Abstract
The basic unit of chromatin, the nucleosome, is an octamer of four core histone proteins (H2A, H2B, H3, and H4) and serves as a fundamental regulatory unit in all DNA-templated processes. The majority of nucleosome assembly occurs during DNA replication when these core histones are produced en masse to accommodate the nascent genome. In addition, there are a number of non-allelic sequence variants of H2A and H3 in particular, known as histone variants, that can be incorporated into nucleosomes in a targeted and replication-independent manner. By virtue of their sequence divergence from the replication-coupled histones, these histone variants can impart unique properties onto the nucleosomes they occupy and thereby influence transcription and epigenetic states, DNA repair, chromosome segregation, and other nuclear processes in ways that profoundly affect plant biology. In this review we discuss the evolutionary origins of these variants in plants, their known roles in chromatin, and their impacts on plant development and stress responses. We focus on the individual and combined roles of histone variants in transcriptional regulation within euchromatic and heterochromatic genome regions. Finally, we highlight gaps in our understanding of plant variants at the molecular, cellular, and organismal level, and we propose new directions for study in the field of plant histone variants.
Introduction
Throughout eons of evolution, the nucleosome has remained a defining characteristic of eukaryotes. As the fundamental unit of chromatin, the nucleosome acts as a barrier between DNA and interacting proteins, making it an integral regulatory component in virtually every DNA-templated process. The nucleosome consists of ~147 bp of DNA wrapped around a histone octamer containing a core (H3-H4)2 tetramer flanked by two H2A-H2B dimers. Histone-histone and histone-DNA interactions contribute to nucleosome stability, while the tails of each histone provide a substrate for posttranslational modifications and protein binding. During replication, DNA content doubles and so does the demand for nucleosomes. To accommodate this demand, canonical histone genes evolved into often intronless multigene families whose expression is tightly linked to the cell cycle with highest expression during S phase. In contrast, histone variants have introns and often show replication-independent expression and deposition. The naming conventions for histone variants generally consist of a prefix indicating the histone protein family (e.g. H2A) to which they belong, followed by a period and a number or letter indicating a specific variant type (e.g. H2A.Z) (130).
Some histone variants such as H2A.Z are conserved throughout eukaryotes while others are lineage-specific, such as the flowering plant-specific H2A.W variant. By changing nucleosome composition, histone variants can change the internal stability of a nucleosome, DNA-histone interactions, internucleosomal interactions, accessibility to chromatin binding proteins, as well as potential posttranslational modifications. All of these changes alter the chromatin landscape and influence key nuclear processes. Therefore, histone variants represent a wealth of currently untapped information that will contribute to answering several of the outstanding questions of eukaryotic epigenetics.
In this review, we assess the current understanding of plant variant histones with a focus on the roles they play in transcriptional control. The eukaryotic genome can be partitioned into transcriptionally permissive euchromatic regions and transcriptionally repressive heterochromatic regions of both facultative and constitutive types. Histone variants H3.3, H2A.Z and H2A.X are found in euchromatic regions. Recent reviews of H3.3, H2A.Z, and H2A.X can be found in Borg et al. and Lei and Berger (9, 69). Here, we discuss how H3.3 is implicated in promoting chromatin accessibility in ways potentially unique to plants. H2A.Z has a more complex relationship with gene expression and we discuss evidence that implicates the variant as both a transcriptional activator and repressor. While H2A.X is known primarily for its role in DNA damage response, we focus on recent evidence pointing toward a role for H2A.X in transcriptional control. Other histone variants contribute to heterochromatin function and we highlight H2A.W and H1, which have also been recently reviewed in Lei and Berger, Kotlinski et al., and Probst et al. (63, 69, 107). H2A.W serves as the heterochromatic counterpart to H2A.X with respect to the DNA damage response, and its structure is thought to promote chromatin condensation. Finally, we call attention to the oft overlooked linker histone H1 and analyze how chromatin structure is dependent on H1 in both heterochromatin and euchromatin (See Figure 1).
Figure 1. The nature of histone variants and their distribution in chromatin.
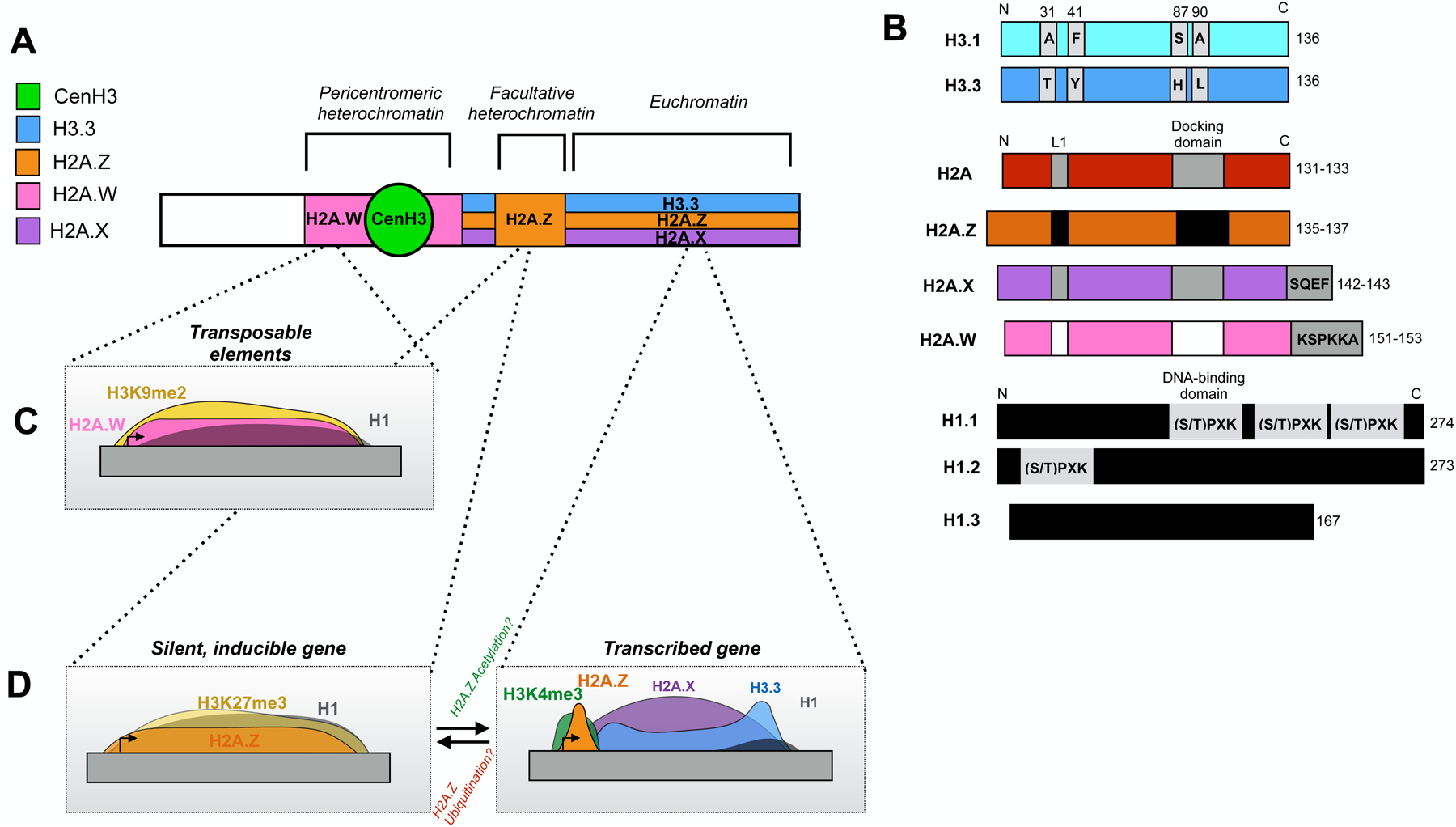
(A) Schematic diagram of a chromosome, showing the distribution of major chromatin types and the histone variants associated with each. (B) Diagrams comparing histone variants and their canonical counterparts. Regions of sequence differences or additions between variants and canonical types are shown as boxes. H3.1 and H3.3 differ by only four amino acid substitutions: two in the N-terminal tail and two in the histone-fold domain. H2A variants are distinguished mainly by sequence variation in the L1 loop and docking domain, while H2A.X and H2A.W have variant-specific C-terminal extensions. H1 subtypes vary in the occurrence of DNA-binding domains and their overall length. (C) Diagram showing the distribution of H2A.W on silent transposable elements and association with H1 and H3K9me2. (D) Two distinct, and perhaps interconvertible, distribution patterns of H2A.Z on silent genes in facultative heterochromatin (left) and active euchromatic genes (right). Silent genes show ubiquitinated H2A.Z nucleosomes across the gene body and are associated with H3K27me3 and H1, while active genes show acetylated H2A.Z in the +1 nucleosome and H3.3 in the gene body.
Euchromatin-Associated Histone Variants
H3.3
In plants, histone H3 proteins are categorized into 4 groups: canonical histone H3.1, H3.3 variants, centromeric H3 variants, and H3-like histones. Centromeric H3 defines the centromere and is essential for kinetochore assembly and proper cell division, while the function of H3-like variants is largely unknown (152). Plant H3.3 contains many features typical of histone variants including introns, replication-independent deposition into chromatin, and expression in terminally differentiated tissue (52, 99, 127). Despite these differences, H3.3 differs from H3.1 at only 4 amino acids (written H3.1➞H3.3): A31T, F41Y, S87H and A90L (Figure 1B). The Arabidopsis genome possesses three H3.3 genes encoding identical proteins (Table 1) (130). Evolutionary analysis of H3 proteins shows that H3.3 evolved independently in plants and animals (139, 140). Despite their independent origins, H3.3 differs from H3.1 at three of the same amino acids in both plants and animals, with H3.1 in flowering plants having an additional amino acid substitution at residue 41 (139). This evidence of convergent evolution strongly points toward the importance of H3.3 to the function of the eukaryotic genome.
Table 1.
Histone variant genes, proteins, and functions in Arabidopsis thaliana
| Histone variants | Arabidopsis Genes | ArabidopsisLoci | Protein | Chaperones/remodelers | Function | Knockout/knockdown Arabidopsis phenotype | Modification | ||
|---|---|---|---|---|---|---|---|---|---|
| General | development | Stress responses | |||||||
| H3.3 | HTR4 HTR5 HTR8 |
At4g40030 At4g40040 At5g10980 |
H3.3 H3.3 H3.3 |
HIRA (95) ATRX(33) |
Transcriptional activation (123, 127, 144, 156) | Flowering time(156); Male Gametogenesis (54)); Post-germination development (5); Cell proliferation and organogenesis (101) |
Regulate the expression of responsive and hypervariable genes(145) | htr5/htr8, are phenotypically normal ; Triple mutants cause lethality; htr4/htr4;htr8/htr8 plants that carried either amiR-HTR5-I or amiR-HTR5-II (h3.3kd) exhibit leaf serration, incomplete sterility, and growth defects (145) | H3.3K36me3 (89) |
| H2A.Z | HTA8 HTA9 HTA11 |
At2g38810 At1g52740 At3g54560 |
H2A.Z.8 H2A.Z.9 H2A.Z.11 |
SWR1 complex (42, 137) | transcriptional activation and repression | Flowering time (16, 29, 86, 98, 124); vegetative to reproductive phase transition (42, 148); Inflorescence architecture (11); Germline development (157); Circadian clock (133) | Temperature response (24, 66, 132); Phosphate deficiency (40, 126, 151) Drought stress (11, 128); Immunity (6); Salt stress (93) |
Double/ triple mutants show developmental aberrations (83, 94); Triple mutants are viable but show reduced fertility (20); Knockdown of all three H2AZ genes cause early flowering (16) |
H2A.Z acetylation (25); H2A.Z monoubiquitination (H2A.Zub) (44); H2A.Z SUMOylation and methylation? |
| H2A.X | HTA3 HTA5 |
At1g54690 At1g08880 |
H2A.X.3 H2A.X.5 |
FACT? (45) | DNA damage response (13, 67, 112); transcription activation (146) | H2A.X is unessential for Arabidopsis development (50) | genotoxic stress(141) | The single/ double mutants are viable, fertile and indistinguishable from wild type (50) | phosphorylated H2A.X (67, 112) |
| H2A.W | HTA6 HTA7 HTA12 |
At5g59870 At5g27670 At5g02560 |
H2A.W.6 H2A.W.7 H2A.W.12 |
DDM1 (100) | Chromatin condensation (150) | H2A.W is unessential for Arabidopsis development (10) |
Not determined | H2A.W triple mutants are indistinguishable from the wild type (10) | phosphorylated H2A.W (75, 112) |
| H1 | HON1 HON2 HON3 |
At1g06760 At2g30620 At2g18050 |
H1.1 H1.2 H1.3 |
NRP1 and NRP2?(91, 102) | Chromatin condensation (14, 114) | Flowering time, Seed dormancy, Lateral root, Stomata and callus development (114) male and female gametogenesis (120) |
Drought stress (2) Combined light and water deficiency (116) |
Triple mutants are viable but show extended dormancy, early flowering, increased root density and lateral root numbers, and altered stomata pattern (114) | phosphorylation, acetylation, mono- and dimethylation, formylation, ,crotonylation and propionylation (64) |
Few studies have investigated exactly how H3.3 is deposited into plant chromatin. In mammals, H3.3 variants are incorporated into genic regions by the HIRA (Histone Transcriptional Regulator A) and in nongenic regions such as pericentromeric repeats and telomeres by ATRX/DAXX (Alpha Thalassemia-mental Retardation X-linked syndrome/Death-domain Associated protein) (Table 1) (70, 111). Arabidopsis ATRX mutants do indeed have altered global H3.3 levels (33, 95). While atrx mutants are viable, hira atrx double mutants result in partial lethality and show strong developmental defects in the surviving plants, indicating a potential cooperation between ATRX and HIRA. (33). Interestingly, atrx mutants display loss of H3.3 in genic regions while H3.3 enrichment at transposable elements (TEs) and pericentromeric regions is unchanged (33). This is counter to observations in mammals where ATRX deposits H3.3 at non-genic regions, suggesting a functional divergence of ATRX-dependent H3.3 localization between plants and mammals (33).
H3.3 localization and relationship with gene expression
While the H3.3 genomic distribution pattern is different from H3.1 in both plants and animals, their respective distribution patterns are highly similar across species (127). In Arabidopsis, immunofluorescence and ChIP-seq experiments show that H3.1 is generally enriched at transposable elements, pericentromeric heterochromatin, and heterochromatin domains in the arms, while H3.3 is associated with euchromatic and nucleosome-depleted regions (NDR) (122, 123, 127, 144). Recent evidence indicates that this distinction in H3.1 and H3.3 distribution is caused in part by sequence variation at amino acid 41: Phe in H3.1 and Tyr in H3.3. Alignment analysis of monocot, dicot and ancient plant histone H3 reveals that the Phe41 residue first appeared in fern H3.1 and established in land plants (76). Lu et al. showed that while Tyr41 is not important for the genomic distribution of H3.3, a Phe41Tyr point mutation in H3.1 causes the protein to lose its heterochromatin-specific localization and spread into active regions (76). This is especially surprising considering that animal H3.1 and H3.3 both have Tyrosine at position 41, and are still able to maintain distinct localization patterns. Tyrosine differs from Phenylalanine in its ability to be phosphorylated. In human cells, H3 is known to be phosphorylated at Tyr41 and this is thought to help prevent heterochromatic proteins from binding active regions (27). Therefore, one hypothesis drawn from these results is that Phe41 evolved to differentiate H3.1 from H3.3 in plants where phosphorylation at Tyr41 has not yet been reported. Alternatively, these results could indicate that H3.1 Phe41 evolved to achieve an additional degree of chromatin targeting unique to vascular plants.
Highly expressed genes have enrichment of H3.3 over the transcribed region, or gene body, with a bias toward the 3’ end (123, 127, 144). However there is no correlation between H3.3 occupancy and transcriptional changes in h3.3 knockdown (h3.3kd) plants, and H3.3 appears to be dispensable for general transcription. This is particularly surprising considering that complete loss of H3.3 is lethal (145). However, a reduction in H3.3 in some stress-responsive genes was associated with reduced transcript levels in h3.3kd mutants. Thus, H3.3 likely plays a specific role in the activation of groups of genes that are involved in environmental responses, while not impacting transcription globally (145). Also, a recent study demonstrated that H3.3 inhibits flowering by increasing H3K4me3 and H3K36me3 levels at the FLOWERING LOCUS C (FLC) gene (156). The authors found that an interaction between FRIGIDA (FRI) and the HIRA chaperones results in the deposition of H3.3 at the 3’ end of FLC. Consequently, increased H3.3 at the 3’ of FLC aids in formation of a gene loop, increasing the interaction between the 5’ and 3’ end, thereby promoting transcriptional activation (156).
While H3.1 overlaps with several repressive chromatin modifications including DNA methylation, H3K9me2, and H3K27me1/3, H3.3 overlaps with several active chromatin marks like H3K4me3, H3K36me3, H3K9me3, H2B ubiquitylation, and RNA Pol II occupancy (127, 144). Despite these correlations, genome-wide patterns of H3K4me3 and H3K36me3 are relatively unchanged between h3.3kd mutants and wild type Arabidopsis (145). However, H3.3 was shown to promote H3K4me3 at a subset of genes with shorter length (<1 kb) (156). Interestingly, loss of H3.3, specifically over gene bodies, is associated with a decrease in DNA methylation and an increase in H1 occupancy (Figure 2A) (145). Additionally, chromatin accessibility assays showed that H3.3-containing nucleosomes are more sensitive to DNase I activity (123). Since H1 has been shown to prevent binding of DNA methyltransferases in pericentromeric heterochromatin, H3.3 may serve as a foil to H1 in euchromatic regions, with gene body H3.3 increasing chromatin accessibility to DNA methyltransferases by preventing H1 deposition (145, 153). Crystal structures of H3 methyltransferases ATXR5/6 reveal their ability to methylate lysine 27 of H3.1 but not H3.3. Therefore, H3.3 could also attenuate the Polycomb pathway of gene repression, of which H3K27 methylation is a key element (56). This difference also suggests that H3.3 can not only stimulate relaxed chromatin but can also perpetuate this chromatin state across cell divisions by preventing the establishment of heterochromatic marks.
Figure 2. Chromatin landscape changes in response to histone variant depletion.
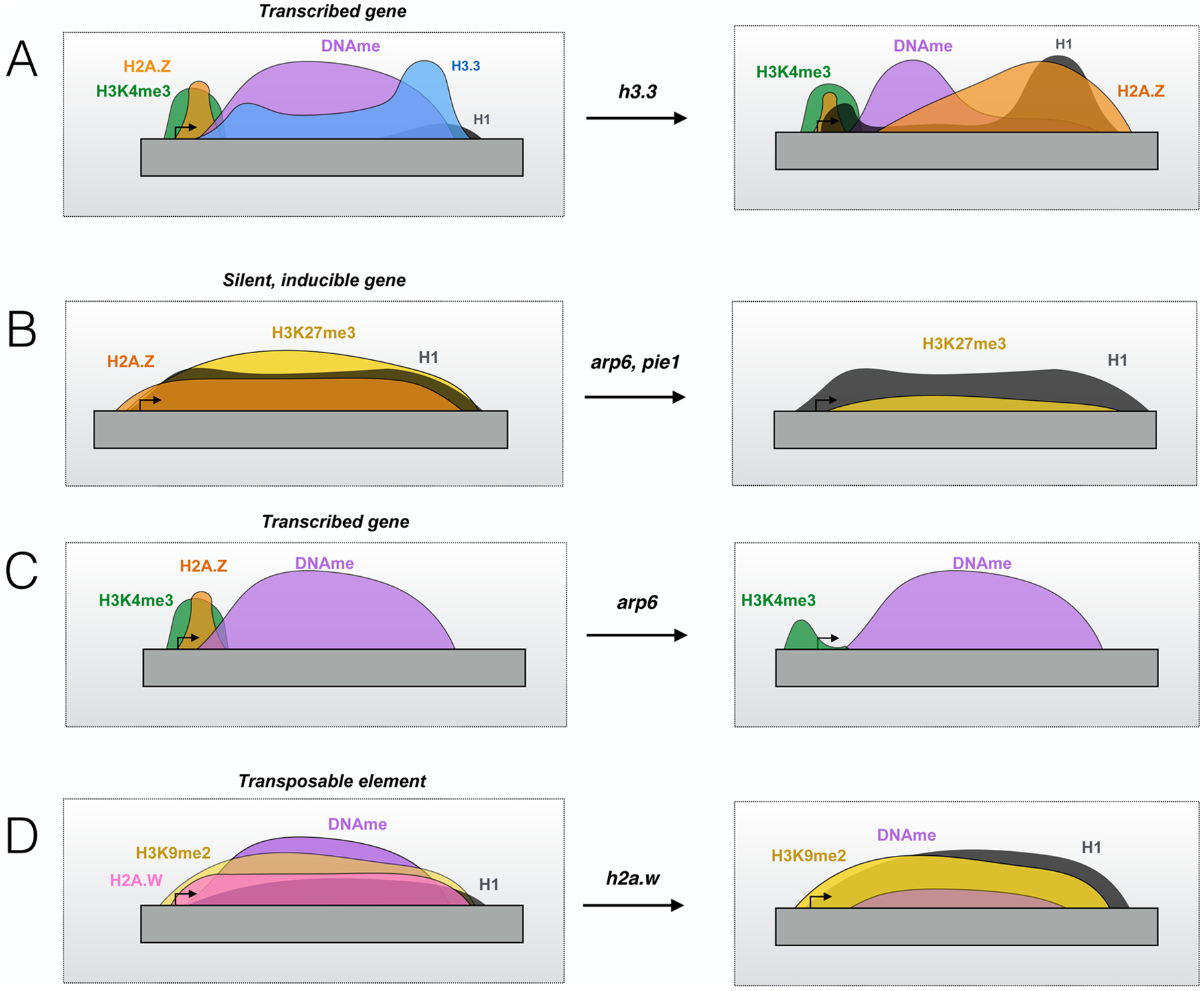
(A) H3.3 loss at transcribed genes results in reduced DNA methylation (DNAme) in the CG context, and increased H2A.Z and H1 in the downstream regions previously occupied by H3.3. Active histone marks such as H3K4me3 and H4K36me3 are generally unaffected. Given that H3.3 does not seem to be methylated at K27, genes targeted for polycomb repression may be subject to silencing when H3 nucleosomes become predominant in the absence of H3.3. (B) H2A.Z loss from silent, inducible genes in SWR1 mutants (arp6 and pie1) results in reduced H3K27me3 without affecting H1 levels. These silent genes also lose H3K27me3 and generally become active upon H2A.Z loss. (C) In transcribed genes, H2A.Z loss in SWR1 mutants results in a reduction of H3K4me3, particularly around the +1 nucleosome, without changes in DNA methylation. H2A.Z loss from these genes generally corresponds to decreased Pol II occupancy and transcription as well. (D) Loss of H2A.W at transposable elements (TEs) results in reduced DNA methylation and increased H1 occupancy, while the repressive mark H3K9me2 is generally unaffected. Interestingly, one study showed that loss of H2A.W in h2a.w mutants did not result in widespread expression of silent TEs (10), while another study showed that H2A.W loss resulting from mutation of DDM1 did result in increased TE expression (98). In these ddm1 mutants, there was also a reduction in H3K9me2 without changes in H1 enrichment. These contrasting results suggest that H2A.W and the DDM1 remodeler have overlapping and distinct functions in heterochromatin.
H3.1 replacement by H3.3 is also a marker for cell fate transitions. Cells undergoing their last cell cycle before differentiation have a lower H3.1/H3.3 ratio and a higher rate of H3.1 eviction compared to dividing cells. This ratio is thought to change in the cells exiting the root meristem because H3.1 replacement with H3.3 occurs during the G2 phase, a phase that is longer in this last cell cycle than in earlier cycles, allowing more time for H3.1 eviction (101). This phenomenon is found in several plant developmental processes, including the stomatal and hypocotyl cell lineages, suggesting H3.1 eviction is a general feature in cell proliferation and organogenesis (49, 101).
H2A.Z
H2A.Z can be traced to a single evolutionary origin and its maintenance through nearly all branches of Eukarya underscore its vital role in multicellular development (80). Since the discovery of H2A.Z, it has been linked with numerous biological processes like plant immunity, germline development, and stress response as well as cellular processes including genome stability, DNA repair, as well as both transcriptional activation and repression (Table 1) (57, 82, 84, 85, 108, 113, 149). H2A.Z composes on average 15% of total H2A cellular content and loss of H2A.Z is lethal in most multicellular and some unicellular eukaryotes including Tetrahymena, Drosophila, mice and humans (17, 37, 57, 74, 136). This surprisingly is not the case for plants, where loss of H2A.Z in Arabidopsis is not lethal but does lead to a severe and pleiotropic phenotype including stunted growth, early flowering, and reduced fertility (16, 20, 82, 94). This tolerance of H2A.Z loss makes plants an exciting model to probe the mechanisms of this histone variant in transcriptional regulation.
H2A.Z is deposited into the post-replicated nucleosome as an H2A.Z/H2B dimer by the SWI2/SNF2-related 1 complex (SWR1), a member of the INO80 subfamily of chromatin remodelers (Table 1) (62, 87, 137). At the level of primary structure, H2A.Z varies from H2A in three prominent ways; the docking domain, the L1 loop and the acidic patch (Figure 1B). The implications of these differences with respect to chromatin binding and gene regulation have been reviewed by Bönisch and Hake (8). Briefly, the extended acidic patch of H2A.Z is speculated to increase the opportunity for interactions between adjacent nucleosomes as well as secondary protein binding (31, 38, 104). Taken individually, the changes to the docking domain and the L1 loop appear to have opposing effects on nucleosome stability. The docking domain of H2A.Z exhibits less hydrogen bonding with H3, suggesting nucleosome destabilization, while the 4 amino acid substitutions found in the L1 loop have been shown to increase histone octamer stability (1, 8, 129). Additionally, amino acid substitutions in the H2A.Z C-terminus reduce binding of linker histone H1 to the core nucleosome particle (158).
While all plants appear to have H2A.Z, they do differ in the number of H2A.Z paralogs, and in some cases distinct splice variants exist within organisms (32, 61). The Arabidopsis genome encodes three expressed H2A.Z proteins; HTA8, HTA9, and HTA11 (Table 1). Although mutant analysis in plants indicates substantial redundancy between isoforms, they do exhibit distinct expression levels and patterns (124). As sub functionalization has been shown in some animals, future investigations will reveal any unique roles between paralogs in plants (34, 97). Interestingly, we found that the expression pattern across tissue types of the various Arabidopsis H2A.Z paralogs is synchronized with corresponding somatic H2B isoforms, with HTA11 and HTB2 having matched expression profiles, as do HTA9 and HTB4. This suggests that H2A.Z isoforms have preferred dimerization partners when deposited in the nucleosome (124).
H2A.Z localization and relationship with gene expression
There is a wealth of evidence that implicates H2A.Z as an essential player in transcriptional responses. However, understanding the exact mechanisms dictating H2A.Z-dependent transcriptional regulation are complicated by the fact that H2A.Z has reported roles as both a transcriptional activator and a repressor. In this section, we discuss how analyses of H2A.Z mutants, structural features, and localization patterns support a role for H2A.Z as a transcriptional activator. H2A.Z’s role as a repressor is discussed in a later section.
In Arabidopsis, most genes contain a prominent H2A.Z peak at the +1 nucleosome beyond the TSS. With respect to this enrichment pattern in plants, no gene has been studied more than FLOWERING LOCUS C (FLC). The most prominent and unifying phenotype of all H2A.Z and SWR1 mutants is the accelerated transition from vegatative to reproductive growth. Expression analysis reveals that these mutants display decreased transcript levels of the floral repressor FLC which leads to early flowering (15, 16, 28, 29, 68, 82, 86, 98, 124). H2A.Z-containing nucleosomes at this locus follow the pattern expected for expressed genes with a characteristic peak of enrichment directly downstream of the TSS (Figure 1D). ChIP analysis revealed that SWR1 subunits PIE1 and ARP6 are required for deposition of H2A.Z at FLC as well as FLC paralogs MAF4 and MAF5 (29). This finding, coupled with the observation that loss of HTA9 and HTA11 also result in decreased expression of these genes indicates that H2A.Z itself is required for their proper activation (82).
Recently, several protein interaction assays from independent groups have provided new insight into the composition of the plant SWR1 complex and H2A.Z interactors (77, 94, 106, 124). Understanding how these new subunits influence SWR1 activity will help in deconvoluting the various and sometimes contradictory functions of H2A.Z in plant transcription. Of several newfound subunits, the most studied in relation to H2A.Z deposition has been Methyl-CpG Binding Domain 9 (MBD9). ChIP-seq analysis of H2A.Z enrichment in Arabidopsis seedlings shows that about 20% of H2A.Z enriched sites become depleted in mbd9 mutants (124). Comparison of FLAG-tagged MBD9 enrichment with corresponding ATAC-seq data indicate that MBD9 localizes primarily to areas of open chromatin and suggests that MBD9 promotes H2A.Z deposition at the 5’ end of highly active genes (106). Future experiments will be needed to determine exactly where and when during plant development this MBD9-containing SWR1 complex is performing its function, and whether specific SWR1 subtypes may be related to the activating and repressive roles of H2A.Z.
H2A.Z enrichment at the +1 nucleosome of genes in most organisms leads many to speculate that H2A.Z may help in the targeted recruitment of transcription initiation machinery. However, the presence of a second H2A.Z peak at the −1 nucleosome in some organisms suggests that H2A.Z may serve as a mere mark of transcription as opposed to a targeting factor. Bagchi et al found that while the level of H2A.Z at the +1 nucleosome did not correlate with gene activity in yeast, it did correlate with upstream antisense transcript levels, indicating that the bimodal profile of H2A.Z at the +1 and −1 nucleosomes found in yeast is the reflection of a transcription event rather than an initiator of that event (4). This observation is corroborated by H2A.Z enrichment patterns in other organisms. For instance in humans, where bidirectional transcription is common, H2A.Z peaks are found on both sides of the NDR (134). Whereas in Drosophila, where bidirectional transcription is not frequently observed, there is a pronounced lack of H2A.Z enrichment at the −1 nucleosome (23). Colino-Sanguino et. al reviewed recent work in mammals highlighting the contradictory results found from experiments aimed at uncovering the relationship between RNA polymerase pausing at the TSS and H2A.Z (21). In Arabidopsis, GRO-seq data indicate a lack of bidirectional transcription and we again observe a lack of H2A.Z enrichment at the −1 nucleosome (48). While H2A.Z has been implicated in gene activation across organisms, the idea that promoter H2A.Z enrichment reflects the direction of transcription implies that H2A.Z incorporation perhaps does not facilitate targeted initiation but may help reinforce existing transcription patterns.
While H2A.Z is clearly required for high level transcription of many genes, the presence of an H2A.Z-containing nucleosome alone is likely not sufficient for transcriptional activation. For instance, recent evidence points toward acetylation of H2A.Z as a modulator of flowering time. Crevillen et al revealed for the first time in plants the occurrence of H2A.Z acetylation and showed that this acetylation is required for proper FLC expression (25). While this study marks an exciting insight into H2A.Z mediated activation, future studies are needed to determine how universal this mechanism of activation is across the plant genome. NAP1-RELATED PROTEIN 1 and 2 were recently identified as inhibitors of H2A.Z deposition (138). Unexpectedly, nap1;nap2 double mutants displayed an increase in H2A.Z enrichment at the TSS of FLC but a decrease in FLC expression. Wang et al. use an observed increase in nucleosome density around the TSS to explain this reduced expression and thus provide further evidence that TSS H2A.Z enrichment is likely not sufficient for activation. Additionally, arp6 mutants also display alterations in H3K4me3 enrichment at FLC, but it is unclear whether this observation is a direct consequence of H2A.Z loss in the arp6 mutant (Figure 2C) (86). Based on current evidence it is still unclear if H2A.Z’s role in active transcription is one of initiation, maintenance, or both. Indeed, various seemingly contradictory results have been reported regarding the role of H2A.Z nucleosomes as a barrier to Pol II elongation and in modulating chromatin accessibility (19, 90, 92, 142).
H2A.X
H2A.X, the most similar histone variant to H2A, differs from canonical H2A only via a C-terminal SQL(E/D) motif in animals and a SQEF motif in plants (Figure 1B). H2A.X is present in most eukaryotes; however, unlike H2A.Z, it has evolved multiple times (61, 131). Arabidopsis and rice each encode two constitutively expressed and functionally redundant H2A.X genes (Table 1) (67). The H2A.X variant is best known for its role in coordinating DNA damage responses in both animals and plants. The presence of phosphorylated H2A.X is considered a hallmark of DNA damage repair (110). However, a number of studies suggest that phosphorylated H2A.X is also required for gene activation (30, 125, 146).
The mechanisms underlying the genome-wide distribution of H2A.X remain largely unknown in both plants and animals (105). In humans, FACT (FAcilitates Chromatin Transcription) plays an important role in both removal and incorporation of H2A.X (Table 1) (35, 46). Studies in the mammalian system showed that H2A.X is incorporated de novo into damaged chromatin by FACT (46, 105). However the involvement of FACT chaperone in plant H2A.X deposition has not been investigated and we do not know if de novo deposition of H2A.X in response to DNA damage occurs in plants. Since the FACT chaperone is conserved among eukaryotes and domain organization of both FACT proteins, SSRP1 and SPT16, is similar between plant and mammalian, FACT may also play a role in plant H2A.X deposition (45).
H2A.X localization and relationship with gene expression
Cytologically, Arabidopsis H2A.X is excluded from chromocenters and primarily enriched over euchromatin (75). Chromatin immunoprecipitation experiments reinforce this observation showing an enrichment of H2A.X over the bodies of expressed genes (150). The relatively ubiquitous distribution of H2A.X in euchromatin is consistent with its role as a platform for DNA damage repair (DDR), as sites of DNA damage preloaded with H2A.X allow for a rapid response.
Although H2A.X is best known as a platform for DDR in plants and other eukaryotes, for the first time in plants, phosphorylated H2A.X (yH2A.X) was recently found to be required for transcriptional activation. Xiao et. al found that expression of the ABA Insensitive 4 (ABI4) gene is repressed by Oxidative Stress 3 (OXS3) family proteins during seed germination. They went on to show that this repression is in fact due to an interaction between OXS3s and H2A.X that prevents H2A.X phosphorylation and subsequent ABI4 activation. Given yH2A.X’s well characterized role in DNA damage response, it will be interesting to investigate whether this yH2A.X-dependent activation involves other elements of DNA damage repair. Future experiments measuring the occurrence of double-stranded breaks (DSBs) and the localization of DDR machinery around the ABI4 promoter during activation will help determine how yH2A.X-dependent activation relates to our previous understanding of yH2A.X function (146).
Although rare, there is some evidence of a non-canonical role for H2A.X in gene regulation from other eukaryotes as well. In the mammalian fibroblast cell, the High Mobility Group AT-Hook 2 (HMGA2) gene also depends on yH2A.X for activation (30). Dobersch et al found that yH2A.X precedes DNA demethylation and transcription initiation. These results would indicate that chromatin conformation changes during activation involve DNA breakage. However, not all studies support a role for H2A.X in gene activation. Recently, Eleuteri et al found that H2A.X curbs embryonic stem cell proliferation by repressing rRNA transcripts. They found that H2A.X, independent of H2A.X phosphorylation, at rDNA promoters is responsible for the targeted recruitment of the nucleolar remodeling complex, a complex known to establish heterochromatic features at rDNA (36).
While there is still no unifying mechanism for H2A.X/yH2A.X in transcription, evidence does suggest that H2A.X involvement in transcription is highly dependent upon cell type. Interestingly, H2A.X is maximally enriched in highly proliferative cell types compared to differentiated cell types and the enrichment patterns tend to favor transcribed genes (117, 118). Seo et al have shown that endogenous H2A.X occupancy is positively correlated with Pol II density at a given TSS in the proliferative Jurkat cancer cell line, while they are inversely correlated in differentiated CD4 cells. Thus, non-canonical functions of H2A.X may arise from unique enrichment patterns present in unique cell types.
Given the apparent connection between DDR and H2A.X phosphorylation, there are surprisingly few studies profiling the mark in plants. However, profiling in mammalian cells shows yH2A.X spreads in cis over large domains surrounding a DSB (53). Interestingly, the boundaries of yH2A.X domains often correspond to the native topological associated domains (TADs), suggesting yH2A.X propagation is compartmentalized by the 3D conformation of chromatin in the nucleus (22). This will be a particularly interesting avenue to explore in plants considering the fact that Arabidopsis has significantly fewer TAD boundaries than animal models, or even other plant species like rice (72).
Heterochromatin-associated histone variants
H2A.Z in facultative heterochromatin
Since H2A.Z incorporation at the TSS has been shown to be important for proper transcription in many organisms, it is puzzling at first to realize that the mere level of H2A.Z at this site does not reliably reflect expression level. Coupling H2A.Z ChIP-seq data with RNA-seq in Arabidopsis seedlings reveals that this TSS enrichment has a parabolic correlation with expression. That is, the highest and lowest expressed genes have lower levels of TSS H2A.Z enrichment than those that are moderately expressed (20, 151, 159). This correlation is also found to a lesser extent in rice, where total genic H2A.Z is parabolically correlated with expression similar to promoter H2A.Z in Arabidopsis (151). Looking at H2A.Z in the promoter region of genes offers a limited perspective. Recent works discussed in the following section analyzing genic H2A.Z beyond promoter enrichment help to paint a more complete picture of H2A.Z as a transcriptional regulator.
Several recent studies in plants have revealed a role for H2A.Z in gene repression. While initial experiments revealed some H2A.Z-dependent repression within specific genes or gene families, no genome-wide relationship between H2A.Z and repression had been established (66, 126). However, in 2012 Coleman-Derr and Zilberman found that H2A.Z enrichment beyond the TSS and into the gene body is anticorrelated with transcriptional output and that these lowly transcribed genes are enriched in pathways involving environmental or developmental responses (20). Since then, several papers have been published validating a repressive role of H2A.Z in gene transcription. Particularly, gene body H2A.Z was shown to play a repressive role in response to light, drought stress, salt stress, and heat stress response in Arabidopsis, as well as phosphate deficiency in rice and heat stress in Brachypodium distachyon (7, 24, 81, 93, 128, 151). A recent study in rice showed that reductions in both H2A.Z and H3K4me3 correlated with increased expression under phosphate starvation while decreases in H3K4me3 alone did not (40). Additionally, loss of the INO80 chromatin remodeling complex (responsible for H2A.Z eviction from chromatin) leads to decreases in both the deposition of H3K4me3 and transcription elongation typically observed at thermomorphogenesis genes during a high temperature induction (147). These results suggest that a coordination between H3K4me3 and H2A.Z may be required for proper activation of certain responsive genes.
Despite this recent focus on H2A.Z-mediated repression, no model has been proposed to sufficiently account for the genome-wide association between H2A.Z and repression. One idea is that gene body H2A.Z facilitates repression in a reversible manner serving as a more dynamic alternative to DNA methylation (20). This notion is supported by findings that the SWR1 complex is required for trimethylation of H3K27 at most H2A.Z enriched sites, a key step in the Polycomb pathway of gene silencing (Figures 1D and 2B) (12). However, several recent reports indicate that H2A.Z may use Polycomb proteins to achieve silencing outside of the accepted Polycomb pathway, raising several questions about the canonical pathway of Polycomb silencing. While SWR1 is required for H3K27me3, the small number of overlapping upregulated genes between hta9 hta11 and PRC2 catalytic subunit CURLY LEAF (CLF) mutants suggest that H2A.Z-mediated repression is independent of PRC2 activity (43, 65). Even more evidence that H2A.Z achieves repression via an unexplored Polycomb pathway comes from Cai et al, who found that H2A.Z enrichment is required for repression of several anthocyanin biosynthesis genes. Interestingly, while H2A.Z is required for the deposition of H3K27me3 at these genes, H3K27me3 is not necessary for their repression (11). Recently, our group, as well as others, identified an interaction between the SWR1 complex and several ALFIN1-LIKE family proteins (AL5, AL6, and AL7) (77, 106, 124). Little is known about this plant-specific family of proteins but the few studies of ALFIN1-LIKE proteins in Arabidopsis implicate them in Polycomb-mediated silencing. Molitor et. al. identified the same AL proteins found in SWR1 pulldowns as interactors with POLYCOMB REPRESSIVE COMPLEX 1 (PRC1) (88). They went on to show that al6/al7 double mutants cause a delay in the chromatin state switch from active H3K4me3 to repressive H3K27me3 in key seed developmental genes (88). This led authors to propose that ALs bind H3K4me3 via a plant-homeodomain (PHD) and recruit PRC1 to initiate Polycomb mediated silencing. How exactly the ALs are targeted to these genes destined for repression is still unclear, and given H2A.Z’s relatively newfound role in repression of responsive genes, it will be interesting to see how the interaction between SWR1 and ALs influences where silencing occurs.
Monoubiquitination of H2A.Z by the PRC1 catalytic subunit atBMI1 provides yet another connection between H2A.Z and Polycomb silencing with 68% of genes upregulated in hta9/hta11 mutants being enriched for both H2A.Z and H2A121ub in WT (43). H2A.Z was also found as a mark of inactive enhancers in plants, with its presence being associated with lower expression of putative target genes and increased enrichment of H3K27me3, a finding that is in contrast to humans where enhancer H2A.Z instead colocalizes with activating marks H3K4me3 and H3K27ac (26, 51).
H2A.W in constitutive heterochromatin
H2A.W variants are exclusive to the plant lineage and are defined by an extended C-terminal tail containing an SPKK motif (61, 150)(Figure 1B). Since green algae and non-flowering land plants lack H2A.W variants, it is proposed that H2A.W evolved from early spermatophytes (61). Liverworts, mosses and lycophytes possess the novel H2A variant H2A.M as a potential alternative to H2A.W, with commonalities in the C-terminal tail and L1 loop (61). In contrast to other histone variants, H2A.W has S-phase expression in Arabidopsis. (152). Additionally, disruption of CAF-1, which regulates chromatin assembly after replication, results in reduced H2A.W levels, implying that its deposition is replication-dependent (Table 1) (5).
Phosphorylation dynamics of H2A.W variant HTA7 were found to play an essential role in the effective response to DNA damage in heterochromatic regions. Therefore, one proposed function of H2A.W is to serve as a functional complement to H2A.X in heterochromatin, providing a platform for phosphorylation in response to DNA damage (75). Monocot H2A.W contains multiple copies of the SPKK motif while eudicots have a single copy (61). This SPKK is known to promote chromatin condensation by binding to A/T rich sites on DNA generally found in the satellite repeats of constitutive heterochromatin. The presence of this motif as well as in vitro nucleosome assembly results indicate that H2A.W generally promotes chromatin condensation (61, 150). However, recent studies highlighted below indicate that H2A.W’s role in heterochromatin is perhaps more nuanced than previously expected.
H2A.W localization and relationship with heterochromatin accessibility
H2A.W is located primarily in constitutive heterochromatin, with correlation between the variant and H3K9me2, DNA methylation, and linker histone H1 (Figure 1C) (10, 75, 150). However, H2A.W deposition into heterochromatic regions does not depend on DNA methylation or H3K9me2 (150). New H2A.W triple mutants, h2a.w-2, created by crossing a CRISPR generated null hta6 allele with hta7 and hta12 T-DNA lines reveal a potentially unique role for H2A.W in maintaining a level of accessibility in constitutive heterochromatin (10). Using ATAC and bisulfite sequencing analysis of h1, h2a.w-2, and h1/h2a.w-2 double mutants, Bourguet et al concluded that H2A.W actually antagonizes the binding of H1 to linker DNA in constitutive heterochromatin (Figure 2D). They propose the SPKK motif of H2A.W competes with the two SPKK motifs found in H1 for binding on linker DNA. Therefore, the SPKK motif of H2A.W, which was thought to promote chromatin condensation when compared to other H2A variants, may actually function to prevent even further condensation by H1. This allows regions occupied by H2A.W to maintain a heterochromatic state while still being accessible to maintenance factors like DNA methyltransferases (10).
Of course, an analysis of H2A.W alone is incomplete without considering the chromatin remodelers that act on it. Recently, Osakabe et al identified DDM1 (DECREASE IN DNA METHYLATION 1) as a depositor of H2A.W in Arabidopsis. In stark contrast with h2a.w-2, ddm1 mutants had significant derepression (40%) of pericentromeric TEs and no reported changes in H1 enrichment (Table 1) (100). While H3K9me2 and DNA methylation were reduced in ddm1, their effects on silencing TEs were found to be secondary to that of ddm1. The results of these two studies raise several exciting questions, namely; How does total H2A.W loss in h2a.w-2 have a lesser effect on TE silencing than DDM1 loss? Is DDM1 acting independently of H2A.W to silence TEs in h2a.w-2 mutants? Genomic profiling analysis of DDM1 enrichment in WT and h2a.w-2 is one of many future experiments that will help answer these questions. Furthermore, deconvoluting the mechanisms behind DDM1 and H2A.W function may help to inform human disease, where Lymphocyte-Specific Helicase (LSH) and macroH2A appear to play a similar role in mammalian silencing (96).
H1
The linker histone H1 binds both the nucleosome core particle and the linker DNA to facilitate internucleosomal interactions and chromatin compaction. Interestingly, H1 and associated variants have a separate evolutionary origin from core histones, having evolved from bacterial proteins rather than archaeal ones (60). H1 histones are also more divergent across species compared to core histones (60). However, the general structure of a lysine rich C-terminal tail, a flexible and short N-terminus and a central globular domain are conserved across eukaryotes (158).
Due to the importance of H1 variants in chromatin dynamics, it is surprising to observe that H1 depletions in Arabidopsis, yeast, worms and fungi are viable while mutation of H1 variants in mouse and Drosophila are lethal (3, 39, 58, 78, 103, 119, 121, 135). Plant H1 variants are classified into two groups, main variants with ubiquitous and stable expression and minor variants, which accumulate in response to stress (59, 63, 130). In contrast to mammals with 11 H1 variants, only three nonallelic H1 variants are found in Arabidopsis: two highly similar major variants H1.1 and H1.2 and the shorter stress-induced minor variant H1.3 (Table 1) (2, 59). The key structural differences between H1.3 and H1.1/H1.2 are a decreased positive charge in H1.3, a shorter C-terminal domain, and a lack of (S/T)PXK DNA-binding motifs in both N- and C-terminal domains (Figure 1B) (115). While H1.1/2 variants are expressed in all cell types, H1.3 is expressed constitutively in guard cells with induced expression in other cell types during stress.
H1 localization
Genome-wide analysis of H1.1/2 in Arabidopsis show that linker histones are found in both heterochromatic and euchromatic regions and generally associate with methylated DNA sequences, with the strongest enrichment over hypermethylated TEs and lowly expressed genes. Genic H1 enrichment however is linked with methylation status rather than expression level, with similarly expressed genes only being enriched for H1 if methylated (14). At a closer look, gene body H1 enrichment is characterized by peaks at the 5’ and 3’ ends, just inside the nucleosome depleted regions. However, as genes increase in expression, total H1 occupancy falls as expected but enrichment takes on a new asymmetrical shape, with 5’ ends having lower H1 levels with increasing enrichment towards the 3’ ends. This asymmetry is not reported in Drosophila or mammals, and future investigations may uncover whether and how this pattern affects transcription in plants. The localization pattern of H1.3 is similar to H1.1/2 variants. However, compared to H1.1/2, H1.3 association with chromatin is far more dynamic and is more frequently associated with active chromatin marks such as H3K4me3 (116). Additionally, increased levels of DNA methylation, which are normally observed in response to stress, were significantly decreased in h1.3 mutants under stress conditions. These distinctions suggest that H1.3 may outcompete H1.1/2 under stress allowing for increased accessibility to regulatory machinery like DNA methyltransferases (116).
H1-dependent silencing in euchromatin and heterochromatin
Recent evidence indicates that plant H1 contributes to the structural organization of both constitutive heterochromatin and euchromatin. Independent studies using H1 triple mutants (3h1) and double mutants both found chromocenter decondensation in Arabidopsis (14, 114). Despite this observation, H1 double and triple mutants had minimal TE derepression. This evidence is in conflict with the common view that chromatin compaction is required for efficient TE silencing and suggests that loss of H1 contributes to heterochromatin structure without any functional impact on silencing.
H1 variants impact the pattern of heterochromatic DNA methylation in CG, CHH and CHG contexts (109, 115, 154). h1.1 and h1.2 mutants both show increased DNA methylation in heterochromatic TEs, suggesting that H1 variants inhibit heterochromatin accessibility to DNA methyltransferases. While further investigation is still needed, considering the relationship between DNA methylation and H1 in plants as well as other eukaryotes may help to explain the surprisingly minimal impact H1 has on TE silencing. In mice, the situation is similar to plants, where h1 mutants (mutation in both H1.1 and H1.2) show only partial TE upregulation (39). However in Drosophila, where cytosine methylation is absent, H1 loss does indeed induce general TE expression (55, 79). This suggests that while H1 contributes to TE silencing, organisms with DNA methylation are able to maintain this silencing despite H1-dependent changes in chromatin structure (14). This theory is supported by a small number of TEs in Arabidopsis that were found to be derepressed more in met1;h1 double mutants than in either single mutant alone (14). Additionally, a recent study revealed that a family of TEs located in pericentromeric heterochromatin (where evidence suggests that silencing is achieved independently of DNA methylation) depend on H1 for their repression under heat stress. By contrast, a family of non-pericentromeric TEs affected by heat rely on DNA methylase CMT2 together with H1 for stable repression (73). H1 overexpression in vegetative Arabidopsis cells also predominantly leads to the repression of pericentromeric TEs (47). Similar to their effect on TE silencing, it was shown that loss of H1 intensifies the activation of antisense transcripts only at genes hypomethylated in met1 (14).
As in heterochromatin, euchromatic H1 loss causes profound changes in chromatin structure with surprisingly little impact on gene expression. In WT plant cells there is a strong inverse correlation between nucleosome occupancy and transcription, with highly expressed genes having the lowest occupancy (114). Low nucleosome occupancy is often interpreted as a requirement for increasing accessibility of a transcribed gene to transcriptional machinery and lowering the energy barrier presented by nucleosomes to RNA polymerase procession. In H1-depleted cells this correlation is almost completely lost, with all genes having similar nucleosome occupancy regardless of expression level (114). Surprisingly, gene expression is relatively unchanged in these cells, with only about 3% of genes being misregulated. This result indicates that H1-mediated nucleosome occupancy is a consequence rather than a driver of steady state transcription. However, H1-depleted plants do have defects in several developmental and cellular transitions including seed dormancy control, flowering time control, and lateral root initiation (114). Collectively, these observations indicate that the massive structural alterations found in H1 mutants likely affect tight control of developmental and cellular transitions. Therefore, H1-dependent chromatin structures may have a more prominent role in transcriptional reprogramming rather than in fundamental expression. Supporting a role for H1 in transcriptional reprogramming is the observation that 3h1 mutant cells also have a dramatic reduction of nuclear H3K27me3, a hallmark of epigenetic silencing memory across plants and animals (114).
Conclusion and future directions
Most studies investigate histone variants by observing their genome-wide distributions before and after a disruption or exposure. However, it is clear that future studies will need to be performed at a higher temporal resolution to determine the exact order of events that take place during variant-mediated gene regulation. For instance, there is mounting evidence for a role of H2A.Z in regulating a majority of environmental responses but no data currently exists to explain how this repressive state comes about, or how it may change during activation. Excitingly, Willige et al used temporally resolved H2A.Z profiling to find that gene body H2A.Z loss actually precedes activation of select red:far-red light sensitive genes, indicating that H2A.Z loss is not merely a consequence of their activation (143). Additionally, following enrichment of H3.3 and H1 through precise time points during cell fate determinations will help explain why these histones play fundamental roles in transcriptional reprogramming during development while being dispensable for general transcription. Similarly, conclusions in variant research have often been limited by assays profiling large cell populations. Emerging single cell data indicates that cells within these heterogeneous populations do not behave uniformly and meaningful changes in variant deposition may be masked by homogenized tissue samples. As chromatin profiling techniques advance and read depth requirements fall, single cell type profiling will uncover how these variants behave within uniform cell types and even single cells.
Modification of variants
It is reasonable to imagine that the apparent multifunctionality of H2A.Z is due in part to modifications to the histone itself. For instance, we know that H2A.Z acetylation is sufficient for gene activation at FLC. But what about acetylation at other genes with similar H2A.Z distribution profiles that appear inactive? Are those genes simply upstream of others in the process of activation or is there a compounding modification like methylation that is stifling activation? Future studies profiling these variant modifications genome-wide will be essential to closing the current knowledge gap between H2A.Z mediated activation and repression. Additionally, H3.3 K4 plays an essential role in mammalian embryonic stem cell differentiation, likely as a platform for methylation (41). This essential role for H3.3K4 in the stem cell could help explain the observation in plants that H3.3 is essential for viability while being dispensable for general transcription. This residue and others known to be modified in other species are conserved in plants meaning there is great potential for the future study of plant H3.3 modifications.
Role of chromatin remodelers in regulation
Histone variant chaperones are often used as proxies for the study of histone variants. However, these chaperones often have functions outside of just histone deposition. For instance, swr1 mutants show a global depletion of H3K27me3 while this phenotype is much less severe in hta9 hta11 double mutants (12, 43). Similarly, ddm1 mutants have significant TE derepression while h2aw-2 mutants do not. Future studies are needed to decouple the functions of these chromatin remodelers from the variants themselves. Additionally, while conservation and mutant analysis implicate other chromatin remodelers as variant chaperones in plants, H2A.X and H3.3 still do not have confirmed interactions with a chromatin remodeler or chaperone.
DNA methylation and histone variants
Each histone variant discussed in this review has some relationship with DNA methylation. H2A.Z and DNA methylation are mutually exclusive in the Arabidopsis genome, suggesting that gene body H2A.Z may serve to protect responsive genes from the more permanent effects of DNA methylation (20, 159). However, while loss of H2A.Z does cause hypermethylation over select regions, overall methylation patterns are unaffected (20, 94). On the contrary, global reductions in DNA methylation in met1 mutants result in an increase in H2A.Z enrichment at those sites, implying that it is DNA methylation which excludes H2A.Z rather than the inverse (159).
DNA methylation and H3.3 are both enriched over the body of active Arabidopsis genes (18, 71, 155). Detailed characterization of Arabidopsis h3.3kd mutants revealed that the level of DNA methylation decreases exclusively at regions where H3.3 and DNA methylation overlap on active gene bodies (145). In the same h3.3kd mutants, these active gene bodies are also invaded by H1 and H2A.Z (Figure 2A). Therefore, reduced gene body methylation in h3.3kd might allow ectopic recruitment of H2A.Z-containing nucleosomes to gene bodies. Given H2A.Z’s role in transcriptional repression, H3.3 enrichment over genes may be required to maintain suitable chromatin structure for transcription by antagonizing H1 invasion of active genes. Low H1 levels will therefore provide sufficient accessibility to DNA methyltransferases that methylate gene bodies and prevent invasion by H2A.Z. Similarly, h2a.w-2 mutants also show an increase in H1 enrichment and a decrease in DNA methylation in constitutive heterochromatin. Therefore, H2A.W may serve as a functional complement to H3.3 with respect to maintaining the balance between H1 and DNA methylation specifically in constitutive heterochromatin.
Summary Points.
H3.3 promotes DNA accessibility in part through an antagonistic relationship with H1.
Phe41 is an amino acid substitution unique to plant H3.1 and may impart functions on H3.1 that are plant-specific.
H3.3 cannot be methylated at K27, implying that H3.3 can interrupt the Polycomb pathway of gene repression and potentially perpetuate the euchromatic chromatin state across cell divisions.
Eukaryotes without bidirectional transcription have peak H2A.Z enrichment downstream of the TSS while organisms with bidirectional transcription have bimodal H2A.Z enrichment. H2A.Z is therefore a marker for transcriptional direction.
For the first time in plants, phosphorylation of H2A.X was found to be required for transcriptional activation of ABI4. It will be interesting to investigate whether this yH2A.X-dependent activation involves other elements of DNA damage response and repair.
Recent evidence shows that H2A.Z enrichment within the gene body contributes to transcriptional repression likely through a non-canonical Polycomb pathway of gene silencing.
H2A.W is a histone variant unique to plants which may promote accessibility of constitutive heterochromatin by competing with H1 for binding to linker DNA.
Nucleosome occupancy depends on linker histone H1 and together with DNA methylation, promotes the silencing of TEs.
H1-dependent chromatin structures may have a more prominent role in transcriptional reprogramming than in steady state expression.
References
- 1.Andrews AJ, Luger K. 2011. Nucleosome structure(s) and stability: variations on a theme. Annu. Rev. Biophys 40:99–117 [DOI] [PubMed] [Google Scholar]
- 2.Ascenzi R, Gantt JS. 1997. A drought-stress-inducible histone gene in Arabidopsis thaliana is a member of a distinct class of plant linker histone variants. Plant Mol. Biol 34(4):629–41 [DOI] [PubMed] [Google Scholar]
- 3.Ausió J 2000. Are linker histones (histone H1) dispensable for survival? Bioessays. 22(10):873–77 [DOI] [PubMed] [Google Scholar]
- 4.Bagchi DN, Battenhouse AM, Park D, Iyer VR. 2020. The histone variant H2A. Z in yeast is almost exclusively incorporated into the+ 1 nucleosome in the direction of transcription. Nucleic Acids Res. 48(1):157–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit M, Simon L, Desset S, Duc C, Cotterell S, et al. 2019. Replication-coupled histone H3. 1 deposition determines nucleosome composition and heterochromatin dynamics during Arabidopsis seedling development. New Phytol. 221(1):385–98 [DOI] [PubMed] [Google Scholar]
- 6.Berriri S, Gangappa SN, Kumar SV. 2016. SWR1 Chromatin-Remodeling Complex Subunits and H2A . Z Have Non-overlapping Functions in Immunity and Gene Regulation in Arabidopsis. Mol. Plant 9(7):1051–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden SA, Kavanová M, Finnegan EJ, Wigge PA. 2013. Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome Biol. 14(6):R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bönisch C, Hake SB. 2012. Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res. 40(21):10719–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borg M, Jiang D, Berger F. 2021. Histone variants take center stage in shaping the epigenome. Curr. Opin. Plant Biol 61:101991. [DOI] [PubMed] [Google Scholar]
- 10.Bourguet P, Picard CL, Yelagandula R, Pélissier T, Lorković ZJ, et al. 2021. The histone variant H2A.W and linker histone H1 co-regulate heterochromatin accessibility and DNA methylation. Nat. Commun 12(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai H, Zhang M, Chai M, He Q, Huang X, et al. 2019. Epigenetic regulation of anthocyanin biosynthesis by an antagonistic interaction between H2A . Z and H3K4me3. . 1:295–308 [DOI] [PubMed] [Google Scholar]
- 12.Carter B, Bishop B, Ho KK, Huang R, Jia W, et al. 2018. The Chromatin Remodelers PKL and PIE1 Act in an Epigenetic Pathway that Determines H3K27me3 Homeostasis in Arabidopsis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charbonnel C, Allain E, Gallego ME, White CI. 2011. Kinetic analysis of DNA double-strand break repair pathways in Arabidopsis. DNA Repair. 10(6):611–19 [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Lyons DB, Kim MY, Moore JD, Zilberman D. 2020. DNA Methylation and Histone H1 Jointly Repress Transposable Elements and Aberrant Intragenic Transcripts. Mol. Cell 77(2):310–23. e7 [DOI] [PubMed] [Google Scholar]
- 15.Choi K, Kim S, Kim SY, Kim M, Hyun Y, et al. 2005. SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell. 17(10):2647–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi K, Park C, Lee J, Oh M, Noh B, Lee I. 2007. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development. 134(10):1931–41 [DOI] [PubMed] [Google Scholar]
- 17.Clarkson MJ, Wells JR, Gibson F, Saint R, Tremethick DJ. 1999. Regions of variant histone His2AvD required for Drosophila development. Nature. 399(6737):694–97 [DOI] [PubMed] [Google Scholar]
- 18.Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, et al. 2008. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 452(7184):215–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole L, Kurscheid S, Nekrasov M, Domaschenz R, Vera DL, et al. 2021. Multiple roles of H2A.Z in regulating promoter chromatin architecture in human cells. Nat. Commun 12(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman-Derr D, Zilberman D. 2012. Deposition of Histone Variant H2A.Z within Gene Bodies Regulates Responsive Genes. PLoS Genet. 8(10): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colino-Sanguino Y, Clark SJ, Valdes-Mora F. 2021. The H2A.Z-nucleosome code in mammals: emerging functions. Trends Genet. [DOI] [PubMed] [Google Scholar]
- 22.Collins PL, Purman C, Porter SI, Nganga V, Saini A, et al. 2020. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nat. Commun 11(1):3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Core LJ, Waterfall JJ, Gilchrist DA, Fargo DC, Kwak H, et al. 2012. Defining the status of RNA polymerase at promoters. Cell Rep. 2(4):1025–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortijo S, Charoensawan V, Brestovitsky A, Buning R, Ravarani C, et al. 2017. Transcriptional Regulation of the Ambient Temperature Response by H2A.Z Nucleosomes and HSF1 Transcription Factors in Arabidopsis. Mol. Plant 10(10):1258–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crevillén P, Gómez-Zambrano Á, López JA, Vázquez J, Piñeiro M, Jarillo JA. 2019. Arabidopsis YAF9 histone readers modulate flowering time through NuA4-complex-dependent H4 and H2A.Z histone acetylation at FLC chromatin. New Phytol. 222(4):1893–1908 [DOI] [PubMed] [Google Scholar]
- 26.Dai X, Bai Y, Zhao L, Dou X, Liu Y, et al. 2018. H2A.Z Represses Gene Expression by Modulating Promoter Nucleosome Structure and Enhancer Histone Modifications in Arabidopsis. Mol. Plant 11(4):635. [DOI] [PubMed] [Google Scholar]
- 27.Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, et al. 2009. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature. 461(7265):819–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deal RB, Kandasamy MK, McKinney EC, Meagher RB. 2005. The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell. 17(10):2633–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deal RB, Topp CN, McKinney EC, Meagher RB. 2007. Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell. 19(1):74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobersch S, Rubio K, Singh I, Günther S, Graumann J, et al. 2021. Positioning of nucleosomes containing γ-H2AX precedes active DNA demethylation and transcription initiation. Nat. Commun 12(1):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorigo B, Schalch T, Bystricky K, Richmond TJ. 2003. Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J. Mol. Biol 327(1):85–96 [DOI] [PubMed] [Google Scholar]
- 32.Dryhurst D, Ishibashi T, Rose KL, Eirín-López JM, McDonald D, et al. 2009. Characterization of the histone H2A. Z-1 and H2A. Z-2 isoforms in vertebrates. BMC Biol. 7(1):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duc C, Benoit M, Détourné G, Simon L, Poulet A, et al. 2017. Arabidopsis ATRX modulates H3. 3 occupancy and fine-tunes gene expression. Plant Cell. 29(7):1773–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn CJ, Sarkar P, Bailey ER, Farris S, Zhao M, et al. 2017. Histone hypervariants H2A. Z. 1 and H2A. Z. 2 play independent and context-specific roles in neuronal activity-induced transcription of Arc/Arg3. 1 and other immediate early genes. Eneuro. 4(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Y-C, Gu S, Zhou J, Wang T, Cai H, et al. 2006. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca2+/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell. Proteomics 5(6):1033–44 [DOI] [PubMed] [Google Scholar]
- 36.Eleuteri B, Aranda S, Ernfors P. 2018. NoRC Recruitment by H2A.X Deposition at rRNA Gene Promoter Limits Embryonic Stem Cell Proliferation. Cell Rep. 23(6):1853–66 [DOI] [PubMed] [Google Scholar]
- 37.Faast R, Thonglairoam V, Schulz TC, Beall J, Wells JRE, et al. 2001. Histone variant H2A . Z is required for early mammalian development. Curretnt Biology. 11(June):1183–87 [DOI] [PubMed] [Google Scholar]
- 38.Fan JY, Rangasamy D, Luger K, Tremethick DJ. 2004. H2A.Z Alters the Nucleosome Surface to Promote HP1α-Mediated Chromatin Fiber Folding. Mol. Cell 16(4):655–61 [DOI] [PubMed] [Google Scholar]
- 39.Fan Y, Nikitina T, Zhao J, Fleury TJ, Bhattacharyya R, et al. 2005. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 123(7):1199–1212 [DOI] [PubMed] [Google Scholar]
- 40.Foroozani M, Zahraeifard S, Oh D-H, Wang G, Dassanayake M, Smith AP. 2020. Low-Phosphate Chromatin Dynamics Predict a Cell Wall Remodeling Network in Rice Shoots. Plant Physiol. 182(3):1494–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehre M, Bunina D, Sidoli S, Lübke MJ, Diaz N, et al. 2020. Lysine 4 of histone H3.3 is required for embryonic stem cell differentiation, histone enrichment at regulatory regions and transcription accuracy. Nat. Genet 52(3):273–82 [DOI] [PubMed] [Google Scholar]
- 42.Gómez-Zambrano Á, Crevillén P, Franco-Zorrilla JM, López JA, Moreno-Romero J, et al. 2018. Arabidopsis SWC4 binds DNA and recruits the SWR1 complex to modulate histone H2A. Z deposition at key regulatory genes. Mol. Plant 11(6):815–32 [DOI] [PubMed] [Google Scholar]
- 43.Gómez-Zambrano Á, Merini W, Calonje M. 2019. The repressive role of Arabidopsis H2A.Z in transcriptional regulation depends on AtBMI1 activity. Nat. Commun 10(1):2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gómez-Zambrano Á, Merini W, Calonje M. 2019. The repressive role of Arabidopsis H2A. Z in transcriptional regulation depends on AtBMI1 activity. Nat. Commun 10(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grasser KD. 2020. The FACT Histone Chaperone: Tuning Gene Transcription in the Chromatin Context to Modulate Plant Growth and Development. Front. Plant Sci 11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heo K, Kim H, Choi SH, Choi J, Kim K, et al. 2008. FACT-mediated exchange of histone variant H2AX regulated by phosphorylation of H2AX and ADP-ribosylation of Spt16. Mol. Cell 30(1):86–97 [DOI] [PubMed] [Google Scholar]
- 47.He S, Vickers M, Zhang J, Feng X. 2019. Natural depletion of histone H1 in sex cells causes DNA demethylation, heterochromatin decondensation and transposon activation. Elife. 8:e42530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hetzel J, Duttke SH, Benner C, Chory J. 2016. Nascent RNA sequencing reveals distinct features in plant transcription. Proc. Natl. Acad. Sci. U. S. A 113(43):12316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann NR. 2016. Last Exit to Differentiation: Histone Variants as Signposts [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huefner ND, Friesner JD, Britt AB. 2009. Characterization of Two H2AX Homologues in Arabidopsis thaliana and their Response to Ionizing Radiation. Induced Plant Mutat. Genomics Era [Google Scholar]
- 51.Hu G, Cui K, Northrup D, Liu C, Wang C, et al. 2013. H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 12(2):180–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y, Lai Y. 2015. Identification and expression analysis of rice histone genes. Plant Physiol. Biochem 86:55–65 [DOI] [PubMed] [Google Scholar]
- 53.Iacovoni JS, Caron P, Lassadi I, Nicolas E, Massip L, et al. 2010. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 29(8):1446–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingouff M, Rademacher S, Holec S, Šoljić L, Xin N, et al. 2010. Zygotic resetting of the HISTONE 3 variant repertoire participates in epigenetic reprogramming in Arabidopsis. Curr. Biol 20(23):2137–43 [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki YW, Murano K, Ishizu H, Shibuya A, Iyoda Y, et al. 2016. Piwi modulates chromatin accessibility by regulating multiple factors including histone H1 to repress transposons. Mol. Cell 63(3):408–19 [DOI] [PubMed] [Google Scholar]
- 56.Jacob Y, Bergamin E, Donoghue MTA, Mongeon V, LeBlanc C, et al. 2014. Selective methylation of histone H3 variant H3.1 regulates heterochromatin replication. Science. 343(6176):1249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarillo JA, Piñeiro M. 2015. H2A.Z mediates different aspects of chromatin function and modulates flowering responses in Arabidopsis. Plant J. 83(1):96–109 [DOI] [PubMed] [Google Scholar]
- 58.Jedrusik MA, Schulze E. 2001. A single histone H1 isoform (H1. 1) is essential for chromatin silencing and germline development in Caenorhabditis elegans. Development. 128(7):1069–80 [DOI] [PubMed] [Google Scholar]
- 59.Jerzmanowski A, Przewłoka M, Grasser KD. 2000. Linker histones and HMG1 proteins of higher plants. Plant Biol. 2(06):586–97 [Google Scholar]
- 60.Kasinsky HE, Lewis JD, Dacks JB, Ausló J. 2001. Origin of H1 linker histones. The FASEB journal. 15(1):34–42 [DOI] [PubMed] [Google Scholar]
- 61.Kawashima T, Lorković ZJ, Nishihama R, Ishizaki K, Axelsson E, et al. 2015. Diversification of histone H2A variants during plant evolution. Trends Plant Sci. 20(7):419–25 [DOI] [PubMed] [Google Scholar]
- 62.Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, et al. 2004. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol. 2(5):E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotliński M, Knizewski L, Muszewska A, Rutowicz K, Lirski M, et al. 2017. Phylogeny-based systematization of Arabidopsis proteins with histone H1 globular domain. Plant Physiol. 174(1):27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotliński M, Rutowicz K, Kniżewski Ł, Palusiński A, Olędzki J, et al. 2016. Histone H1 Variants in Arabidopsis Are Subject to Numerous Post-Translational Modifications, Both Conserved and Previously Unknown in Histones, Suggesting Complex Functions of H1 in Plants. PLoS One. 11(1):e0147908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kralemann LEM, Liu S, Trejo-Arellano MS, Muñoz-Viana R, Köhler C, Hennig L. 2020. Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable Polycomb-mediated gene repression in Arabidopsis. Genome Biol. 21(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar SV, Wigge PA, Centre JI, Lane C, Nr N. 2010. H2A.Z-Containing Nucleosomes Mediate the Thermosensory Response in Arabidopsis. Cell. 140(1):136–47 [DOI] [PubMed] [Google Scholar]
- 67.Lang J, Smetana O, Sanchez-Calderon L, Lincker F, Genestier J, et al. 2012. Plant γH2AX foci are required for proper DNA DSB repair responses and colocalize with E2F factors. New Phytol. 194(2):353–63 [DOI] [PubMed] [Google Scholar]
- 68.Lázaro A, Gómez-Zambrano A, López-González L, Piñeiro M, Jarillo JA. 2008. Mutations in the Arabidopsis SWC6 gene, encoding a component of the SWR1 chromatin remodelling complex, accelerate flowering time and alter leaf and flower development. J. Exp. Bot 59(3):653–66 [DOI] [PubMed] [Google Scholar]
- 69.Lei B, Berger F. 2020. H2A Variants in Arabidopsis: Versatile Regulators of Genome Activity. Plant Communications. 1(1):100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis PW, Elsaesser SJ, Noh K-M, Stadler SC, Allis CD. 2010. Daxx is an H3. 3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proceedings of the National Academy of Sciences. 107(32):14075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, et al. 2008. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 133(3):523–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C, Cheng Y-J, Wang J-W, Weigel D. 2017. Prominent topologically associated domains differentiate global chromatin packing in rice from Arabidopsis. Nature Plants. 3(9):742–48 [DOI] [PubMed] [Google Scholar]
- 73.Liu S, de Jonge J, Trejo-Arellano MS, Santos-González J, Köhler C, Hennig L. 2021. Role of H1 and DNA methylation in selective regulation of transposable elements during heat stress. New Phytol. 229(4):2238–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Li B, Gorovsky MA. 1996. Essential and nonessential histone H2A variants in Tetrahymena thermophila. Mol. Cell. Biol 16(8):4305–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorković ZJ, Park C, Goiser M, Jiang D, Kurzbauer M-T, et al. 2017. Compartmentalization of DNA damage response between heterochromatin and euchromatin is mediated by distinct H2A histone variants. Curr. Biol 27(8):1192–99 [DOI] [PubMed] [Google Scholar]
- 76.Lu L, Chen X, Qian S, Zhong X. 2018. The plant-specific histone residue Phe41 is important for genome-wide H3. 1 distribution. Nat. Commun 9(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo Y-X, Hou X-M, Zhang C-J, Tan L-M, Shao C-R, et al. 2020. A plant-specific SWR1 chromatin-remodeling complex couples histone H2A. Z deposition with nucleosome sliding. EMBO J. 39(7):e102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu X, Wontakal SN, Emelyanov AV, Morcillo P, Konev AY, et al. 2009. Linker histone H1 is essential for Drosophila development, the establishment of pericentric heterochromatin, and a normal polytene chromosome structure. Genes Dev. 23(4):452–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu X, Wontakal SN, Kavi H, Kim BJ, Guzzardo PM, et al. 2013. Drosophila H1 regulates the genetic activity of heterochromatin by recruitment of Su (var) 3–9. Science. 340(6128):78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malik HS, Henikoff S. 2003. Phylogenomics of the nucleosome. Nat. Struct. Biol 10(11):882–91 [DOI] [PubMed] [Google Scholar]
- 81.Mao Z, Wei X, Li L, Xu P, Zhang J, et al. 2021. Arabidopsis cryptochrome 1 controls photomorphogenesis through regulation of H2A.Z deposition. Plant Cell. 33(6):1961–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.March-Díaz R, García-Domínguez M, Lozano-Juste J, León J, Florencio FJ, Reyes JC. 2008. Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J. 53(3):475–87 [DOI] [PubMed] [Google Scholar]
- 83.March-Díaz R, García-Domínguez M, Lozano-Juste J, León J, Florencio FJ, Reyes JC. 2008. Histone H2A. Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J. 53(3):475–87 [DOI] [PubMed] [Google Scholar]
- 84.March-Díaz R, Reyes JC. 2009. The Beauty of Being a Variant: H2A.Z and the SWR1 Complex in Plants. Mol. Plant 2(4):565–77 [DOI] [PubMed] [Google Scholar]
- 85.Marques M, Laflamme L, Gervais AL, Gaudreau L. 2010. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics. 5(4):267–72 [DOI] [PubMed] [Google Scholar]
- 86.Martin-Trillo M, Lázaro A, Poethig RS, Gómez-Mena C, Piñeiro MA, et al. 2006. EARLY IN SHORT DAYS 1 (ESD1) encodes ACTIN-RELATED PROTEIN 6 (AtARP6), a putative component of chromatin remodelling complexes that positively regulates FLC accumulation in Arabidopsis. Development. 133(7):1241–52 [DOI] [PubMed] [Google Scholar]
- 87.Mizuguchi G, Shen X, Landry J, Wu W-H, Sen S, Wu C. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 303(5656):343–48 [DOI] [PubMed] [Google Scholar]
- 88.Molitor AM, Bu Z, Yu Y, Shen WH. 2014. Arabidopsis AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes. PLoS Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moraes I, Yuan Z-F, Liu S, Souza GM, Garcia BA, Casas-Mollano JA. 2015. Analysis of Histones H3 and H4 Reveals Novel and Conserved Post-Translational Modifications in Sugarcane. PLoS One. 10(7):e0134586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy KE, Meng FW, Makowski CE, Murphy PJ. 2020. Genome-wide chromatin accessibility is restricted by ANP32E. Nat. Commun 11(1):5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muto S, Senda M, Akai Y, Sato L, Suzuki T, et al. 2007. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc. Natl. Acad. Sci. U. S. A 104(11):4285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mylonas C, Lee C, Auld AL, Cisse II, Boyer LA. 2021. A dual role for H2A.Z.1 in modulating the dynamics of RNA polymerase II initiation and elongation. Nat. Struct. Mol. Biol 28(5):435–42 [DOI] [PubMed] [Google Scholar]
- 93.Nguyen NH, Cheong J-J. 2018. H2A.Z-containing nucleosomes are evicted to activate AtMYB44 transcription in response to salt stress. Biochem. Biophys. Res. Commun 499(4):1039–43 [DOI] [PubMed] [Google Scholar]
- 94.Nie W-F, Lei M, Zhang M, Tang K, Huang H, et al. 2019. Histone acetylation recruits the SWR1 complex to regulate active DNA demethylation in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 116(33):16641–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nie X, Wang H, Li J, Holec S, Berger F. 2014. The HIRA complex that deposits the histone H3. 3 is conserved in Arabidopsis and facilitates transcriptional dynamics. Biol. Open 3(9):794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ni K, Ren J, Xu X, He Y, Finney R, et al. 2020. LSH mediates gene repression through macroH2A deposition. Nat. Commun 11(1):5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishibuchi I, Suzuki H, Kinomura A, Sun J, Liu N-A, et al. 2014. Reorganization of damaged chromatin by the exchange of histone variant H2A.Z-2. Int. J. Radiat. Oncol. Biol. Phys 89(4):736–44 [DOI] [PubMed] [Google Scholar]
- 98.Noh Y-S, Amasino RM. 2003. PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. Plant Cell. 15(7):1671–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okada T, Endo M, Singh MB, Bhalla PL. 2005. Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J. 44(4):557–68 [DOI] [PubMed] [Google Scholar]
- 100.Osakabe A, Jamge B, Axelsson E, Montgomery SA, Akimcheva S, et al. 2021. The chromatin remodeler DDM1 prevents transposon mobility through deposition of histone variant H2A.W. Nat. Cell Biol 23(4):391–400 [DOI] [PubMed] [Google Scholar]
- 101.Otero S, Desvoyes B, Peiró R, Gutierrez C. 2016. Histone H3 dynamics reveal domains with distinct proliferation potential in the Arabidopsis root. Plant Cell. 28(6):1361–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park Y-J, Luger K 2006. The structure of nucleosome assembly protein 1. Proc. Natl. Acad. Sci. U. S. A 103(5):1248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patterton HG, Landel CC, Landsman D, Peterson CL, Simpson RT. 1998. The biochemical and phenotypic characterization of Hho1p, the putative linker histone H1 of Saccharomyces cerevisiae. J. Biol. Chem 273(13):7268–76 [DOI] [PubMed] [Google Scholar]
- 104.Paul S 2021. Histone “acidic patch”: a hotspot in chromatin biology. Nucleus [Google Scholar]
- 105.Piquet S, Le Parc F, Bai S-K, Chevallier O, Adam S, Polo SE. 2018. The Histone Chaperone FACT Coordinates H2A.X-Dependent Signaling and Repair of DNA Damage. Mol. Cell 72(5):888–901.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Potok ME, Wang Y, Xu L, Zhong Z, Liu W, et al. 2019. Arabidopsis SWR1-associated protein methyl-CpG-binding domain 9 is required for histone H2A.Z deposition. Nat. Commun 10(1):3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Probst AV, Desvoyes B, Gutierrez C. 2020. Similar yet critically different: the distribution, dynamics and function of histone variants. J. Exp. Bot 71(17):5191–5204 [DOI] [PubMed] [Google Scholar]
- 108.Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, et al. 2005. Histone variant H2A.Z marks the 5’ ends of both active and inactive genes in euchromatin. Cell. 123(2):233–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rea M, Zheng W, Chen M, Braud C, Bhangu D, et al. 2012. Histone H1 affects gene imprinting and DNA methylation in Arabidopsis. Plant J. 71(5):776–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, Bonner WM. 2011. Recent developments in the use of γ-H2AX as a quantitative DNA double-strand break biomarker. Aging. 3(2):168–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ricketts MD, Frederick B, Hoff H, Tang Y, Schultz DC, et al. 2015. Ubinuclein-1 confers histone H3. 3-specific-binding by the HIRA histone chaperone complex. Nat. Commun 6(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roitinger E, Hofer M, Köcher T, Pichler P, Novatchkova M, et al. 2015. Quantitative phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol. Cell. Proteomics 14(3):556–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosa M, Von Harder M, Cigliano RA, Schlögelhofer P, Mittelsten Scheid O. 2013. The Arabidopsis SWR1 chromatin-remodeling complex is important for DNA repair, somatic recombination, and meiosis. Plant Cell. 25(6):1990–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rutowicz K, Lirski M, Mermaz B, Teano G, Schubert J, et al. 2019. Linker histones are fine-scale chromatin architects modulating developmental decisions in Arabidopsis. Genome Biol. 20(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rutowicz K, Puzio M, Halibart-Puzio J, Lirski M, Kotliński M, et al. 2015. A specialized histone H1 variant is required for adaptive responses to complex abiotic stress and related DNA methylation in Arabidopsis. Plant Physiol. 169(3):2080–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rutowicz K, Puzio M, Halibart-Puzio J, Lirski M, Kotliński M, et al. 2015. A Specialized Histone H1 Variant Is Required for Adaptive Responses to Complex Abiotic Stress and Related DNA Methylation in Arabidopsis. Plant Physiol. 169(3):2080–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seo J, Kim SC, Lee H-S, Kim JK, Shon HJ, et al. 2012. Genome-wide profiles of H2AX and γ-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic Acids Res. 40(13):5965–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shechter D, Chitta RK, Xiao A, Shabanowitz J, Hunt DF, David Allis C. 2009. A distinct H2A.X isoform is enriched in Xenopus laevis eggs and early embryos and is phosphorylated in the absence of a checkpoint. Proc. Natl. Acad. Sci. U. S. A 106(3):749–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen X, Yu L, Weir JW, Gorovsky MA. 1995. Linker histories are not essential and affect chromatin condensation in vivo. Cell. 82(1):47–56 [DOI] [PubMed] [Google Scholar]
- 120.She W, Baroux C. 2015. Chromatin dynamics in pollen mother cells underpin a common scenario at the somatic-to-reproductive fate transition of both the male and female lineages in Arabidopsis. Front. Plant Sci 6:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.She W, Grimanelli D, Rutowicz K, Whitehead MWJ, Puzio M, et al. 2013. Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development. 140(19):4008–19 [DOI] [PubMed] [Google Scholar]
- 122.Shi L, Wang J, Hong F, Spector DL, Fang Y. 2011. Four amino acids guide the assembly or disassembly of Arabidopsis histone H3. 3-containing nucleosomes. Proceedings of the National Academy of Sciences. 108(26):10574–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shu H, Nakamura M, Siretskiy A, Borghi L, Moraes I, et al. 2014. Arabidopsis replacement histone variant H3. 3 occupies promoters of regulated genes. Genome Biol. 15(4):R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sijacic P, Holder DH, Bajic M, Deal RB. 2019. Methyl-CpG-binding domain 9 (MBD9) is required for H2A.Z incorporation into chromatin at a subset of H2A.Z-enriched regions in the Arabidopsis genome. PLoS Genet. 15(8):e1008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh I, Ozturk N, Cordero J, Mehta A, Hasan D, et al. 2015. High mobility group protein-mediated transcription requires DNA damage marker γ-H2AX. Cell Res. 25(7):837–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, et al. 2010. Histone H2A.Z Regulates the Expression of Several Classes of Phosphate Starvation Response Genes But Not as a Transcriptional Activator. Plant Physiol. 152(1):217–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stroud H, Otero S, Desvoyes B, Ramírez-Parra E, Jacobsen SE, Gutierrez C. 2012. Genome-wide analysis of histone H3.1 and H3.3 variants in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A 109(14):5370–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sura W, Kabza M, Karlowski WM, Bieluszewski T, Kus-Slowinska M, et al. 2017. Dual Role of the Histone Variant H2A.Z in Transcriptional Regulation of Stress-Response Genes. Plant Cell. 29(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suto RK, Clarkson MJ, Tremethick DJ, Luger K. 2000. Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat. Struct. Biol 7(12):1121–24 [DOI] [PubMed] [Google Scholar]
- 130.Talbert PB, Ahmad K, Almouzni G, Ausió J, Berger F, et al. 2012. A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Talbert PB, Henikoff S. 2010. Histone variants—ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol 11(4):264–75 [DOI] [PubMed] [Google Scholar]
- 132.Tasset C, Singh Yadav A, Sureshkumar S, Singh R, van der Woude L, et al. 2018. POWERDRESS-mediated histone deacetylation is essential for thermomorphogenesis in Arabidopsis thaliana. PLoS Genet. 14(3):e1007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong M, Lee K, Ezer D, Cortijo S, Jung J, et al. 2020. The Evening Complex Establishes Repressive Chromatin Domains Via H2A.Z Deposition. Plant Physiol. 182(1):612–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trinklein ND, Aldred SF, Hartman SJ, Schroeder DI, Otillar RP, Myers RM. 2004. An abundance of bidirectional promoters in the human genome. Genome Res. 14(1):62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ushinsky SC, Bussey H, Ahmed AA, Wang Y, Friesen J, et al. 1997. Histone H1 in Saccharomyces cerevisiae. Yeast. 13(2):151–61 [DOI] [PubMed] [Google Scholar]
- 136.Van Daal A, Elgin SCR. 1992. A Histone Variant , H2AvD , is Essential in Drosophila melanogaster. Mol. Biol. Cell 3(June):593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verbsky ML, Richards EJ. 2001. Chromatin remodeling in plants. Curr. Opin. Plant Biol 4(6):494–500 [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Zhong Z, Zhang Y, Xu L, Feng S, et al. 2020. NAP1-RELATED PROTEIN1 and 2 negatively regulate H2A.Z abundance in chromatin in Arabidopsis. Nat. Commun 11(1):2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waterborg JH. 2012. Evolution of histone H3: emergence of variants and conservation of post-translational modification sites. Biochem. Cell Biol 90(1):79–95 [DOI] [PubMed] [Google Scholar]
- 140.Waterborg JH, Robertson AJ. 1996. Common features of analogous replacement histone H3 genes in animals and plants. J. Mol. Evol 43(3):194–206 [DOI] [PubMed] [Google Scholar]
- 141.Waterworth WM, Wilson M, Wang D, Nuhse T, Warward S, et al. 2019. Phosphoproteomic analysis reveals plant DNA damage signalling pathways with a functional role for histone H2 AX phosphorylation in plant growth under genotoxic stress. Plant J. 100(5):1007–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weber CM, Ramachandran S, Henikoff S. 2014. Nucleosomes Are Context-Specific, H2A.Z-Modulated Barriers to RNA Polymerase. Mol. Cell 53(5):819–30 [DOI] [PubMed] [Google Scholar]
- 143.Willige BC, Zander M, Yoo CY, Phan A, Garza RM, et al. 2021. PHYTOCHROME-INTERACTING FACTORs trigger environmentally responsive chromatin dynamics in plants. Nat. Genet, pp. 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wollmann H, Holec S, Alden K, Clarke ND, Jacques P-E, Berger F. 2012. Dynamic deposition of histone variant H3. 3 accompanies developmental remodeling of the Arabidopsis transcriptome. PLoS Genet. 8(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wollmann H, Stroud H, Yelagandula R, Tarutani Y, Jiang D, et al. 2017. The histone H3 variant H3.3 regulates gene body DNA methylation in Arabidopsis thaliana. Genome Biol. 18(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao S, Jiang L, Wang C, Ow DW. 2021. Arabidopsis OXS3 family proteins repress ABA signaling through interactions with AFP1 in the regulation of ABI4 expression. J. Exp. Bot [DOI] [PubMed] [Google Scholar]
- 147.Xue M, Zhang H, Zhao F, Zhao T, Li H, Jiang D. 2021. The INO80 chromatin remodeling complex promotes thermomorphogenesis by connecting H2A.Z eviction and active transcription in Arabidopsis. Mol. Plant 14(11):1799–1813 [DOI] [PubMed] [Google Scholar]
- 148.Xu M, Leichty AR, Hu T, Poethig RS. 2018. H2A.Z promotes the transcription of MIR156A and MIR156C in Arabidopsis by facilitating the deposition of H3K4me3. Development. 145(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Y, Ayrapetov MK, Xu C, Gursoy-Yuzugullu O, Hu Y, Price BD. 2012. Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol. Cell 48(5):723–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yelagandula R, Stroud H, Holec S, Zhou K, Feng S, et al. 2014. The histone variant H2A. W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell. 158(1):98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zahraeifard S, Foroozani M, Sepehri A, Oh D-H, Wang G, et al. 2018. Rice H2A.Z negatively regulates genes responsive to nutrient starvation but promotes expression of key housekeeping genes. J. Exp. Bot 69(20):4907–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zambrano-Mila MS, Aldaz-Villao MJ, Casas-Mollano JA. 2019. Canonical Histones and Their Variants in Plants: Evolution and Functions. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications, pp. 185–222. Springer [Google Scholar]
- 153.Zemach A, Kim MY, Hsieh P-H, Coleman-Derr D, Eshed-Williams L, et al. 2013. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 153(1):193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zemach A, Kim MY, Hsieh P-H, Coleman-Derr D, Eshed-Williams L, et al. 2013. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 153(1):193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW-L, et al. 2006. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 126(6):1189–1201 [DOI] [PubMed] [Google Scholar]
- 156.Zhao F, Zhang H, Zhao T, Li Z, Jiang D. 2021. The histone variant H3.3 promotes the active chromatin state to repress flowering in Arabidopsis. Plant Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao L, Cai H, Su Z, Wang L, Huang X, et al. 2018. KLU suppresses megasporocyte cell fate through SWR1-mediated activation of WRKY28 expression in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 115(3):E526–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou B-R, Feng H, Kato H, Dai L, Yang Y, et al. 2013. Structural insights into the histone H1-nucleosome complex. Proc. Natl. Acad. Sci. U. S. A 110(48):19390–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S. 2008. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature. 456(7218):125–29 [DOI] [PMC free article] [PubMed] [Google Scholar]


