Abstract
West African Aspergillus flavus S isolates differed from North American isolates. Both produced aflatoxin B1. However, 40 and 100% of West African isolates also produced aflatoxin G1 in NH4 medium and urea medium, respectively. No North American S strain isolate produced aflatoxin G1. This geographical and physiological divergence may influence aflatoxin management.
Aflatoxins are fungal metabolites that suppress animal immune systems, are probable human carcinogens, and frequently contaminate foods and feeds (15, 17). Diverse communities of aflatoxin-producing fungi occupy soils where aflatoxin contamination is common (2). Aflatoxin producers are asexual fungi belonging to Aspergillus section Flavi (9). These fungi vary in genetic, morphological, and physiological traits (3, 4). North American communities of section Flavi differ by region in both species composition and aflatoxin-producing potential (8).
The most common aflatoxin-producing species, Aspergillus flavus, can be divided into two strains. The S strain produces numerous small sclerotia (average diameter, <400 μm) and high levels of aflatoxins. The L strain produces fewer, larger sclerotia and, on average, less aflatoxin (4, 13, 18). S strain isolates have been referred to as atypical (18), microsclerotium producing (14), and Aspergillus flavus var. parvisclerotigenus (19). Within the S strain, some isolates, termed SB, produce only B aflatoxins, while others, termed SBG, produce both B and G aflatoxins (12–14, 18). Molecular phylogenetics suggests that SB isolates are closely related to the A. flavus type culture and other L strain isolates (12).
In the United States, S strain incidence within section Flavi communities ranges from less than 5% to more than 90% (8). S strain isolates from Indonesia, Thailand, the Philippines, and Africa are also known (13, 14, 18). From North America, only SB isolates have been reported (4, 8). However, in Thailand both SB and SBG isolates occur (18). The only S strain isolates previously reported from Africa are two SBG isolates collected three decades ago in Nigeria (13). Our objectives in this study were, firstly, to compare S strain communities in West Africa with those previously characterized in North America and, secondly, to compare ammonium- and urea-based media for assessing the S strain phenotype.
We recovered 67 S strain isolates from 15 agricultural soils collected during 1994 and 1995 in the Republic of Benin by the dilution plate technique on modified rose bengal agar (5). Isolates were evaluated for aflatoxin-producing ability (8) in a medium containing 3 g of (NH4)2SO4/liter (27 mM) as the sole N source (1). Initial screens (limit of detection, 1 μg of aflatoxin B1/fermentation) of African isolates detected both SB (40 isolates) and SBG (27 isolates) phenotypes. All 374 S strain isolates from North America previously examined in the same medium had the SB phenotype (4, 8). The African SB isolates produced less aflatoxin B1 than was reported for North American SB isolates. Most (59%) North American SB isolates produced >100 μg of aflatoxin B1 per 70-ml fermentation (8), whereas just 2% of African SB isolates produced that quantity. Two percent of North American SB isolates produced <0.5 μg of aflatoxin B1 per fermentation, whereas 15% of African SB isolates produced <0.5 μg.
African SB isolates were reevaluated in medium with 3 g of urea/liter (50 mM) substituted for NH4. All 24 isolates examined had the SBG phenotype in urea. To determine if the two phenotypes were dependent on the nitrogen source or concentration, four representatives of each phenotype were assayed in independent tests in medium with either 45 or 100 mM N supplied as either (NH4)2SO4 or urea (Table 1). Cultures were fermented on a rotary shaker (30°C, 150 rpm, 5 days) in 250-ml flasks containing 70 ml of medium seeded with approximately 5,000 conidia (8). Media were adjusted to pH 4.7 prior to autoclaving. After fermentation, the final filtrate pH was measured prior to the addition of 70 ml of acetone. Mycelia were caught on Whatman no. 4 filter paper during filtration, dried (55°C, 3 days), and weighed. Filtrates were diluted to 50% with water and were extracted twice with 25 ml of methylene chloride. Extracts were passed through anhydrous sodium sulfate, combined, and evaporated to dryness, and the residue was dissolved in 4 ml of methylene chloride (8). Residues were concentrated or diluted as appropriate, applied with aflatoxin standards to thin-layer chromatography plates, and developed with ether–methanol–water (96:3:1). Aflatoxins were quantified with fluorescence densitometry (8, 16).
TABLE 1.
Production of aflatoxins B1 and G1 by representative West African and North American S strain isolates in liquid media containing either ammonium or urea as the sole nitrogen source
| Test | Origin | Isolate | N concn (mM) | Result in NH4 or urea mediuma
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoxin B1 (μg)
|
Aflatoxin G1 (μg)
|
Final pH
|
Mass (g)
|
||||||||
| NH4 | Urea | NH4 | Urea | NH4 | Urea | NH4 | Urea | ||||
| 1 | Benin | BN008R | 45 | 6 B | 940 A | 0 B | 270 A | 3.2 B | 3.1 B | 790 B | 700 B |
| 100 | 16 B | 1,400 A | 0.04 B | 1,900 A | 2.3 C | 3.6 A | 830 B | 1,000 A | |||
| 2 | Benin | BN038G | 45 | 400 E | 1,900 D | 6 E | 240 D | 2.4 E | 3.4 DE | 850 D | 770 E |
| 100 | 370 E | 2,000 D | 4 E | 290 D | 2.4 DE | 3.9 E | 730 E | 870 D | |||
| 3 | Benin | BN026G | 45 | 35 G | 240 F | 0.9 G | 71 F | 2.3 H | 3.3 G | 970 F | 620 G |
| 100 | 13 G | 180 F | 0.7 G | 69 F | 2.4 H | 4.0 F | 990 F | 760 FG | |||
| 4 | Benin | BN040B | 45 | 30 J | 890 I | 1 J | 210 I | 2.2 K | 3.3 J | 990 I | 690 J |
| 100 | 28 J | 1,900 I | 1 J | 930 I | 2.4 K | 3.9 I | 910 I | 920 I | |||
| 5 | United States | YV2-19 | 45 | 620 L | 1,400 L | 0 | 0 | 2.5 M | 3.9 L | 310 M | 580 M |
| 100 | 780 L | 1,600 L | 0 | 0 | 2.5 M | 4.1 L | 310 M | 890 L | |||
| 6 | United States | LA2-5 | 45 | 940 N | 220 O | 0 | 0 | 2.4 P | 3.3 O | 580 P | 950 O |
| 100 | 900 N | 380 O | 0 | 0 | 2.5 P | 4.6 N | 450 P | 1,100 N | |||
| 7 | United States | MS22-41 | 45 | 830 Q | 940 Q | 0 | 0 | 2.5 S | 3.4 R | 300 S | 680 R |
| 100 | 620 Q | 1,200 Q | 0 | 0 | 3.0 RS | 4.2 Q | 260 S | 960 Q | |||
| 8 | United States | AL3-22 | 45 | 680 T | 590 T | 0 | 0 | 2.7 V | 3.5 U | 250 V | 640 U |
| 100 | 1,100 T | 820 T | 0 | 0 | 2.5 V | 4.0 T | 360 V | 870 T | |||
Results are averages of three replicates. Values for a variable within a test followed by a common letter are not significantly different (P = 0.05) according to Fisher’s least significant difference test. Each Benin isolate originated from a separate agricultural field. Isolates YV2-19, LA2-5, MS22-41, and AL3-22 originated from agricultural fields in Arizona, Louisiana, Mississippi, and Alabama, respectively.
Increasing the NH4 concentration from 45 to 100 mM did not significantly increase aflatoxin production by any of the eight isolates examined (Table 1). However, African S strain isolates produced at least 7-fold more aflatoxin B1 and at least 58-fold more aflatoxin G1 with urea than with NH4. Most North American isolates produced statistically similar quantities of aflatoxin with NH4 and urea. However, one North American isolate (LA 2-5) produced significantly less aflatoxin with urea. The growth of African isolates was not inhibited by NH4. Indeed, African isolates produced greater biomass than North American isolates in NH4 medium, and frequently the mass produced by African isolates in NH4 medium was similar to that in the molar equivalent urea medium (Table 1). All isolates modified the pH of the ammonium medium similarly. However, African isolates reduced the pH of the urea medium slightly more than North American isolates.
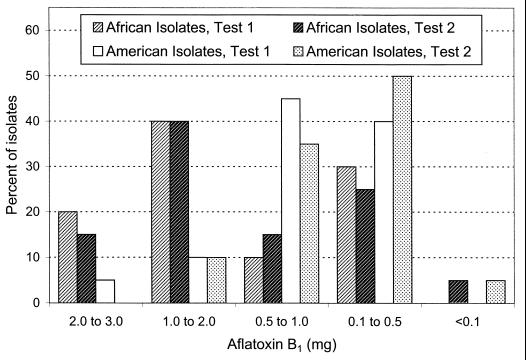
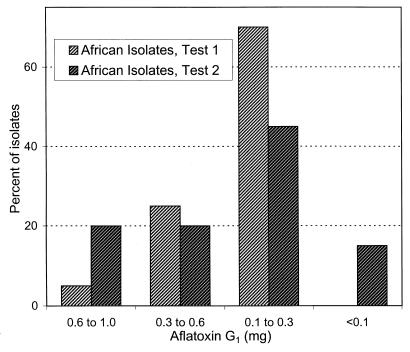
In two additional tests, African and North American isolates were compared in 50 mM urea medium. For each test 20 African and 20 North American isolates were evaluated as described above in a single fermentation. Different isolates were used in each test, and test 2 included A-11612 and A-11611 from Nigeria (13). All African isolates had the SBG phenotype, and all North American isolates had the SB phenotype. More than 50% of African isolates and more than 10% of North American isolates produced >1 mg of aflatoxin B1/fermentation (Fig. 1), and more than 90% of African isolates produced between 0.1 and 1.0 mg of aflatoxin G1 (Fig. 2).
FIG. 1.
Percentage of A. flavus S strain isolates producing various quantities of aflatoxin B1 in a chemically defined medium containing urea as the sole nitrogen source. Results of two tests are shown. Each test evaluated production by 20 West African and 20 North American isolates. Values are expressed in milligrams of aflatoxin B1 per 70-ml fermentation.
FIG. 2.
Percentage of A. flavus S strain isolates producing various quantities of aflatoxin G1 in a chemically defined medium containing urea as the sole nitrogen source. Each test evaluated production by 20 West African and 20 North American isolates. No North American isolate produced aflatoxin G1. Values are expressed in milligrams of aflatoxin G1 per 70-ml fermentation.
The urea tests suggest that the SB phenotypes detected during initial screens of African isolates resulted from a reduced capacity of ammonium to support aflatoxin production and not from an innate inability of the African isolates to produce aflatoxin G1. No African isolate expressed the SB phenotype in urea. In North America, SB isolates predominate, and no SBG isolates have been detected among the several hundred S strain isolates checked on NH4 medium (4, 8) or among the >50 isolates checked either on other media (10, 13) or on crops (4, 11). The present results demonstrate that communities of section Flavi in North America are different from those in West Africa. The causes of the divergence are unclear. SBG isolates may be ancestral to SB isolates (12), and the SB phenotype may have arisen outside of Africa. Thus, SB isolates might never have become established in West Africa. However, it is also possible that as yet unidentified selective forces active in West Africa and/or North America are responsible for observed differences in community composition.
The occurrence of aflatoxins in food creates international concern (9). Both the North American SB and the West African SBG isolates have great aflatoxin-producing potential. The aflatoxin-producing potentials of A. flavus communities can impact crop contamination (6, 8). Therefore, incidences of both S strain types should be of interest. SB isolates can be major contributors to the contamination of cottonseed with aflatoxin (7). However, the extent to which SBG isolates contribute to aflatoxin contamination in West Africa, and elsewhere, is unknown. Adapting aflatoxin management strategies from North America for use in Africa requires consideration of different cultures and agronomic systems. The divergence in fungal communities described here is an additional factor to consider.
Acknowledgments
We thank Darlene Downey, Kerrilee Kobbeman, and Nicole Hurban for technical assistance.
REFERENCES
- 1.Adye J, Mateles R I. Incorporation of labeled compounds into aflatoxins. Biochim Biophys Acta. 1964;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- 2.Bayman P, Cotty P J. Vegetative compatibility and genetic diversity in the Aspergillus flavus population of a single field. Can J Bot. 1991;69:1707–1711. [Google Scholar]
- 3.Bayman P, Cotty P J. Genetic diversity in Aspergillus flavus: association with aflatoxin production and morphology. Can J Bot. 1993;71:23–31. [Google Scholar]
- 4.Cotty P J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology. 1989;79:808–814. [Google Scholar]
- 5.Cotty P J. Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia. 1994;125:157–162. doi: 10.1007/BF01146521. [DOI] [PubMed] [Google Scholar]
- 6.Cotty P J. Influence of field application of an atoxigenic strain of Aspergillus flavus on the populations of A. flavus infecting cotton bolls and on aflatoxin content of cottonseed. Phytopathology. 1994;84:1270–1277. [Google Scholar]
- 7.Cotty P J. Aflatoxin contamination of commercial cottonseed caused by the S strain of Aspergillus flavus. Phytopathology. 1996;86:S71. [Google Scholar]
- 8.Cotty P J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol Res. 1997;101:698–704. [Google Scholar]
- 9.Cotty P J, Bayman P, Egel D S, Elias K S. Agriculture, aflatoxins, and Aspergillus. In: Powell K A, Renwick A, Peberdy J F, editors. The genus Aspergillus: from taxonomy and genetics to industrial applications. New York, N.Y: Plenum Press; 1994. pp. 1–27. [Google Scholar]
- 10.Doster M A, Michailides T J. Development of Aspergillus molds in litter from pistachio trees. Plant Dis. 1994;78:393–397. [Google Scholar]
- 11.Doster M A, Michailides T J, Morgan D P. Aspergillus species and mycotoxins in figs from California orchards. Plant Dis. 1996;80:484–489. [Google Scholar]
- 12.Egel D S, Cotty P J, Elias K S. Relationships among isolates of Aspergillus sect. Flavi that vary in aflatoxin production. Phytopathology. 1994;84:906–912. [Google Scholar]
- 13.Hesseltine C W, Shotwell O, Smith M, Ellis J J, Vandegraft E, Shannon G. Production of various aflatoxins by strains of the Aspergillus flavus series. In: Herzberg M, editor. Proceedings of the First U.S.-Japan Conference on Toxic Microorganisms. U.S. Washington, D.C: Government Printing Office; 1970. pp. 202–210. [Google Scholar]
- 14.Nozawa K, Sekita S, Harada M, Udagawa S, Kawai K. Isolation and structures of two new indoloterpenes related to aflavine from a microsclerotium-producing strain of Aspergillus flavus. Chem Pharm Bull. 1989;37:626–630. [Google Scholar]
- 15.Park D L, Stoloff L. Aflatoxin control—how a regulatory agency managed risk from an unavoidable natural toxicant in food and feed. Regul Toxicol Pharmacol. 1989;9:109–130. doi: 10.1016/0273-2300(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 16.Pons W A, Jr, Robertson J A, Goldblatt L A. Collaborative study on the determination of aflatoxins in cottonseed products. J Am Oil Chem Soc. 1966;43:655–669. [Google Scholar]
- 17.Robens J F, Richard J L. Aflatoxins in animal and human health. Rev Environ Contam Toxicol. 1992;127:69–94. doi: 10.1007/978-1-4613-9751-9_3. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Tsuruta O, Siriacha P, Kawasugi S, Manabe M, Buangsuwon D. Distribution and aflatoxin productivity of the atypical strains of Aspergillus flavus isolated from soils in Thailand. Proc Jpn Assoc Mycotoxicol. 1986;24:41–46. [Google Scholar]
- 19.Saito M, Tsurata O. A new variety of Aspergillus flavus from tropical soil in Thailand and its aflatoxin productivity. Proc Jpn Assoc Mycotoxicol. 1993;37:31–36. [Google Scholar]