Abstract
Herpes Simplex Virus-1 (HSV-1) is a neurotropic virus that can infect humans in the eye and travel to the trigeminal ganglion to establish latency. HSV-1 causes various disease states in both the primary and secondary sites of infection including the eye and the nervous system. This DNA virus exploits various adaptive measures to infect host cells, hijack host cell proteins, evade host immune response and spread from cell-to-cell to avoid immune detection. Recent data suggest that Optineurin (OPTN), a host protein, is a key restriction factor that prevents cell-to-cell spread of HSV-1 and guards against serious damage to the nervous system during infection. In recent years OPTN has gained increased attention because of its involvement in cellular mechanisms that promote homeostasis and prevent neurodegeneration. At the center of it all is the role OPTN plays as a receptor for selective autophagy. This review summarizes our latest understanding of the viral lifecycle, disease pathologies, and OPTN-mediated protective mechanisms during HSV-1 infection of the eye and the nervous system. We specifically highlight recent discoveries that implicate OPTN as crucial in the prevention of ocular and neurodegenerative diseases.
Keywords: HSV-1, optineurin, neuroprotection, selective autophagy
Introduction
HSV-1 belongs to the Alpha subfamily of Herpesviridae family. It is a double-stranded DNA (dsDNA) virus that is a significant health concern to humans since approximately 66.6% of people ages 15-49 are seropositive for the virus (Koganti et al., 2019; Koujah et al., 2019; Krishnan & Stuart, 2021). Infection by HSV-1 can undertake several pathologies, which are not limited to the site of primary infection. HSV-1 infection can lead to distressing pathologies of ocular as well as the orolabial, and genital regions (Gangappa et al., 2000; Koganti et al., 2019; Koujah et al., 2019, 2021; Liesegang, 2001). The phenotypic signs of disease that follow HSV-1 infection include herpes labialis (cold sores), gingivostomatitis, herpetic whitlow and genital herpes (Koujah et al., 2019). HSV-1 infection of the eye can lead to herpetic stromal keratitis (HSK) and is the leading cause of blindness by an infectious agent in developed nations (Agelidis et al., 2017). Moreover, asymptomatic shedding often contributes to the transmission of HSV-1, leaving new hosts unaware of the virus transmission and infection.
In most cell types, HSV-1 undergoes a productive or lytic mode of infection. Similarly, a primary infection in the corneal epithelium also results in productive infection, which maximizes HSV-1's ability to replicate locally and spread to the innervating nerves while causing damage to the ocular surface. Infection of nerves results in the viral progeny traveling to the trigeminal ganglion (TG) and establishing latency (Farooq & Shukla, 2011). Latency is established by HSV-1 virions entering the axons of sensory neurons innervating the affected area and, through retrograde travel to neuronal cell bodies of the TG. By establishing latency, the virus effectively evades host immune mechanisms for the entirety of the host's life (Koujah et al., 2019).
During latency the virus may occasionally reactivate and travel back to the primary site of infection through anterograde transport. HSV-1 reactivation, in most cases, happens randomly, but certain internal and external stimulants have been linked to inciting reactivation, such as psychological stressors, UV exposure, and temperature-dependent stressors (Ludema et al., 2014; Herpetic Eye Disease Study Group, 2000). Phenotypic signs of HSV-1 reactivation are often lesions, sores and inflammation at primary infection sites such as the mouth, eye, and genital area. The symptoms are treatable by common antivirals such as acyclovir and its analogs. However, in rare situations, reactivation can result in neuronal inflammation known as herpes simplex encephalitis (HSE) (Ames, Yadavalli, Suryawanshi, et al., 2021; Wang et al., 2020). Similarly, HSK, which involves corneal stroma, can turn into a chronic inflammatory disease that may lead to non-healing herpetic lesions, associated immune responses, and neovascularization, causing scarring, opaque cellular aggregation, and immense corneal damage, resulting in vision loss (Gangappa et al., 2000; Liesegang, 2001). Although acyclovir and its analogs effectively combat the phenotypic signs of HSV-1 infection, current treatment options only combat productive HSV-1 infection, notably viral DNA synthesis, and over time, acyclovir-resistant strains of HSV-1 emerge as the virus keeps reactivating (Agelidis et al., 2017; Hadigal et al., 2015; Rousseau et al., 2022; Zhou et al., 2020). Therefore, it is essential to identify targets and mechanisms that may help reduce latency or more effectively combat ocular HSV-1 infection and hinder viral dissemination and inflammation in the nervous system tissues.
OPTN, which is abundantly expressed in neurons as well as many ocular cell types, is a conserved ubiquitin-binding protein involved in many signaling pathways and cellular processes. Patients suffering from neurodegenerative disorders such as Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Dementia (FTD) and Glaucoma have been found to have one or more mutations in the optn gene (Minegishi et al., 2016; Rezaie et al., 2002a, 2002b; Richter et al., 2016; Toth & Atkin, 2018; Wiggs & Pasquale, 2017). Of novel interest is OPTN's autophagic role in host protection against ocular and neurotropic infections by HSV (Ames et al., 2021; Patil et al., 2022; Richter et al., 2016). Coincidentally, HSV pathogenesis has been linked in the past to neuroinflammation, necroptosis and the disease pathology of common neurodegenerative disorders (Oakes et al., 2017; Slowicka & van Loo, 2018). This review highlights novel findings in OPTN's protective role against HSV-1 infection of the eye and brings to light OPTN's potential in hindering neurodegeneration of the CNS caused by a neurotropic virus and neurodegenerative diseases.
HSV-1 entry in the cornea
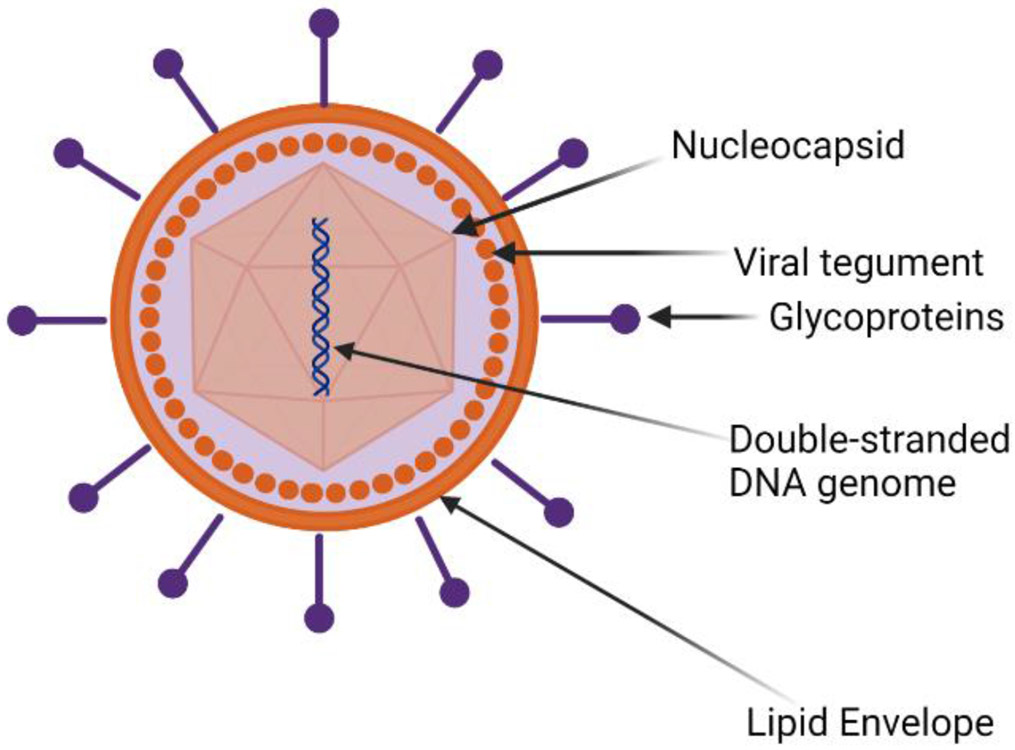
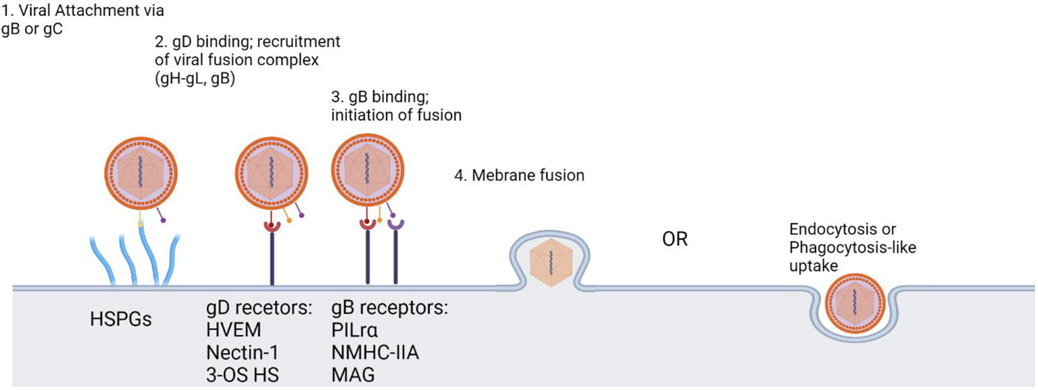
The structure of HSV-1 includes an icosahedral capsid which contains a linear, double-stranded viral DNA genome (Figure 1). Enclosing the capsid is a layer comprised of mRNAs and proteins known as the tegument, which is further covered by an outermost, lipid bilayer envelope containing several glycoproteins. There are 12 known glycoproteins on the surface of the viral envelope. Four glycoproteins, gB, gD, gH and gL are required for efficient viral attachment, fusion, entry and egress. The molecular mechanism of HSV-1 entry into the host cell includes the attachment of gB or gC to Heparan Sulfate Proteoglycan (HSPG)(Agelidis et al., 2017; Shukla & Spear, 2001). This attachment leads to an inwards viral movement along F-actin-rich membrane projections called filopodia to the cell body and is commonly known as viral surfing. After migration to the cell body, HSV-1 gD binds to one of its cognate receptors located on the host cell membrane (Figure 2). Over the years, various gD receptors have been identified but their presence and abundance on the cell surface is cell type dependent (Koujah et al., 2021). Briefly, Nectin-1, Herpes Virus Entry Mediator (HVEM) and 3-O-sulfated HS (3-OS HS) are the known receptors for HSV-1 gD and they all are expressed on the surface of human corneal epithelial cells (Agelidis et al., 2017; Hadigal et al., 2015). Binding of gD to its receptor catalyzes a conformational change that signals the recruitment of the membrane fusion complex, which includes gB, gH and gL. The association of gH-gL heterodimer initiates a conformational change in gB that ensues viral membrane insertion into the host cell membrane (Atanasiu et al., 2010; Cairns et al., 2011; Eisenberg et al., 2012). The merging of the viral and host cell membrane allows for the transportation of the viral capsid and tegument proteins into the cytoplasm. The viral capsid then travels to the cell nucleus for replication of viral genes and creation of viral progeny (Jambunathan et al., 2021).
Figure 1:
HSV-1 structure includes an icosahedral capsid that contains a linear, double-stranded viral DNA genome. Enclosing the capsid is a layer comprised of viral proteins known as the tegument, an outermost, lipid bilayer envelope containing several glycoproteins.
Figure 2.
The molecular interactions that enable HSV-1 entry. HSV-1's gB or gC attaches to filopodia like projections (HSPGs) from the cell surface and unilaterally moves towards cell membrane. Then, gD binds to one of its three receptors and this allows for the recruitment of the viral fusion complex (gH-gL and gB). gB then to one of its three receptors on the cell surface and conformational change occurs which mediates membrane fusion and entry into the cell. An alternate entry route is via endocytosis or phagocytosis-like uptake.
HSV-1 Replication and Translation
After successful fusion into the cell, HSV-1 releases its viral capsids and tegument proteins which travel to the cell’s nucleus, inhibit host transcription and suppress innate immune response. Microtubules (MT) are used in transporting the viral capsid from the cell surface to the nucleus. The MT-mediated transport of viral capsid to the nucleus is facilitated by cytoplasmic motor protein, dynein. In the nucleus, the viral mRNA is transcribed by host RNA polymerase II. After synthesis it is transported to the cytoplasm for translation (Agelidis et al., 2017; Koganti et al., 2019; Sodeik et al., 1997). Post translational viral protein modifications occur in Rough Endoplasmic Reticulum and Golgi Apparatus. Two subcategories of viral genes are essential for synthesizing all virally encoded proteins and replicating the viral genome. The viral genes required for protein synthesis and viral DNA replication are categorized as α or Immediate Early genes and β or Early genes. There are five identifiable Immediate Early gene products known as the Infected Cell Peptides (ICPs): ICP0, ICP4, ICP22, ICP27, ICP47 and US 1.5. Out of this list of proteins, ICP22 was recently studied in context with ocular infection and shown to have strong immune modulatory roles (Matundan & Ghiasi, 2019; Matundan et al., 2019). In combination, these six gene products act as transactivators of the Early genes. The Early genes include viral DNA polymerase, ICP8, DNA-helicase-primase, and the origin binding protein(Boehmer & Lehman, 1997; Koganti et al., 2019). Following the translation of the Early gene products, viral DNA synthesis embarks. Viral DNA synthesis relinquishes through the expression and presence of the third category of HSV-1 gene products known as γ or Late genes(Boehmer & Lehman, 1997; Koganti et al., 2019).
At the start of replication, the linear DNA of HSV-1 becomes circular, which facilities DNA replication to occur at one of the three origin sites. After that, viral DNA polymerase starts replication at an origin site by creating a lengthy DNA concatemer consisting of several extensive, branched networks of replication forks (Boehmer & Lehman, 1997; Koganti et al., 2019). The concatemeric DNA is then cleaved and packaged, by Late gene products, into viral capsids in a well-regulated process that ensures each new capsid is filled with one genome equivalent of viral DNA. Afterward, the packaged HSV-1 viral capsid can exit the nucleus via the nuclear envelope either by nuclear pores or budding (Boehmer & Lehman, 1997).
HSV-1 Egress from Infected Cells
After viral protein synthesis and DNA replication, some of the structural proteins and glycoproteins are transported back into the nucleus. They participate in viral nucleocapsid synthesis in the nucleus. The glycoproteins facilitate capsid transport out of the nucleus into the cytoplasm, where the capsid, tegument proteins and glycoproteins are packaged in vesicles and sent to the surface of the host cell membrane. The viral capsid acquires its envelope from budding out of the plasma membrane (Lv et al., 2019). Viral progenies can then leave the host cell to infect neighboring cells.
HSV-1 glycoproteins are also important for cell-to-cell spread of HSV-1 virions, which often requires a fusion pore formation. Along with its surface receptors, gD as well as gB, gH-gL were shown to be important for the fusion pore formation. Interestingly, gK takes over an important function during viral egress as it coordinates the recruitment of other viral glycoproteins into intracellular virus assembly and transport of the virions across the membrane. In context with ocular infection it is known to exacerbate eye disease (Jaggi et al., 2018) and in the process, likely compromises the tissue protective and homeostatic functions of OPTN, a possibility that future studies will need to address.
Recent data show that Heparanase (HPSE), a host enzyme, plays a vital role in HSV-1 egress. In human corneal epithelial cells (HCEs) HPSE is upregulated upon HSV-1 infection. It is believed that HPSE assists in the pathogenesis of HSV-1 by alleviating viral egress by cleaving HS residues on the surface of infected cells (Agelidis et al., 2017; Hadigal et al., 2015). Removal of HS ensures that virions are not trapped by the mother cell, instead they are released freely to find other, non-infected cells (Agelidis et al., 2017; Hadigal et al., 2015). In addition, HPSE has been implicated in perturbing specific anti-viral innate immune responses such as Type-1 Interferon response while stimulating proinflammatory cytokine production. It is also shown to interfere with DNA damage response (Agelidis et al., 2021a; Agelidis et al., 2021b). Taken together, HPSE has hence been implicated as a host virulence factor that is a significant driver of HSV-1 pathogenesis by inducing a diseased state.
Due to the efficiency by which HSV-1 can infect ocular cells, hijack host cellular mechanisms and spread to nearby cells for viral dissemination, a primary infection in the eye can easily allow the virus to spread to the TG and possibly, the CNS (Farooq & Shukla, 2011). The latter, however, is not normally observed. Recent data show that host protein, OPTN plays a major role in reducing viral spread from the eye to the CNS and preventing the death of neurons (Ames, Yadavalli, Patil, et al., 2021; Ames, Yadavalli, Suryawanshi, et al., 2021).
OPTN
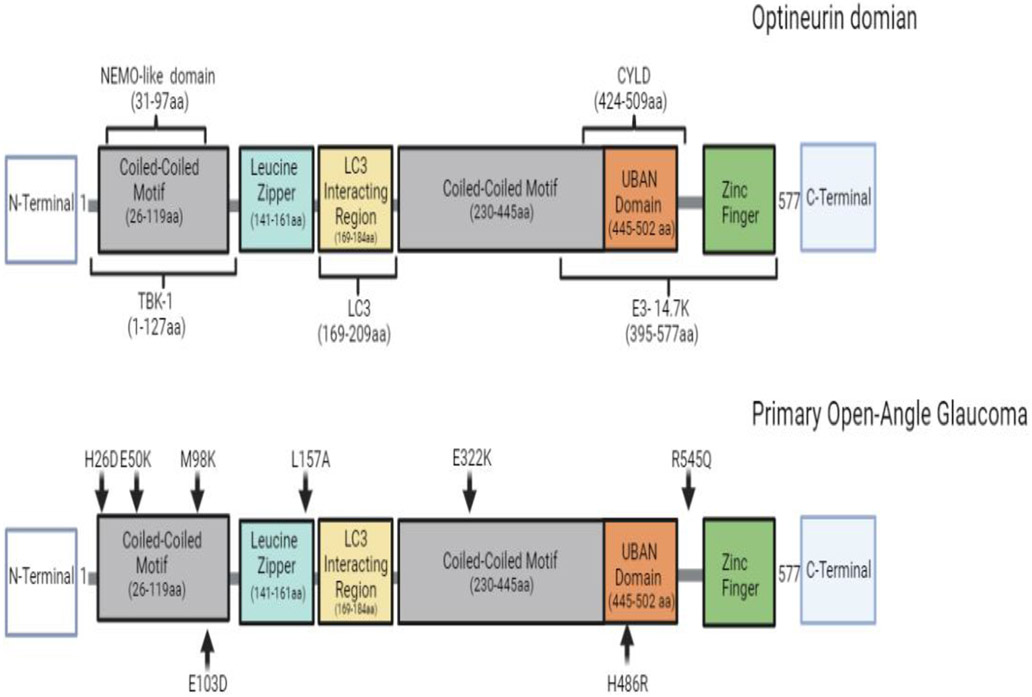
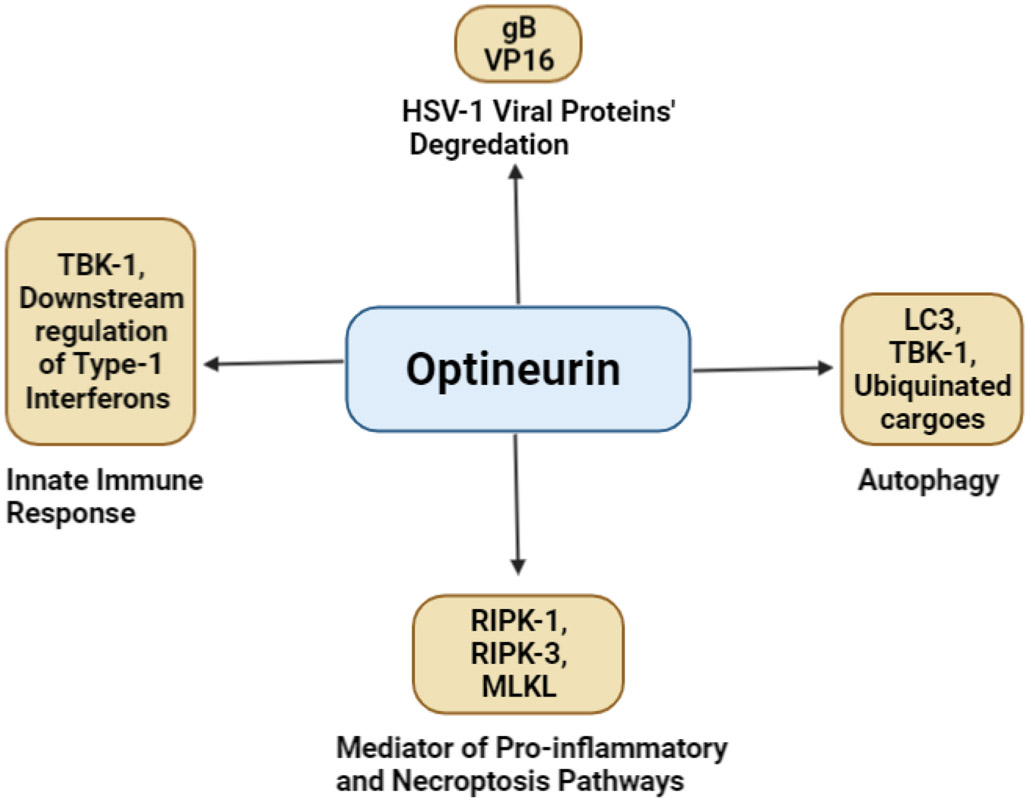
OPTN is a ubiquitous protein found in all human organs such as the brain, eye, heart, liver, and kidney. It is highly conserved and contains 577 amino acids which form a 67 kDa (Ying & Yue, 2012; Slowicka & van Loo, 2018) (Figure 3). OPTN was first revealed at the nuclear membrane as a binding partner for adenoviral protein E3-14.7K and previously named “FIP2” (E3-14.7K-interacting protein). However, the protein’s name was changed to Optineurin (optic neuropathy inducing) because of its clinical relevance in primary open-angle glaucoma(Li et al., 1998; Slowicka & van Loo, 2018). Patients suffering from primary open-angle glaucoma were later revealed to have a mutation in the optn gene. To date, mutations in the optn gene or deficiency in the expression or binding domains of OPTN have also been implicated in several neurodegenerative diseases such as ALS, Paget's disease of the bone, and FTD (Ito et al., 2016; McCauley & Baloh, 2019; Minegishi et al., 2016; Oakes et al., 2017; Rezaie et al., 2002b; Toth & Atkin, 2018). Over time, OPTN has been recognized as multifunctional proteins involved in maintaining homeostasis. It functions in several cellular processes, namely, autophagy, cell division, inflammatory signaling, vesicular trafficking, and anti-pathogen signaling and responses (Figure 4). To pursue such functions OPTN has various binding domains and partners. Very recently OPTN was shown to have a protective role as an autophagy receptor in HSV-1 ocular and neuronal infections (Ames, Yadavalli, Patil, et al., 2021; Ames, Yadavalli, Suryawanshi, et al., 2021). These findings not only highlighted OPTN’s antiviral role in the eye, but the results also suggested that in the absence of OPTN, HSV-1 infection worsens and can induce a clinical manifestation of the disease in the CNS that shares phenotypic similarity to several neurodegenerative disease such as HSE and ALS (Ames, Yadavalli, Suryawanshi, et al., 2021). This study’s findings put OPTN at the forefront of the future investigation of ocular and neurotropic diseases. More specifically, this study’s findings highlight the continued need to investigate the multifunctional properties that enable OPTN to act as a potential protective host factor against HSV-1.
Figure 3.
Optineurin domain structure and loss-of function point mutations. OPTN is a 65-kDa hexameric protein and is comprised of 577 amino acids. Loss-of function point mutations in OPTN's domain that have been implicated in patients with POAG.
Figure 4.
Optineurin’s multifaceted role in cellular mechanisms that promote homeostasis and prevent Herpes Simplex Virus-1 infection.
OPTN in Innate and Adaptive Immune Responses
A major innate immune mechanism regulated by OPTN is mediated by TANK-binding kinase 1 (TBK1) pathway. The first 127 amino acids of OPTN contain a TBK-1 binding site. TBK1 functions in the innate immune response by regulating the production of Type 1 Interferons (IFNα and IFNβ). Type 1 Interferons in a well-regulated state act as pro-survival cytokines responsible for inducing cells’ antipathogenic state in-self and neighboring cells and recruiting immune cells to areas of impeding danger (Ahmad et al., 2016). The down regulation of interferons can cause advancement of the manifestation of damage or disease (Ahmad et al., 2016; Meena et al., 2016; Oakes et al., 2017). Pathogen Associated Molecular Pattern recognition induces the polyubiquitination and activation via transautophorylation of TBK-1. This induction of TBK-1 results in the upregulation and secretion of Type 1 Interferons. This mechanism is used in the innate response to bacterial and viral infections (Bakshi et al., 2017; Meena et al., 2016; Richter et al., 2016).
It is intriguing that loss of function mutations in human TBK-1 have been linked to the increased susceptibility and development of HSE during childhood (Oakes et al., 2017). However, there has been much controversy over the relationship and mechanism by which OPTN influences TBK-1 and Type 1 Interferon response. There have been distinct differences in the pathogen-induced cytokine production and immune response that results in the presence and absence of OPTN, and much of it can be attributed to the differences in investigation models. For example, an earlier investigation reported that during RNA virus infection, the overexpression of OPTN in HEK293-HTLR3 cells resulted in perturbed TBK-1 activity and IFN-β response whereas, silencing of Optineurin enhanced IFN-β production. This finding was later supported by an investigation that identified OPTN as a negative regulator of TBK-1 and INF-β secretion during RNA virus infection of HeLa cells (Génin et al., 2015; Mankouri et al., 2010). On the other hand, two mouse model investigations reported that OPTN was needed for efficient TBK-1 activation and IFN-β production. In short, both models obstructed the ubiquitin-binding domain of OPTN, one model had a point mutation in the ubiquitin-binding domain (optnD477N), and the other model deleted the entire C-terminal region (optn470T). Their combined results showed that in vivo, mutations to the ubiquitin-binding domain of OPTN diminished bone marrow-derived macrophages and dendritic cells’ TBK-1 activity and IFN-β production upon TLR stimulation (Gleason et al., 2011; Munitic et al., 2013). A more recent investigation using mutant mice with the TBK-1 interacting N-terminus residues 1-157 removed (optn Δ157) had reduced TBK-1 activity, no phosphorylation of OPTN at Ser187 and expressed low levels of INF-β responses (Meena et al., 2016). Taken together, the in vivo results reveal that OPTN is required for optimal TBK-1 activity.
Ocular herpes infection in an immunocompetent host results in the induction and lifelong persistence of robust antiviral adaptive immune response. Virus-specific as well as non-specific T lymphocytes (CD4+ and CD8+ T cells) play important roles in protecting the host against serious consequences of HSV-1 especially during the virus reactivation. At the same time, they also contribute to the immune pathologies associated with HSV keratitis. More specifically, CD4+ T cells are considered as the key drivers of herpetic lesion development in the corneal stroma (Lobo, Agelidis & Shukla, 2019). While adaptive immunity against HSV-I is clearly important, very little knowledge exists on the role of OPTN in adaptive immune system. One of the initial evidence that OPTN may have a role in T cell functions against ocular HSV was reported recently. It was demonstrated that mice lacking OPTN showed a marked decrease in the recruitment of CD4+ and CD8+ T cells to the brain (Ames et al., 2021; Patil & Shukla, 2022), which resulted in a significant increase in animal death, partly due to encephalitis. This possibility was given further credence by Patil et al. (2022) who demonstrated dysregulated T cell frequencies in HSV-2 infected tissues and lymph nodes of animals lacking OPTN. Future studies are likely to shed more light on OPTN’s role in modulating ocular HSV infection.
OPTN in Pro-inflammatory Pathway
Recent studies have focused on OTPN’s role in neuroinflammation and neurodegenerative pathologies (Slowicka & van Loo, 2018). More recently, OPTN was shown to play a role in ocular HSV-1 induced neuroinflammation leading to necroptosis (Ames, Yadavalli, Patil, et al., 2021; Ames, Yadavalli, Suryawanshi, et al., 2021; Ito et al., 2016; Mifflin et al., 2021). OPTN's role in the NF-κB regulated inflammation pathway may be complex. NF-κB is a transcription factor considered an integrated regulator of inflammation and immune response in the cellular environment. In a normal state IKB typically is bound to, and inhibits, NF-κB in the cytoplasm. Upon inflammatory stimulus, NF-κB translocates from the cytoplasm to the nucleus to stimulate the transcription of proinflammatory cytokines (Baldwin, 1996; Schwamborn et al., 2000). NF-κB is regulated by the NF-κB essential modulator (NEMO) in NEMO-dependent and NEMO-independent pathways. The NEMO-dependent pathway is regulated by a kinase complex which consists of the NEMO adaptor protein (IKKγ) and two IκB kinases (IκKα and IκKβ). Upon inflammatory signaling, the NEMO-IKB kinases complex is activated. After that, the IκKα and IκKβ subunit phosphorylate IκB which has NF-κB bound, this phosphorylation causes the release of NF-κB and allows for its nuclear translocation (Baldwin, 1996; Schwamborn et al., 2000).
OPTN and NEMO have homology in more than half of their amino acid sequence, so it was initially believed that OPTN would directly affect the functionality of NF-KB. It was previously hypothesized that this affinity might competitively inhibit NEMO's binding with the IKB kinases; thus, obstructing the NEMO-dependent NF-κB pathway. However, it was discovered that OPTN does not bind to either of the IKB kinases or NEMO (Slowicka & van Loo, 2018; Zhu et al., 2007). Hence, earlier studies failed to correlate OPTN and NF-KB activation directly. Interestingly, some parallel studies have implicated OPTN as a negative regulator of a NEMO regulated NF-κB activation pathway. This pathway is dependent on Tumor Necrosis Factor (TNF-α) binding to the TNF receptor (TNFR1). NEMO binds to K63-linked polyubiquitinated (poly Ub) receptor interacting protein kinase (RIPK-1), which binds to TNFR1. In one interesting study, investigators were able to show that in the presence of polyubiquitinated RIPK-1, both NEMO and highly expressed OPTN bound to distinct complexes of polyUb RIPK-1. This finding acknowledged OPTN as a competitive inhibitor of NEMO. Additionally, investigators showed that in HeLa cells stimulated by TNF-α, OPTN and NEMO both bind to the signaling complex of TNFR1 (Zhu et al., 2007). Since NEMO binding to polyUb RIPK-1 is essential for TNF-α induced NF-κB activation, the study implicated OPTN for having influence on the NF-κB activation pathway. However, continuous TNF-α stimulation, caused by prolonged cell damage, pathogenic infection, or disease, can cause dysregulation of this mechanism resulting in RIPK-1’s de-ubiquitination by deubiquitinases. RIPK-1 regulates necroptosis through inducing the activation of two downstream targets, RIPK-3 and mixed lineage kinase domain-like protein (MLKL). A paradigm shift occurs in which cellular pro-survival and pro-inflammatory responses become mediators of cell death. Thus, RIPK-1 can be implicated as a major regulator in the process of necrotic cell death, necroptosis (Ito et al., 2016; Mifflin et al., 2021).
OPTN as an Autophagy Receptor
Amongst the various cellular functions that OPTN contributes to, OPTN’s role as an autophagy receptor has garnered immense interest. Autophagy is a pro-survival mechanism of the cell to combat intracellular and extra cellular stressors. However, dysregulation of this cellular process can cause neurodegenerative pathologies (Levine et al., 2011). Selective autophagy is a tightly regulated process that relies on the contribution of autophagy proteins that recognize specific cargoes that need to be selectively degraded and recycled (Khaminets et al., 2016; Stolz et al., 2014). Induction of autophagy includes a double-membraned structure called a phagophore that develops into an autophagosome, which eventually fuses with the lysosome to form an autolysosome to degrade the cargo (Khaminets et al., 2016; Levine et al., 2011; Stolz et al., 2014). The cargo is initially recruited using autophagy receptors. Then, autophagy receptors connect ubiquitin-like microtubule-associated protein light chain (LC3) with identified ubiquitinated cargo. As aforementioned, OPTN is an autophagy receptor, and it interacts with LC3 via its LC3 interacting motif (LIR), and phosphorylation of OPTN increases its affinity for LC3 binding. Notably, phosphorylation of OPTN by TBK-1 promotes its function in autophagy-mediated clearance of pathogens and mutant protein aggregates(Oakes et al., 2017; Richter et al., 2016; Wild et al., 2011).
OPTN in Glaucoma
The eye, like other organs of the body, rely on autophagy to regulate its cellular content and maintain homeostasis (Frost et al., 2014; Lee et al., 2013; Trichonas et al., 2010). Glaucoma, a blinding disease, is another degenerative disease that OPTN has been implicated for playing a role in its disease pathology (Minegishi et al., 2016; Rezaie et al., 2002b; Toth & Atkin, 2018; Wiggs & Pasquale, 2017). Mutations in OTN have been implicated as a contributor to the onset of glaucoma. A glaucoma-associated mutant of OPTN, E50K, induces abnormal autophagy signaling that plays a role in the death of Retinal Ganglion Cells (RGCs) and thus glaucoma pathogenesis (Figure 3). Previously, E50K-OPTN expressing transgenic mice models have been developed, these mice only experience the death of RGCs similar to the patients with glaucoma, but they do not experience increased optical pressure. More recently, a knock-in, E50K-OPTN expressing mouse model was developed that utilized CRISPR/Cas9. Unlike the E50K-OPTN transgenic mice, these mice do not show thinning of the retina, but experience loss of RGC fiber layer near the optic nerve head (Minegishi et al., 2016; Rezaie et al., 2002b; Wiggs & Pasquale, 2017; Zhang et al., 2021). Additionally, M98K-OPTN mutation was also implicated in patients with glaucoma, and it is believed that this mutation may attribute to autophagy-dependent retinal cell death. However, this OPTN mutation is implicated more with Asian patients with glaucoma than Caucasian patients, which indicates that M98K polymorphism functions with an additional, unique genotypic change that stimulates glaucoma (He et al., 2019).
OPTN: an Integral Player in Ocular and Neuronal Survival from HSV-1 infection
Earlier studies implicated OPTN as potentially detrimental to cells during RNA virus infection (Mankouri et al., 2010); however, a contrasting result was reported with Salmonella infection (Slowicka et al., 2016). Interestingly, last year it was demonstrated that OPTN was critical to ocular and neuronal cell survival during HSV-1 infection, and this was accomplished through selective autophagy (Ames, Yadavalli, Suryawanshi, et al., 2021). The study results revealed that optn−/− cells had higher VP16 and gB, but not ICP0, implicating that the lack of OPTN contributed to the enhanced presence of some viral proteins but not all. In contrast, VP16 levels in optn+/+ cells were significantly higher when autophagy was blocked, suggesting that OPTN-mediated autophagy played a role in the degradation of viral proteins (Ames, Yadavalli, Suryawanshi, et al., 2021). Also of note, is that investigators showed that TBK-1 first interacted with OPTN then OPTN interacted with VP16 and when TBK-1 was inhibited, the total level of OPTN and phosphorylated OPTN was decreased in an infection dependent manner, thus implicating that OPTN is regulated by TBK-1.
To further support their in vitro studies, the investigators used optn+/+ and optn−/− mouse models in which the animals were infected in the eye with HSV-1. The brain, brain stem and TG from infected mice were examined. The optn−/− mice experienced far more severe infection pathologies than optn+/+ mice. Although both groups of mice experienced infection in all three brain regions, optn−/− mice demonstrated significant infection dissemination to the brainstem and brain. Additionally, optn−/− mice experienced significant weight loss and increased mortality compared to optn+/+. Neuronal cell degeneration in the brainstem was also a consequence of the severe disease pathology optn−/− mice experienced, whereas optn+/+ mice did not show signs of neuronal cell death in their brainstem, implicating that having OPTN in the brainstem was advantageous and protective during HSV-1 infection of the CNS (Ames, Yadavalli, Suryawanshi, et al., 2021). The finding was further supported when the RIPK-1 inhibitor, necrostatin, was given to another experimental group of optn−/− mice during infection in that they did not experience the same neuronal degeneration pathology as the untreated optn−/− mouse group that remained untreated. Necrostatin treatment salvaged neuronal cells of HSV-1 infected optn−/− mice implicates that in the absence of OPTN, HSV-1 induces necroptosis in the CNS (Ames, Yadavalli, Suryawanshi, et al., 2021). Since the investigators established that the RIPK-1 necroptosis pathway was being induced during HSV-1 infection and previous studies have implicated mutations in OPTN and RIPK-1 contributing to neurodegeneration in ALS, the investigators wanted to identify if there is a similarity in the disease pathologies of brain stem tissues of patients with ALS and patients HSE. Compared to normal brain tissue, the brainstem tissue of the patient with ALS and the patient with HSE had an accumulation of OPTN aggregates, whereas the control tissue did not (Ames, Yadavalli, Suryawanshi, et al., 2021). Moreover, investigators used a novel-object recognition test to identify if the neuronal degeneration that HSV-1 infected optn−/− mice experienced affected their cognitive ability to explore new objects. By 30 days post infection the HSV-1 infected optn−/− mice failed the test (Ames, Yadavalli, Suryawanshi, et al., 2021). The investigators were also able to identify that optn+/+ could proliferate and recruit T cells to the CNS more than optn−/− mice (Ames, Yadavalli, Suryawanshi, et al., 2021).
Manifestation of HSK pathologies is attributed to an over activated inflammatory response which can lead to blindness (Buela & Hendricks, 2015). In a separate study, optn+/+ and optn−/− mice were infected with HSV-1 in the eye, and their ocular disease pathology was observed for 30 days. The study revealed that optn−/− experienced a complete loss of cornea and bilateral whisker sensitivity from 4dpi until 26dpi, whereas optn+/+ mice experienced slight loss (Ames, Yadavalli, Patil, et al., 2021). Although optn−/− presented a more severe HSK, there was no significant difference between viral replication nor virus titers in the eye at 4dpi and 8dpi, and the histology revealed that both groups of mice experience similar levels of corneal inflammation and thickening(Ames, Yadavalli, Patil, et al., 2021). Additionally, optn−/− mice experience more distressing oral disease pathologies such as complete opacification of the infected eye by 8dpi optn−/− mice. Taken together, these results suggested that in the eye, OPTN’s influence prioritized protecting corneal sensitivity and possibly CNS infiltration(Ames, Yadavalli, Patil, et al., 2021). Since the eye is an immune-privileged organ, it should be noted that OPTN and the immune responses are possibly regulated by a different mechanism than other regions of the body; thus, there may be some organ-specific mechanisms at play(Ames, Yadavalli, Patil, et al., 2021).
Conclusions
As HSV-1 continues to present distressing ocular and neurodegenerative disease pathologies in a large section of human population, there exists an extreme need to continue to seek out host cellular pathways, which positively or negatively regulate viral replication and transmission (Farooq & Shukla, 2011, 2012; Giménez et al., 2013; Koujah et al., 2019; Krishnan & Stuart, 2021; Liesegang, 2001; Steiner, 2013). OPTN was identified as a multifaceted host protein that regulates several essential host processes such as vestibular trafficking, interferon signaling, inflammation, necroptosis, and autophagy (Slowicka & van Loo, 2018). Notably, new interest in OPTN’s role as an autophagy receptor has revealed that OPTN plays a crucial protective role in clearance of HSV-1 from the host’s eye. In the absence of OPTN, HSV-1 infection in the eye of experimental mice presents severe disease pathologies locally as well as in the CNS. Future studies should thus continue incorporating various models that explore OPTN’s role in ocular cell and tissue types to expand current knowledge and gain a firm understanding of OPTN’s role in ocular health and neurotropic HSV-1 infection.
Highlights:
Herpes simplex virus (HSV) is a leading cause of infection-associated blindness.
It is a DNA virus capable of replicating in the nuclei of virtually all ocular cell types.
The virus exploits several different cellular proteins to establish a productive infection.
Optineurin, a selective autophagy receptor, is required for maintaining ocular homeostasis.
Optineurin is required for protection against HSV-induced nerve damage in the eye and the brain.
Acknowledgement
This work was supported by the NIH grants RO1 EY029426, P30 EY001792 and RO1 EY024710 (to D.S.). E.G. was supported by R25 GM-121212. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agelidis AM, Hadigal SR, Jaishankar D, & Shukla D (2017). Viral Activation of Heparanase Drives Pathogenesis of Herpes Simplex Virus-1. Cell Reports, 20(2), 439–450. 10.1016/j.celrep.2017.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agelidis A, Suryawanshi RK, Patil CD, Campeau A, Gonzalez DJ, & Shukla D (2021). Dissociation of DNA damage sensing by endoglycosidase HPSE. iScience. Feb 27;24(3):102242. doi: 10.1016/j.isci.2021.102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agelidis A, Turturice BA, Suryawanshi RK, Yadavalli T, Jaishankar D, Ames J, Hopkins J, Koujah L, Patil CD, Hadigal SR, Kyzar EJ, Campeau A, Wozniak JM, Gonzalez DJ, Vlodavsky I, Li JP, Perkins D,L,, Finn PW, & Shukla D (2021). Disruption of innate defense responses by endoglycosidase HPSE promotes cell survival. JCI Insight. Apr 8;6(7):e144255. doi: 10.1172/jci.insight.144255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad L, Zhang S-Y, Casanova J-L, & Sancho-Shimizu V (2016). Human TBK1: A Gatekeeper of Neuroinflammation. Trends in Molecular Medicine, 22(6), 511–527. 10.1016/j.molmed.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames J, Yadavalli T, Patil C, Hopkins J, Bhattacharya I, & Shukla D (2021). OPTN limits herpes stromal keratitis severity and demyelination through negative regulation of IL-17 and hyperinflammatory T-cell response. BioRxiv, 1–14. [Google Scholar]
- 6.Ames J, Yadavalli T, Suryawanshi R, Hopkins J, Agelidis A, Patil C, Fredericks B, Tseng H, Valyi-Nagy T, & Shukla D (2021). OPTN is a host intrinsic restriction factor against neuroinvasive HSV-1 infection. Nature Communications, 12(1), 5401. 10.1038/s41467-021-25642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atanasiu D, Saw WT, Cohen GH, & Eisenberg RJ (2010). Cascade of Events Governing Cell-Cell Fusion Induced by Herpes Simplex Virus Glycoproteins gD, gH/gL, and gB. Journal of Virology, 84(23), 12292–12299. 10.1128/JVI.01700-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakshi S, Taylor J, Strickson S, McCartney T, & Cohen P (2017). Identification of TBK1 complexes required for the phosphorylation of IRF3 and the production of interferon β. Biochemical Journal, 474(7), 1163–1174. 10.1042/BCJ20160992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin AS (1996). THE NF-κB AND IκB PROTEINS: New Discoveries and Insights. Annual Review of Immunology, 14(1), 649–681. 10.1146/annurev.immunol.14.1.649 [DOI] [PubMed] [Google Scholar]
- 10.Boehmer PE, & Lehman IR (1997). HERPES SIMPLEX VIRUS DNA REPLICATION. Annual Review of Biochemistry, 66(1), 347–384. 10.1146/annurev.biochem.66.1.347 [DOI] [PubMed] [Google Scholar]
- 11.Buela K-AG, & Hendricks RL (2015). Cornea-Infiltrating and Lymph Node Dendritic Cells Contribute to CD4 + T Cell Expansion after Herpes Simplex Virus-1 Ocular Infection. The Journal of Immunology, 194(1), 379–387. 10.4049/jimmunol.1402326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, & Cohen GH (2011). Capturing the Herpes Simplex Virus Core Fusion Complex (gB-gH/gL) in an Acidic Environment. Journal of Virology, 85(13), 6175–6184. 10.1128/JVI.00119-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, & Cohen GH (2012). Herpes virus fusion and entry: a story with many characters. Viruses, 4(5), 800–832. 10.3390/v4050800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooq A. v, & Shukla D (2011). Corneal latency and transmission of herpes simplex virus-1. Future Virology, 6(1), 101–108. 10.2217/fvl.10.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooq A. v., & Shukla D (2012). Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Survey of Ophthalmology, 57(5), 448–462. 10.1016/j.survophthal.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost LS, Mitchell CH, & Boesze-Battaglia K (2014). Autophagy in the eye: Implications for ocular cell health. Experimental Eye Research, 124, 56–66. 10.1016/j.exer.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangappa S, Deshpande SP, & Rouse BT (2000). Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Investigative Ophthalmology & Visual Science, 41(2), 453–459. [PubMed] [Google Scholar]
- 18.Génin P, Cuvelier F, Lambin S, Côrte-Real Filipe J, Autrusseau E, Laurent C, Laplantine E, & Weil R (2015). Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner. PLOS Pathogens, 11(4), e1004877. 10.1371/journal.ppat.1004877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giménez F, Suryawanshi A, & Rouse BT (2013). Pathogenesis of herpes stromal keratitis – A focus on corneal neovascularization. Progress in Retinal and Eye Research, 33, 1–9. 10.1016/j.preteyeres.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleason CE, Ordureau A, Gourlay R, Arthur JSC, & Cohen P (2011). Polyubiquitin Binding to Optineurin Is Required for Optimal Activation of TANK-binding Kinase 1 and Production of Interferon β. Journal of Biological Chemistry, 286(41), 35663–35674. 10.1074/jbc.M111.267567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadigal SR, Agelidis AM, Karasneh GA, Antoine TE, Yakoub AM, Ramani VC, Djalilian AR, Sanderson RD, & Shukla D (2015). Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nature Communications, 6(1), 6985. 10.1038/ncomms7985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He JN, Lu S, Chen LJ, Tam POS, Zhang BN, Leung CKS, Pang CP, Tham CCY, & Chu WK (2019). Coding Region Mutation Screening in Optineurin in Chinese Normal-Tension Glaucoma Patients. Disease Markers, 2019, 1–5. 10.1155/2019/5820537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, Geng J, Amin P, DeWitt JP, Mookhtiar AK, Florez M, Ouchida AT, Fan J, Pasparakis M, Kelliher MA, … Yuan J (2016). RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science, 353(6299), 603–608. 10.1126/science.aaf6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaggi U, Wang S, Tormanen K, Matundan H, Ljubimov AV, & Ghiasi H (2018). Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8+ T Cells in Exacerbation of Eye Disease. Front Immunol. 9:2895. doi: 10.3389/fimmu.2018.02895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jambunathan N, Clark CM, Musarrat F, Chouljenko VN, Rudd J, & Kousoulas KG (2021). Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion. Viruses, 13(9). 10.3390/v13091849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaminets A, Behl C, & Dikic I (2016). Ubiquitin-Dependent And Independent Signals In Selective Autophagy. Trends in Cell Biology, 26(1), 6–16. 10.1016/j.tcb.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Koganti R, Yadavalli T, & Shukla D (2019). Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms, 7(10), 429. 10.3390/microorganisms7100429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koujah L, Allaham M, Patil CD, Ames JM, Suryawanshi RK, Yadavalli T, Agelidis A, Mun C, Surenkhuu B, Jain S, & Shukla D (2021). Entry receptor bias in evolutionarily distant HSV-1 clinical strains drives divergent ocular and nervous system pathologies. The Ocular Surface, 21, 238–249. 10.1016/j.jtos.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koujah L, Suryawanshi RK, & Shukla D (2019). Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cellular and Molecular Life Sciences, 76(3), 405–419. 10.1007/s00018-018-2938-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer T, & Enquist L (2013). Directional Spread of Alphaherpesviruses in the Nervous System. Viruses, 5(2), 678–707. 10.3390/v5020678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan R, & Stuart PM (2021). Developments in Vaccination for Herpes Simplex Virus. Frontiers in Microbiology, 12. 10.3389/fmicb.2021.798927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y-S, Dayma Y, Park M-Y, Kim K. il, Yoo S-E, & Kim E (2013). Daxx is a key downstream component of receptor interacting protein kinase 3 mediating retinal ischemic cell death. FEBS Letters, 587(3), 266–271. 10.1016/j.febslet.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Mizushima N, & Virgin HW (2011). Autophagy in immunity and inflammation. Nature, 469(7330), 323–335. 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Kang J, & Horwitz MS (1998). Interaction of an Adenovirus E3 14.7-Kilodalton Protein with a Novel Tumor Necrosis Factor Alpha-Inducible Cellular Protein Containing Leucine Zipper Domains. Molecular and Cellular Biology, 18(3), 1601–1610. 10.1128/MCB.18.3.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liesegang TJ (2001). Herpes Simplex Virus Epidemiology and Ocular Importance. Cornea, 20(1), 1–13. 10.1097/00003226-200101000-00001 [DOI] [PubMed] [Google Scholar]
- 36.Lobo AM, Agelidis AM, & Shukla D (2019). Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 17(1):40–49. doi: 10.1016/j.jtos.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludema C, Cole SR, Poole C, Smith JS, Schoenbach VJ, & Wilhelmus KR (2014). Association Between Unprotected Ultraviolet Radiation Exposure and Recurrence of Ocular Herpes Simplex Virus. American Journal of Epidemiology, 179(2), 208–215. 10.1093/aje/kwt241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv Y, Zhou S, Gao S, & Deng H (2019). Remodeling of host membranes during herpesvirus assembly and egress. Protein & Cell, 10(5), 315–326. 10.1007/s13238-018-0577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mankouri J, Fragkoudis R, Richards KH, Wetherill LF, Harris M, Kohl A, Elliott RM, & Macdonald A (2010). Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection. PLoS Pathogens, 6(2), e1000778. 10.1371/journal.ppat.1000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matundan H, Ghiasi H, (2019). Herpes Simplex Virus 1 ICP22 Suppresses CD80 Expression by Murine Dendritic Cells. J Virol. 17;93(3):e01803–18. doi: 10.1128/JVI.01803-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matundan HH, Jaggi U, Wang S, & Ghiasi H (2019). Loss of ICP22 in HSV-1 Elicits Immune Infiltration and Maintains Stromal Keratitis Despite Reduced Primary and Latent Virus Infectivity. Invest Ophthalmol Vis Sci. Aug 1;60(10):3398–3406. doi: 10.1167/iovs.19-27701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCauley ME, & Baloh RH (2019). Inflammation in ALS/FTD pathogenesis. Acta Neuropathologica, 137(5), 715–730. 10.1007/s00401-018-1933-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meena NP, Zhu G, Mittelstadt PR, Giardino Torchia ML, Pourcelot M, Arnoult D, Ashwell JD, & Munitic I (2016). The TBK1-binding domain of optineurin promotes type I interferon responses. FEBS Letters, 590(10), 1498–1508. 10.1002/1873-3468.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mifflin L, Hu Z, Dufort C, Hession CC, Walker AJ, Niu K, Zhu H, Liu N, Liu JS, Levin JZ, Stevens B, Yuan J, & Zou C (2021). A RIPK1-regulated inflammatory microglial state in amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences, 118(13), e2025102118. 10.1073/pnas.2025102118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minegishi Y, Nakayama M, Iejima D, Kawase K, & Iwata T (2016). Significance of optineurin mutations in glaucoma and other diseases. Progress in Retinal and Eye Research, 55, 149–181. 10.1016/j.preteyeres.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 46.Munitic I, Giardino Torchia ML, Meena NP, Zhu G, Li CC, & Ashwell JD (2013). Optineurin Insufficiency Impairs IRF3 but Not NF-κB Activation in Immune Cells. The Journal of Immunology, 191(12), 6231–6240. 10.4049/jimmunol.1301696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oakes JA, Davies MC, & Collins MO (2017). TBK1: a new player in ALS linking autophagy and neuroinflammation. Molecular Brain, 10(1), 5. 10.1186/s13041-017-0287-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patil CD, Suryawanshi R, Ames J, Koganti R, Agelidis A, Kapoor D, Yadavalli T, Koujah L, Tseng HC, & Shukla D (2022). Intrinsic Antiviral Activity of Optineurin Prevents Hyperproliferation of a Primary Herpes Simplex Virus Type 2 Infection. J Immunol. Jan 1;208(1):63–73. doi: 10.4049/jimmunol.2100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patil CD, & Shukla D (2022), OPTN (optineurin)-mediated selective autophagy prevents neurodegeneration due to herpesvirus infection. Autophagy. 15:1–2. doi: 10.1080/15548627.2022.2037223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psychological Stress and Other Potential Triggers for Recurrences of Herpes Simplex Virus Eye Infections. (2000). Archives of Ophthalmology, 118(12), 1617. 10.1001/archopht.118.12.1617 [DOI] [PubMed] [Google Scholar]
- 51.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, & Sarfarazi M (2002a). Adult-Onset Primary Open-Angle Glaucoma Caused by Mutations in Optineurin. Science, 295(5557), 1077–1079. 10.1126/science.1066901 [DOI] [PubMed] [Google Scholar]
- 52.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, & Sarfarazi M (2002b). Adult-Onset Primary Open-Angle Glaucoma Caused by Mutations in Optineurin. Science, 295(5557), 1077–1079. 10.1126/science.1066901 [DOI] [PubMed] [Google Scholar]
- 53.Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, Zaffagnini G, Wild P, Martens S, Wagner SA, Youle RJ, & Dikic I (2016). Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proceedings of the National Academy of Sciences, 113(15), 4039–4044. 10.1073/pnas.1523926113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rousseau A, Burrel S, Gueudry J, Deback C, Haigh O, Schweitzer C, Boutolleau D, Labetoulle M, Rousseau A, Burrel S, Gueudry J, Deback C, Orignac I, Vabres B, Mouriaux F, Labalette P, Bazard M-C, Gabison E, Guindolet D, … Touboul D (2022). Acyclovir-resistant HSV-1 keratitis: a concerning and emerging clinical challenge. American Journal of Ophthalmology. 10.1016/j.ajo.2022.01.010 [DOI] [PubMed] [Google Scholar]
- 55.Schwamborn K, Weil R, Courtois G, Whiteside ST, & Israël A (2000). Phorbol Esters and Cytokines Regulate the Expression of theNEMO-related Protein, a Molecule Involved in a NF-κB-independent Pathway. Journal of Biological Chemistry, 275(30), 22780–22789. 10.1074/jbc.M001500200 [DOI] [PubMed] [Google Scholar]
- 56.Shukla D, & Spear PG (2001). Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. Journal of Clinical Investigation, 108(4), 503–510. 10.1172/JCI13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slowicka K, & van Loo G (2018). Optineurin Functions for Optimal Immunity. Frontiers in Immunology, 9. 10.3389/fimmu.2018.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slowicka K, Vereecke L, Mc Guire C, Sze M, Maelfait J, Kolpe A, Saelens X, Beyaert R, & van Loo G (2016). Optineurin deficiency in mice is associated with increased sensitivity to Salmonella but does not affect proinflammatory NF-κB signaling. European Journal of Immunology, 46(4), 971–980. 10.1002/eji.201545863 [DOI] [PubMed] [Google Scholar]
- 59.Sodeik B, Ebersold MW, & Helenius A (1997). Microtubule-mediated Transport of Incoming Herpes Simplex Virus 1 Capsids to the Nucleus. Journal of Cell Biology, 136(5), 1007–1021. 10.1083/jcb.136.5.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steiner I (2013). Herpes virus infection of the peripheral nervous system (pp. 543–558). 10.1016/B978-0-444-52902-2.00031-X [DOI] [PubMed] [Google Scholar]
- 61.Stolz A, Ernst A, & Dikic I (2014). Cargo recognition and trafficking in selective autophagy. Nature Cell Biology, 16(6), 495–501. 10.1038/ncb2979 [DOI] [PubMed] [Google Scholar]
- 62.Toth RP, & Atkin JD (2018). Dysfunction of Optineurin in Amyotrophic Lateral Sclerosis and Glaucoma. Frontiers in Immunology, 9. 10.3389/fimmu.2018.01017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, & Vavvas DG (2010). Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proceedings of the National Academy of Sciences, 107(50), 21695–21700. 10.1073/pnas.1009179107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Wang R, Xu C, & Zhou H (2020). Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators. Frontiers in Immunology, 11, 766. 10.3389/fimmu.2020.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiggs JL, & Pasquale LR (2017). Genetics of glaucoma. Human Molecular Genetics, 26(R1), R21–R27. 10.1093/hmg/ddx184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wild P, Farhan H, McEwan DG, Wagner S, Rogov V. v., Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dötsch V, Bumann D, & Dikic I (2011). Phosphorylation of the Autophagy Receptor Optineurin Restricts Salmonella Growth. Science, 333(6039), 228–233. 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ying H, & Yue BY (2012). Cellular and molecular biology of optineurin. International review of cell and molecular biology, 294, 223–258. 10.1016/B978-0-12-394305-7.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang S, Shao Z, Liu X, Hou M, Cheng F, Lei D, & Yuan H (2021). The E50K optineurin mutation impacts autophagy-mediated degradation of TDP-43 and leads to RGC apoptosis in vivo and in vitro. Cell Death Discovery, 7(1), 49. 10.1038/s41420-021-00432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H, Gao S, Gong Y, Lin T, Tong S, Xiong W, Shi C, Wang W, & Fang J (2020). Anti-HSV-1 effect of dihydromyricetin from Ampelopsis grossedentata via the TLR9-dependent anti-inflammatory pathway. Journal of Global Antimicrobial Resistance, 23, 370–376. 10.1016/j.jgar.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 70.Zhu G, Wu C-J, Zhao Y, & Ashwell JD (2007). Optineurin Negatively Regulates TNFα- Induced NF-κB Activation by Competing with NEMO for Ubiquitinated RIP. Current Biology, 17(16), 1438–1443. 10.1016/j.cub.2007.07.041 [DOI] [PubMed] [Google Scholar]