, documenting induced expression of genes encoding a broad suite of antimicrobial peptides in response to infectious challenge.
- [30]. Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B: Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. eLife 2019, 8:e44341. •• Although D. melanogaster strongly induce the expression of over a dozen antimicrobial peptides in response to infection, this paper shows that control of any given bacterial infection is typically due to one or a few of those peptides and the rest are superfluous. This results in a wasteful investment in antimicrobial peptide production, which is presumably borne because the host lacks the capacity to sufficiently discriminate among infections and mount a specific response.
- [31].Mellroth P, Steiner H: PGRP-SB1: an N-acetylmuramoyl L-alanine amidase with antibacterial activity. Biochem Biophys Res Commun 2003, 350:994–999. [DOI] [PubMed] [Google Scholar]
- [32].Zaidman-Rémy A, Hervé M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B: The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 2006, 24:463–473. [DOI] [PubMed] [Google Scholar]
- [33].Paredes JC, Welchman DP, Poidevin M, Lemaitre B: Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 2011, 35:770–9. [DOI] [PubMed] [Google Scholar]
- [34].Cressler CE, Graham AL, Day T: Evolution of hosts paying manifold costs of defence. Proc Biol Sci 2015, 282:20150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Råberg L, Graham AL, Read AF: Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci 2009, 364:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Louie A, Song KH, Hotson A, Tate AT, Schneider DS: How many parameters does it take to describe disease tolerance? PLoS Biol. 2016, 14:e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Longo DM, Selimkhanov J, Kearns JD, Hasty J, Hoffmann A, Tsimring LS: Dual delayed feedback provides sensitivity and robustness to the NF-κB signaling module. PLOS Computational Biology 2013, 9: e1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Howick VM, Lazzaro BP: Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol Biol 2014, 14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Parker BJ, Garcia JR, Gerardo NM: Genetic variation in resistance and fecundity tolerance in a natural host-pathogen interaction. Evolution 2014, 68:2421–9. [DOI] [PubMed] [Google Scholar]
- [40].Howick VM, Lazzaro BP: The genetic architecture of defence as resistance to and tolerance of bacterial infection in Drosophila melanogaster. Mol Ecol 2017. 26:1533–1546. [DOI] [PubMed] [Google Scholar]
- [41].Kutzer MAM, Kurtz J, Armitage SAO: Genotype and diet affect resistance, survival, and fecundity but not fecundity tolerance. J Evol Biol 2018, 31:159–171. [DOI] [PubMed] [Google Scholar]
- [42]. Kutzer MAM, Kurtz Armitage SAO: A multi-faceted approach teasting the effects of previous bacterial exposure on resistance and tolerance. J Animal Ecol 2019, 88:566–578. • This recent article, along with the related cited reference [41], document genetic variation in tolerance of bacterial infection by Drosophila melanogaster.
- [43].Ayres JS, Freitag N, Schneider DS: Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics 2008, 178:1807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Metcalf CJE, Tate AT, Graham AL: Demographically framing trade-offs between sensitivity and specificity illuminates selection on immunity. Nat Ecol Evol 2017, 1:1766–1772. [DOI] [PubMed] [Google Scholar]
- [45].Khan I, Agashe D, Rolff J: Early-life inflammation, immune response and ageing. Proc Biol Soc 2017, 284: 20170125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Badinloo M, Nguyen E, Suh W, Alzahrani F, Castellanos J, Klichko VI, Orr WC, Radyuk SN: Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Arch Insect Biochem Physiol 2018, 98:e21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miller MR, White A, Boots M: Host life span and the evolution of resistance characteristics. Evolution 2007, 61:2–14. [DOI] [PubMed] [Google Scholar]
- [48].Mayer A, Mora T, Rivoire O, Walczak AM: Diversity of immune strategies explained by adaptation to pathogen statistics. Proc Natl Acad Sci USA 2016, 113:8630–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pigeault R, Garnier R, Rivero A, Gandon S: Evolution of transgenerational immunity in invertebrates. Proc Biol Sci. 2016, 283:20161136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Long GH, Boots M: How can immunopathology shape the evolution of parasite virulence? Trends Parasitol. 2011, 27:300–305. [DOI] [PubMed] [Google Scholar]
- [51].Day T, Graham AL, Read AF: Evolution of parasite virulence when host responses cause disease. Proc Biol Sci 2007, 274:2685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Long GH, Graham AL: Consequences of immunopathology for pathogen virulence evolution and public health: malaria as a case study. Evol Appl 2011, 4:278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vale PF, Fenton A, Brown SP Limiting damage during infection: lessons from infection tolerance for novel therapeutics. PLoS Biology 2014, 12:e1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Haine ER, Moret Y, Siva-Jothy MT, Rolff JL: Antimicrobial defense and persistent infection in insects. Science 2008, 322:1257–9. [DOI] [PubMed] [Google Scholar]
- [55].Tate AT, Andolfatto P, Demuth JP, Graham AL: The within-host dynamics of infection in trans-generationally primed flour beetles. Mol Ecol 2017, 26:3794–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]