Abstract
With 9 million hogs, North Carolina (NC) is the second leading hog producer in the United States. Most hogs are housed at concentrated animal feeding operations (CAFOs), where millions of tons of hog waste can pollute air and water with fecal pathogens that can cause diarrhea, vomiting, and/or nausea (known as acute gastrointestinal illness (AGI)). We used NC’s ZIP code-level emergency department (ED) data to calculate rates of AGI ED visits (2016–2019) and swine permit data to estimate hog exposure. Case exposure was estimated as the inverse distances from each hog CAFO to census block centroids, weighting with Gaussian decay and by manure amount per CAFO, then aggregated to ZIP code using population weights. We compared ZIP codes in the upper quartile of hog exposure (“high hog exposed”) to those without hog exposure. Using inverse probability of treatment weighting, we created a control with similar demographics to the high hog exposed population and calculated rate ratios using quasi-Poisson models. We examined effect measure modification of rurality and race using adjusted models. In high hog exposed areas compared to areas without hog exposure, we observed a 11% increase (95% CI: 1.06, 1.17) in AGI rate and 21% increase specifically in rural areas (95% CI: 0.98, 1.43). When restricted to rural areas, we found an increased AGI rate among American Indian (RR=4.29, 95% CI: 3.69, 4.88) and Black (RR=1.45, 95% CI: 0.98, 1.91) residents. The association was stronger during the week after heavy rain (RR=1.41, 95% CI: 1.19, 1.62) and in areas with both poultry and swine CAFOs (RR=1.52, 95% CI: 1.48, 1.57). Residing near CAFOs may increase rates of AGI ED visits. Hog CAFOs are disproportionally built near rural Black and American Indian communities in NC and are associated with increased AGI most strongly in these populations.
Keywords: CAFOs, swine, hog production, animal farming, environmental justice, gastrointestinal illness
Graphical Abstract
INTRODUCTION
North Carolina (NC) is the second leading hog producer in the United States, with approximately 9 million hogs. Most of the state’s hogs are housed, by the thousands, at large concentrated animal feeding operations (CAFOs) in eastern NC (Nicole, 2013). The massive amount of waste produced by these hogs, which exceeds the fecal waste produced by NC’s human population, is collected in uncovered pits, or lagoons, and sprayed on land as a fertilizer (Environmental Working Group & Waterkeeper Alliance, 2016). However, as the land cannot absorb all of the manure, these practices often spread pathogens and chemicals that invariably pollute the air and water (Wing et al., 2002). Communities that live near hog CAFOs have reported numerous health problems compared to residents who do not live near a hog CAFO, including throat, eye, and nose irritation, headaches, coughing, sore throats, diarrhea, methicillin-resistant S. aureus-related (MRSA) infections, higher risk of cardiovascular mortality, and reduced quality of life (Son et al., 2021a; Wing et al., 2008; Wing and Wolf, 2000). Drinking water contaminated with waterborne pathogens from hog waste or inhaling the sprayed waste in the air can result in diarrhea, vomiting, nausea, or other gastrointestinal tract distress in humans, known collectively as acute gastrointestinal illness (AGI)(Wing et al., 2000; Wing and Wolf, 2000). AGI can be severely painful and can disrupt work and school attendance for several days. In the US, approximately 2,330,000 AGI cases caused by waterborne pathogens occurred in 2014, with a direct healthcare cost of roughly $160 million (Collier et al., 2021). Despite the harm caused by AGI and the potential association between hog CAFOs and AGI, few studies have examined the effect of hog CAFO proximity and concentration on human AGI.
Numerous pathogens found in hog manure can cause AGI, including Escherichia coli O157:H7, Salmonella spp., Campylobacter spp., Yersinia enterocolitica, Cryptosporidium parvum, Giardia spp, and Clostridium difficile (Guan and Holley, 2003; Hooda et al., 2000; Keessen et al., 2013). After heavy rain events, surface water and groundwater near pigs or swine manure have been found to have higher concentrations of E. coli, indicating that heavy rain transports pathogens from hog CAFOs (Eisenhauer et al., 2016; Thurston-Enriquez et al., 2005). This suggests that the rate of AGI of residents near hog CAFOs may be especially high after heavy precipitation, especially for residents who have drunk contaminated well water or touched surfaces contaminated by runoff. While healthy humans are usually able to recover from AGI in 1–3 days without medical care, young children, older adults, and immunosuppressed people are at higher risk for severe illness from AGI (Jones et al., 2007; Lin et al., 2019; Schwartz et al., 1997; Stuempfig and Seroy, 2021).
Hog CAFOs, and the accompanying health issues related to living near hog CAFOs, are not distributed equally across NC; industrialized hog operations have been disproportionally built near communities of people of color (POC) in NC (Wing and Johnston, 2014). NC hog CAFOs are densely concentrated in several counties in the flood-prone eastern part of the state that are predominantly rural and are also home to many other harmful exposures such as poultry CAFOs and landfills (Figure 1) (Norton et al., 2007; Stingone and Wing, 2011; Wing and Johnston, 2014). Many of the NC counties with a high density of hog CAFOs also have poor healthcare access and a high percent of uninsured residents, which means reduced access to preventative care and increased risk for health issues (Hardy, 2012; “North Carolina County Health Profiles,” 2018). Because of the area’s rurality, many residents near CAFOs use private wells, which stand at higher risk of contamination than community water supplies (DeFelice et al., 2016; Wing et al., 2000). Each year, over $40 million are spent in NC on AGI emergency department (ED) visits due to microbial contamination in drinking water (DeFelice et al., 2016). Given that rural POC communities in eastern NC have decreased healthcare access, worse overall health, and a higher risk of private well water contamination than the rest of the state, the disproportionate effect of hog CAFOs on these communities exacerbates existing health problems and health inequities (“North Carolina County Health Profiles,” 2018).
Figure 1.
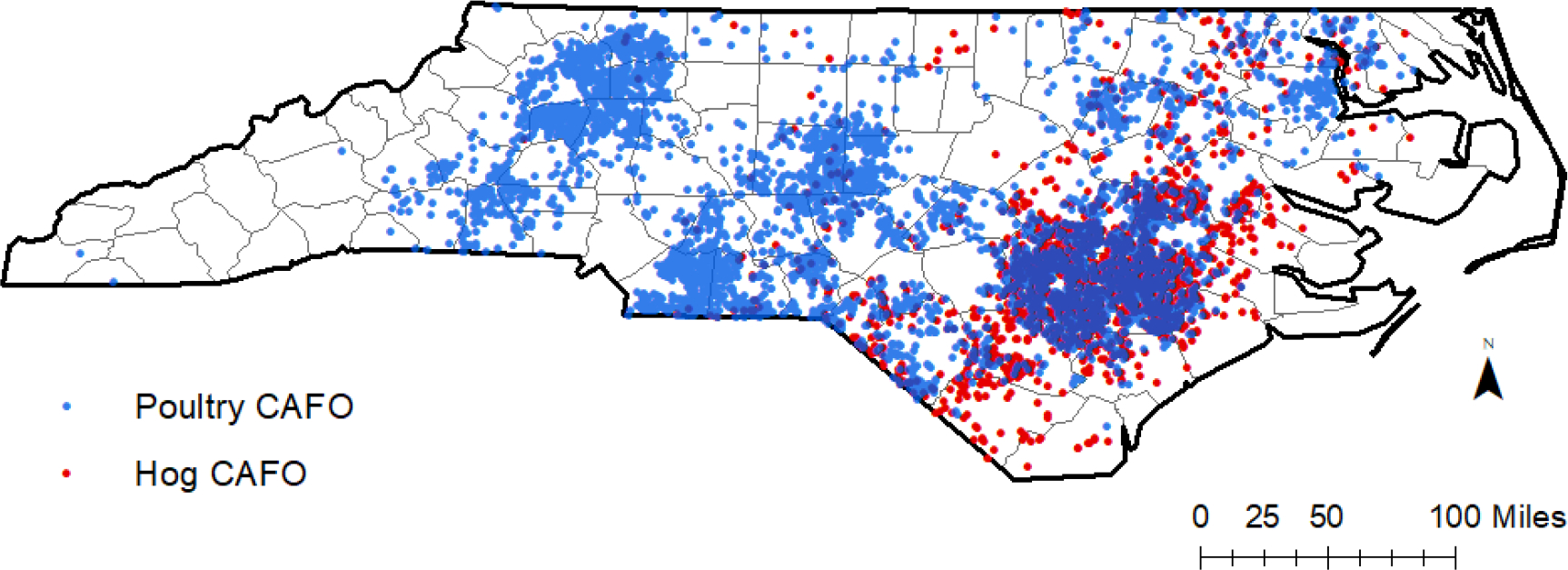
Locations of swine and poultry concentrated animal feeding operations (CAFOs) in North Carolina (NC) counties, according to 2019 NC Department of Environmental Quality swine permit data and poultry estimates from Environmental Working Group and Waterkeepers Alliance.
This paper investigates how the relationship between hog CAFO exposure and AGI ED visit rate varies by race, age, rurality, and precipitation. While some studies have examined the association between CAFO exposure and AGI rates, they found mixed results and none have assessed this relationship in NC (Febriani et al., 2010, 2009; Hooiveld et al., 2016; Levallois et al., 2014). This is the first study, to our knowledge, that investigates how race may modify the relationship between hog CAFO exposure and AGI ED visit rate. Additionally, very few studies have examined the combined effect of industrial swine operations and precipitation on AGI (Febriani et al., 2010; O’Brien and Xagoraraki, 2019).
METHODS
Hog Exposure
We used 2019 swine permit data from the NC Department of Environmental Quality (DEQ) (“List of Permitted Animal Facilities,” 2020). The data included the number of animals, type/life stage of animals, and location of each permitted animal facility. The number of hogs has remained relatively constant at 9 million over the past 20 years, and our examination of the 2016 and 2019 swine permit data showed very few changes (“National Agriculture Statistics Service,” 2020). We calculated the steady state live weight (SSLW) of each hog CAFO using the North Carolina Department of Environment and Natural Resources’ formula that incorporates the number of hogs, growth stage of the hogs, and average weight of each growth stage (see Supplementary Table 1 for list of growth stage/production phase of hogs and mean weight used to calculate SSLW)(Pietrosemoli et al., 2012). SSLW is an indicator of the amount of waste produced at each hog CAFO and has been used in other studies (Williams, 2009; Wing and Johnston, 2014).
We measured the distance between hog CAFOs and census block centroids and used a Gaussian distance decay function , distance threshold of 10 miles with α=3) to convert distances to weights. Our parameterization of the Gaussian decay and distance restriction were based on literature that suggests an association between living within half a mile to two miles of hog CAFO and various health outcomes, with weaker associations at three miles and five miles (Casey et al., 2014; Hooiveld et al., 2016; Kilburn, 2012; Kravchenko et al., 2018; Williams et al., 2011). For each block centroid, we multiplied the distance-based weights by each hog CAFO’s SSLW to create a block-level exposure measure, based on both hog density and distance to CAFO. We summed the exposure values for all CAFOs within 10 miles of each block centroid. We then aggregated the block-level hog CAFO exposure estimates to the ZIP code level using population weights created from 2017 American Community Survey (ACS) five-year block group-level estimates, the 2010 block-level census data, and 2017 NC polygon ZIP code boundaries from ESRI. While there are 1080 distinct ZIP codes in NC, there are only 750 ZIP code polygons as several hundred ZIP codes are point ZIP codes, mostly for PO boxes. We used ZIP code polygons for this analysis, and categorized patients with a point ZIP code as living in the polygon ZIP code that encompasses their point ZIP code. We categorized ZIP codes in the upper quartile of hog CAFO exposure as high hog CAFO exposed and compared them to ZIP codes with no hog CAFOs within 10 miles of any nested block centroid, thus excluding areas with low/medium hog CAFO exposure from the main analyses (Figure 2). We believe 10 miles to be an appropriate distance for control areas as studies have indicated that when hog manure is transported from CAFOs, it is typically applied on land within about 10 miles or 15 km from the CAFO (Bergström et al., 2005; Son et al., 2021a). While the majority of residents affected by CAFOs likely live within a mile or two of the CAFO, we chose a 10-mile buffer for the control to reduce exposure misclassification of residents who live near manure-applied land.
Figure 2.
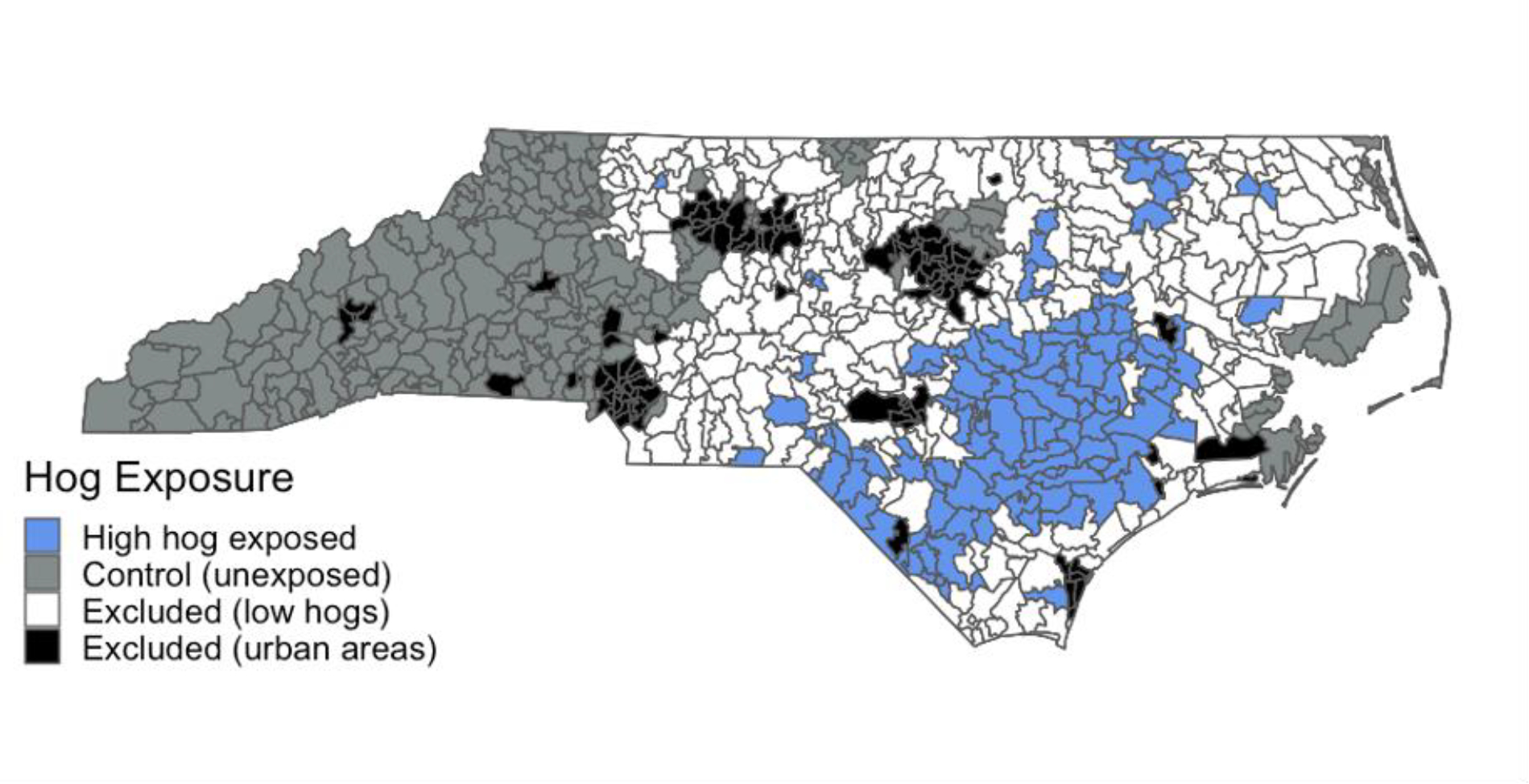
North Carolina ZIP codes with high hog CAFO exposure (>75th percentile of hog CAFO exposure measure), ZIP codes with no hog CAFO exposure (control areas), and ZIP codes excluded from analyses (urban areas and low hog CAFO exposed areas).
Acute Gastrointestinal Illness
Acute gastrointestinal illness (AGI) was measured using data from the North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT), a public health surveillance system containing all civilian ED visits in NC. We calculated 2016–2019 AGI ED visit rates at the ZIP code level, the finest geographic level available for these data. Diagnostic codes (International Classification of Diseases, Tenth Revision; ICD-10) were used to identify intestinal infectious illness (A00-A09), unspecified noninfectious gastroenteritis and colitis (K52.3, K52.89, K52.9), diarrhea (R19.7), and nausea and vomiting (R11.10-R11.12) as AGI ED visits. Similar diagnosis codes have been used in other studies of AGI ED visits (DeFelice et al., 2016; Drayna et al., 2010; Wade et al., 2014). While Clostridium difficile infections (A04.7) are often acquired in hospitals, we included C. difficile infections in our AGI definition because C. difficile infections are common at swine operations and some C. difficile infections in humans have been linked to pigs (“Clostridium difficile on U.S. Swine Operations,” 2011; Keessen et al., 2013). Our main analyses focused on all-cause AGI ED visit rate because specific pathogens are seldom tested for and/or included in the ED discharge report.
Covariates
For the main analyses, we adjusted for ZIP code-level rurality, health insurance status, median income, and race. We identified rurality, health insurance status, and median income as the minimally sufficient set of confounders using a directed acyclic graph (DAG, see Supplementary Figure 1). We incorporated race when we created our control pseudo-population because race is strongly correlated with the exposure and we found it necessary to include a race variable in order to create balanced groups (Austin and Stuart, 2015). Data on median income, number of White residents, number of uninsured residents, and total number of residents were available at the block group-level from the 2017 ACS. We assigned these values to the centroids of each 2010 census block based on the proportion of the block group population within that block and then aggregated these block centroid data to create ZIP code-level population estimates for population size, median income, percent of ZIP code population uninsured, and percent of ZIP code population White. Rurality was measured using a continuous geographic isolation scale that classifies ZIP codes according to their access to resources (Doogan et al., 2018).
To examine effect measure modification (EMM), we used individual-level covariates on patients’ race, ethnicity, age, and health insurance status, and we used area-level covariates for rurality, median income, and well water usage. The 2015 U.S. Geological Survey estimates the number of people in each county on private well water, and we used this data to create ZIP code-level well water usage estimates (Dieter et al., 2018). For race/ethnicity, patients were categorized as “White non-Hispanic” if their reported race in the ED data was White and they were not reported to be Hispanic. We analyzed Black, American Indian, Hispanic, and Asian patients separately, but due to insufficient case counts, we combined Pacific Islander patients and Other Race patients into an Other Race category.
We estimated the NC population (by age category, race/ethnicity, health insurance status) using 2017 ACS block group estimates aggregated to the ZIP code level. We did not use census data at the ZIP code tabulation area (ZCTA) level due to the spatiotemporal mismatch between ZCTAs and ZIP codes (Grubesic and Matisziw, 2006; Krieger et al., 2002). We examined all changes in ZIP codes from 2016–2019 and assigned all ZIP codes to the 2017 ZIP code polygon in which they were contained (2017 was chosen as a study period midpoint and because 2017 ZIP code polygons were the dominant polygons throughout the study period). The continuous geographic isolation scale was split into quartiles (labeled as metropolitan, micropolitan, small towns, rural) when examining EMM by rurality (Doogan et al., 2018). As urban areas lack hog CAFOs and have different ED visit patterns than areas with hog CAFOs, we excluded the most urban ZIP codes from analyses by removing ZIP codes with a geographic isolation scale below 5.6 (273 ZIP codes excluded), as we observed this point to be the natural gap where almost all CAFOs were in areas with a higher geographic isolation score. The remaining metropolitan ZIP codes were classified as micropolitan. Data on the location of poultry CAFOs and estimated number of birds at each poultry CAFO was provided by the Environmental Working Group and Waterkeepers Alliance, as permits are not required for most poultry CAFOs. They identified poultry facility locations via highresolution satellite data and aerial photographs and estimated number of birds at each poultry CAFO using the National Agriculture Imagery Program (2008, 2012, 2016, and 2019) and the United States National Agricultural Statistics Service’s 2017 Census of Agriculture (Environmental Working Group & Waterkeeper Alliance, 2016). We categorized a ZIP code as unexposed to poultry CAFOs if all its constituent block centroids were >5 miles from a poultry CAFO. We used a smaller distance for poultry (5 miles) than swine (10 miles) because poultry CAFOs produce less waste than swine CAFOs and because poultry waste is dry and may not be transported as far (“Animal Manure Management,” n.d.; Environmental Working Group & Waterkeeper Alliance, 2016). Additionally, because of the large number of poultry CAFOs throughout NC, using a larger distance to represent areas unexposed to poultry CAFOs would prevent us from creating an adequate control group. We also created a distance-weighted poultry exposure variable similar to our hog exposure variable, but with the Gaussian decay threshold of 5 miles and using number of birds instead of SSLW of hogs.
Analysis
For the main analysis, we used inverse probability of treatment weighting (IPTW) to estimate the average treatment effect on the treated (ATT, or, in this study, the average exposure effect on the high hog exposed). IPTW can create a weighted (i.e., synthetic) population in which measured confounders are balanced between exposure groups (Austin, 2011). Using IPTW, we created a control group with similar demographics as the high hog CAFO-exposed population (based on the ZIP codes’ median income, rurality, percent of non-Hispanic White residents, and percent of uninsured residents) but with no hog CAFO exposure. We truncated the weights of control group at the 1st and 99th percentile due to a few large weights (see Supplementary Table 2)(Cole and Hernán, 2008). We chose to compare areas with high hog CAFO exposure to areas with no hog CAFO exposure because these areas had relatively similar demographics before IPTW; areas with low hog CAFO exposure had higher median incomes and a larger percent of non-Hispanic White residents than NC overall and the high hog CAFO exposed areas. We used quasi-Poisson models to account for overdispersion in the ED visit data. Robust standard errors were used to calculate 95% confidence intervals (95% CI) using the sandwich package in R.
To examine how the association between high hog CAFO exposure and AGI rate may vary according to antecedent rain, we conducted restricted analyses according to the ZIP code precipitation during the previous week. We obtained daily precipitation data from the PRISM Climate Group as 4k-mby-4km raster data (“PRISM Climate Group,” n.d.), which we subsampled into 1km raster data and used the centroids of the 1 km grids to aggregated to 2017 ZIP code polygons, assigning the ZIP code the maximum precipitation recorded in the ZIP code for the day. We identified the days (day 0) and ZIP codes where the precipitation was above the 80th, 90th, 95th, and 99th percentile of daily NC precipitation 2016–2019 (to represent high precipitation time periods and areas) and all AGI ED visits within the next seven days (days 1–7) were included in each analysis of high hog CAFO exposure and AGI ED rate. To represent low precipitation time periods and areas, we identified days and ZIP codes where the precipitation during the prior seven days fell below the 50th percentile of daily NC precipitation 2016–2019 and included all AGI ED visits from these days in a separate analysis of high hog CAFO exposure and AGI. We created new IPTWs for each analysis, matching for median income, rurality, percent uninsured, and percent White. We also conducted a supplementary analysis where this precipitation analysis was restricted by the race/ethnicity of the patient. Lastly, we examined whether total precipitation over the entire study period by ZIP code was an EMM in the relationship between high hog CAFO exposure and AGI, to assess whether this relationship was stronger in areas that consistently received heavy cumulative rain. When examining EMM for various covariates, we adjusted for percent uninsured, median income, and rurality, which we had identified as confounders using a directed acyclic graph. All analyses were performed in R (Version 3.6.2)(“R: A language and environment for statistical computing,” 2019).
Sensitivity analyses
While our main analysis examined the association between high hog CAFO exposure and AGI ED visit rate compared to no hog CAFO exposure using dichotomous categories, in sensitivity analyses we examined the association between hog CAFO exposure and AGI ED visit rate using alternate methods to categorize hog CAFO exposure. Using our continuous ZIP code-level hog CAFO exposure variable, we created tertiles of all ZIP codes with any hog CAFO exposure and separately compared the AGI ED visit rates in high, medium, and low hog exposed ZIP codes to the hog unexposed ZIP codes, using IPTW and quasi-Poisson models to calculate rate ratios (we created a different control pseudo-population for each tertile of hog CAFO exposure, so each control had similar demographics to the compared exposure tertile). We also examined the association between the continuous hog CAFO exposure (which had been log transformed because it was highly skewed) and AGI ED visit rate, adjusting for median income and percent uninsured. Additionally, we assessed the sensitivity of our primary findings to choice of the distance threshold and the alpha parameter for the Gaussian decay hog CAFO exposure metric.
Because hog CAFOs and poultry CAFOs are frequently co-located and living near either type of CAFO may increase one’s risk for AGI (Figure 1), we conducted sensitivity analyses where ZIP codes with any poultry CAFOs within 5 miles were excluded from the control group. As poultry CAFOs are located in the majority of areas hog CAFOs are located, we were unable to conduct analyses with poultry CAFOs excluded from the exposed group. We also assessed the association between high poultry CAFO exposure (>75th percentile of the distance weighted poultry CAFO measure) and AGI ED visit rate to better understand how poultry CAFOs may influence the association between high hog CAFO exposure and AGI ED visit rate. We examined this relationship using IPTW controls (ZIP codes >5 miles from a poultry CAFO, urban areas excluded), and we assessed the relationship both when control ZIP codes included hog CAFOs and when control ZIP codes did not include hog CAFOs. We also examined the joint effect of hog and poultry CAFOs by identifying ZIP codes with medium/high exposure of both (>50th percentile of both poultry and hog CAFO measures) and compared to jointly unexposed ZIP codes (>10 miles from hog CAFOs and >5 miles from poultry CAFOs) using IPTW.
We conducted separate analyses restricted to ICD-10 codes that indicated specific pathogens that may be found in hog feces that could cause AGI, including enterotoxigenic or enterohemorrhagic E. coli, Salmonella, Campylobacter, C. difficile, and rotavirus. We also examined the association between hog CAFO exposure and overall bacterial AGI, viral AGI, and protozoal AGI. As many C. difficile infections are nosocomial infections, we conducted a sensitivity analysis of all-cause AGI excluding C. difficile ED visits. Additionally, as we observed strong EMM by rurality, we conducted analyses restricted to rural areas (the highest quartile of the continuous geographic isolation scale) where we examined EMM by race, age, and insurance status.
RESULTS
Of the 750 polygon ZIP codes in NC, we categorized 104 ZIP codes as high hog exposed and 242 as control ZIP codes (no hog CAFO exposure, see Figure 2), with 272 urban ZIP codes excluded and 132 low/medium hog exposed ZIP codes excluded from main analysis. High hog exposed ZIP codes had an average hog density of 830 hogs/mile2 per ZIP code and a median of 36,092 hogs per ZIP code and a maximum of 716,829 hogs (housed within 213 hog CAFOs) in one ZIP code. In 2016–2019, there were 868,691 AGI ED visits in NC by residents with a NC residential ZIP code, with 79,293 AGI ED visits (259 AGI ED visits per 10,000 people) in high hog exposed ZIP codes and 205,116 (224 AGI ED visits per 10,000 people) in control ZIP codes. High hog exposed areas had higher proportions of American Indian, Hispanic, and Black people and lower proportions of White non-Hispanics and Asians than areas with no hog CAFO exposure (Table 1). Among Asian Americans in NC, high hog exposed ZIP codes have a larger proportion of Filipino, Japanese, and Vietnamese residents and a lower proportion of Indian and Chinese residents compared to ZIP codes with no hog CAFO exposure (Supplementary Table 3). High hog exposed areas also have a higher proportion of people without health insurance, lower median household incomes, and higher poultry CAFO density than the control. With IPTW, we were able to create a control with similar demographics as the high hog exposed ZIP codes, although the control continues to have a much lower bird density than the high hog exposed area.
Table 1.
Comparison of demographics and characteristics of the high hog exposed ZIP codes (>75th percentile of the distance weighted hog measure), the unweighted control ZIP codes with no hog CAFO exposure, and the inverse probability of treatment weighted (IPTW) control. The IPTW control was matched on rurality, percent white, percent uninsured, and median income (data from 2017 American Community Survey, weights truncated at the 1st and 99th percentile).
| Characteristic | Unweighted Control | IPT-Weighted Control (modeled control) | High Hog Exposed (>75th percentile) |
|---|---|---|---|
| Total Population | 2,293,170 | 654,293 | 765,602 |
| American Indian, N (%) | 17,683 (0.8) | 18,246 (2.8) | 34,156 (4.5) |
| Asian, N (%) | 38,209 (1.7) | 6,172 (0.9) | 5,409 (0.7) |
| Black, N (%) | 207,737 (9.1) | 155,106 (23.7) | 203,438 (26.6) |
| Hispanic, N (%) | 147,184 (6.4) | 47,484 (7.3) | 90,418 (11.8) |
| White non-Hispanic, N (%) | 1,933,070 (84.3) | 435,252 (66.5) | 476,674 (62.3) |
| Other race, N (%) | 48,264 (2.1) | 20,147 (3.1) | 23,062 (3.0) |
| Uninsured, N (%) | 237,228 (10.3) | 71,908 (11.0) | 98,119 (12.8) |
| Median Income (U.S. dollars) | 45,835 | 39,725 | 38,226 |
| Rurality Score1 | 7.56 | 8.00 | 8.06 |
| Hogs, N | 0 | 0 | 7,834,422 |
| Average Hog Density (hogs/sqmi) | 0 | 0 | 831 |
| Poultry, N | 8,685,8060 | 27,969,195 | 194,548,824 |
| Average Poultry Density (birds/sqmi) | 5,788 | 4,066 | 22,446 |
| Total ED2 Visits | 3,101,694 | 1,041,486 | 1,630,070 |
| Total AGI3 Visits | 205,116 | 61,301 | 79,293 |
| ED Rate per 10,000 people | 3,381 | 3,979 | 5,323 |
| AGI ED Rate per 10,000 people | 224 | 234 | 259 |
| Sum of Weights | 242 | 95 | 104 |
| Number of ZIP Codes | 242 | 241 | 104 |
Rurality was measured using a continuous geographic isolation scale that classifies ZIP codes according to their access to resources, higher value indicates greater isolation (more rural)(Doogan et al., 2018)
ED: emergency department
AGI: acute gastrointestinal illness
In high hog exposed areas compared to areas without hog CAFO exposure, we observed an 11% higher (rate ratio [RR]=1.11, 95% CI: 1.06, 1.17, Table 2) AGI ED visit rate overall. We found modification by rurality and observed a rate ratio of 1.21 (95% CI: 0.98, 1.43) in rural areas, while we did not observe positive associations in small towns (RR=0.95, 95% CI: 0.75, 1.16) or micropolitan areas (RR=0.86, 95% CI: 0.71, 1.02). We observed an elevated rate ratio in the highest ($47,500–103,000: RR=1.31, 95% CI: 1.18, 1.42) median income category and a slightly elevated estimate in the lowest income category ($21,900–36,099: RR=1.07, 95% CI: 0.96, 1.18). Similarly, we saw a positive association in the lowest (1–9.2% uninsured: RR=1.35, 95% CI: 1.19, 1.51) and highest (14.8–32.7% uninsured: RR=1.11, 95% CI: 0.96, 1.34) categories of percent of population uninsured, with no positive association in the middle categories (Table 2). We did not observe a positive association between high hog CAFO exposure and AGI ED visit rate in ZIP codes with the highest amounts of precipitation during the study period. We did not observe patterns in the association by well water usage. We found race to be an effect measure modifier between high hog CAFO exposure and AGI ED visit rate. We observed positive associations for American Indian (RR=1.60, 95% CI: 1.28, 1.92) and Asian (RR=1.95, 95% CI: 1.30, 2.61) patients and negative associations among White non-Hispanic (RR=0.88, 95% CI: 0.66, 1.00) and Other Race (RR=0.45, 95% CI: 0.04, 0.86) patients (Table 2).
Table 2.
The association between high hog CAFO exposure (>75th percentile of distance weighted hog CAFO measure) and AGI ED visit rate (2016–2019). For the main effect, high hog exposed ZIP codes were compared to IPTW control ZIP codes with no hog CAFO exposure.1 Effect measure modification models do not use IPTW; these adjusted models had a product interaction term between the effect measure modifier and the dichotomous high hog CAFO/no hog CAFOs exposure variable.2
| Analysis3 | Rate Ratio (95% CI) | # AGI ED Visits in High Hog CAFO Exposed ZIP Codes | # AGI ED Visits in ZIP Codes with No Hog CAFO Exposure |
|---|---|---|---|
| Main analysis (hog exposed: >75th percentile)1 | 1.11 (1.06, 1.17) | 82,762 | 205,116 |
| Effect measure modification:2 | |||
| Rurality4,5 | |||
| Micropolitan | 0.86 (0.71, 1.02) | 37,259 | 98,787 |
| Small Towns | 0.95 (0.75, 1.16) | 30,963 | 42,712 |
| Rural | 1.21 (0.98, 1.43) | 13,876 | 16,543 |
| Income4,6 | |||
| $21,900–36,099 | 1.07 (0.96, 1.18) | 38,671 | 29,680 |
| $36,100–41,599 | 0.86 (0.67, 1.05) | 13,450 | 67,188 |
| $41,600–47,499 | 1.01 (0.89, 1.12) | 25,214 | 52,318 |
| $47,500–103,000 | 1.31 (1.11, 1.51) | 4,474 | 54,914 |
| Percent Uninsured4,6 | |||
| 1.0–9.2% | 1.35 (1.19, 1.51) | 6,829 | 47,580 |
| 9.3–11.9% | 0.90 (0.77, 1.02) | 26,266 | 76,787 |
| 12.0–14.7% | 0.92 (0.81, 1.06) | 18,296 | 69,836 |
| 14.8–32.7% | 1.11 (0.92, 1.34) | 30,695 | 9,897 |
| Precipitation (4-year sum of daily rain, inches)4,7 | |||
| 0–21 inches | 1.09 (0.82, 1.36) | 1,481 | 4,346 |
| 22–54 | 1.04 (0.83, 1.25) | 10,488 | 11,645 |
| 55–116 | 1.10 (0.98, 1.23) | 24,602 | 36,681 |
| 117–361 | 0.90 (0.75, 1.04) | 45,527 | 151,422 |
| Percent of people on well water4,8 | |||
| 1–11 | 0.85 (0.74, 0.97) | 27,230 | 142,073 |
| 12–23 | 0.91 (0.78, 1.05) | 20,253 | 109,221 |
| 24–38 | 1.12 (0.98, 1.26) | 17,261 | 83,621 |
| 39–85 | 0.91 (0.78, 1.04) | 16,005 | 38,398 |
| Race/ethnicity9 | |||
| American Indian | 1.60 (1.28, 1.92) | 3,493 | 588 |
| Asian | 1.95 (1.30, 2.61) | 245 | 686 |
| Black | 0.93 (0.79, 1.08) | 28,695 | 23,490 |
| Hispanic | 0.87 (0.66, 1.08) | 6,200 | 9,469 |
| White non-Hispanic | 0.88 (0.76, 1.00) | 40,295 | 149,301 |
| Other | 0.45 (0.04, 0.86) | 4,624 | 17,465 |
| Age9 | |||
| Under 5 | 1.07 (0.80, 1.33) | 12,330 | 25,033 |
| 5–17 | 1.11 (0.87, 1.35) | 8,870 | 20,114 |
| 18–64 | 1.03 (0.72, 1.35) | 46,819 | 116,480 |
| Over 64 | 1.00 (0.78, 1.24) | 11,908 | 37,727 |
| Insurance9 | |||
| Private | 1.31 (1.02, 1.60) | 16,981 | 45,376 |
| Public | 1.00 (0.74, 1.26) | 45,040 | 98,405 |
| Self-pay/none | 1.04 (0.72, 1.35) | 15,441 | 33,596 |
Control ZIP codes were matched on rurality, median income, percent uninsured, percent white to high hog CAFO exposed ZIP codes, and weights were truncated at the 1st and 99th percentile.
Effect measure modification models were adjusted for rurality, median income, and percent uninsured.
Analysis ZIP codes includes ZIP codes with high hog exposure and ZIP codes with no hog exposure, with urban ZIP codes and ZIP codes with some hog CAFO exposure that was below the 75th percentile excluded from analyses.
ZIP code level variables separated into quartiles.
Rurality was measured using a continuous geographic isolation scale that classifies ZIP codes according to their access to resources.(Doogan et al., 2018)
ZIP code-level estimates created from 2017 American Community Survey data.
Precipitation from PRISM Climate Group.
Well water data from the 2016 U.S. Geological Survey at the county level.
Individual-level data from ED visit data.
Because we observed the association only in rural areas, we examined EMM by race/ethnicity, age, and insurance status in analyses restricted to rural areas. In these analyses, we found higher AGI ED visit rates among American Indian (RR=4.29, 95% CI: 3.69, 4.88) and Black (RR=1.45, 95% CI: 0.98, 1.91) patients in rural high hog areas compared to rural areas without hog CAFO exposure (Table 3). Other racial and ethnic groups either showed little evidence of association or had too few cases to produce reliable rate ratios. We did not observe strong differences by age, although the strongest association was among adults aged 18–64 years (RR=1.38, 95% CI: 1.19, 1.61). While we observed a positive association between high hog CAFO exposure and AGI ED visit rate in all insurance categories, the strongest association was found among patients who paid for the ED visit themselves and were likely uninsured (RR=1.72, 95% CI: 1.40, 2.04).
Table 3.
The association between high hog CAFO exposure (>75th percentile of distance weighted hog CAFO measure) and AGI ED rate (2016–2019) compared to areas with no hog CAFO exposure, restricted to rural areas and with various effect measure modifiers (using individual level information from ED visit data).
| Effect Measure Modifier | Rate Ratio (95% CI) | Number of AGI ED Visits in High Hog CAFO Exposed ZIP Codes | Number of AGI ED Visits in ZIP Codes with No Hog CAFO Exposure |
|---|---|---|---|
| Race/ethnicity | |||
| American Indian | 4.29 (3.69, 4.88) | 146 | 187 |
| Asian | 6.15 (5.55, 6.76) | 14 | 16 |
| Black | 1.45 (0.98, 1.91) | 5,211 | 527 |
| Hispanic | 1.00 (0.53, 1.47) | 824 | 588 |
| White non-Hispanic | 1.20 (0.82, 1.57) | 6,979 | 14,519 |
| Other | 1.30 (0.64, 1.96) | 757 | 357 |
| Age Category | |||
| Under 5 | 1.28 (0.94, 1.74) | 1988 | 1884 |
| 5–17 | 1.23 (0.88, 1.73) | 1560 | 1633 |
| 18–64 | 1.38 (1.19, 1.61) | 7658 | 8741 |
| Over 64 | 1.18(0.91, 1.52) | 2293 | 3869 |
| Insurance | |||
| Private | 1.26 (1.00, 1.53) | 2725 | 3684 |
| Public | 1.35 (1.02, 1.67) | 7686 | 8717 |
| Self-pay/none | 1.72 (1.40, 2.04) | 2537 | 2169 |
We observed that the association between high hog CAFO exposure and AGI ED visit rate was higher when restricted to the days and areas when daily precipitation was above the 99th percentile of NC daily precipitation for at least one day during the prior week (RR=1.41, 95% CI: 1.19, 1.62, Figure 3). When we restricted our analyses to only include AGI ED visits during dry periods (no precipitation in the ZIP code during the previous seven days), we did not observe an association between high hog CAFO exposure and AGI ED visit rate (RR=0.94, 95%: 0.28, 1.60). The association between high hog CAFO exposure and AGI ED visit rate was even higher after heavier precipitation events (i.e., during the seven days following precipitation above the 99.9th percentile of NC precipitation: RR=2.86, 95% CI: 2.54, 3.18; see Supplementary Table 4 for different precipitation lags). When restricted by race/ethnicity of the patients, the association between high hog CAFO exposure and AGI ED rate during the week after heavy precipitation was stronger among Black (RR=1.73, 95% CI: 1.35, 2.12) and Hispanic (1.66, 95% CI=1.03, 2.29) patients than among White non-Hispanic patients (RR=1.20, 95% CI=0.89, 2.50; Supplementary Table 5).
Figure 3.
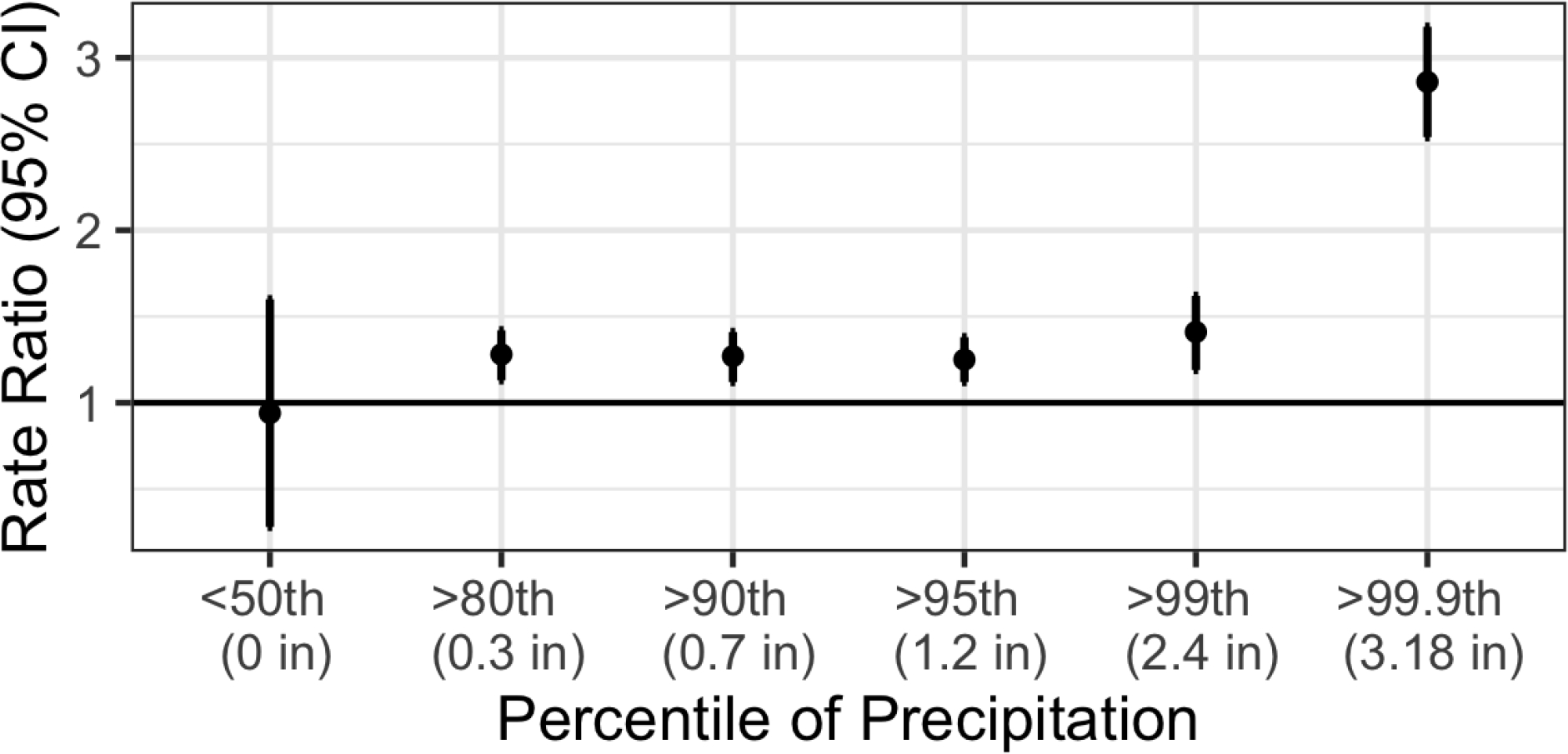
The association between high hog CAFO exposure (>75th percentile of distance weighted hog CAFO measure) and AGI ED rate (2016–2019) restricted across a range of daily precipitation measures. These heavy precipitation analyses include all AGI ED visits during the seven days after each day of precipitation above a given percentile. For example, for the >99th percentile of precipitation analysis, we included only AGI ED visits when the daily ZIP code precipitation was above the 99th percentile of NC daily precipitation during at least one of the prior seven days. For the <50th percentile of precipitation analysis, we only included AGI ED visits when the daily ZIP code precipitation was below the 50th percentile of NC daily precipitation during all of the prior seven days. As the 50th percentile was 0 inches, this analysis included only areas and days with no precipitation in the previous week. CAFO proximity was most associated with increased AGI after periods of heaviest precipitation.
We found that people who lived in high hog exposed ZIP codes were more likely to visit an ED due to a Salmonella infection compared to those who lived in areas without hog CAFO exposure (RR=1.60, 95% CI: 0.94, 2.29, Table 4). We did not observe positive associations between high hog CAFO exposure and pathogenic E. coli, Campylobacter, C. difficile, or rotavirus ED visits, though several of these results were based on a very small number of cases. Our results changed very little when we removed C. difficile infections from our case definition of all-cause AGI (RR=1.12, 95% CI: 1.03, 1.22).
Table 4.
The association between high hog CAFO exposure (>75th percentile of the distance weighted hog CAFO exposure variable) and alternative AGI case definitions by pathogen or pathogen group, compared to areas without hog CAFO exposure (IPTW control, weighted truncated at 1% and 99%; 2016–2019).
| Pathogen | Rate Ratio (95% CI) | Number of AGI ED Visits in High Hog Exposed ZIP Codes | Number of AGI ED Visits in ZIP codes Unexposed to Hogs |
|---|---|---|---|
| All bacteria | 0.80 (0.60, 1.00) | 2419 | 8520 |
| All viruses | 1.05 (0.64, 1.47) | 5242 | 12439 |
| All protozoa | 0.50 (0, 1.66) | 14 | 105 |
| Pathogenic E. coli | 0.09 (0, 1.51) | 8 | 280 |
| Salmonella | 1.61 (0.94, 2.29) | 151 | 393 |
| Campylobacter | 0.37 (0, 0.98) | 64 | 629 |
| C. difficile | 0.79 (0.56, 1.01) | 1913 | 6755 |
| Rotavirus | 0.20 (0, 1.03) | 45 | 512 |
We did not observe an association when we examined the continuous association between log-transformed hog CAFO exposure and AGI ED rate in an adjusted model (RR=1.00, 95%CI: 0.99, 1.00). When we separated our continuous hog CAFO exposure measure into non-zero tertiles and compared each tertile to the reference of areas without hog CAFO exposure (using different IPTWs for each tertile), we found similar positive associations between both medium (RR=1.13, 95% CI: 0.94, 1.32) and high (RR=1.11, 95% CI: 1.92, 1.34) hog CAFO exposure and AGI ED rate, but no association between low hog CAFO exposure and AGI ED rate (RR=0.95, 95% CI: 0.75, 1.16; Table 5). In analyses where we excluded all ZIP Codes with poultry CAFOs (within ZIP code or within 5 miles from ZIP code boundary) from the control, we found a stronger association between high hog CAFO exposure and AGI ED rate (RR=1.43, 95% CI: 1.39, 1.47; Figure 4). Similarly, when we examined the association between high poultry CAFO exposure and AGI ED visit rate, we found a slightly stronger association when areas with hog CAFO exposure were excluded from the control (RR=1.26, 95% CI: 1.21, 1.31) than when we included areas with hog CAFO exposure in the control (RR=1.16, 95% CI: 1.12, 1.19). Areas with medium/high exposure to both hog and poultry CAFOs had a 52% higher AGI ED rate compared to areas without hog and poultry CAFO exposure (RR=1.52, 95% CI: 1.48, 1.57). We observed little difference when we conducted our main analysis with the hog CAFO exposure measure created using different alpha values and distance thresholds in the Gaussian decay function, keeping the control distance constant at >10 miles (see Supplementary Table 6).
Table 5.
The association between low, medium, and high hog CAFO exposure (based on tertiles of exposed ZIP codes) and AGI ED visit rate, compared to ZIP codes with no hog CAFO exposure (IPTW control, weights truncated at 1% and 99%).
| Hog CAFO exposure Category | Mean hog density (hogs/sqmi) | Mean hog measure1 | Rate Ratio (95% CI) | Number of AGI ED visits |
|---|---|---|---|---|
| No hog CAFO exposure | 0 | 0 | (ref) | 205,116 |
| Low hog CAFO exposure2 | 0 | 0.001 | 0.95 (0.75, 1.16) | 107,845 |
| Medium hog CAFO exposure | 30 | 0.31 | 1.13 (0.94, 1.32) | 172,595 |
| High hog CAFO exposure | 654 | 8.32 | 1.11 (0.92, 1.30) | 107,077 |
Mean value of the Gaussian decay hog CAFO measure
Low hog CAFO exposure represents ZIP codes that are within 10 miles of a hog CAFO but there are no hog CAFOs within the ZIP code and no very large hog CAFOs nearby.
Figure 4.
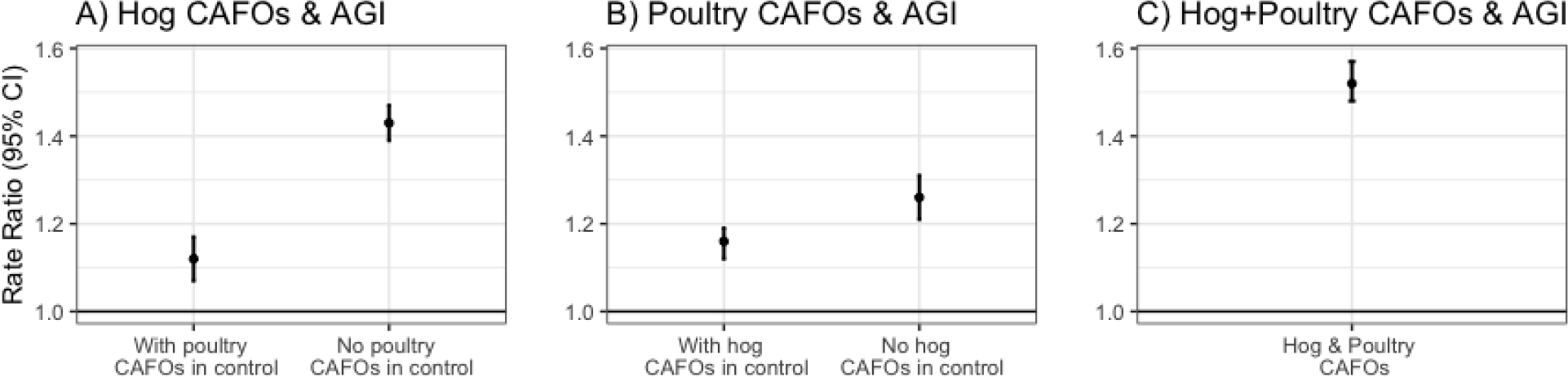
The association between A) high hog exposure (top 75th percentile of hog exposure) and AGI ED rate when including and excluding areas with poultry CAFOs in the control; B) high poultry exposure (top 75th percentile of poultry exposure) and AGI ED rate when including and excluding areas with hog CAFOs in the control; and C) high hog and poultry exposure (top half of both hog and poultry exposure) and AGI ED rate, excluding areas with either hog or poultry CAFO exposure from the control. Separate controls were created for each analysis using IPTW, 2016–2019.
DISCUSSION
Major Findings
Overall, we observed an 11% higher (RR=1.11, 95% CI: 1.06, 1.17) all-cause AGI ED rate in high hog exposed areas than in areas without hog CAFO exposure. The association was stronger in rural areas. In analyses restricted to rural ZIP codes, we observed EMM by race, where the association between high hog CAFO exposure and AGI ED visit rate was highest among American Indian and Black patients. We also observed that the association between high hog CAFO exposure and AGI ED visit rate was stronger during the week after heavy rain, especially among Black and Hispanic patients. The association between high hog CAFO exposure and AGI ED visit rate was also stronger when ZIP codes with poultry CAFO exposure were excluded from the control. The joint effect of poultry and hog CAFOs on AGI ED rate was larger than their individual effects.
Comparison to Extant Literature
Our overall results are consistent with some studies that found increased gastrointestinal symptoms and gastroenteritis hospitalizations near high intensity farming (Febriani et al., 2009; Wing and Wolf, 2000), although other studies found no association (Febriani et al., 2010; Hooiveld et al., 2016). In an ecological study of livestock density and acute gastroenteritis hospitalizations in Quebec, Canada, Febriani et al. observed an increased risk of acute gastroenteritis hospitalizations associated with high intensity farming (Febriani et al., 2009). They observed modification by age and water source, with a particularly strong association in children under age 5 years and in areas that used predominantly private wells and ground water for drinking water. Unlike Febriani et al. (Febriani et al., 2009), we did not find private well water usage or age to be a strong modifier and found the association to be highest among adults 18–64; however, we were limited by the availability of only county-level well water data. To further examine the relationship between intensive farming and AGI, the Febriani et al. group later conducted a cross-sectional telephone survey of 7,006 residents in rural municipalities in Quebec, Canada and found that living in a municipality with intensive farming was inversely associated with self-reported AGI (Febriani et al., 2009). They propose that the differences between these studies may be due to ecological vs. individual-level data and severe AGI hospitalizations vs. self-reported AGI. Another study used electronic medical record data from primary care practices in southern Netherlands and found that the prevalence of gastrointestinal and respiratory symptoms were similar in the high and low CAFO exposed populations (Hooiveld et al., 2016). In the only study that examined this relationship in NC, Wing et al. interviewed 155 residents in eastern NC who lived near a cattle CAFO, a hog CAFO, or no nearby CAFOs, and found self-reported diarrhea, headaches, coughing, and sore throats to be most prevalent among residents living near the hog CAFO (Wing and Wolf, 2000). While literature remains mixed on this general subject, our study supports the positive association between hog CAFO exposure and AGI in NC, especially after heavy precipitation.
Conflicting results on the association between CAFO exposure and AGI rate may be caused by differences in study design, region, precipitation, animal density, and type(s) of animals. We observed that the relationship between high hog CAFO exposure and AGI ED visit rate was stronger when a heavy rain event had occurred within the previous week than when the previous week had been dry. These results are supported by studies that have found increased pathogens and/or increased concentrations of fecal indicator bacteria after heavy rain events in areas with hogs. A study of 59 wells in southwest Guatemala found recent precipitation to be associated with almost 3-fold higher E. coli concentrations, with the strongest association at wells with pigs nearby (Eisenhauer et al., 2016). A study of runoff after land application of cattle and swine manure and after simulated heavy rainfall events found E.coli and enterococci concentrations to be significantly higher than in runoff from control plots with no manure (Thurston-Enriquez et al., 2005). Runoff from swine slurry-applied fields had the highest concentrations of E. coli, Clostridium, and Giardia cysts compared to cattle manure-applied and control fields, possibly because swine manure’s liquid state enables microorganisms in the manure to be transported more readily than does cattle manure or chicken litter (Thurston-Enriquez et al., 2005). Febriani et al. found that high precipitation episodes in the fall increased AGI risk three weeks later and observed effect modification of high intensity farming and season on the association between cumulative precipitation and AGI four weeks later (Febriani et al., 2010).
Joint Exposure of Hog and Poultry CAFOs
Our study focuses on high hog CAFO exposure, partly because hog manure is stored in large, uncovered lagoons that are especially susceptible to flooding, which may allow pathogens to be carried widely (although both hog and poultry waste are stored and applied in under-protected outdoor environments and are associated with water contamination)(Amato et al., 2020; Heaney et al., 2015; Hubbard et al., 2020). However, as thousands of poultry CAFOs are co-located with hog CAFOs in eastern NC, it is difficult to isolate the effect of hog CAFOs from that of poultry CAFOs. For example, our control areas for the main analyses have lower poultry density than the high hog exposed area, which make it challenging to attribute the higher AGI rate entirely to hog CAFOs. When we include ZIP codes with poultry CAFOs in the control, as we did in our main analyses, our results are attenuated as the poultry CAFO exposure seems to increase the AGI ED visit rate in these areas. Our analyses examining the joint effect of hog and poultry CAFOs on AGI suggest that both poultry and hog CAFOs are associated with adverse health effects in humans and that co-location of both may be doubly detrimental. While some studies found no association of industrial poultry and swine production with AGI (Febriani et al., 2010; Hooiveld et al., 2016), Febriani et al. observed an increasing trend in association between quartile of poultry density AGI. The authors noted that the association between poultry density and AGI in children was predominantly from Salmonella infections (Febriani et al., 2009). Another study found Michigan counties with high poultry density to have a higher incidence of C. jejuni enteritis, especially among children, compared with low poultry density counties (Potter et al., 2002).
Effect Measure Modification by Rurality and Race
We also observed a stronger association between high hog CAFO exposure and AGI ED visit rate in rural ZIP codes. In analyses restricted to rural areas, we found the associations to be particularly strong in American Indian, Black, and Asian patients, as well as in patients who paid for their ED visit out of pocket (self-pay, likely uninsured). Although the association is statistically insignificant among Black patients in rural areas (RR=1.45, 95% CI: 0.98, 1.91), the effect estimate is relatively strong with the lower confidence bounds near the null, and many scholars warn against relying purely on statistical significance and p-value thresholds to interpret results (Amrhein and Greenland, 2018; McShane et al., 2019; Wasserstein and Lazar, 2016). While Indian, Chinese, and Vietnamese are the largest Asian ethnic groups in NC, most NC Asians reside in metropolitan areas. In high hog exposed ZIP codes, which are the more rural areas of NC, Filipinos are the largest Asian ethnic group, followed by Japanese and Indian (Supplementary Table 3). While we did not observe a positive association between high hog CAFO exposure and AGI ED visit rate among Black patients in the overall analysis, we saw a positive association among Black patients when restricted to rural ZIP codes. As there are several layers of EMM by rurality, race/ethnicity, age, insurance status, and income, our IPTW analysis and adjusted sub-analyses attempted to disentangle these factors. For the main analysis, IPTW was relatively successful at creating a control pseudo-population with similar levels of rurality, race, insurance status, and median income as the high hog exposed areas. Additional rural-restricted EMM analyses were helpful in better understanding the complex relationship between hog CAFO exposure and AGI in NC. Additionally, the strong positive association between high hog exposure and AGI ED rate among Black patients in rural areas may be due, in part, to occupational exposure, as many workers at meatpacking facilities in rural NC are Black (Supplementary Table 7).
Rural areas in NC have the highest ED rates, the highest proportion of uninsured residents, and the lowest median household incomes (Supplementary Table 8). A recent study examining six EDs in Minnesota and South Dakota found that rural EDs had a higher proportion of Native American patients and patients below the 200% income poverty level compared to urban EDs (Zook et al., 2018). The authors concluded that Native American residents have more barriers to obtaining primary care in rural areas than White residents do (Zook et al., 2018). Similar healthcare barriers may exist in rural NC, as we observed an especially strong association between high hog exposure and AGI ED rate among rural American Indians (many of whom are Lumbee in eastern NC). As healthcare access differs in urban and rural populations, it is difficult to disentangle its effects. We may have observed an elevated rate ratio among those with private insurance because people without adequate health insurance may be less likely to go to the ED for AGI, as most AGI resolves by itself. Additionally, while we observed an elevated rate ratio in the wealthiest areas and a slightly elevated rate ratio in the poorest areas (with no association in the middle-income groups), very few high-income areas were exposed to high hog CAFOs and many low-income areas were exposed to high hog CAFOs.
Strengths and Limitations
This study’s strengths include its use of four years of recent data and its use of distance weighting to account for proximity to hog CAFOs, number and density of hog CAFOs, and approximate manure exposure using SSLW. The study period, 2016–2019, excludes the COVID-19 pandemic, which began in 2020 and greatly changed ED visit patterns (Hartnett et al., 2020). The sensitivity analyses illustrate the results’ robustness to changing model specifications and the complexity of the many correlated variables (e.g., race, income, insurance status, rurality, location of hog CAFOs) and co-location of hog and poultry CAFOs. This study is limited by its use of all-cause AGI ED visits as the main outcome, which is a broad indicator of health effects that may arise from pathogens in hog manure, but that has many possible etiologies and comorbidities, including causes unrelated to hog pathogens in hog waste. Our sensitivity analyses restricted to AGI visits attributable to particular pathogens, attempted to address this limitation, but we lacked the data necessary to determine the source of the pathogen; we observed a positive association between high hog CAFO exposure and Salmonella ED visit rates. While this may be from hog manure-associated Salmonella, the association we see may be confounded by nearby poultry CAFOs or other sources of Salmonella (Foley et al., 2008). Additionally, most ED AGI-related diagnoses are made without laboratory testing and records normally do not report specific pathogens or else identify incorrect pathogens (Scallan et al., 2018). Also, most AGI episodes resolve without a visit to the ED. This study examined AGI cases resulting in ED visits, which represents a small proportion of total AGI in the population, so the associations we observed in potentially severe AGI cases visiting an ED likely underestimate total AGI incidence (Mead et al., 1999). Moreover, rural areas—where the stronger association between high hog CAFO exposure and AGI is found—typically have reduced healthcare access, meaning that residents there may be less inclined to visit a hospital for AGI symptoms than their counterparts residing in micropolitan or metropolitan regions.
While the ZIP code-level resolution of the AGI ED visit data is better than the county-level resolution used previously, the data and analyses are still limited by this relatively coarse geographic granularity (Setzer and Domino, 2004). All residents in high hog exposed ZIP codes are not necessarily exposed, or exposed to the same degree, to pathogens from hog CAFOs, as exposure depends on topography, drainage, manure spraying patterns, and human actions. As rural ZIP codes can be quite large, residents in one part of a ZIP code may be highly exposed to pathogens from hog manure while residents in other parts of the ZIP code may be unexposed or low exposed. We attempted to reduce this misclassification by creating our ZIP code-level continuous hog CAFO exposure using high geographic resolution block-level population weights. Thus, if one sparsely populated area of a ZIP code were exposed to a hog CAFO, but the majority of the ZIP code’s population resided farther from hog CAFOs, then the ZIP code would be unlikely to be categorized as a high hog exposed ZIP code. Because these analyses were conducted at the ZIP code level, we are unable to directly examine the effect of distance from a CAFO on the association between CAFOs and AGI rate. Almost all of the high hog CAFO exposed ZIP codes contained multiple CAFOs, and the two high hog exposed ZIP codes that lacked hog CAFOs had multiple hog CAFOs just outside their borders.
Additionally, our IPTW methods are limited by the geographical clustering of hog CAFOs in NC (Chagas et al., 2011; Davis et al., 2017). The probability of a ZIP code being exposed to CAFOs is affected by various measured and unmeasured factors, including rurality, land price, community resistance and political power, and the location of slaughterhouses and other CAFOs. While IPTW was better able to control for bias than standard model adjustment, it was difficult, even with IPTW, to create an ideal control to areas with high hog CAFO exposure. This resulted in some very large and very small weights that we truncated at the 1st and 99th percentile (see Supplementary Table 2) (Cole and Hernán, 2008). Our approach minimized bias while preventing a few ZIP codes from being severely overweighted.
Environmental Injustice of CAFOs in North Carolina
Without employing IPTW, we would have predominantly compared rural eastern NC, with dense hog CAFOs, to rural western NC, without hog CAFOs, despite their having different populations and environmental exposures. For example, rural eastern NC has a much higher proportion of Black residents than does rural western NC, which has a very high proportion of White non-Hispanic residents. These characteristics are not random; the majority of NC’s enslaved Black population in the 18th and 19th century lived in eastern NC, and industrial hog operations exploded during the 1990s and early 2000s in these same areas that continue to be heavily inhabited by Black and American Indian residents (MacNell, 2015; Son et al., 2021b; Wing and Johnston, 2014). The environmental racism of the hog industry makes it difficult to isolate the effect of hog CAFOs on AGI in NC independent of race, income, and rurality, as these factors are strongly correlated with the exposure (Son et al., 2021b). We are also limited by the ED data, which includes American Indian as a race without distinguishing between individual Indigenous populations. Our American Indian EMM analyses compare AGI rates among American Indians in high hog CAFO exposed areas to American Indians unexposed to hog CAFOs, which effectively compares tribal populations in eastern NC (predominantly Lumbee, Coharie, and Waccamaw Siouan) to the Eastern Band of Cherokee Indians, in western NC. “American Indians” are not a monolith and each Indigenous nations have their own histories, environments, and politics.
The location of private wells and septic systems also reflect environmental injustice as municipal water lines and sewer systems do not reach some peri-urban, Black communities in NC (MacDonald Gibson and Pieper, 2017; Wilson et al., 2008). Septic tank leaks are a frequent cause of waterborne disease outbreaks, compounding the environment justice issues in rural NC (Yates, 1985). Urban areas exploit rural areas for waste disposal and food and energy production, causing pollution and reduced quality of life for rural communities. These environmentally unjust industrial practices disproportionately harm the health of rural populations while disproportionately benefiting urban populations (Kelly-Reif and Wing, 2016). In non-urban North Carolina in 2014, the proportions of American Indian, Black, and Hispanic residents that lived within 3 miles of a permitted hog CAFO were 2.18, 1.54, and 1.39 times higher, respectively, than the proportion of non-Hispanic White residents (Wing and Johnston, 2014). Thus, American Indian, Black, and Hispanic communities in NC are disproportionately at risk for CAFO-related illnesses, including, from this study, AGI-related illness. Many low-income and POC communities in eastern NC lack the political power and financial resources to prevent CAFOs from being built in their communities. Lower-income families may not be able to move away from newly sited polluting industries, a challenge exacerbated by the impact of these operations on their property values (Kim et al., 2010). The environmental injustice of hog CAFOs encompasses racism, classism, poverty, and the urban-rural divide (Kelly-Reif and Wing, 2016). NC should reduce the number and density of CAFOs and strengthen environmental regulations to improve the health of rural POC and low-income communities.
CONCLUSIONS
Results from studies on industrial hog operations and AGI have been inconsistent, possibly due to varying methods, regions, populations, and topography. NC’s 9 million hogs are housed predominantly in its hurricane-prone eastern rural region, where many residents depend on private well water and have limited healthcare access. We observed a 41% increase in AGI ED visit rate in areas with high hog CAFO exposure compared to areas with no hog CAFO exposure during the week after heavy rain (>99th percentile of precipitation). Overall, there was a 11% higher AGI ED visit rate in high hog exposed ZIP codes than in ZIP codes without hog CAFO exposure and a 21% higher AGI ED visit rate when restricted to rural areas. We found a higher AGI ED visit rate among American Indians, Black, and Asian American patients in rural high hog areas compared to rural areas without hogs. Hog CAFOs in NC were built in areas with a higher population of Black, Lumbee, and Filipino residents than the rest of the state. Because hog CAFOs in NC are disproportionally located in and near rural, low-income, POC communities, and near poultry CAFOs, and in areas with a high prevalence of septic systems and private wells, it is difficult to isolate the effect of hog CAFOs independent from these other factors. We are limited in making causal statements about the effect of hog CAFOs on AGI rate in large part because polluting facilities are disproportionately placed near other polluting facilities and in under-resourced communities, or sacrifice zones, which often hides harmful effects (Lerner, 2010). However, the associations presented in this paper—the positive association between high hog CAFO exposure and AGI ED visit after heavy rain and among rural Black, American Indian, and Asian residents (and disproportionately greater exposure to CAFOs for rural Black, American Indian, and Hispanic residents)—highlight the environmental injustice affecting communities in eastern NC.
Supplementary Material
Highlights.
Areas with industrial hog farming may have higher rates of gastrointestinal illness
Heavy rain at industrial hog farms increases gastrointestinal emergency room visits
People of color are disproportionately harmed by industrial hog operations
ACKNOWLEDGEMENTS:
Sources of funding: University of North Carolina Dissertation Completion Fellowship, National Institute of Environmental Health Sciences T32 training grant (T32 ES007018).
The authors would like to acknowledge Timothy J. Wade for his contributions to this manuscript.
The North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT) is an advanced, statewide public health surveillance system. NC DETECT is funded with federal funds by North Carolina Division of Public Health (NC DPH), Public Health Emergency Preparedness Grant (PHEP), and managed through a collaboration between NC DPH and the University of North Carolina at Chapel Hill Department of Emergency Medicine’s Carolina Center for Health Informatics (UNC CCHI). The NC DETECT Data Oversight Committee does not take responsibility for the scientific validity or accuracy of methodology, results, statistical analyses, or conclusions presented.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT Author Statement
Arbor J.L. Quist: Conceptualization, methodology, data curation, software, formal analysis,; writing—original draft
David A. Holcomb: Data curation, software, writing – review and editing
Mike Dolan Fliss: Methodology, software, writing – review and editing
Paul L. Delamater: Supervision, writing – review and editing
David B. Richardson: Methodology, writing – review and editing, supervision
Lawrence S. Engel: Methodology, supervision, writing – review and editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- Amato HK, Wong NM, Pelc C, Taylor K, Price LB, Altabet M, Jordan TE, Graham JP, 2020. Effects of concentrated poultry operations and cropland manure application on antibiotic resistant Escherichia coli and nutrient pollution in Chesapeake Bay watersheds. Sci. Total Environ 735, 139401. 10.1016/j.scitotenv.2020.139401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein V, Greenland S, 2018. Remove, rather than redefine, statistical significance. Nat. Hum. Behav 2, 4. 10.1038/s41562-017-0224-0 [DOI] [PubMed] [Google Scholar]
- Animal Manure Management [WWW Document], n.d. . United States Dep. Agric. URL https://www.nrcs.usda.gov/wps/portal/nrcs/detail/null/?cid=nrcs143_014211 (accessed 2.7.22).
- Austin PC, 2011. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res 46, 399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Stuart EA, 2015. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med 34, 3661–3679. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström L, Bowman BT, Sims JT, 2005. Definition of sustainable and unsustainable issues in nutrient management of modern agriculture. Soil Use Manag. 21, 76–81. 10.1111/j.1475-2743.2005.tb00111.x [DOI] [Google Scholar]
- Casey JA, Shopsin B, Cosgrove SE, Nachman KE, Curriero FC, Rose HR, Schwartz BS, 2014. High-density livestock production and molecularly characterized MRSA infections in Pennsylvania. Environ. Health Perspect 122, 464–470. 10.1289/ehp.1307370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas ALS, Toneto R, Azzoni CR, 2011. A Spatial Propensity Score Matching Evaluation of the Social Impacts of Sugarcane Growing on Municipalities in Brazil. Int. Reg. Sci. Rev 35, 48–69. 10.1177/0160017611400069 [DOI] [Google Scholar]
- Clostridium difficile on U.S. Swine Operations [WWW Document], 2011. . Vet. Serv. Centers Epidemiol. Anim. Heal URL https://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_is_Cdiff.pdf (accessed 9.1.21).
- Cole SR, Hernán MA, 2008. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol 168, 656–664. 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SA, Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, Bruce BB, Derado G, Edens C, Fullerton KE, Gargano JW, Geissler AL, Hall AJ, Havelaar AH, Hill VR, Hoekstra RM, Reddy SC, Scallan E, Stokes EK, Yoder JS, Beach MJ, 2021. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis 27, 140–149. 10.3201/eid2701.190676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ML, Neelon B, Nietert PJ, Hunt KJ, Burgette LF, Lawson AB, Egede LE, 2017. Addressing geographic confounding through spatial propensity scores: a study of racial disparities in diabetes. Stat. Methods Med. Res 28, 734–748. 10.1177/0962280217735700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice NB, Johnston JE, Gibson JM, 2016. Reducing Emergency Department Visits for Acute Gastrointestinal Illnesses in North Carolina (USA) by Extending Community Water Service. Environ. Health Perspect 124, 1583–1591. 10.1289/EHP160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter C, Maupin M, Caldwell R, Harris M, Ivahnenko T, Lovelace J, Barber N, Linsey K, 2018. Estimated use of water in the United States in 2015: U.S. Geological Survey Circular 1441.
- Doogan NJ, Roberts ME, Wewers ME, Tanenbaum ER, Mumford EA, Stillman FA, 2018. Validation of a new continuous geographic isolation scale: A tool for rural health disparities research. Soc. Sci. Med 215, 123–132. 10.1016/j.socscimed.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna P, McLellan SL, Simpson P, Li S-H, Gorelick MH, 2010. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ. Health Perspect. 118, 1439–1443. 10.1289/ehp.0901671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer IF, Hoover CM, Remais JV, Monaghan A, Celada M, Carlton EJ, 2016. Estimating the Risk of Domestic Water Source Contamination Following Precipitation Events. Am. J. Trop. Med. Hyg 94, 1403–1406. 10.4269/ajtmh.15-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Working Group & Waterkeeper Alliance, 2016. Exposing Fields of Filth: Locations of Concentrated Animal Feeding Operations in North Carolina [WWW Document]. URL https://www.ewg.org/research/exposing-fields-filth (accessed 10.9.19).
- Febriani Y, Levallois P, Gingras S, Gosselin P, Majowicz SE, Fleury MD, 2010. The association between farming activities, precipitation, and the risk of acute gastrointestinal illness in rural municipalities of Quebec, Canada: a cross-sectional study. BMC Public Health 10, 48. 10.1186/1471-2458-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febriani Y, Levallois P, Lebel G, Gingras S, 2009. Association between indicators of livestock farming intensity and hospitalization rate for acute gastroenteritis. Epidemiol. Infect 137, 1073–1085. 10.1017/S0950268808001647 [DOI] [PubMed] [Google Scholar]
- Foley SL, Lynne AM, Nayak R, 2008. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates1,2. J. Anim. Sci 86, E149–E162. 10.2527/jas.2007-0464 [DOI] [PubMed] [Google Scholar]
- Grubesic TH, Matisziw TC, 2006. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int. J. Health Geogr 5, 58. 10.1186/1476-072X-5-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan TY, Holley RA, 2003. Pathogen survival in swine manure environments and transmission of human enteric illness--a review. J. Environ. Qual 32, 383–392. [DOI] [PubMed] [Google Scholar]
- Hardy S, 2012. The Price of Pork [WWW Document]. UNC Endeavors. URL http://endeavors.unc.edu/the_price_of_pork (accessed 2.15.21). [Google Scholar]
- Hartnett KP, Kite-Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, Gundlapalli AV, Practice N.S.S.P.C. of, 2020. Impact of the COVID-19 Pandemic on Emergency Department Visits - United States, January 1, 2019-May 30, 2020. MMWR. Morb. Mortal. Wkly. Rep 69, 699–704. 10.15585/mmwr.mm6923e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney CD, Myers K, Wing S, Hall D, Baron D, Stewart JR, 2015. Source tracking swine fecal waste in surface water proximal to swine concentrated animal feeding operations. Sci. Total Environ 511, 676–683. 10.1016/j.scitotenv.2014.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda PS, Edwards AC, Anderson HA, Miller A, 2000. A review of water quality concerns in livestock farming areas. Sci. Total Environ 250, 143–167. 10.1016/s0048-9697(00)00373-9 [DOI] [PubMed] [Google Scholar]
- Hooiveld M, Smit LAM, van der Sman-de Beer F, Wouters IM, van Dijk CE, Spreeuwenberg P, Heederik DJJ, Yzermans CJ, 2016. Doctor-diagnosed health problems in a region with a high density of concentrated animal feeding operations: a cross-sectional study. Environ. Health 15, 24. 10.1186/s12940-016-0123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard LE, Givens CE, Griffin DW, Iwanowicz LR, Meyer MT, Kolpin DW, 2020. Poultry litter as potential source of pathogens and other contaminants in groundwater and surface water proximal to large-scale confined poultry feeding operations. Sci. Total Environ 735, 139459. 10.1016/j.scitotenv.2020.139459 [DOI] [PubMed] [Google Scholar]
- Jones TF, McMillian MB, Scallan E, Frenzen PD, Cronquist AB, Thomas S, Angulo FJ, 2007. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996–2003. Epidemiol. Infect 135, 293–301. 10.1017/S0950268806006765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keessen EC, Harmanus C, Dohmen W, Kuijper EJ, Lipman LJA, 2013. Clostridium difficile infection associated with pig farms. Emerg. Infect. Dis 10.3201/eid1906.121645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Reif K, Wing S, 2016. Urban-rural exploitation: An underappreciated dimension of environmental injustice. J. Rural Stud 47, 350–358. 10.1016/j.jrurstud.2016.03.010 [DOI] [Google Scholar]
- Kilburn KH, 2012. Human impairment from living near confined animal (hog) feeding operations. J. Environ. Public Health 2012, 565690. 10.1155/2012/565690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Goldsmith P, Thomas MH, 2010. Economic impact and public costs of confined animal feeding operations at the parcel level of Craven County, North Carolina. Agric. Human Values 27, 29–42. 10.1007/s10460-009-9193-x [DOI] [Google Scholar]
- Kravchenko J, Rhew SH, Akushevich I, Agarwal P, Lyerly HK, 2018. Mortality and Health Outcomes in North Carolina Communities Located in Close Proximity to Hog Concentrated Animal Feeding Operations. N. C. Med. J 79, 278–288. 10.18043/ncm.79.5.278 [DOI] [PubMed] [Google Scholar]
- Krieger N, Waterman P, Chen JT, Soobader M-J, Subramanian SV, Carson R, 2002. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas--the Public Health Disparities Geocoding Project. Am. J. Public Health 92, 1100–1102. 10.2105/ajph.92.7.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner S, 2010. Sacrifice Zones: The Front Lines of Toxic Chemical Exposure in the United States. The MIT Press, Cambridge, Massachusetts. [Google Scholar]
- Levallois P, Chevalier P, Gingras S, Dery P, Payment P, Michel P, Rodriguez M, 2014. Risk of infectious gastroenteritis in young children living in Quebec rural areas with intensive animal farming: results of a case-control study (2004–2007). Zoonoses Public Health 61, 28–38. 10.1111/zph.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Richardson DB, Hilborn ED, Weinberg H, Engel LS, Wade TJ, 2019. Emergency Department Visits for Acute Gastrointestinal Illness After a Major Water Pipe Break in 2010. Epidemiology 30, 893–900. 10.1097/EDE.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List of Permitted Animal Facilities [WWW Document], 2020. . North Carolina Environ. Qual. URL https://deq.nc.gov/cafo-map (accessed 4.27.21). [Google Scholar]
- MacDonald Gibson J, Pieper KJ, 2017. Strategies to Improve Private-Well Water Quality: A North Carolina Perspective. Environ. Health Perspect 125, 76001. 10.1289/EHP890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNell N, 2015. The influence of slavery on the locations of North Carolina’s industrial hog operations, 17th Annual North Carolina Environmental Justice Network Summit: The Role of International Solidarity in Achieving Environmental Justice. The Franklinton Center at Bricks, Whitakers, North Carolina. [Google Scholar]
- McShane BB, Gal D, Gelman A, Robert C, Tackett JL, 2019. Abandon Statistical Significance. Am. Stat 73, 235–245. 10.1080/00031305.2018.1527253 [DOI] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV, 1999. Food-related illness and death in the United States. Emerg. Infect. Dis 5, 607–625. 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Agriculture Statistics Service [WWW Document], 2020. . United States Dep. Agric URL https://quickstats.nass.usda.gov/#C81021E1-5D2E-384E-9E67-C80622016BB5 (accessed 2.10.21).
- Nicole W, 2013. CAFOs and environmental justice: the case of North Carolina. Environ. Health Perspect 10.1289/ehp.121-a182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North Carolina County Health Profiles [WWW Document], 2018. . North Carolina Inst. Med URL http://nciom.org/map/ (accessed 12.10.18).
- Norton JM, Wing S, Lipscomb HJ, Kaufman JS, Marshall SW, Cravey AJ, 2007. Race, wealth, and solid waste facilities in North Carolina. Environ. Health Perspect 115, 1344–1350. 10.1289/ehp.10161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E, Xagoraraki I, 2019. Understanding temporal and spatial variations of viral disease in the US: The need for a one-health-based data collection and analysis approach. One Heal. 8, 100105. 10.1016/j.onehlt.2019.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosemoli S, Green J, Bordeaux C, Menius L, Curtis J, 2012. Conservation Practices in Outdoor Hog Production Systems: Findings and Recommendations from the Center for Environmental Farming Systems. Raleigh, NC. [Google Scholar]
- Potter RC, Kaneene JB, Gardiner J, 2002. A comparison of Campylobacter jejuni enteritis incidence rates in high- and low-poultry-density counties: Michigan 1992–1999. Vector Borne Zoonotic Dis. 2, 137–143. 10.1089/15303660260613701 [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group [WWW Document], n.d. . Oregon State Univ. URL http://prism.oregonstate.edu (accessed 6.5.20). [Google Scholar]
- R: A language and environment for statistical computing, 2019.
- Scallan E, Griffin PM, McLean HQ, Mahon BE, 2018. Hospitalisations due to bacterial gastroenteritis: A comparison of surveillance and hospital discharge data. Epidemiol. Infect 146, 954–960. 10.1017/S0950268818000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Levin R, Hodge K, 1997. Drinking water turbidity and pediatric hospital use for gastrointestinal illness in Philadelphia. Epidemiology 8, 615–620. 10.1097/00001648-199710000-00001 [DOI] [PubMed] [Google Scholar]
- Setzer C, Domino ME, 2004. Medicaid outpatient utilization for waterborne pathogenic illness following Hurricane Floyd. Public Health Rep. 119, 472–478. 10.1016/j.phr.2004.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J-Y, Miranda ML, Bell ML, 2021a. Exposure to concentrated animal feeding operations (CAFOs) and risk of mortality in North Carolina, USA. Sci. Total Environ 799, 149407. 10.1016/j.scitotenv.2021.149407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J-Y, Muenich RL, Schaffer-Smith D, Miranda ML, Bell ML, 2021b. Distribution of environmental justice metrics for exposure to CAFOs in North Carolina, USA. Environ. Res 195, 110862. 10.1016/j.envres.2021.110862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone J. a, Wing S, 2011. Poultry litter incineration as a source of energy: reviewing the potential for impacts on environmental health and justice. New Solut. 21, 27–42. 10.2190/NS.21.1.g [DOI] [PubMed] [Google Scholar]
- Stuempfig ND, Seroy J, 2021. Viral Gastroenteritis. Treasure Island (FL). [Google Scholar]
- Thurston-Enriquez JA, Gilley JE, Eghball B, 2005. Microbial quality of runoff following land application of cattle manure and swine slurry. J. Water Health 3, 157–171. [PubMed] [Google Scholar]
- Wade TJ, Lin CJ, Jagai JS, Hilborn ED, 2014. Flooding and emergency room visits for gastrointestinal illness in Massachusetts: a case-crossover study. PLoS One 9, e110474. 10.1371/journal.pone.0110474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA, 2016. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat 70, 129–133. 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- Williams CM, 2009. Development of environmentally superior technologies in the US and policy. Bioresour. Technol 100, 5512–5518. 10.1016/j.biortech.2009.01.067 [DOI] [PubMed] [Google Scholar]
- Williams DL, Breysse PN, McCormack MC, Diette GB, McKenzie S, Geyh AS, 2011. Airborne cow allergen, ammonia and particulate matter at homes vary with distance to industrial scale dairy operations: an exposure assessment. Environ. Health 10, 72. 10.1186/1476-069X-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Heaney CD, Cooper J, Wilson O, 2008. Built Environment Issues in Unserved and Underserved African-American Neighborhoods in North Carolina. Environ. Justice 1, 63–72. 10.1089/env.2008.0509 [DOI] [Google Scholar]
- Wing S, Cole D, Grant G, 2000. Environmental injustice in North Carolina’s hog industry. Environ. Health Perspect 108, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Freedman S, Band L, 2002. The potential impact of flooding on confined animal feeding operations in eastern North Carolina. Environ. Health Perspect 110, 387–391. 10.1289/ehp.02110387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Horton RA, Muhammad N, Grant GR, Tajik M, Thu K, 2008. Integrating epidemiology, education, and organizing for environmental justice: Community health effects of industrial hog operations. Am. J. Public Health 98, 1390–1397. 10.2105/AJPH.2007.110486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Johnston J, 2014. Industrial Hog Operations in North Carolina Disproportionately Impact African-Americans, Hispanics and American Indians. Title VI Complain. [Google Scholar]
- Wing S, Wolf S, 2000. Intensive livestock operations, health, and quality of life among eastern North Carolina residents. Environ. Health Perspect 108, 233–238. 10.1289/ehp.00108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M, 1985. Septic Tank Density and Ground-water Contamination. Ground Water 23. [Google Scholar]
- Zook HG, Kharbanda AB, Puumala SE, Burgess KA, Pickner W, Payne NR, 2018. Emergency Department Utilization by Native American Children. Pediatr. Emerg. Care 34, 802–809. 10.1097/PEC.0000000000001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


