Abstract
Background
Obesity is a substantial public health concern; however, gold-standard behavioral treatments for obesity are costly and burdensome. Existing adaptations to the efficacious Diabetes Prevention Program (DPP) demonstrate mixed results. Our prior research applying the Multiphase Optimization Strategy (MOST) to DPP identifies a more parsimonious, less costly intervention (EVO) resulting in significant weight loss.
Objective
The aim of the remotely conducted EVO trial is to test the non-inferiority of EVO against DPP. We will conduct economic evaluations alongside the trial to estimate delivery and patient costs, cost-effectiveness, and lifetime healthcare costs of EVO as compared to DPP. Exploratory analyses will examine maintenance, moderators, and mediators of the treatment effect.
Study Design
The EVO trial will recruit nationally to randomize 524 participants with obesity. Participants will receive either EVO or DPP over a 6 month period. EVO participants will be provided online lessons, a smartphone application to self-monitor diet, physical activity, and weight, and attend 12 brief calls with a Health Promotionist. DPP participants will receive the first 6 months of the Center for Disease Control’s T2D materials and attend 16 one-hour video call sessions with staff certified in DPP delivery. Weight will be measured at baseline, 3-, 6-, and 12-months. Itemized delivery cost will be collected. Staff and participants will also provide information to estimate costs for intervention-related activities.
Significance
The EVO trial could establish evidence supporting dissemination of a scalable, cost-effective behavioral treatment with potential to shift clinical practice guidelines, inform policy, and reduce the prevalence of obesity.
Keywords: Weight loss, Optimization, mHealth, Obesity, Telehealth, Economic evaluation
Introduction
Approximately 40% of US adults have obesity, and the prevalence is projected to worsen.1,2 Obesity is a primary risk factor for all major cardiometabolic conditions, including Type 2 diabetes mellitus, hypertension, sleep disorders, left ventricular hypertrophy, and atherosclerosis, and is a primary contributor to cardiometabolic mortality rates.3 Obesity is similarly linked to increases in economic burdens. In the US, individuals with obesity have approximately $2,741 greater annual medical care costs compared to those without obesity,4 contributing to approximately $150 billion in additional annual health care costs.5 Unaddressed, obesity-related annual health care costs are projected to increase by between $48-$66 billion per year through 2030.6
Weight loss is associated with prevention of diabetes improvements in quality of life, and reduced mortality;7–10 however, efficacious weight loss interventions are out of reach for many Americans due to pervasive barriers such as lack of access to care, burdensome intervention-formats, and cost.7,11 Interventions that are scalable, affordable, and effective are critically needed to curtail obesity in the US.
Though efficacious, the most widely used weight management interventions, based on the Diabetes Prevention Program (DPP), includes various costly and burdensome components that impede widespread dissemination and uptake, particularly when patients have constraints on time, money, childcare, and/or transportation.7,11 Attempts to identify and eliminate DPP components which do not meaningfully contribute to treatment efficacy have been largely ineffective.12–16 Prior efforts to streamline the DPP relied on qualitative information, or pragmatic adjustments to treatment components, by removing costly elements for example. However, such adjustments driven by cost, rather than empirical data to support preserving positive effects can have unintended consequences.17 Systematic optimization research that can empirically inform adjustments is necessary to identify and develop affordable, scalable, and efficient weight management interventions which do not sacrifice effectiveness.18
The Multiphase Optimization Strategy (MOST)19,20 is a highly efficient framework for building interventions optimized around a set of identified parameters and goals. We recently completed a trial using MOST in which we identified an optimal weight loss treatment package for under $500 that included buddy training and reports to a primary care physician.21–23 However, as can occur with factorial trials, our results suggest that significant weight loss is also possible with the most minimal intervention offered, a remotely-delivered mHealth weight loss intervention with 12 coaching sessions, which we refer to herein as EVO (Elements Vital to treat Obesity).24 Recognizing that some treatment contexts may not be well suited for the additional cost and burden that the additional components confer, it is important to evaluate the very lowest cost option when there appears to be an estimated benefit. Despite the considerable promise of the minimal intervention, the relative efficacy of the EVO intervention compared to the gold-standard DPP is unknown.
To directly compare this optimized weight loss intervention, EVO, against the DPP in terms of clinical effectiveness and cost, we designed the EVO study, which employs a 2-arm randomized trial design. We hypothesize that EVO will be non-inferior to DPP on the outcome of weight loss. If such is the case, it could warrant consideration of disseminating EVO as an alternative weight loss program to DPP. The EVO study will also evaluate the cost-effectiveness of EVO vs DPP. As EVO is anticipated to be less costly than DPP, a non-inferior outcome would support the idea that dissemination of the EVO package is warranted. Additionally, the remote delivery of EVO would support immediate scalability. On an exploratory basis, we plan to examine baseline moderators and mediators of treatment response, and long-term (one year) maintenance of treatment improvements. if the EVO treatment is less costly and reduces participant burden, it would have great potential to change the standard of care.
Methods
The EVO study protocol and consent procedures were approved by the Northwestern University Institution Review Board prior to enrolling participants (STU00212742). The trial is registered on clinicaltrials.gov (NCT04708769) and the full study protocol and results will be published on clinicaltrials.gov upon completion of the trial.
Research Design
The EVO trial is a 2-arm randomized clinical trial to evaluate EVO as compared to DPP. Participants will be randomized to receive either the optimized intervention, EVO, or the intensive intervention, DPP, over a 6-month period.
Our optimized treatment arose from our past study, Opt-IN,21,22 in which we conducted a full-factorial study to test components of weight loss treatment (e.g., meal replacements, text messages). We found that a treatment package composed of calorie, fat gram, and physical activity goals, a smartphone application for self-monitoring, online educational materials, and biweekly coaching calls produced estimated weight loss at a level considered to be clinically meaningful.24 This treatment package resulted in an estimated 53% of participants losing 5% and 41% losing 7% of their baseline weight over 6-months.
Aims
The primary aim of the EVO trial is to determine whether EVO is non-inferior to DPP. We hypothesize that EVO is non-inferior to an individually delivered DPP program such that weight loss attained is sufficient to warrant implementation of the EVO package at scale. Throughout the trial, we will also collect detailed cost data and participant quality-of-life data for our secondary aim. This aim seeks to inform policy and practice by conducting a comprehensive economic evaluation to estimate delivery and patient costs, cost-effectiveness, and lifetime healthcare costs of EVO as compared to DPP.
The EVO Trial also has three exploratory aims. First, we will examine weight loss maintenance at 12-months after the baseline evaluation. It is also plausible that EVO or DPP will each work better for some participants under certain conditions or circumstances, and as such, we will examine baseline moderators (age, socioeconomic status, sex). Finally, we will examine self-regulation and related mediators (self-efficacy, self-monitoring, autonomous motivation) to identify potential elements for further treatment optimization.
Participants and Procedures
Eligibility
We will recruit participants for the EVO trial within the United States. For this trial, we aim to randomize 524 participants of any sex, gender, race, or ethnicity between 18–70 years old, with a Body Mass Index (BMI) between 30–45 kg/m2 (see Table 1). Children younger than 18 will not be included as practice guidelines for weight management in children differ and this study heavily relies on self-regulation, a skill not well developed in children. Individuals over age 70 will not be included due to risk of sarcopenia. Enrollees must be weight-stable, not concurrently enrolled in another weight loss program, and own a smartphone with a data plan. Additionally, participants must be willing to use the study smartphone application, as well as video conferencing software. Candidates with unstable medical or psychiatric conditions (e.g., recent myocardial infarction), with diabetes requiring insulin, or with a need for an assistive mobility device will be excluded from the study. We will also exclude those who are pregnant, lactating, or trying to become pregnant.
Table 1.
EVO Trial Inclusion and Exclusion Criteria
| Inclusion Criteria |
| - BMI between 30–45 kg/m2 |
| - Ages 18–70 |
| - Weight stable: weight changed > 25lbs within 6 months |
| - Not enrolled in any formal weight loss program |
| - Not taking anti-obesity medications |
| - Not taking medications that may cause weight gain |
| - Must own a Smartphone and be willing to install study smartphone app |
| - Must be willing to conduct video conference calls with study staff |
|
|
| Exclusion Criteria |
| - Unstable medical conditions |
| - Diabetes requiring insulin supplementation |
| - Crohn’s Disease |
| - Diagnosis of obstructive sleep apnea requiring intervention (i.e., CPAP) |
| - Use of assistive devices for mobility |
| - Hospitalizations for a psychiatric disorder within the past 5 years |
| - CVD symptoms while performing moderate intensity exercise |
| - Pregnancy, lactation, or intended pregnancy |
| - Bulimia or binge eating disorder |
| - Endorses active suicidal ideation |
| - Current substance abuse or dependence besides nicotine and caffeine dependence |
Initial Screening & Run-in
Participants will be recruited through multiple channels, focusing primarily on online platforms such as ResearchMatch, Facebook, and Instagram. Recruitment materials will direct candidates to the study website where they can complete an initial eligibility questionnaire and view an equipoise induction activity that prompts thinking about and explains pros and cons of the two intervention conditions.25 The initial eligibility questionnaire links to a REDCap26 survey that assesses weight, readiness to do physical activity,27 demographics, smartphone ownership, and scheduling availability. If candidates are found to be eligible based on the online screen, study staff will conduct a brief telephone screening interview where they will explain the study and conduct a full verbal informed consent process. Staff will screen candidates for additional exclusion criteria, as noted in table 1. Following this call, we will ask all candidates to download the study smartphone application to start a one-week run-in procedure, during which they track their dietary intake. In addition they will be asked to obtain medical approval from their primary physician to participate in the study, and complete an online baseline questionnaire to measure self-efficacy, autonomous motivation, and health related quality of life. Those candidates who demonstrate commitment to the program by logging 7 consecutive days of intake and completing these additional steps will remain eligible for the study. Participant study flow is displayed in Figure 1.
Figure 1.

EVO Study Design
Baseline and Randomization
Staff will call candidates after the run-in week to assess understanding of study procedures and expectations and to schedule the baseline session. In our previous trials we found that an individual phone call prior to a baseline session can answer additional questions, clarify any study expectations, and help to build rapport between participants and staff, thereby improving retention.23 During the call, staff will also administer the PRIME-MD28 to screen out candidates who meet criteria for bulimia, substance abuse, or report suicidal ideation. At the virtual baseline session, assessors masked to the participant’s treatment assignment will collect anthropometric data (weight and blood pressure), as well as administer two executive functioning tasks, the Stop Signal Task29 and the Oral Trails Making Test.30 Participants are provided with written instructions to set up the scale and blood pressure machine prior to the videoconference. Staff guide participants through taking their own weight and blood pressure and are able to show visual confirmation to the staff to verify the data. Eligible participants will be block-randomized to the EVO or DPP condition in permuted blocks of sizes 4, 6, or 8, stratified by biological sex. If randomized to EVO, study staff will orient the participant to all features of the smartphone application and the online lessons website, and will explain and schedule the first of 12 coaching calls. If randomized to DPP, study staff will remove the run-in application from the participant’s smartphone, provide the participant with a study binder including the Center for Disease Control’s Prevent TD31 curriculum and handouts, and will schedule the first of 16 video conferencing sessions.
Intervention Conditions
Due to the ongoing COVID-19 pandemic, all treatment will be delivered remotely; however, by delivering all treatment remotely, we control for potential confounding effects of differing treatment modality.32
A. The EVO Condition
Participants randomized to this condition will receive access to a smartphone application developed for the express purpose of this trial. The application facilitates participant self-monitoring of diet, physical activity, and weight loss. The app graphically represents progress relative to participant goals in diet, physical activity, and weight. Screenshots from the app are displayed in Figure 2. Physical activity goals are increased on a weekly basis, starting at 100 min of moderate to vigorous physical activity (MVPA) per week, and increasing to 300 min of MVPA by the end of 6 months. Daily calorie and fat gram goals are determined based on baseline weight, as displayed in Table 2.
Figure 2.
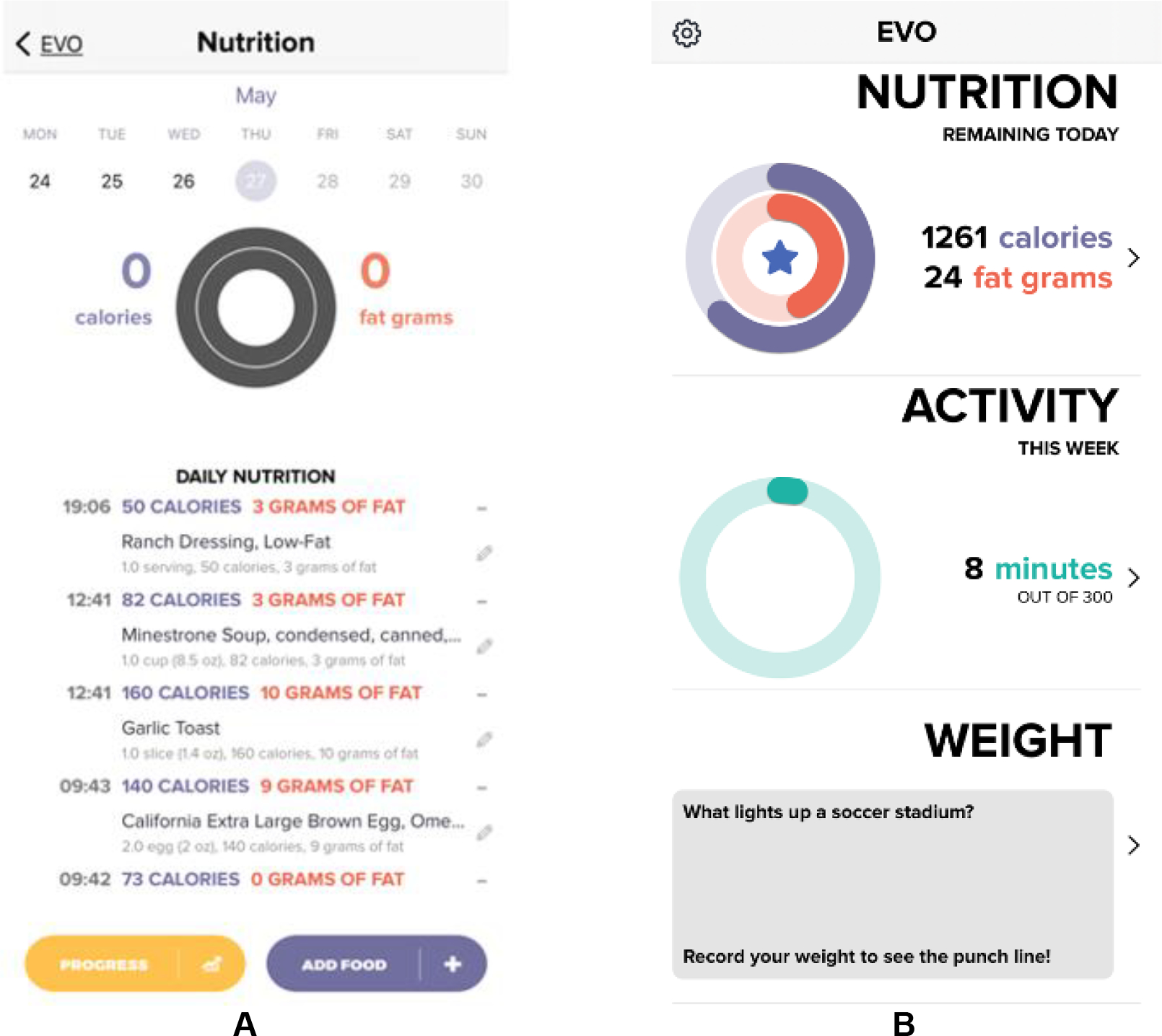
Run-in and main display interface for the custom-built EVO App. Run-in (A) is used as a tool to screen participants interested in the EVO trial. Following randomization to the EVO conditions, the main display (B) allows participants to track their daily calorie and fat gram intake, minutes of physical activity completed, and daily weight.
Table 2.
Daily calorie and fat gram goals
| Baseline Weight | Calorie Goal | Fat Goal |
|---|---|---|
| < 174 lbs. | 1200 kcal/d | 33g |
| 175 – 219 lbs. | 1500 kcal/d | 42g |
| 220– 249 lbs. | 1800 kcal/d | 50g |
| > 250 lbs. | 2000 kcal/d | 55g |
Participants in the EVO condition also receive access to an online site that contains lessons on diet, physical activity, and behavioral strategies for weight loss. They are given a unique login for the study website, which contains psychoeducational strategies for weight management. Each lesson on the study website provides information on a weight loss topic which was used in the Opt-IN study, based on other successful weight loss curricula such as LookAhead and DPP.33,34 We updated the lessons and put them into a web-based format to increase accessibility. The study database tracks and stores number of logins, page visits, and time spent on each lesson for each participant.
Participants in the EVO condition also receive remotely delivered health promotion. Health Promotionists35 will call participants for 10–15 minute program sessions every other week, for a total of 12 coaching calls during the 6-month intervention period. Health Promotionists are trained to review self-monitoring data in a web-based dashboard where all app data is visible, as seen in Figure 3. During calls, the Health Promotionist reviews goal progress with the participant, discusses barriers and facilitators to behavior change, supports the participant in problem solving, and provides recommendations as needed. All Health Promotionists receive training from a clinical psychologist in motivational interviewing and brief behavioral counseling principles. Program sessions are audio-recorded and monitored for treatment fidelity. All Health Promotionists attend a weekly group supervision session with the study psychologist.
Figure 3.

Weight and nutrition goals displayed within the web-based dashboard
B. The DPP Condition
The Center for Disease Control (CDC) now administers the National Diabetes Prevention Program (DPP), which maintains, updates, disseminates, and provides recognition standards of the intensive lifestyle program. Participants in the study DPP condition will receive the first 6 months of the CDC’s current curriculum, Prevent T2,31 which represents the core intervention and aligns with the timing of the EVO treatment course.
Participants are asked to track their physical activity, food intake, body weight, and an action plan for meeting SMART goals on a daily basis. Consistent with the curriculum, participants are asked to log using the tool of their choice, but they are provided with an electronic log to use for convenience. Accompanying the log is a DPP packet, which includes session outlines, worksheets, and psychoeducational materials. For the purpose of this trial, we will provide the materials corresponding to the first 6 months of the Prevent T2 curriculum, which are freely available from the CDC. The curriculum guides participants towards two goals: a goal to lose 7% of their starting weight, and a goal to complete 150 minutes of MVPA per week.
Per the CDC’s standard protocol, participants in the DPP condition will meet via videoconferencing with a Health Promotionist for one hour each, for 16 sessions. As part of the Prevent T2 curriculum, the CDC also includes a detailed coaching manual to guide each session. All Health Promotionists will be trained to use the curriculum through a American Association of Diabetes Educators lifestyle coach training program, specifically a CDC recognized National DPP Lifestyle training entity. In line with the curriculum, during virtual program sessions, participants are asked to weigh themselves on a study-provided Fitbit Aria scale. Health Promotionists will verify this weight measurement by observing the scale’s reading, either by viewing the scale display directly via video or by observing the participant’s Fitbit app reading via the web-based dashboard. All logs are reviewed and discussed during each call.
Fidelity
We have successfully used REDCap26 to administer checklists during sessions such that coaches find it easy to adhere to study protocol.26 To evaluate fidelity, program sessions will be audio recorded. On a quarterly basis, 15% of all calls will be sampled and rated. Manuals and checklists will be developed to ensure consistency and fidelity, modified from our previously published fidelity checklist.36 Although the curriculum and approach to lifestyle coaching are similar and there are no conflicting cocepts being taught in one curriculum versus the other, there are important differences in the frequency and duration of each treatment session, the order of topics to be covered, and the focus of the sessions. We will retrain and recertify any Promotionist who receives a rating below 95% fidelity. Per this method, the Opt-IN trial achieved 99.3% average fidelity and no Promotionist required retraining over the course of the trial.
Outcome assessments
Participant anthropometrics will be assessed remotely at 3-, 6- and 12-months by trained staff masked to condition. Participants will be paid $15 for completing their 3-month assessment, $25 for completing their 6-month assessment, and $50 for completing their 12-month assessment.
Height, weight, BMI, and blood pressure
Height will be collected via a report from each participant’s physician prior to study enrollment, recorded to the nearest 0.25 inch. Weight and blood pressure will be measured at each assessment remotely over video conferencing with the guidance of study staff. Weight will be measured after participants are asked to wear light clothing and no shoes. Body weight will be measured to the nearest 0.1 lb. using an Aria Air wireless scale. 91 BMI will be calculated using the Quetelet Index37 as weight in pounds / (height in inches)2 × 704.5. Blood pressure is measured three times using an Omron 3 Series Upper Arm blood pressure machine positioned on the participant’s non-dominant arm. A Welch Allyn 1700 series blood pressure machine will be provided to any participant requiring an extra-large arm cuff.
Costs
Cost data will be collected using a micro-costing approach to capture all costs associated with the treatments in both arms.38 Specific cost items will be collected for each arm: (1) personnel (e.g. staff salaries + fringes, measured as time spent × hourly wage) and non-personnel (e.g. intervention materials, overhead) costs for implementing the designed interventions, both of which will be marked as fixed (costs not varying by number of participants) or variable (costs varying by number of participants) costs; (2) participant time and travel costs as well as program-related purchase expenses (e.g. clothing or shoes for exercise); and (3) participants’ lost productivity due to program participation. Personnel and non-personnel costs will be collected via REDCap surveys on a weekly basis. Participant time and expense questionnaires collected on a biweekly basis in REDCap will be utilized to collect the data for (2) and (3).
Health-related-quality-of life
Health-related-quality-of-life (HrQoL) will be measured using the EuroQol 5-dimension 5-level descriptive system.39 This questionnaire has five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels: no problem, slight problems, moderate problems, severe problems, and unable to do (or extreme pain/anxiety). EQ-5D is one of the health indices that can be used to compute quality-adjusted life years (QALYs).40 Participants’ QOL data will be collected at baseline, 3-month, 6-month, and 12-month.
Measurement of moderators
We will measure moderators at the baseline time point. Age, gender, and ethnicity will be collected by a demographic questionnaire, while socioeconomic status will be measured using the MacArthur Scale of Subjective Social Status.41 This is a single-item measure that assesses a person’s perceived rank relative to others in their group. Food insecurity will be measured using a 2-item insecurity questionnaire,42 and a weight loss history questionnaire43 will be used to collect information about previous participant experiences with diet and weight loss.
Measurement of mediators
We will measure mediators at baseline, as well as the 3-, 6-, and 12-month time points of the study. This will include self-monitoring adherence, collected by the smartphone application (EVO condition) or manual log entry (DPP condition) and measured in terms of the quantity, frequency, and consistency of a participant entering diet, weight, and exercise data. Self-efficacy will be measured for both diet and physical activity. Diet self-efficacy will be assessed from the scenario-based Dieting Self- Efficacy Scale (DIET-SE).44 The DIET-SE includes 11 items that are broken down into three subscales to reflect different challenges to eating self-control: 1) high caloric food (HCF, 4 items), 2) social and internal factors (SIF, 4 items), and 3) negative emotional events (NEE, 3 items). The internal consistency of the DIET-SE is satisfactory (α = 0.77 for HCF; α = 0.79 for SIF; α = 0.79 for NEE; α = 0.87 for total score). Test–retest correlations for a 2- to 3-week interval were r = 0.83 for the DIET-SE scale (r = 0.75 for HCF, r = 0.77 for SIF, and r = 0.80 for NEE), indicating good test–retest reliability. Physical activity self-efficacy will be evaluated using a modified version of The Self-Efficacy of Exercise Behavior Change scale.45 Participants use a 5-point Likert scale (1 = not at all confident; 5 = completely confident) to rate how confident they are that they will be able to exercise when “other things get in the way” such as being depressed, anxious, busy, or fatigued. A higher score indicates higher self-efficacy for physical activity. The abbreviated scale asks 18 items, and shows satisfactory internal consistency (α =.77).46
Autonomous Motivation will be measured by an adapted and abbreviated version of the Treatment Self-Regulation Questionnaire.47,48 Four statements will be rated on a 5-point scale ranging from 1 (not at all true) to 5 (very true). Each item begins with the same stem: “The reason I want to achieve a healthier weight is…,” followed by, for example, “…because I personally believe it is the best thing for my health.” The abbreviated scale shows satisfactory internal consistency (α =0.77).
Self-regulation will be measured in multiple ways. First, to evaluate changes in the self-regulation skills necessary for weight loss, we will use adapted versions49,50 of a scale developed by Saelens and colleagues.51 The adapted scale measures skills for appropriate eating and physical activity, each using 10 items on a scale of 1 (Never) to 5 (Often). The scales have a reported internal consistency ranging from α=.75 - .81, and test-retest reliability between .74 - .77. Secondly, to align with areas likely to yield useful information related to treatment response as recommended by the ADOPT Working Group,92 we will use executive functioning measures to evaluate participant ability to use self-regulation skills, focusing on commonly used measures of working memory, inhibition, and shifting. The Stop-Signal Task52–54 measures inhibition by asking participants to use a response key to respond as quickly as possible to stimuli on a computer screen, and to refrain from responding when a signal noise is presented.52 The outcome is total reaction time. The Oral Trail Making Test30 measures attention shifting by asking the participant to list sequential numbers, and then shift to completing a slightly different task listing alternating sequential numbers and letters. The outcome is the difference in completion time of the two administrations of the task.
Statistical and Economic Analyses
The primary outcome measure for the trial is change in weight (kilograms) from baseline to 6-months. The secondary outcome measure is cost-effectiveness of EVO. For exploratory analyses, we are interested in change in weight from baseline to 12-months. Each participant, once randomized, will be included in the intent-to-treat sample. Thus, every effort will be made to collect all primary and secondary outcomes even if a participant does not engage in the assigned treatment.
Primary Aim Analysis
We will determine whether EVO is non-inferior to DPP in terms of weight change from baseline to 6-months. A Covariance Pattern Model (CPM) using SAS PROC MIXED will be used to analyze the longitudinal weight data. CPMs use all available outcome data allowing for unequal numbers of observations at each time point and, under full-likelihood estimation, accommodate missingness when observations are missing at random (MAR).55 Thus, all available data from weight at baseline, 6-, and 12-months will be used. Pattern-mixture models will be used to perform sensitivity analyses to investigate how robust our inferences are to departures from the MAR assumption.56,57 The CPM will include fixed effects for time (treated as a nominal variable with baseline as the reference cell), treatment group, and treatment group by time interaction. All fixed effects will be tested using Wald statistics. The CPM will include an unstructured residual variance-covariance matrix to allow for differing variances and covariances across time, and will adjust for sex and baseline age. Model diagnostics (likelihood ratio tests and AIC) will be used to determine the suitability of more parsimonious covariance structures. From the fitted CPMs, the primary hypothesis will be examined by attending to the coefficient of the treatment group by month 6 interaction term (the difference in weight loss between EVO and DPP at 6-months). Non-inferiority will be established if the upper limit of a (1–2α) × 100% confidence interval for the mean difference in weight loss between EVO and DPP is below δ, where α is the Type I error, and δ is the non-inferiority margin.58 Jones et al.59 recommend a non-inferiority margin to be 50% of the difference between a treatment and control condition. The primary DPP trial resulted in a 6.5kg weight loss after 6 months compared to 0.1kg in the control condition,11,60 which would give us a 6.4kg difference and a 3.2kg margin. Thus, relative to results from DPP and other non-inferiority margins set in prior research, we set a 3.2kg margin such that if EVO loses up to 3.2kg less than our DPP condition, we will conclude that EVO is non-inferior to DPP as an obesity treatment.
Sample Size and Power
Sample size calculations were based on the primary outcome contrast: change in weight from baseline to 6-months in EVO versus DPP. Based on our previous studies,14,24,36 we assume an overall SD of 13.6kg, an intra-class correlation of 0.93, and 15% attrition at 6-months and 20% attrition at 12-months. Using formulas from Hedeker et al.56,61 for longitudinal designs, to obtain 80% statistical power for a 1-sided test with an alpha equal to 0.05, a total of 262 participants in each treatment group (for a total of 524 participants) will be randomized to establish the non-inferiority of EVO compared to DPP.
Secondary Aim Analysis
An analytic plan was made following economic evaluation guidelines62–64 at the planning stage of this trial. All analyses will be conducted based on intention-to-treat principle for the primary economic evaluation and per-protocol principle for the secondary economic evaluation.65 All economic evaluations will be conducted from both the health system perspective and the societal perspective based on the cost-effectiveness analysis recommendations.66 Missing and censored data (particularly for participants lost to follow-up) will be summarized and evaluated to determine the appropriateness for exclusion or imputation. If the latter, multiple imputation will be performed.67,68
Economic evaluation 1
Cost analysis A: For each arm, we will separately analyze the variable costs associated with the number of participants served and the fixed costs associated with the program itself regardless of how many participants are served. These will allow us to estimate costs when the program expands to accommodate more participants for a future budget impact analysis.
Cost analysis B: For each perspective, aggregated costs for each arm will be divided by the number of participants to yield the per-participant costs. Per-participant costs in the EVO arm will be compared to those in the DPP arm.
Economic evaluation 2
Cost-effectiveness analysis A: We will compare the two treatment arms: EVO versus DPP in the one-year time horizon (trial time period) from both perspectives.
Cost-effectiveness analysis B: We will compare the two treatment arms (EVO versus DPP) in the lifetime horizon (from time of treatment to death) from the health system perspective. We will use the BMI change trajectory observed in the trial under different scenarios: (1) ideal scenario – lifetime maintenance of weight loss (2) second-ideal scenario: long-term BMI change trajectory based on published data on a similar behavioral intervention69 followed by lifetime maintenance; and (3) real-life scenario: long-term BMI trajectory based on published data on similar behavioral intervention followed by predicted BMI trajectory based on participant characteristics and weight loss trajectory in the trial. More scenarios informed by the collected data will be considered at the time of analysis. Since the BMI change trajectory under different scenarios are projected based on the assumed scenarios, as opposed to real data, the analyses in Cost-effectiveness analysis B will be performed using simulation models.
Sensitivity analyses
We will perform sensitivity analyses to explore the variation resulting from (1) sampling uncertainties from the trial; (2) parameter uncertainties from the simulation model; (3) imputation uncertainties, if imputation is determined to be the approach to fill in data missingness; and (4) baseline time horizon. For (4), if we extend the time period beyond the baseline time horizon, we will combine the trial data with long-term observational data published by other studies.
Threshold and subgroup analyses
Threshold analyses will be conducted to answer important research questions identified during the analyses.For example, when the cost per EVO participant is greater than a threshold at which both DPP and EVO yield the same value, then DPP would be preferred over EVO. For Economic evaluation 2, the value can be determined by net monetary benefit, which is computed by QALYs × willingness-to-pay per QALY – cost. If some subgroups are considered to be important and likely to drive decision making, subgroup analyses will also be conducted. These subgroups can be demographic subgroups (e.g., women or men, older or younger) or any subgroup that are of interest (e.g., mildly obese individuals with a BMI 30–34.9 kg/m 2).
Exploratory Aims: Maintenance, Moderators, and Mediators
Maintenance at 12-months will be investigated using the model from the primary analysis, focusing on the treatment group by month-12 interaction term, which measures the difference in weight loss between the two treatment groups at 12-months.
We will identify moderating variables at baseline (e.g., SES, baseline self-efficacy, baseline self-regulation) that predict who will benefit more or less from EVO versus DPP. To do this, we will use models similar to those in the primary analysis, including a main effect for the moderator, its interaction with time, and a three-way treatment group by time by moderator interaction term.
We will identify mechanisms (mediators; e.g., self-monitoring during the initial treatment period, changes in self-efficacy) by which the treatments affect weight loss. Models to assess the effect of potential mediators of the treatment effect will follow the approach for estimating mediation in multilevel models as outlined by Krull and Mackinnon,70 wherein mediation is assessed by fitting two models. The first mediation model is similar to that for the primary aim and estimates the treatment effect on weight but also includes the mediating variable as a time-varying covariate. The second mediation model estimates the effect of the components on the mediator itself over time (using CPM with the same covariates as in the primary aim analysis).
Discussion
Obesity prevalence, estimated to be 40% in the US,1,2 is blamed for stagnant mortality rates,71 increased health care spending,4,5 decreased employment, and lower wages.72,73 Current gold standard weight loss treatments are based on the Diabetes Prevention Program.11,74 Despite its promise of success, DPP is burdensome to attend and costly to deliver,7 rendering the program inaccessible to most of the American public. The EVO study follows a systematic optimization process and tests the effectiveness of a remotely delivered intervention designed to be less burdensome and costly than currently available evidence-based weight loss programs. In this non-inferiority randomized controlled trial – EVO, we will first compare EVO to a remotely delivered version of DPP on weight loss at 6-months in parallel to a comprehensive economic evaluation. By using the MOST framework,75 the goal of this study is to provide a sound evidence base to judge whether a less intensive and costly intervention is a viable option to address the grave public health problem of obesity.
Many studies have adapted the DPP to reduce cost or increase access, with mixed success in achieving weight loss. For example, researchers have tested varying group sizes, in an effort to create efficiency, but larger groups did not lose as much weight.76 Others have adapted the DPP to be delivered remotely through mobile health technologies as a way to increase access, but although promising, results have not always been favorable.16 In the only published study directly comparing a remote versus in-person version of the DPP,77 the remotely delivered intervention performed just as well as in-person on weight loss, but so did the control group. All intervention conditions in this study shared the requirement of in-person weigh-in visits, obscuring which common versus shared components were responsible for weight loss. These attempts to test adapted versions of the DPP have failed to empirically, efficiently, and clearly identify which obesity treatments are essential and which are unnecessary, largely due to a lack of guiding optimization research framework.17,78,79 Although we have some evidence to support aspects of weight loss treatment that are effective (e.g. small groups or individual treatment, technology supported intervention) we lack clarity on what parts of the DPP can be reduced to cut cost and burden without sacrificing weight loss effects compared to the DPP as originally packaged.
Our prior work was the first to use MOST to systematically improve efficiency of an effective obesity treatment by reducing resource use. After a preparation phase to develop a conceptual model and pilot test treatment components,80 we conducted an optimization trial (Opt-IN) with the objective of assembling a treatment package that included only treatment components that contributed to weight loss and could be delivered for less than $500.21,22 A full-factorial design testing five components and a component level in addition to a core intervention revealed that the core intervention alone, without additional components, produced an estimated weight loss that was clinically meaningful and under the cost threshold.24 Opt-IN also directly compared weight loss resulting from 12 coaching calls versus 24 and findings supported the notion that increasing intervention sessions does not confer additional benefit as suggested by Ali and colleagues.17 After this optimization phase during which we identified an optimal treatment package, the next phase requires evaluation against a reasonable comparator, in this case the most widely used an existing treatment, as is our goal in the current study.20,81 To our knowledge, there has not been an evaluation phase trial following the systematic optimization process of MOST, for the purpose of improving weight loss treatment.
Alongside an evaluation trial, given that decreasing cost and burden is a goal, assessing cost-effectiveness is necessary to inform treatment decision making. Economic evaluation provides information on efficient use of available resources for maximizing health benefits and informs policy and clinical guidelines, yet there is a lack of cost data systematically collected or reported as part of behavioral intervention trials for weight loss.82 Only 1.7% of the cost-effectiveness studies (n=71028) searched through Pubmed in 2017 were listed under the behavioral interventions heading.83 Among the studies that did report cost-effectiveness results for behavioral studies (n=188), only 50% reported the results according to current guidelines.63,64,66 Among those, economic evaluation was often conducted after the conclusion of a trial as a by-product, decreasing the credibility of the findings. Head-to-head comparisons of the value of the target interventions within the trial, or even other competing interventions outside of the trial, improve understanding of health care resource utilization. As a result, there is a critical need for economic evaluations to inform implementation, clinical practice, health insurance, and health policy related to treatments for obesity. Our study thus was designed to conduct economic evaluation alongside this trial to fill this gap.
Mechanisms of behavioral weight loss treatment are poorly understood, limiting further optimization of treatment, particularly to improve long-term treatment effects. Higher autonomy, self-monitoring, and self-regulation are consistently associated with weight loss in lifestyle interventions.84 In particular, self-monitoring behavior is a significant predictor of weight loss during behavioral interventions and is associated with energy balance.85,86 However, there are mechanisms related to self-monitoring, such as support received from an interventionist, which may help amplify the effect. In fact, session attendance increases the likelihood of self-monitoring both before and after sessions.85,87 Technology-supported self-monitoring supports greater adherence when compared to traditional paper and pencil methods, perhaps in part due to increased autonomous motivation.39 Thus, to improve weight loss interventions, more information is needed about why behavioral interventions work.
Limitations of the current project warrant discussion. First, there remain open questions about the components of the intervention. For example, 24 calls from a Health Promotionist were not better than 12 but a lower number call condition was not compared. Thus, we might ask how many calls are truly necessary. This was not tested in Opt-IN and is an empirical question worth exploring.36 However, given the research to dissemination gap and the prevalence of obesity we face, this work seeks to push forward the systematic evaluation of a treatment package that has likelihood of uptake due to its low cost, sooner. The use of smartphone technology, in particular an app that uses data, could limit the utility for those without adequate access or resources. Given smartphone ownership, we contend that EVO would be a viable option for many Americans with obesity should it show effectiveness. Providing a scalable remote option may free up needed resources to offer currently available in-person interventions to those that need them. Additionally, while health care utilization may be an important metric to include in a comprehensive economic evaluation, the time horizon limits the number of events likely. Finally, as this study is conducted during the era of Covid-19, the methods for the EVO intervention do not perfectly match what was done during the Opt-IN trial on which it was based.32 Participants in Opt-IN, recruited from the Chicagoland area, were asked to attend in person clinic visits for assessments at baseline, 3-months, and 6-months. Due to the uncertainty of the ongoing pandemic, this trial is conducted completely remotely, including assessments. Assessments of behavioral consequences and outcomes like weight are likely to exert demand characterists. We emulate the in-person assessment and its effects to the extent possible by having participants weigh themselves while on video conference being observed by the study staff. Adjusting protocol in this way has opened the door to recruiting beyond a local sample. Hence, what we lose in the precision of in person weight assessment, we hope to gain in more robust generalizability.
Randomized controlled trials of intensive behavioral weight loss interventions produce an average of 8kg weight loss.88–90 Behavioral weight loss trials have attempted to improve scalability, cost, and patient burden, yet many are pre-post and few are RCTs with appropriate comparators.17,78,79 The only study to our knowledge that directly compared a less intensive treatment to the gold standard, intensive treatment produced results that suggested that intensive treatments may not be necessary to produce sufficient weight loss.77 Adaptations to treatment that are not guided by evidence from trials designed to test such additions or subtractions of components, has left us with multicomponent behavioral treatments that fail to be useful in clinical practice. More rigorous trials such as dismantling or factorial trials are needed to elucidate necessary treatment components. Doing so can support identifying interventions that balance effectiveness against affordability, scalability, and efficiency.20 Furthermore, translation of effective interventions to practice is also limited by a lack of cost data on which to make decisions. Our prior work sought to improve upon the DPP, primarily through using MOST as a framework and a highly efficient factorial research design to identify components necessary to confer a weight loss effect. However, the limitation of Opt-IN’s research design is that it was not powered to test effects of a treatment package. The EVO trial builds on the preponderance of evidence for effective behavioral weight loss programs and our rigorous optimization trial to move weight loss intervention science towards reducing obesity in more of the population. The results of this trial have the potential to inform clinical practice guidelines, policy, and provide essential information needed to further optimize treatment. Critically, this work together with the work that preceded it 21–23,80 offers a path for other optimization work to follow, such that the proliferation of RCTs of disparate multicomponent treatment packages is no longer the norm, and systematic work to develop effective scalable interventions using the MOST framework prevails.
Acknowledgements
Funding: This work was supported in part by National Institutes of Health grants R01DK125749, R01DK108678, UL1TR001422.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. Jama. 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden C Trends in obesity among adults in the United States, 2. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lorenzo A, Gratteri S, Gualtieri P, Cammarano A, Bertucci P, Di Renzo L. Why primary obesity is a disease? J Transl Med. 2019;17(1):169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–230. [DOI] [PubMed] [Google Scholar]
- 5.Kim DD, Basu A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value Health. 2016;19(5):602–613. [DOI] [PubMed] [Google Scholar]
- 6.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. [DOI] [PubMed] [Google Scholar]
- 7.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143(4):251–264. [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ (Clinical research ed). 2017;359:j4849–j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172–1191. [DOI] [PubMed] [Google Scholar]
- 10.Hamman RF, Wing RR, Edelstein SL, et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care. 2006;29(9):2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring). 2008;16(11):2462–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):69, 74–65, 77–68. [DOI] [PubMed] [Google Scholar]
- 14.Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity (Silver Spring). 2017;25(7):1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of Smartphone-Based Behavioral Obesity Treatment With Gold Standard Group Treatment and Control: A Randomized Trial. Obesity (Silver Spring). 2019;27(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Preventive medicine. 2017;100:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health affairs. 2012;31(1):67–75. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini CA, Pfammatter AF, Conroy DE, Spring B. Smartphone applications to support weight loss: current perspectives. Advanced health care technologies. 2015;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins L Optimization of Behavioral, Biobehavioral, and Biomedical Interventions: The Multiphase Optimization Strategy (MOST). New York: Springer; 2018. [Google Scholar]
- 20.Collins LM. Conceptual introduction to the multiphase optimization strategy (MOST). In: Optimization of behavioral, biobehavioral, and biomedical interventions. Springer; 2018:1–34. [Google Scholar]
- 21.Pellegrini CA, Hoffman SA, Collins LM, Spring B. Optimization of remotely delivered intensive lifestyle treatment for obesity using the Multiphase Optimization Strategy: Opt-IN study protocol. Contemporary clinical trials. 2014;38(2):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrini CA, Hoffman SA, Collins LM, Spring B. Corrigendum to “Optimization of remotely delivered intensive lifestyle treatment for obesity using the Multiphase Optimization Strategy: Opt-IN study protocol” [Contemp. Clin. Trials 38 (2014) 251–259]. Contemporary Clinical Trials. 2015;45:468–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spring B, PFammatter AF, Marchese SH, et al. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: Results of the Opt-IN study. Obesity. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spring B, Pfammatter AF, Marchese SH, et al. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: results of the Opt-IN Study. Obesity. 2020;28(9):1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health education research. 2005;20(4):439–447. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Canadian journal of sport sciences. 1992. [PubMed] [Google Scholar]
- 28.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 29.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological science. 1997;8(1):60–64. [Google Scholar]
- 30.Mrazik M, Millis S, Drane DL. The oral trail making test: effects of age and concurrent validity. Arch Clin Neuropsychol. 2010;25(3):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruss SA, Luman E, Wittner J, Keenan J, Conner D, Moore T, Bieber-Tregear MA Proven Program to Prevent or Delay Type 2 Diabetes. 2018. [Google Scholar]
- 32.Turner-McGrievy G, Halliday TM, Moore JB. COVID-19 Messed Up My Research: Insights from Physical Activity and Nutrition Translational Research. Translational Journal of the American College of Sports Medicine. 2021;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group DPPR. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Group LAR. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spring B, Stump T, Penedo F, Pfammatter AF, Robinson JK. Toward a health-promoting system for cancer survivors: Patient and provider multiple behavior change. Health Psychology. 2019;38(9):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfammatter AF, Nahum-Shani I, DeZelar M, et al. SMART: study protocol for a sequential multiple assignment randomized controlled trial to optimize weight loss management. Contemporary clinical trials. 2019;82:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hortobagyi T, Israel R, O’Brien K. Sensitivity and specificity of the Quetelet index to assess obesity in men and women. European journal of clinical nutrition. 1994;48(5):369–375. [PubMed] [Google Scholar]
- 38.Ritzwoller DP, Sukhanova A, Gaglio B, Glasgow RE. Costing behavioral interventions: a practical guide to enhance translation. ann behav med. 2009;37(2):218–227. [DOI] [PubMed] [Google Scholar]
- 39.Group. E. EQ-5G: A standardized instrument for use as a measure of health outcomes. 1990; http://www.euroqol.org/. Accessed January 29, 2014.
- 40.Hunink MM, Weinstein MC, Wittenberg E, et al. Decision making in health and medicine: integrating evidence and values. Cambridge University Press; 2014. [Google Scholar]
- 41.Adler N, Stewart J, Group wtPR. The MacArthur scale of subjective social Status. 2007. Psychosocial Research Notebook. 2016. [Google Scholar]
- 42.Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–32. [DOI] [PubMed] [Google Scholar]
- 43.Myers VH, McVay MA, Champagne CM, et al. Weight loss history as a predictor of weight loss: results from Phase I of the weight loss maintenance trial. J Behav Med. 2013;36(6):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stich C, Knauper B, Tint A. A scenario-based dieting self-efficacy scale: the DIET-SE. Assessment. 2009;16(1):16–30. [DOI] [PubMed] [Google Scholar]
- 45.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Research quarterly for exercise and sport. 1992;63(1):60–66. [DOI] [PubMed] [Google Scholar]
- 46.Vinkers CD, Adriaanse MA, de Ridder DT. In it for the long haul: characteristics of early and late drop out in a self-management intervention for weight control. J Behav Med. 2013;36(5):520–530. [DOI] [PubMed] [Google Scholar]
- 47.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of personality and social psychology. 1996;70(1):115. [DOI] [PubMed] [Google Scholar]
- 48.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Education Research. 2006;22(5):691–702. [DOI] [PubMed] [Google Scholar]
- 49.Dishman RK, Motl RW, Sallis JF, et al. Self-management strategies mediate self-efficacy and physical activity. American journal of preventive medicine. 2005;29(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annesi JJ, Johnson PH, McEwen KL. Changes in Self-Efficacy for Exercise and Improved Nutrition Fostered by Increased Self-Regulation Among Adults With Obesity. The Journal of Primary Prevention. 2015;36(5):311–321. [DOI] [PubMed] [Google Scholar]
- 51.Saelens BE, Gehrman CA, Sallis JF, Calfas KJ, Sarkin JA, Caparosa S. Use of self-management strategies in a 2-year cognitive-behavioral intervention to promote physical activity. Behavior Therapy. 2000;31(2):365–379. [Google Scholar]
- 52.Eisenberg IW, Poldrack R, Bissett P, Enkavi AZ, Kim SJ, King JW, Riddle MW, . Applying novel techonologies and methods to inform the ontology of self-regulation. . OSF. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(2):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological review. 1984;91(3):295. [DOI] [PubMed] [Google Scholar]
- 55.Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5(3):445–464. [DOI] [PubMed] [Google Scholar]
- 56.Hedeker D, Gibbons RD. Longitudinal data analysis. Vol 451: Wiley. com; 2006. [Google Scholar]
- 57.Daniels MJ, Hogan JW. Missing data in longitudinal studies: Strategies for Bayesian modeling and sensitivity analysis. Chapman and Hall/CRC; 2008. [Google Scholar]
- 58.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. Journal of general internal medicine. 2011;26(2):192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones B, Jarvis P, Lewis J, Ebbutt A. Trials to assess equivalence: the importance of rigorous methods. Bmj. 1996;313(7048):36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hedeker D, Gibbons RD, Waternaux C. Sample Size Estimation for Longitudinal Designs with Attrition: Comparing Time-Related Contrasts Between Two Groups. Journal of Educational and Behavioral Statistics. 1999;24(1):70–93. [Google Scholar]
- 62.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 63.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. [DOI] [PubMed] [Google Scholar]
- 64.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value in health. 2013;16(2):231–250. [DOI] [PubMed] [Google Scholar]
- 65.Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR Good Research Practices Task Force report. Value in Health. 2015;18(2):161–172. [DOI] [PubMed] [Google Scholar]
- 66.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. Jama. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 67.Briggs A, Clark T, Wolstenholme J, Clarke P. Missing… presumed at random: cost-analysis of incomplete data. Health Econ. 2003;12(5):377–392. [DOI] [PubMed] [Google Scholar]
- 68.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Apolzan J, Venditti E, Edelstein S, et al. Diabetes Prevention Program Research G Long-Term Weight Loss With Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study. Ann Intern Med. 2019;170:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krull J, MacKinnon D. Multilevel modeling of individual and group level mediated effects. Multivariate Behavioral Research. 2001;36(2):249–277. [DOI] [PubMed] [Google Scholar]
- 71.Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2018;115(5):957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkelstein EA, DiBonaventura M, Burgess SM, Hale BC. The costs of obesity in the workplace. J Occup Environ Med. 2010;52(10):971–976. [DOI] [PubMed] [Google Scholar]
- 73.McCormick B, Stone I, Team CA. Economic costs of obesity and the case for government intervention. Obes Rev. 2007;8 Suppl 1:161–164. [DOI] [PubMed] [Google Scholar]
- 74.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: the multiphase optimization strategy (MOST). Springer; 2018. [Google Scholar]
- 76.Dutton GR, Nackers LM, Dubyak PJ, et al. A randomized trial comparing weight loss treatment delivered in large versus small groups. International Journal of Behavioral Nutrition and Physical Activity. 2014;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of smartphone-based behavioral obesity treatment with gold standard group treatment and control: a randomized trial. Obesity. 2019;27(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pellegrini CA, Pfammatter A, Conroy D, Spring B. Smartphone applications to support weight loss: current perspectives. Advanced health care technologies. 2015;1:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pfammatter AF, Marchese SH, Pellegrini C, Daly E, Davidson M, Spring B. Using the Preparation Phase of the Multiphase Optimization Strategy to Develop a Messaging Component for Weight Loss: Formative and Pilot Research. JMIR Formative Research. 2020;4(5):e16297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freedland KE, King AC, Ambrosius WT, et al. The selection of comparators for randomized controlled trials of health-related behavioral interventions: recommendations of an NIH expert panel. Journal of clinical epidemiology. 2019;110:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y, You W, Almeida F, Estabrooks P, Davy B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: a systematic review and meta-analysis. Journal of the Academy of Nutrition and Dietetics. 2017;117(3):404–421. e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaplan RM, Gold M, Duffy SQ, et al. Economic analysis in behavioral health: Toward application of standardized methodologies. Health Psychology. 2019;38(8):672. [DOI] [PubMed] [Google Scholar]
- 84.Teixeira PJ, Carraça EV, Marques MM, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC medicine. 2015;13(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fitzpatrick SL, Bandeen-Roche K, Stevens VJ, et al. Examining behavioral processes through which lifestyle interventions promote weight loss: results from PREMIER. Obesity. 2014;22(4):1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Painter SL, Ahmed R, Hill JO, et al. What matters in weight loss? An in-depth analysis of self-monitoring. Journal of medical Internet research. 2017;19(5):e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sevick MA, Stone RA, Zickmund S, Wang Y, Korytkowski M, Burke LE. Factors associated with probability of personal digital assistant-based dietary self-monitoring in those with type 2 diabetes. J Behav Med. 2010;33(4):315–325. [DOI] [PubMed] [Google Scholar]
- 88.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. [DOI] [PubMed] [Google Scholar]
- 89.Wing RR, Greeno CG. 9 Behavioural and psychosocial aspects of obesity and its treatment. Baillière’s clinical endocrinology and metabolism. 1994;8(3):689–703. [DOI] [PubMed] [Google Scholar]
- 90.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatric Clinics. 2011;34(4):841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krukowski RA, Ross KM. Measuring Weight with Electronic Scales in Clinical and Research Settings During the Coronavirus Disease 2019 Pandemic. Obesity (Silver Spring). 2020;28(7):1182–1183. doi: 10.1002/oby.22851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacLean PS, Rothman AJ, Nicastro HL, et al. The Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) Core Measures Project: Rationale and Approach. Obesity (Silver Spring). 2018;26 Suppl 2(Suppl 2):S6–S15. doi: 10.1002/oby.22154 [DOI] [PMC free article] [PubMed] [Google Scholar]


