Abstract
Preeclampsia (PE) is a common syndrome of pregnancy, characterized by new-onset hypertension and proteinuria after gestational week 20, or new onset of hypertension and significant end-organ dysfunction. In the worst cases, it can threaten the survival of both mother and baby. Extracellular vesicles (EVs) are lipid-bilayer nanoparticles released from cells. They are involved in cell-cell communication and transport of diverse cargo molecules. Small extracellular vesicles (sEVs, exosomes) are defined by their size and biogenesis within the endocytic compartment of the cell or reverse budding of the plasma membrane. The function of circulating gestational EVs, released from maternal organs or the placenta, remains to be explored. Here, we focused on sEVs that circulate in the maternal blood in the third trimester of human pregnancy and hypothesized that sEVs from pregnant women with PE play a role in regulation of vessel tone. When compared to sEVs from women with uncomplicated pregnancies, ex vivo exposure of isolated mouse mesenteric arteries to sEVs purified from the plasma of pregnant women with PE led to constriction in response to intraluminal pressure. This effect was not observed using microvesicles from the plasma of women with PE or using PE plasma that was depleted of EVs. Blood vessels exposed to sEVs from women with PE were also more resistant to methacholine-stimulated relaxation. Immunofluorescence microscopy confirmed the presence of sEVs within the vessel wall. Together, these data support the notion that circulating sEVs from pregnant women play a role in the regulation of arterial tone.
Keywords: pregnancy, preeclampsia, small extracellular vesicles, myogenic reactivity
INTRODUCTION
Preeclampsia (PE) is a major complication of pregnancy that affects approximately 3-8% of pregnancies in the late second or third trimester [1, 2]. PE is the most common cause of perinatal mortality [3]. It is clinically characterized by new-onset high blood pressure and proteinuria and, when it worsens, can result in life-threatening damage to maternal target organs, fetal growth restriction, and death [1, 4–6]. Although the etiology of PE remains unclear, shallow invasion of placental interstitial and endovascular trophoblasts into the decidua and uterine wall seems to play an important role in early-onset disease [1, 7]. Other pathways that have been implicated in disease pathogenesis include inadequate trophoblast differentiation, placental malperfusion, oxidative stress, and abnormal immune and inflammatory responses, culminating in maternal systemic vasoconstriction and related clinical manifestations [1, 8, 9].
Extracellular vesicles (EVs) are micro- and nano-size particles that are released from most cell types. The EV family encompasses a growing number of particle subtypes [10–12]. Most studied are the larger sized (1-5 μm) apoptotic bodies, which are formed by cell disintegration during apoptotic death, the smaller (0.1-1 μm) microvesicles (MVs), which are formed by budding off from the plasma membrane, and the even smaller (30-150 nm) small extracellular vesicles (sEVs), also referred to as exosomes, formed as intraluminal vesicles within endocytic multivesicular bodies or by budding from the cell membrane [10–12]. A growing number of biological processes are linked to sEV-based cell-cell communication, such as cancer metastatic spread and cardiovascular and neurodegenerative diseases [13–16].
Several lines of evidence link sEVs to hypertension and, possibly, PE. Otani et al. found that intravascular injection of plasma exosomes isolated from hypertensive rats caused increased blood pressure in normotensive rats [17]. Additionally, Tong et al. reported that sEVs derived from first trimester human placentas can impair nitric oxide-mediated relaxation in exposed maternal arteries [18]. Women with PE have more sEVs in their blood compared to women with uncomplicated pregnancy [19, 20]. Our laboratory has previously shown that sEVs from the plasma of women with PE contain different miRNA cargo when compared to sEVs from healthy controls or from pregnancies complicated by fetal growth restriction [21]. Biro et al. found that levels of sEV-bound miR-210 are elevated in placentas from women with PE, implicating sEV miRNAs in PE pathology [22]. Wu et al. found that miR-302a expression is upregulated in tissues from PE patients, with increasing expression in more severe disease presentation [23]. Others suggested that sEVs contribute to the pathogenesis of PE by contributing to impaired angiogenesis, dysfunction of trophoblast cells, and increases in inflammation and immunological pathways [24–26]. However, the involvement of sEVs in regulation of vascular tone remains to be defined.
Placental sEVs, released from trophoblast cells, appear in the maternal circulation roughly 6 weeks post conception and remain circulating in the maternal blood several hours after birth [26, 27]. Work from our laboratory suggests a role for trophoblastic EVs in conferring viral resistance to non-trophoblastic cells [28–30]. Additionally, during pregnancy, sEVs are involved in feto-maternal communication, the inflammatory response, and parturition [31–33]. However, the role of sEVs in normal and pathological pregnancy remains to be further explored [34, 35].
Here, we surmised that plasma sEVs from women with PE interact with vascular endothelial cells and promote vessel constriction. Using an ex vivo system of perfused mouse mesenteric arteries, we hypothesized that, when compared to sEVs from healthy pregnancies, sEVs isolated from the plasma of women with PE increase arterial vessel tone and reduce endothelial cell-mediated vasorelaxation.
MATERIALS AND METHODS
Study subjects and sample collection
Clinical data and plasma samples were obtained from twenty nulliparous women, 10 with PE and 10 with uncomplicated pregnancies, enrolled in the Prenatal Exposures and Preeclampsia Prevention: Mechanisms of Preeclampsia and the Impact of Obesity (PEPP3) project, which recruited subjects either early in pregnancy or at the time of admission to labor and delivery at Magee-Womens Hospital from 2008 to 2014 [36]. All women provided written informed consent, and the study was approved by the University of Pittsburgh’s Institutional Review Board (IRB #PRO08050339). PE was defined using the criteria of the American College of Obstetricians and Gynecologists (ACOG) guidelines of 2002, and as updated in 2013 [4, 37]. Subjects were adjudicated by the PEPP3 research team through chart review. Proteinuria was defined as >300 mg per 24h urine collection, or ≥2+ (100 mg/dL) on a voided or ≥1+ (30 mg/dL) on a catheterized random urine specimen, or protein:creatinine ratio of ≥ 0.3. Women with uncomplicated pregnancies were normotensive throughout gestation, did not have proteinuria, and delivered at term. Women with PE or uncomplicated pregnancies were group-matched on the basis of age, pre-pregnancy body mass index (BMI), race, and gestational age at blood sample collection. The mean maternal age, pre-pregnancy BMI, blood pressure prior to 20 weeks gestation, and time of blood sampling among these women were not different between the groups. All women were non-smokers. Blood samples were obtained prior to labor and therapeutic intravenous administration of MgSO4. Samples were also obtained from 10 non-pregnant subjects at 10-14 months after normotensive uncomplicated pregnancy (as described and adjudicated above, and who had no history of pregnancy complications) enrolled in the Glycocalyx Pathways Linking Pregnancy Profile with Microvascular Dysfunction Postpartum (the Pathways Study, IRB #PRO16030582). For all groups, patients with positive toxicology screen, chronic hypertension, renal disease, or a previous history of metabolic disorders were excluded. The plasma was separated by centrifugation within 60 min of blood collection and immediately frozen at −70°C until use for isolation of sEVs.
Small extracellular vesicle isolation
To ensure a sufficient number of sEVs for the experiments, plasma samples (2 ml total) derived from pooled plasma samples (n=10) were centrifuged for 15 min at 2,500 g to remove debris. Samples were then diluted with 2 mL PBS (Sigma-Aldrich, St. Louis, MO, 0.1 μm filtered) and filtered through a 0.2 μm syringe filter. Samples were diluted again to 10 mL and passed three times through gelatin-agarose columns (Sigma, G5384). PEG6000 (Sigma, 81253-250g) was added to the filtrate, and samples were incubated at room temperature (RT) for 10 min, then centrifuged at 10,000 g for 15 min at RT. The pellet was resuspended in 0.4 mL PBS. A 6-30% OptiPrep (Sigma, D1556) gradient was created using a gradient formation chamber and peristaltic pump. The sample was centrifuged for 22 h at 100,000 g at 4°C. Fractions 1-12 (1 mL each) were collected, fractions 4-6 were diluted 10-fold with PBS and concentrated using Vivaspin 20 (100 kDa cutoff) (Sigma, GE28-9323-63). Protein concentration was measured using microBCA assay (Thermo Fisher, Waltham, MA, 23235), and particle size was assessed by nanoparticle tracking analysis (NTA; Malvern Panalytical, Malvern, UK, Supplemental Fig. 2). [21]. For each vesicle type, the same number of EVs were added to each vessel.
For experiments with labeled sEVs, BeWo cells (from American Type Culture Collection) were cultured in Kaighn’s modified medium F-12K (Gibco, Thermo Fisher) supplemented with 10% v/v GemCell SuperCalf Serum (Gemini Bioproducts, West Sacramento, CA), 20 mM HEPES, 1% v/v penicillin/streptomycin, then changed to EV-free F-12K medium supplemented with EV-free FBS (Thermo Fisher, A2720801) for 72 h. The medium was collected and centrifuged at 500 g for 10 min at 4°C. The supernatant was transferred to new tubes and centrifuged at 1,800 g for 20 min at 4°C to remove cells and apoptotic fragments. The supernatant was transferred to new tubes and centrifuged at 12,000 g for 30 min at 4°C to remove microvesicles, and then filtered through 0.22-μm filters (Genessee Scientific, San Diego, CA). Samples were concentrated using Vivacell 100 (100 kDa cutoff, Sartorius VC1042), and the concentrate was diluted to 14 mL with EV-free PBS and ultracentrifuged overnight at 4°C and 100,000 g. For staining of BeWo sEVs, the pellet was resuspended in 0.5 mL PBS, and 1μg/μl of CM-DiI lipophilic dye (Invitrogen 2272596, Thermo Fisher) was added. The samples were incubated for 10 min at 37°C and 30 min at 4°C. Each sample was then processed as noted above, with removal of unbound dye during the OptiPrep gradient step. Staining of plasma sEVs was performed in a similar fashion, yet the final sEV samples were stained with CM-DiI at the end of the isolation, and the unbound dye was removed using an additional OptiPrep gradient centrifugation step.
MV Isolation
Plasma samples (2 ml) were centrifuged for 15 min at 2,500 g to remove cell and apoptotic fragments. Supernatant was then centrifuged at 12,000 g for 15 min at 4°C to pellet MVs. MVs were washed three times by resuspending pellets in PBS and centrifuging 15 min at 12,000 g at RT.
Preparation of EV-depleted plasma
Plasma samples (2 ml) were first centrifuged at 2,500 g for 15 min at RT to remove apoptotic bodies. Supernatant was then centrifuged at 10,000 g for 15 min at RT to remove microvesicles. Finally, samples were centrifuged at 100,000 g for 2 h at 4°C to remove sEVs. The supernatant was retained as EV-depleted plasma. The depletion of vesicles was confirmed by NTA.
Establishment of the ex vivo mouse mesenteric artery system
Female C57BI/6J mice, 7-12 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained on a 12:12-hour light-dark cycle with food and water available ad libitum. Mice were euthanized by CO2 asphyxiation, using cervical dislocation as a secondary method, and mesenteric arteries were immediately removed. The protocol was approved by the Magee-Womens Research Institute and University of Pittsburgh Animal Care and Use Committee protocol (IACUC #19085595).
Mesenteric arterial bioassay
Mesenteric arteries (inner diameter <300 μm) were used, given that vessels of this caliber constitute the major site of generation of systemic (peripheral) vascular resistance [38]. These arteries receive 30% of cardiac output and therefore contribute significantly to overall cardiovascular homeostasis. A portion of the mesenteric arcade 1 cm distal from the pylorus was placed in a HEPES buffered physiological saline solution (pH 7.4). Second order branches arising from the mesenteric arcade were dissected free, and two branches arising from the same artery were mounted in the dual chamber myograph described below. The inner diameter of these vessels at 60 mm Hg was 200-300 μm, which is consistent with the notion that approximately 50% of the flow resistance between the aorta and precapillary arterioles occurs in arteries of this diameter [38].
The vessels were transferred to a dual-chamber pressurized arteriograph (Living Systems, Burlington, VT) and mounted on two glass microcannulas suspended inside each chamber. Residual blood was flushed from the lumen. Buffer containing either 1% plasma or an EV sample was introduced into the lumen at approximately 1.5 x 109 sEVs/mL of buffer. The distal cannula was occluded to prevent flow. The proximal cannula was attached to a flow-through pressure transducer and pressure servo control unit. This system allowed the intraluminal pressure to be maintained and controlled. A video dimension-analyzing system (Living Systems) processed a selected vidicon line to provide lumen diameter and wall thickness measurements. Both the pressure and dimensional parameters were calibrated at the beginning of each experiment. Further description of this system is reported elsewhere [39]. sEVs at a matched concentration were added to the bath, and this began the 2-h exposure period, during which the vessels were equilibrated at 60 mm Hg transmural pressure. A conditioning stretch [38, 40] was performed by slowly increasing the transmural pressure from 60 to 100 mm Hg (1mm Hg/sec) and decreasing back to 60 mm Hg 15 min before the end of the equilibration and exposure. After the 2-h incubation, the vessels were washed with HEPES-PSS, such that sEVs were removed from the bath but remained in the vessel lumen for the remainder of the experiment.
Myogenic assessment
Immediately following refreshment of HEPES-PSS in the vessel bath, intraluminal pressure was reduced to 20 mm Hg for 10 min. The arteries were then subjected to rapid (~2 s) pressure steps of 20 mm Hg every 4 min, from 20 to 120 mmHg in the absence of flow [40]. The 20 mm Hg pressure step was chosen to approximate the type of stimuli that occurs in vivo [41, 42]. At the end of the relaxation responsiveness experiments (described below), the smooth muscle was inactivated by a 10-min incubation in Ca2+-free buffer with EGTA and 10−4 M papaverine, and the pressure steps were repeated, providing the passive (inactivated vascular smooth muscle) internal diameter response needed for the percent tone calculation. The percent tone of individual arteries was calculated, at each pressure, as % tone = [(Dr –DPSS)/Dr] x100, where Dr is the passive internal diameter (relaxed and inactivated by Ca2+-free buffer with EGTA and papaverine) and DPSS is the stable internal diameter in normal HEPES-PSS [40]. Calculating tone in this manner adjusts for differences that exist in the size of individual arteries.
Endothelium-dependent relaxation responsiveness
The alpha-adrenergic agonist phenylephrine (0.1-10 μM, Sigma) was used to constrict arteries to 50% of their initial diameter. Relaxation response curves were generated to cumulative doses of the endothelium-dependent agonist methacholine (0.001-1.0 μM, Sigma). Vessels were washed and buffer was replaced with a calcium-free buffer to inactivate vascular smooth muscle. The rapid pressure steps were repeated to determine the maximum vessel diameter at each intraluminal pressure step, which was needed for the percent myogenic tone calculation.
Immunofluorescence microscopy
Following 2 h exposure of mesenteric arteries to CM-DiI-labeled sEVs (described above), the arteries were rinsed and mounted in Optimal Cutting Temperature Compound (OCT, Thermo Fisher), snap-frozen in 2 methyl-butane, pre-chilled in liquid nitrogen, and stored at −80°C until use. Frozen tissues were sectioned (8 μm) with a cryostat and mounted on slides pre-treated with Vectabond (Vector Laboratories, Burlingame, CA). Tissue sections were fixed with 4% paraformaldehyde in PBS (15 min, RT) and blocked with 5% goat serum (G9023-10ML, Sigma) followed by the avidin/biotin blocking kit (Vector, PK-7200). Artery sections were incubated with biotin-CD31 antibody (BD Pharmingen, # 558737, BD Biosciences, San Jose, CA, 1:100) in 5% goat serum at 4°C overnight, followed by AF647-streptavidin (Thermo Fisher S-21374, 1:400 in 5% goat serum at RT for 45 min). The slides were washed with PBS, and then streptavidin AF647 secondary antibody (Thermo Fisher S-21374), diluted 1:400 in 5% goat serum, was added and incubated, protected from light, for 45 min at RT. The slides were washed with PBS, and cell nuclei were counterstained with DAPI (0.1μg/ul, D9542, Sigma) was added for 15 min at RT. Tissue sections were fixed again in 4% paraformaldehyde in PBS (15 min at RT). Coverslips were mounted with Gelvatol (81365, Sigma). The tissues were imaged using a Nikon E800 microscope with Zeiss Axiocam 506 camera.
Data analysis
Unpaired analyses were used to compare characteristics between PE and uncomplicated pregnancy groups. Continuous, normally distributed variables were compared by one-way analysis of variance (ANOVA). Clinical characteristics were compared using either the Kruskal-Wallis or Holm-Sidak test. Myogenic and relaxation responses were compared using two-way repeated measures ANOVA with post hoc Tukey Test for pairwise comparisons. The total sample size was estimated on the basis of previous publications demonstrating differential effects of PE vs normal pregnancy plasma-derived sEVs on vascular function [43] and our preliminary vascular data and estimates of the total amount of plasma required for sEV isolation and arterial bioassay experiments.
RESULTS
Participants
Pregnant women in the PE group were group-matched as detailed in Materials and Methods. As expected, the mean systolic and diastolic blood pressure before delivery was higher in women with PE, and women with PE delivered earlier and had smaller newborns (Table 1).
Table 1.
Clinical characteristics of participants from whom plasma samples were obtained.
| Uncomplicated pregnancy (n=10) | Preeclampsia (n=10) | Non-pregnant (n=10) | |
|---|---|---|---|
| Maternal age (years) | 24 (21,27) | 28 (25,31) | 29 (24,30) |
| Race (n, % Black) | 2 (20%) | 2 (20%) | 2 (20%) |
| Pre-pregnancy BMI (kg/m2) | 28 (24, 34) | 26 (24, 34) | 25 (20, 31) |
| Gestational age at delivery (wks) | 39.7 (39.2, 40.7) | 37.3 (36.0, 41.0) | 39.6 (39.0, 40.8) |
| Gestational age at venipuncture (wks) | 37.4 ± 0.78 | 37.2 ± 1.1 | N/A |
| Third trimester, pre-delivery BP | |||
| Systolic | 122 ± 3** | 148 ± 3* | 108 ± 3# |
| Diastolic | 69 ± 3 | 92 ± 2* | 69 ± 3# |
| Infant weight (grams) | 3538 ± 157 | 2658 ± 238* | 3551 ± 109# |
| Infant birth weight centile | 52 ± 11 | 31 ± 10 | 59 ± 7 |
| Infant sex (n, % female) | 4 (40%) | 6 (60%) | 6 (60%) |
| Postpartum interval (wks) | N/A | N/A | 53 (46-62) |
| Postpartum BP (1 year) | |||
| Systolic | N/A | N/A | 108 ± 2 |
| Diastolic | N/A | N/A | 69 ± 3 |
Continuous data are given as mean ±SEM if normally distributed or median (25th percentile, 75th percentile). Categorical data are given as number (percent). Groups were compared by either the Kruskal-Wallis or Holm-Sidak tests. BMI: Body mass index was prepregnancy or at study visit for nonpregnant subjects (approximately 1 year after the index uncomplicated pregnancy).
P≤0.003 Preeclampsia vs. Uncomplicated pregnancy,
P<0.003 Uncomplicated pregnancy vs Nonpregnant,
P<0.004 Preeclampsia vs. Nonpregnant.
N/A: Not applicable.
Plasma from women with PE increased myogenic tone
We exposed mouse mesenteric arteries to individual samples of whole plasma from 10 pregnant women with PE or to individual plasma samples from pregnant women with uncomplicated pregnancy. We then used pressurized arteriography to examine the myogenic response of the arteries. We found that exposure to individual plasma samples from pregnant women with PE increased the vessel tone, when compared to arteries exposed to plasma from women with uncomplicated pregnancies. Arteries exposed to plasma from women with PE had an average peak of 30% constriction, whereas arteries exposed to plasma from women with uncomplicated pregnancies had a peak constriction of approximately 6% (Fig. 1).
Figure 1: The myogenic response (% tone) of mesenteric arteries exposed to plasma.
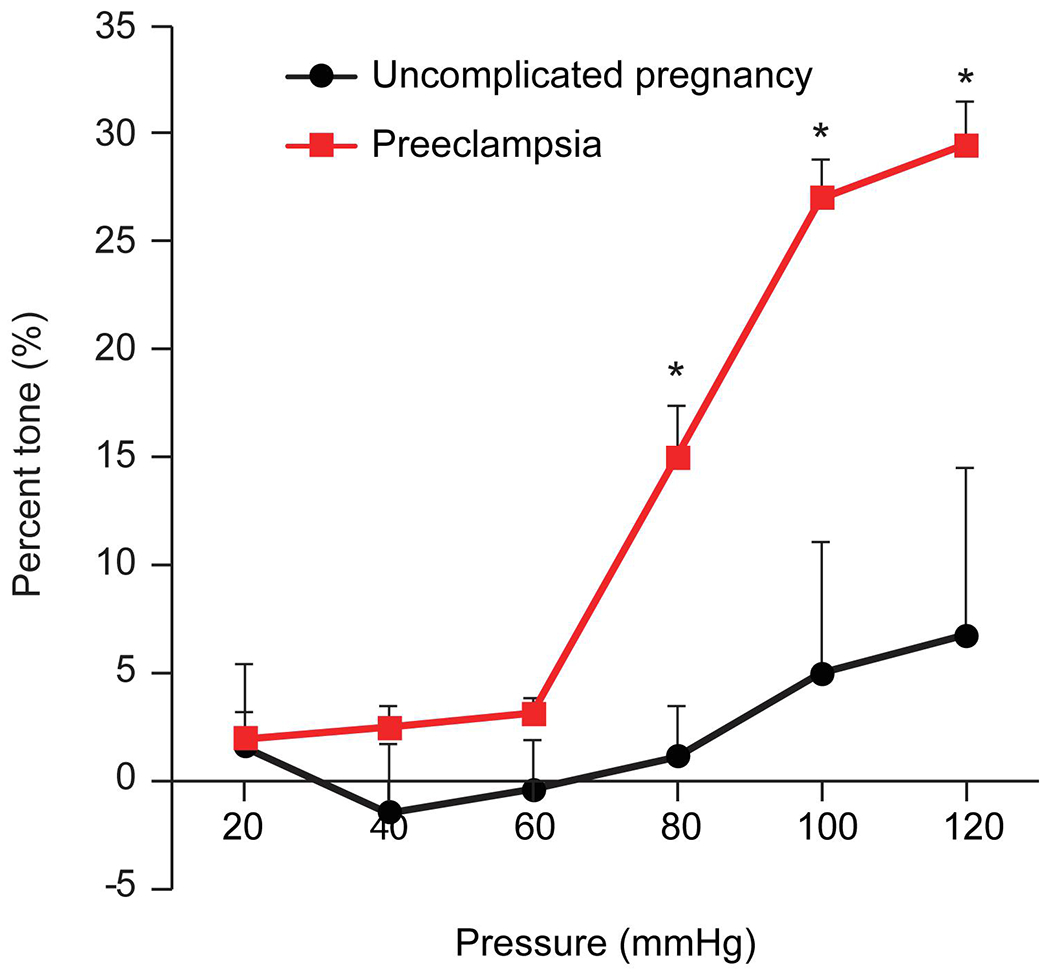
The figure shows the percent tone generated (Y-axis), calculated at each pressure as [(Di–Da)/Di]x100, where Da is the steady-state arterial diameter achieved after each 20-mm Hg increase in pressure (x-axis) and Di is the passive arterial diameter measured at the same pressure steps when the smooth muscle is inactivated (detailed in Materials and Methods). The data are presented as mean ± SEM, n=10 plasma samples from individual participants. *denotes p<0.0001 for PE vs healthy pregnancy (control), calculated by 2-way repeated measures ANOVA with post hoc Tukey Test for pairwise comparisons.
Arterial tone is increased when exposed to sEVs from plasma of women with PE
To investigate whether isolated sEVs reproduce the effect of plasma on vessel tone, we isolated sEVs from pooled plasma of pregnant women with PE and pooled plasma from uncomplicated pregnant women or healthy women at 1 year postpartum. We found that the application of sEVs from women with PE led to an approximate 15% increase in arterial tone at the 100- and 120- mmHg pressure steps when compared to sEVs from women with uncomplicated pregnancies (Fig. 2). Interestingly, sEVs from non-pregnant women led to increased tone in a manner near that of sEVs from women with PE, suggesting a vessel tone-relaxing effect of sEVs during healthy pregnancies.
Figure 2: The myogenic response (% tone) of mesenteric arteries exposed to sEVs.
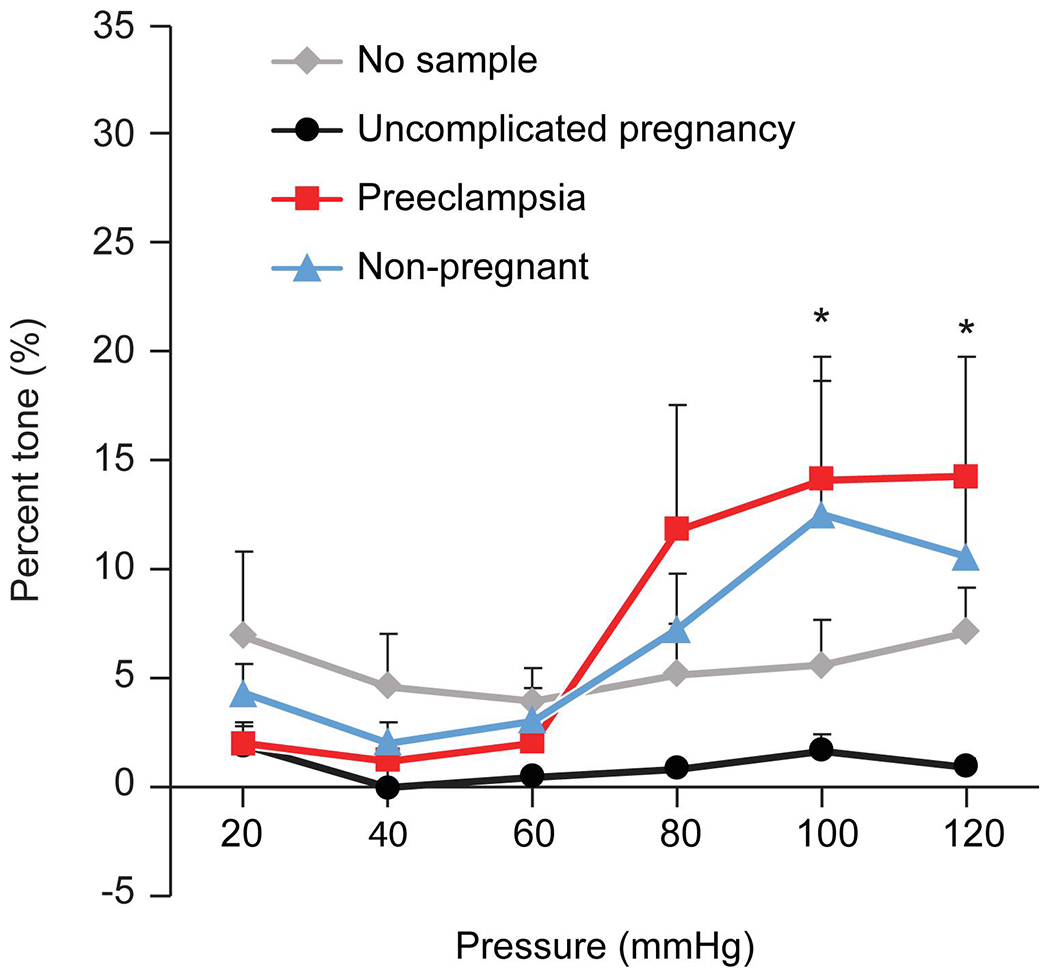
The figure shows the percent tone (Y-axis), calculated as shown in Fig. 1 and detailed in Materials and Methods. The data are presented as mean ± SEM, n=4 replicates using sEVs from pooled plasma samples (n=10). *denotes p<0.05 for PE vs healthy pregnancy (control) or no sEVs, calculated by 2-way repeated measures ANOVA with post hoc Tukey Test for pairwise comparisons.
The effect of EV-depleted plasma or isolated MVs on vessel tone
Using plasma from women with PE or from women with uncomplicated pregnancies, we depleted all EVs as described in Materials and Methods and tested the effect of these EV-depleted plasma samples on vessel tone. Arteries exposed to EV-depleted plasma from women with PE exhibited no significant increase in tone (Fig. 3A). Interestingly, EV-depleted plasma from healthy pregnancies exhibited a 15% increase in arterial tone, supporting the notion that sEVs may contribute to the myogenic tone-reducing effect of normal-pregnancy plasma. To assess whether the effect of sEVs can be recapitulated using MVs, we isolated MVs from women with PE and from plasma from healthy pregnant women. Exposure of the isolated mesenteric arteries to MVs obtained from the plasma of women with PE or from those with uncomplicated pregnancies did not significantly affect myogenic tone compared to vehicle (no treatment, Fig. 3B), suggesting the observed effect is unique to sEVs.
Figure 3: The myogenic responses (% tone) of mesenteric arteries exposed to plasma that was depleted of all EVs, or to MVs.
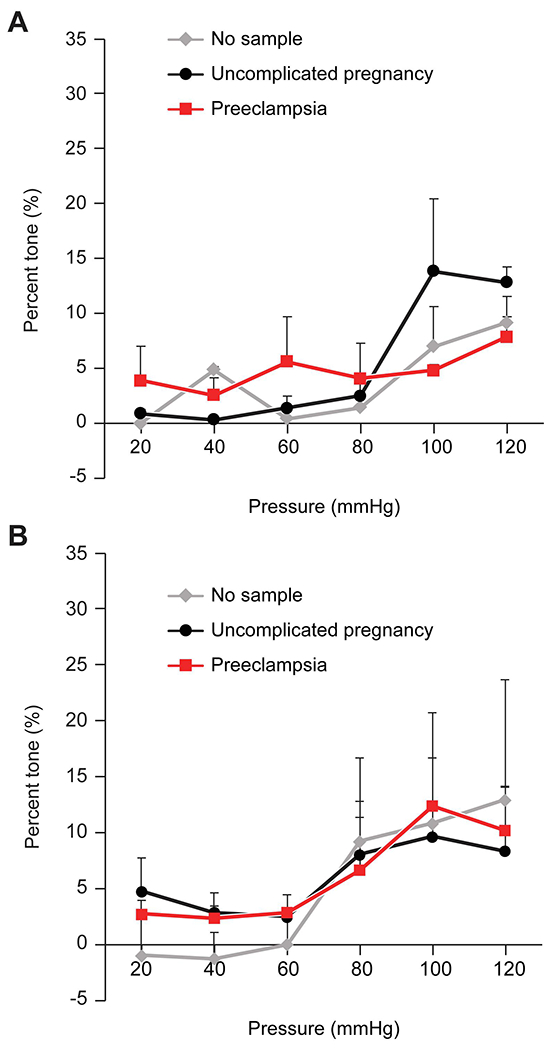
The figure shows the percent tone (Y-axis), calculated as detailed in Materials and Methods and in Fig 1. (A) plasma that was depleted of EVs. (B) Exposure to MVs. The data are presented as mean ± SEM, n=4. None of the differences were significant, calculated by 2-way repeated measures ANOVA.
The effect of sEVs on endothelium-mediated vessel relaxation
To provide additional support for our observations, we assessed the effect sEVs on arterial endothelium-dependent relaxation response. In these experiments, the vessels were initially constricted to 50% of their initial diameter at 60 mmHg using the alpha-adrenergic agonist phenylephrine (0.1-10 μM). We exposed the vessels to increasing concentrations of methacholine (0.001-1.0 μM) in the absence or presence of sEVs from women with PE vs sEVs from women with uncomplicated pregnancies. We found that exposure to sEVs from women with PE led to an impairment of methacholine-induced relaxation, with a more complete relaxation response when exposed to sEVs from uncomplicated pregnancies (Fig. 4). These data suggest that sEVs from women with PE blunt the relaxation capacity and increase the myogenic tone generated in response to pressure stimuli.
Figure 4: Endothelium-dependent methacholine-induced relaxation response (% relaxation of preconstriction) of mesenteric arteries exposed to sEVs.
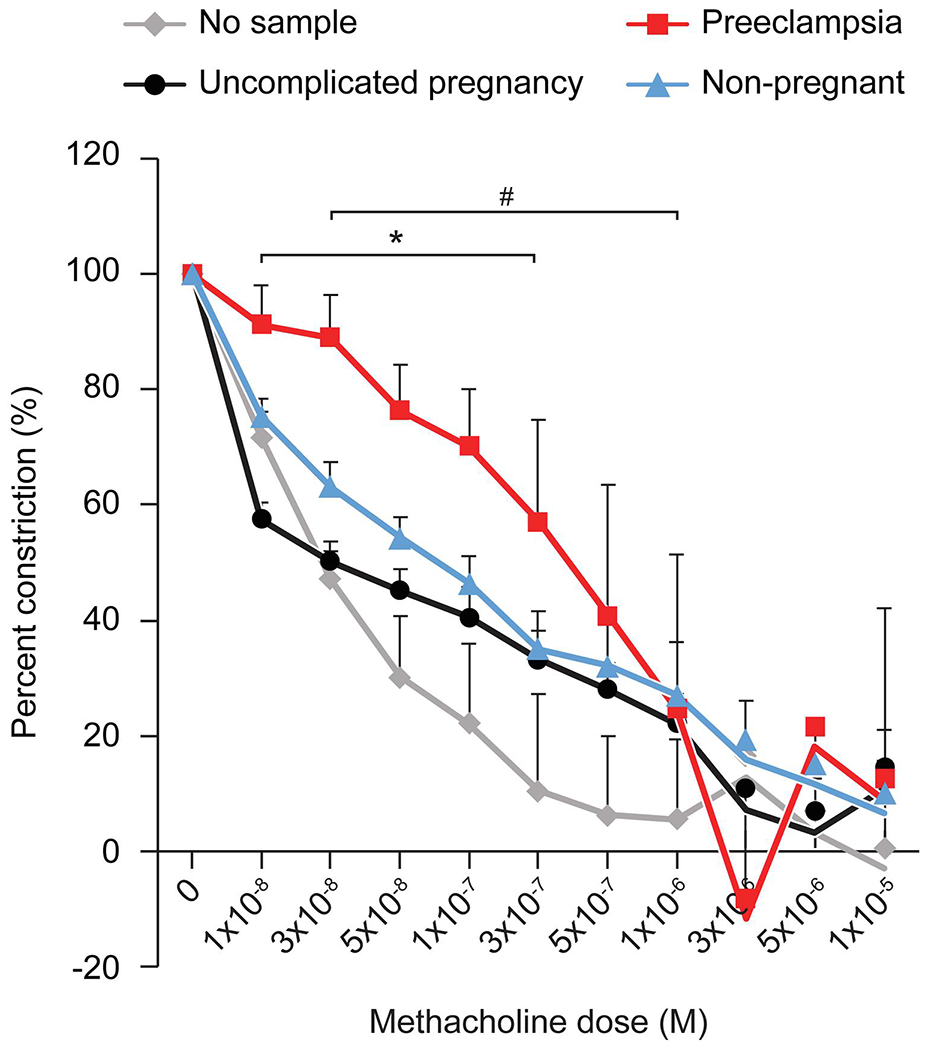
See text for details. The figure shows the percent preconstriction remaining after consecutive doses of methacholine (Y-axis), calculated as detailed in Materials and Methods. The data are presented as mean ± SEM, n=4. *denotes p<0.05 for PE vs healthy pregnancy (control), and # denotes p<0.05 for PE vs no EVs, calculated by 2-way repeated measures ANOVA with post hoc Tukey Test for pairwise comparisons.
Labeled sEVs localize to the endothelial cell layer of mouse mesenteric artery
To test whether trophoblast-derived sEVs interact with endothelial cells, we perfused mouse mesenteric arteries with buffer solution containing sEVs isolated from the culture supernatant of the human trophoblast cell line BeWo and labeled red with the CM-DiI dye. As shown in Fig. 5, we found that perfused sEVs localized to CD31+ endothelial cells. These results were consistent when arteries were exposed to sEVs isolated from human plasma and labeled with CM-DiI (supplemental Figure 1). These data, along with the effect of sEVs on endothelial cell relaxation, suggest that sEVs can be trafficked to endothelial cells, and this may be a mechanism by which these particles affect mesenteric arterial tone.
Figure 5: Localization of sEVs in mesenteric artery endothelial cells.
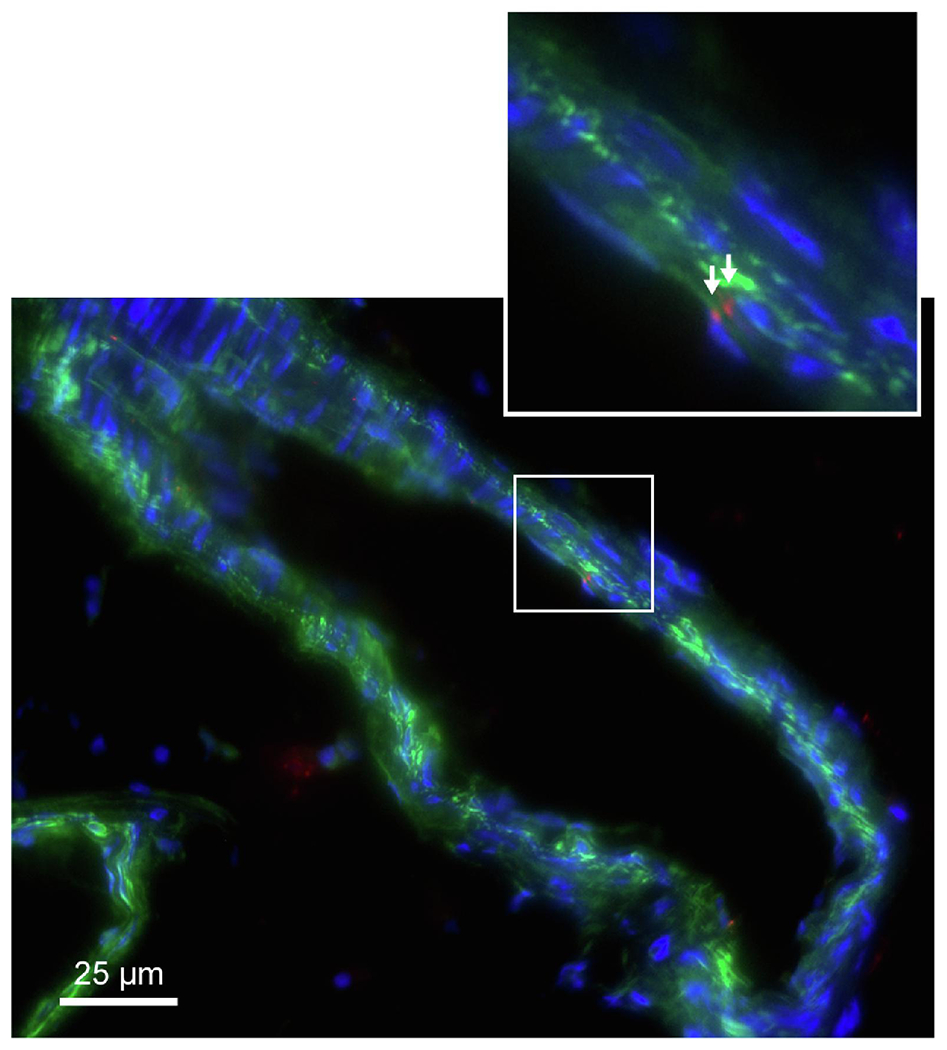
CM-DiI-labeled sEVs (red) are shown in CD31-labeled endothelium (green) of mesenteric artery from a 12-week-old female virgin C57BI6J mouse. sEVS were derived from BeWo trophoblast line. Cell nuclei are stained blue with DAPI (n=4).
DISCUSSION
Using an ex vivo pressurized arteriography system, we found that exposing arteries to whole plasma from women with PE led to an increase in arterial vessel myogenic (pressure step-induced) tone, compared to arteries exposed to whole plasma from women with uncomplicated pregnancy, as previously shown [44]. We obtained similar results when we exposed mouse mesenteric arteries to sEVs isolated from the plasma of women with PE compared to sEVs from plasma of women with uncomplicated pregnancies. These data suggest sEVs from women with PE contribute to the vasoconstrictive effect and, thus, high blood pressure that is characteristic of PE. Supporting this notion, the vascular contractile effects of PE sEVs was lessened when we exposed vessels to EV-depleted plasma from women with PE, suggesting that sEVs are involved in the vasoconstrictive effect of plasma from women with PE. Interestingly, these vascular effects were specific to sEVs and not recapitulated in experiments with the larger MVs alone. This may be related to differences in the ability of sEVs to enter and interact with vascular endothelial cells [45, 46] or could also be related to variation in EV content. Possible differences in cargo molecules may involve RNA, including miRNA, DNA, proteins, lipids, or pro-inflammatory and anti-inflammatory cytokines [21, 23, 43, 47–58]. Notably, our data suggest that non-EV mediated plasma signals contribute to the arterial vessel myogenic tone in our system.
Among our control samples we used sEVs that were purified from the plasma of non-pregnant women one-year postpartum. Interestingly, these sEVs also caused some increase in arterial tone, although the differences were statistically insignificant compared to sEVs from women with uncomplicated pregnancies. Such differences might point to a vasodilatory component in sEVs from the plasma of healthy pregnant women, and possibly the lack of a vasodilatory factor in sEVs from women with PE. In future studies it might be intriguing to examine the myogenic effects of sEVs from non-pregnant women, with and without hypertension, to investigate the role of sEVs in this condition [59, 60].
Previous data have shown that myometrial arteries from women with normotensive pregnancies exhibit impaired endothelium-dependent relaxation when incubated with plasma from women with PE [61–63]. Our vessel relaxation data and the immunofluorescence analysis of the arterial wall suggest that short-term exposure to plasma-derived sEVs can act directly on the arterial endothelial cell layer, leading to impaired endothelium-mediated relaxation in response to sEVs from women with preeclampsia. We recently showed that human trophoblastic sEVs enter target cells (endothelial cells, fibroblasts) through discrete endocytic pathways and are processed through early and late endosomes and lysosomes [46]. Previous studies have also shown that placental EVs containing miRNAs are taken up into arterial endothelial cells in vitro [46, 64]. Future experiments may allow us to detect the origin of the vasoactive circulating sEVs during pregnancy, and possibly inhibit their target cell entry, which may have therapeutic implications. Our data may also support the use of sEVs designed to carry synthetic cargo for the treatment of hypertension. Additional research into sEV cargo during disorders of pregnancy, including PE, is needed to assess this potential application.
There were several limitations to our study. First, although we observed a significant increase in arterial tone stimulated by sEVs from women with PE, these sEVs were isolated from whole plasma, and thus, their origin is not known and may include placental trophoblasts, platelets, diverse endothelial beds, and other cellular sources. Second, the sEV cargo or surface molecules contributing to these effects remain to be identified. Third, using a cross-species system (human sEVs and mouse blood vessels) may not capture the full spectrum of the phenomenon analyzed, even though an immune-based response is unlikely because of the short incubation time (2 h). In addition, the ex vivo artery system from non-pregnant mice, may not recapitulate the physiology of these vessels during pregnancy and may not resemble the response of human arterioles in vivo. These notions highlight the need for additional investigations into the function sEVs in uncomplicated human pregnancies or pregnancies complicated by PE.
Supplementary Material
Highlights.
Maternal plasma small extracellular vesicles (sEVs) may affect arterial vessel tone
Third trimester sEVs from preeclamptic women’s plasma constrict vessels ex vivo
Microvesicles or sEV-depleted plasma do not exhibit this effect on vessel tone
sEVs from preeclamptic women’s plasma attenuate methacholine-stimulated relaxation
Immunofluorescent sEVs localize ex vivo to arterial vessel walls
ACKNOWLEDGEMENTS
We thank Marcia Gallaher and William J. Shufesky for technical assistance, Lori Rideout for assistance in manuscript preparation, and Bruce Campbell for editing. Data and/or materials were obtained with assistance from the Preeclampsia Prevention: Mechanisms of Preeclampsia and the Impact of Obesity (PEPP3) project Clinical Data Core, headed by Arun Jeyabalan, MD.
FUNDING
The project was supported by Eunice Kennedy Shriver NICHD grant R37HD086916 (to Y.S.), NIAID grant R01AI148690 (to A.M. and Y.S.), Eunice Kennedy Shriver NICHD grant P01HD030367 (to C.A.H., R.W.P., and R.E.G.), Pennsylvania Department of Health Formula Research Funds (to C.A.H., R.W.P., and R.E.G,), American Heart Association Go Red for Women Strategically Focused Research Network grant 16SFRN28340000 (to C.A.H., R.W.P., and R.E.G.), and the Margaret Ritchie R. Battle Family Charitable Fund (to C.A.H. and Y.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
Yoel Sadovsky is a consultant at Illumina, Inc.
REFERENCES
- [1].Burton GJ, Redman CW, Roberts JM, Moffett A, Pre-eclampsia: pathophysiology and clinical implications, BMJ 366 (2019) l2381. 10.1136/bmj.12381. [DOI] [PubMed] [Google Scholar]
- [2].Sutton ALM, Harper LM, Tita ATN, Hypertensive disorders in pregnancy, Obstet. Gynecol. Clin. North Am 45(2) (2018) 333–347. 10.1016/j.ogc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- [3].Chappell LC, Cluver CA, Kingdom J, Tong S, Pre-eclampsia, Lancet 398(10297) (2021) 341–354. 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- [4].Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, Obstet. Gynecol 122(5) (2013) 1122–1131. 10.1097/01.A0G.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- [5].Staff AC, The two-stage placental model of preeclampsia: An update, J. Reprod. Immunol 134-135 (2019) 1–10. 10.1016/i.iri.2019.07.004. [DOI] [PubMed] [Google Scholar]
- [6].Myatt L, Roberts JM, Preeclampsia: Syndrome or disease?, Curr. Hypertens. Rep 17(11) (2015) 83. 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- [7].Fisher SJ, Why is placentation abnormal in preeclampsia?, Am. J. Obstet. Gynecol 213(4 Suppl) (2015) S115–22. 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karumanchi SA, Granger JP, Preeclampsia and pregnancy-related hypertensive disorders, Hypertension 67(2) (2016) 238–42. 10.1161/HYPERTENSIONAHA.115.05024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rana S, Lemoine E, Granger JP, Karumanchi SA, Preeclampsia: Pathophysiology, challenges, and perspectives, Circ. Res 124(7) (2019) 1094–1112. 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- [10].Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, Reassessment of exosome composition, Cell 177(2) (2019) 428–445 e18. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat. Cell Biol 20(3) (2018) 332–343. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Thery C, Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9, Nat Commun 12(1) (2021) 4389. 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wortzel I, Dror S, Kenific CM, Lyden D, Exosome-mediated metastasis: Communication from a distance, Dev. Cell 49(3) (2019) 347–360. 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- [14].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature 527(7578) (2015) 329–35. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang ZG, Chopp M, Exosomes in stroke pathogenesis and therapy, J. Clin. Invest 126(4) (2016) 1190–7. 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hill AF, Extracellular vesicles and neurodegenerative diseases, J. Neurosci 39(47) (2019) 9269–9273. 10.1523/JNEUROSCI.0147-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M, Yamawaki H, Plasma exosomes regulate systemic blood pressure in rats, Biochem. Biophys. Res. Commun 503(2) (2018) 776–783. 10.1016/j.bbrc.2018.06.075. [DOI] [PubMed] [Google Scholar]
- [18].Tong M, Stanley JL, Chen Q, James JL, Stone PR, Chamley LW, Placental nano-vesicles target to specific organs and modulate vascular tone in vivo, Hum. Reprod 32(11) (2017) 2188–2198. 10.1093/humrep/dex310. [DOI] [PubMed] [Google Scholar]
- [19].Pillay P, Maharaj N, Moodley J, Mackraj I, Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies, Placenta 46 (2016) 18–25. 10.1016/j.placenta.2016.08.078. [DOI] [PubMed] [Google Scholar]
- [20].Gao X, Shao L, Ge X, Zhang L, Chen D, He R, The potential role of serum exosomes in preeclampsia, Curr Drug Metab 21(5) (2020) 352–356. 10.2174/1389200221666200525152441. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Ouyang Y, Sadovsky E, Parks WT, Chu T, Sadovsky Y, Unique microRNA signals in plasma exosomes from pregnancies complicated by preeclampsia, Hypertension 75(3) (2020) 762–771. 10.1161/HYPERTENSIONAHA.119.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Biro O, Fothi A, Alasztics B, Nagy B, Orban TI, Rigo J Jr., Circulating exosomal and Argonaute-bound microRNAs in preeclampsia, Gene 692 (2019) 138–144. 10.1016/j.gene.2019.01.012. [DOI] [PubMed] [Google Scholar]
- [23].Wu M, Zhao Y, Li L, Wang G, Xing L, Exosomal microRNA302a promotes trophoblast migration and proliferation, and represses angiogenesis by regulating the expression levels of VEGFA in preeclampsia, Mol Med Rep 24(6) (2021). 10.3892/mmr.2021.12504. [DOI] [PubMed] [Google Scholar]
- [24].Wang Z, Zhao G, Zeng M, Feng W, Liu J, Overview of extracellular vesicles in the pathogenesis of preeclampsiadagger, Biol. Reprod 105(1) (2021) 32–39. 10.1093/biolre/ioab060. [DOI] [PubMed] [Google Scholar]
- [25].Gilani SI, Weissgerber TL, Garovic VD, Jayachandran M, Preeclampsia and extracellular vesicles, Curr. Hypertens. Rep 18(9) (2016) 68. 10.1007/s11906-016-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C, Placental exosomes in normal and complicated pregnancy, Am. J. Obstet. Gynecol 213(4 Suppl) (2015) S173–81. 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- [27].Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y, Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity, Placenta 47 (2016) 86–95. 10.1016/j.placenta.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB, Human placental trophoblasts confer viral resistance to recipient cells, Proc. Natl. Acad. Sci. U. S. A 110(29) (2013) 12048–53. 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ouyang Y, Mouillet JF, Sorkin A, Sadovsky Y, Trophoblastic extracellular vesicles and viruses: Friends or foes?, Am. J. Reprod. Immunol 85(2) (2021) e13345. 10.1111/aji.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sadovsky Y, Ouyang Y, Powell JS, Li H, Mouillet JF, Morelli AE, Sorkin A, Margolis L, Placental small extracellular vesicles: Current questions and investigative opportunities, Placenta 102 (2020) 34–38. 10.1016/j.placenta.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Czernek L, Duchler M, Exosomes as messengers between mother and fetus in pregnancy, Int. J. Mol. Sci 21(12) (2020). 10.3390/ijms21124264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F, Extracellular vesicles in human reproduction in health and disease, Endocr. Rev 39(3) (2018) 292–332. 10.1210/er.2017-00229. [DOI] [PubMed] [Google Scholar]
- [33].Hashimoto A, Sugiura K, Hoshino A, Impact of exosome-mediated feto-maternal interactions on pregnancy maintenance and development of obstetric complications, J. Biochem 169(2) (2021) 163–171. 10.1093/jb/mvaa137. [DOI] [PubMed] [Google Scholar]
- [34].Salomon C, Rice GE, Role of Exosomes in placental homeostasis and pregnancy disorders, Prog. Mol. Biol. Transl. Sci 145 (2017) 163–179. 10.1016/bs.pmbts.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [35].Tannetta D, Collett G, Vatish M, Redman C, Sargent I, Syncytiotrophoblast extracellular vesicles - Circulating biopsies reflecting placental health, Placenta 52 (2017) 134–138. 10.1016/j.placenta.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sween LK, Althouse AD, Roberts JM, Early-pregnancy percent body fat in relation to preeclampsia risk in obese women, Am. J. Obstet. Gynecol 212(1) (2015) 84 e1–7. 10.1016/j.ajog.2014.07.055. [DOI] [PubMed] [Google Scholar]
- [37].American College of Obstetrics and Gynecology (ACOG), ACOG Practice Bulletin No. 33: Diagnosis and Management of Preeclampsia and Eclampsia, Obstet. Gynecol 99(1) (2002) 159–167. [DOI] [PubMed] [Google Scholar]
- [38].Crandall ME, Keve TM, McLaughlin MK, Characterization of norepinephrine sensitivity in the maternal splanchnic circulation during pregnancy, Am. J. Obstet. Gynecol 162(5) (1990) 1296–301. 10.1016/0002-9378(90)90040-e. [DOI] [PubMed] [Google Scholar]
- [39].Halpern W, Osol G, Coy GS, Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system, Ann. Biomed. Eng 12(5) (1984) 463–79. 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- [40].Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, McLaughlin MK, Pitt BR, Levitan ES, Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein, Proc. Natl. Acad. Sci. U. S. A 97(1) (2000) 477–82. 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gandley RE, Conrad KP, McLaughlin MK, Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats, Am. J. Physiol. Regul. Integr. Comp. Physiol 280(1) (2001) R1–7. 10.1152/ajpregu.2001.280.1.R1. [DOI] [PubMed] [Google Scholar]
- [42].MacPherson RD, McLeod LJ, Rasiah RL, Myogenic response of isolated pressurized rabbit ear artery is independent of endothelium, Am. J. Physiol 260(3 Pt 2) (1991) H779–84. 10.1152/ajpheart.1991.260.3.H779. [DOI] [PubMed] [Google Scholar]
- [43].Chang X, Yao J, He Q, Liu M, Duan T, Wang K, Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (soluble fms-like tyrosine kinase)-1 and sEng (soluble endoglin) to endothelial cells, Hypertension 72(6) (2018) 1381–1390. 10.1161/HYPERTENSIONAHA.118.11706. [DOI] [PubMed] [Google Scholar]
- [44].Ashworth JR, Warren AY, Johnson IR, Baker PN, Plasma from pre-eclamptic women and functional change in myometrial resistance arteries, Br. J. Obstet. Gynaecol 105(4) (1998) 459–61. 10.1111/j.1471-0528.1998.tb10134.x. [DOI] [PubMed] [Google Scholar]
- [45].Baruah J, Wary KK, Exosomes in the regulation of vascular endothelial cell regeneration, Front Cell Dev Biol 7 (2019) 353. 10.3389/fcell.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li H, Pinilla-Macua I, Ouyang Y, Sadovsky E, Kajiwara K, Sorkin A, Sadovsky Y, Internalization of trophoblastic small extracellular vesicles and detection of their miRNA cargo in P-bodies, J Extracell Vesicles 9(1) (2020) 1812261. 10.1080/20013078.2020.1812261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang Y, Du X, Wang J, Transfer of miR-15a-5p by placental exosomes promotes pre-eclampsia progression by regulating PI3K/AKT signaling pathway via CDK1, Mol. Immunol 128 (2020) 277–286. 10.1016/j.molimm.2020.10.019. [DOI] [PubMed] [Google Scholar]
- [48].Konecna B, Tothova L, Repiska G, Exosomes-associated DNA—New marker in pregnancy complications?, Int. J. Mol. Sci 20(12) (2019). 10.3390/ijms20122890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tong M, Abrahams VM, Chamley LW, Immunological effects of placental extracellular vesicles, Immunol. Cell Biol (2018). 10.1111/imcb.12049. [DOI] [PubMed] [Google Scholar]
- [50].Ayala-Ramirez P, Machuca-Acevedo C, Gamez T, Quijano S, Barreto A, Silva JL, Olaya CM, Garcia-Robles R, Assessment of placental extracellular vesicles-associated Fas Ligand and TNF-related apoptosis-inducing ligand in pregnancies complicated by early and late onset preeclampsia, Front. Physiol 12 (2021) 708824. 10.3389/fphys.2021.708824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gong RQ, Nuh AM, Cao HS, Ma M, Roles of exosomes-derived lncRNAs in preeclampsia, Eur. J. Obstet. Gynecol. Reprod. Biol 263 (2021) 132–138. 10.1016/j.ejogrb.2021.06.015. [DOI] [PubMed] [Google Scholar]
- [52].Pillay P, Moodley K, Vatish M, Moodley J, Duarte R, Mackraj I, Exosomal Th1/Th2 cytokines in preeclampsia and HIV-positive preeclamptic women on highly active anti-retroviral therapy, Cytokine 125 (2020) 154795. 10.1016/j.cyto.2019.154795. [DOI] [PubMed] [Google Scholar]
- [53].Ellis R, Katerelos M, Choy SW, Cook N, Lee M, Paizis K, Pell G, Walker S, Power DA, Mount PF, Increased expression and phosphorylation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoforms in urinary exosomes in pre-eclampsia, J. Transl. Med 17(1) (2019) 60. 10.1186/s12967-019-1806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hu CC, Katerelos M, Choy SW, Crossthwaite A, Walker SP, Pell G, Lee M, Cook N, Mount PF, Paizis K, Power DA, Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles, PLoS One 13(9) (2018) e0204514. 10.1371/journal.pone.0204514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Biro O, Alasztics B, Molvarec A, Joo J, Nagy B, Rigo J Jr., Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension, Pregnancy Hypertens. 10 (2017) 207–212. 10.1016/j.preghy.2017.09.002. [DOI] [PubMed] [Google Scholar]
- [56].Tong M, Chen Q, James JL, Stone PR, Chamley LW, Micro- and nano-vesicles from first trimester human placentae carry Flt-1 and levels are increased in severe preeclampsia, Front. Endocrinol. (Lausanne) 8 (2017) 174. 10.3389/fendo.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Motta-Mejia C, Kandzija N, Zhang W, Mhlomi V, Cerdeira AS, Burdujan A, Tannetta D, Dragovic R, Sargent IL, Redman CW, Kishore U, Vatish M, Placental vesicles carry active endothelial nitric oxide synthase and their activity is reduced in preeclampsia, Hypertension 70(2) (2017) 372–381. 10.1161/HYPERTENSIONAHA.117.09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, Truong G, Duncombe G, Rice G, Salomon C, Role of extracellular vesicles and microRNAs on dysfunctional angiogenesis during preeclamptic pregnancies, Front. Physiol 7 (2016) 98. 10.3389/fphys.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tan PPS, Hall D, Chilian WM, Chia YC, Mohd Zain S, Lim HM, Kumar DN, Ching SM, Low TY, Md Noh MF, Pung YF, Exosomal microRNAs in the development of essential hypertension and its potential as biomarkers, Am. J. Physiol. Heart Circ. Physiol 320(4) (2021) H1486–H1497. 10.1152/aipheart.00888.2020. [DOI] [PubMed] [Google Scholar]
- [60].Perez-Hernandez J, Riffo-Campos AL, Ortega A, Martinez-Arroyo O, Perez-Gil D, Olivares D, Solaz E, Martinez F, Martinez-Hervas S, Chaves FJ, Redon J, Cortes R, Urinary- and plasma-derived exosomes reveal a distinct microRNA signature associated with albuminuria in hypertension, Hypertension 77(3) (2021) 960–971. 10.1161/HYPERTENSIONAHA.120.16598. [DOI] [PubMed] [Google Scholar]
- [61].Myers J, Mires G, Macleod M, Baker P, In preeclampsia, the circulating factors capable of altering in vitro endothelial function precede clinical disease, Hypertension 45(2) (2005) 258–63. 10.1161/01.HYP.0000153461.58298.a4. [DOI] [PubMed] [Google Scholar]
- [62].Hayman R, Warren A, Brockelsby J, Johnson I, Baker P, Plasma from women with pre-eclampsia induces an in vitro alteration in the endothelium-dependent behaviour of myometrial resistance arteries, BJOG 107(1) (2000) 108–15. 10.1111/j.1471-0528.2000.tb11586.x. [DOI] [PubMed] [Google Scholar]
- [63].Hayman R, Warren A, Johnson I, Baker P, The preliminary characterization of a vasoactive circulating factor(s) in preeclampsia, Am. J. Obstet. Gynecol 184(6) (2001) 1196–203. 10.1067/mob.2001.113130. [DOI] [PubMed] [Google Scholar]
- [64].Cronqvist T, Tannetta D, Morgelin M, Belting M, Sargent I, Familari M, Hansson SR, Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells, Sci. Rep 7(1) (2017) 4558. 10.1038/s41598-017-04468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


