Abstract
Background:
Cocoa extract and multivitamins have been proposed to reduce the risk of cardiovascular disease (CVD) and cancer, respectively. However, few randomized clinical trials have tested their long-term effects on these outcomes.
Methods:
The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) is a randomized, double-blind, placebo-controlled, 2×2 factorial trial of a cocoa extract supplement and a multivitamin supplement to reduce the risk of CVD and cancer. Here we describe the pragmatic, hybrid design of the trial and baseline characteristics of the trial participants.
Results:
The nationwide study population includes 21,442 U.S. women aged ≥65 years and men aged ≥60 years without baseline myocardial infarction (MI), stroke, or a recent (within the past 2 years) cancer diagnosis. Participants were randomized in a 2×2 factorial design to one of four groups: (1) cocoa extract (containing 500 mg/d flavanols, including 80 mg (−)-epicatechins) and a multivitamin (Centrum Silver©); (2) cocoa extract and multivitamin placebo; (3) multivitamin and cocoa extract placebo; or (4) both placebos. Randomization successfully distributed baseline demographic, clinical, behavioral, and dietary characteristics across treatment groups. Baseline biospecimens were collected from 6867 participants, with at least one follow-up biospecimen from 2142 participants. The primary outcome for the cocoa extract intervention is total CVD (a composite of MI, stroke, cardiovascular mortality, coronary revascularization, unstable angina requiring hospitalization, carotid artery surgery, and peripheral artery surgery); the primary outcome for the multivitamin intervention is total invasive cancer.
Conclusion:
COSMOS will provide important information on the health effects of cocoa extract and multivitamin supplementation in older U.S. adults.
Introduction
Cocoa is made from the bean of the cacao tree, Theobroma cacao, and has a long history of medicinal use1 and potential health benefits based upon its flavanol and procyanidin content,2 along with other flavanol-containing foods and beverages such as grapes, wine, tea, and others. Several smaller-scale, shorter-term clinical dietary intervention studies have investigated various flavanol-containing foods, beverages, and purified flavanols and procyanidins in the context of vascular function and cardiovascular health.3–5 Within this body of literature, studies based on cocoa and cocoa products are prominent and often use chemically and nutritionally well-characterized test materials that have been consistently linked with cardiovascular6,7 and cardiometabolic8 health with beneficial effects on endothelium-dependent vasodilation,9–12 blood pressure (BP),9,13–15 inflammation,16,17 and platelet activation.18,19 These data have also provided important insights into the absorption, distribution, metabolism, and excretion of flavanols in humans20–22 and the potential vascular effects due to intake of the flavanol (−)-epicatechin.12,23 However, there have been no large-scale trials testing cocoa extract for prevention of clinical cardiovascular disease (CVD) events.
Multivitamins, which typically contain a broad range of essential vitamins and minerals, are the most common dietary supplement taken by at least one-third of US adults24,25 and an even larger percentage of older adults.26,27 Studies have reported that adults take multivitamins to promote general health and well-being28 or to reduce chronic diseases.29 Yet findings from longitudinal studies of long-term multivitamin use and total and site-specific cancer30–36 and CVD33,37–40 are inconsistent. In the Physicians’ Health Study (PHS) II, a randomized trial testing a multivitamin in 14,641 men aged ≥50 years, there was a modest but statistically significant 8% reduction in total incident cancer41 and no effect on CVD.42 Notably, among 1,312 men with a baseline history of cancer in PHS II, a daily multivitamin significantly reduced cancer by 27%. With no corresponding randomized trial data in women, a confirmatory large-scale randomized trial was warranted given the potential clinical and public health impact of any benefits associated with multivitamin use.
We therefore initiated the COcoa Supplement and Multivitamin Outcomes Study (COSMOS), a pragmatic, large-scale, 2×2 factorial randomized trial testing a cocoa extract supplement and a standard multivitamin in the prevention of CVD and cancer events among older women and men. COSMOS also represents an important collaboration among academic, government, and industry partners as a cost-effective clinical trial that leverages existing cohort infrastructure including that of the Women’s Health Initiative (WHI) and the VITamin D and OmegA-3 TriaL (VITAL).43 We describe the overall methodology of COSMOS and present baseline characteristics of the COSMOS trial cohort.
Methods
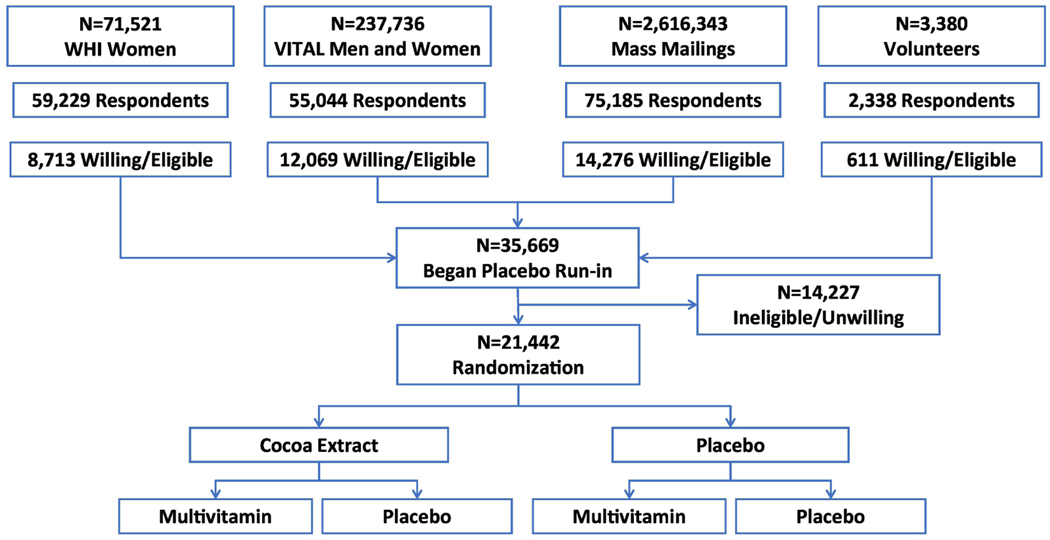
The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) [clinicaltrials.gov NCT02422745] is a randomized, double-blind, placebo-controlled, 2×2 factorial trial testing a cocoa extract supplement (containing 500 mg/d cocoa flavanols, including 80 mg (−)-epicatechins) and a multivitamin supplement (Centrum Silver®) to reduce the risk of cardiovascular disease (CVD) and cancer in 21,442 U.S. adults, including 12,666 women aged ≥65 years and 8,776 men aged ≥60 years who are free of self-reported myocardial infarction, stroke, and recently diagnosed cancer within the past 2 years. Figure 1 summarizes the overall recruitment and 2×2 factorial design for the COSMOS trial.
Figure 1.
Recruitment and 2×2 factorial design for the COcoa Supplement and Multivitamin Outcomes Study (COSMOS)
Integration of WHI and VITAL cohorts as part of recruitment
We enhanced recruitment for COSMOS by inviting potential participants with available data from existing cohorts (“embedded” recruitment). We initially mailed to 71,521 active participants from the Women’s Health Initiative (WHI) Extension Study44 and then to 237,736 participants (including 140,454 women and 97,282 men) contacted for, but not randomized into, the VITamin D and OmegA-3 TriaL (VITAL) at Brigham and Women’s Hospital (BWH).45 To ultimately reach our recruitment targets, BWH sent mass mailings with recruitment invitations using commercially available mailing tapes to 2,616,343 U.S. men and women plus 3,380 volunteers who heard about the study through multiple sources, including advertisements and word of mouth.
Additional Recruitment Efforts
The nationwide trial recruitment process for COSMOS began in June 2015 and was completed by March 2018. The overall process of recruitment and determining eligibility had 4 steps, including (1) a brief initial screening questionnaire completed by 191,796 participants; (2) for those expressing interest and having initial eligibility, a baseline questionnaire and informed consent were sent; (3) for those eligible, willing, and consenting, a placebo run-in of at least 2 months was begun by 35,669 participants; and (4) for all run-in participants, final compliance, eligibility, expanded baseline characteristics, and semi-quantitative food frequency questionnaires were completed.46 All CSMOS subjects read and remotely signed an informed consent. When deciding whether to sign the consent form, potential participants were given multiple opportunities to speak with study staff about the risks and benefits of study participation. All trial activities were overseen by the Human Subjects Committee at BWH/Mass General Brigham.
Eligibility criteria
Eligible participants did not report a history of MI or stroke and were not diagnosed with invasive cancer (except non-melanoma skin cancer) in the last 2 years. Participants had to agree to forgo supplements of cocoa extract and multivitamins during the trial (chocolate intake was not restricted), and limit vitamin D to ≤1000 IU/day and calcium to ≤1200 mg/day from all supplemental sources during the trial, and complete at least a 2-month placebo run-in phase in which they missed ≤8 days of pills per month (equivalent to taking ~75% of study pills). Safety exclusions included renal failure or dialysis or other serious conditions that would preclude participation. We also excluded participants with extreme sensitivity to caffeine given the modest theobromine (50 mg/day) and caffeine (15 mg/day) content of the cocoa extract supplement.
Interventions and calendar packs
Active cocoa extract and matching inert placebo was provided by Mars Edge. The cocoa extract intervention was selected based on extensive prior testing of similar products for quality, patient safety, and vascular and cardiometabolic outcomes in small mechanistic trials. Participants were asked to take two capsules per day. Upon initiation of the COSMOS trial, the cocoa flavanol content totaled 600 mg/day, including 80 mg/d (−)-epicatechin. After the COSMOS trial began, an advanced method to analyze cocoa flavanols was accredited by AOAC International as a First Action Official Method of Analysis.47 This updated method relied on a reference material (RM8403) recently standardized and made commercially available by the U.S. National Institute of Standards and Technology. Although the actual cocoa flavanol content of the COSMOS intervention remained unchanged throughout the trial, the application of this new analytical method led to expected changes in how the total cocoa flavanol content is now reported. Applying AOAC 2020.05/RM8403 to the COSMOS intervention, the total cocoa flavanol content of the COSMOS intervention is now determined to be 500 mg/day. Reporting of (−)-epicatechin content remained unaffected.
Active multivitamin (currently marketed as Centrum Silver®) and matching inert placebo was provided by Pfizer Inc (Pfizer Consumer Healthcare is now part of GSK Consumer Healthcare). We selected the multivitamin intervention for greater generalizability as a commonly used formulation, along with prior safety and consistency with that tested in PHS II, despite minor formulation differences (See Supplemental Table 1 for the composition of the cocoa extract supplement and Supplemental Table 2 for the composition of the multivitamin.) Participants took one active or placebo multivitamin tablet per day. Participants were provided with blinded calendar packs containing all study pills.
Run-in and randomization
A total of 35,669 potential participants who remained eligible and interested after the second mailing were asked to complete at least a 2-month single-blind placebo run-in to eliminate poor compliers prior to randomization, increase study power, and improve the internal validity of a trial.48 Eliminating non-compliers prior to randomization through the use of a placebo run-in mitigates the impact of noncompliance on exposure misclassification and the dilution of the intervention’s effects. In addition, by using an inert placebo during the run-in period, we avoided excluding non-compliant participants due to adverse intervention effects that can occur from giving active pills during the run-in phase.
We excluded 14,227 (39.9%) of 35,669 participants after the run-in due to noncompliance (defined as not taking study pills on >8 days per month during the run-in) or becoming unwilling or ineligible to participate in COSMOS. Among those randomized, median run-in was 4.6 (4.1–5.5) months. From April 2016 to March 2018, 21,442 participants meeting all eligibility requirements were randomized via a 2×2 factorial design to one of four treatment groups with equal probabilities: (1) active cocoa extract (containing 500 mg/d flavanols, including 80 mg (−)-epicatechins) and an active daily multivitamin (Centrum Silver®) (n=5,360); (2) active cocoa extract and multivitamin placebo (n=5,359); (3) active multivitamin and cocoa extract placebo (n=5,360); or (4) both placebos (n=5,363). Randomization was computer generated using a permuted block approach and stratified by sex (women, men), age (for women, 65-<70, 70-<75, 75-<80, 80-<85, and ≥85 years; for men, 60-<65, 65-<70, 70-<75, 75-<80, and ≥80 years), and recruitment source (WHI or BWH) in blocks of twelve. Double-blinded calendar packs containing their assigned pills were centrally mailed to all randomized COSMOS participants, with resupplies of their blinded calendar packs mailed as needed and typically coinciding with the receipt of follow-up questionnaires.
Follow-up
Approximately 6 and 12 months following randomization and semi-annually thereafter, COSMOS participants were sent follow-up questionnaires via REDCap or mail to assess compliance with randomized treatments, use of non-trial cocoa supplements and/or multivitamins, potential side effects of the interventions, updated medical history including study outcomes (confirmed by medical records, as described below), and other relevant lifestyle, clinical, and dietary risk factors. A second food frequency questionnaire was sent at 2 years follow-up. COSMOS participants from the WHI received separate annual mailings from the WHI to update medical history and outcome follow-up. The COSMOS randomized treatments continued through December 31, 2020, ending as scheduled, and with a median (IQR) treatment period of 3.6 (3.2 – 4.2) years.
Primary Outcomes
The primary outcome for the cocoa extract intervention when COSMOS was initiated was a composite total cardiovascular outcome including incident myocardial infarction (MI), stroke, coronary revascularization, and cardiovascular mortality. However, during the active COSMOS intervention period, we observed that the overall rates of CVD were less than originally projected due to (a) the impact of COVID-19 on 2020 reported CVD event rates; (b) WHI women comprising 21.5%, versus the original plan for 66.7%, of the COSMOS trial cohort, resulting in younger non-WHI women with lower CVD event rates; and (c) lower CVD rates due to increasing use of statins and other preventive treatments. As a result, in November 2020 we obtained approval from the COSMOS Data and Safety Monitoring Board (DSMB) to expand the primary total CVD outcome to add carotid artery surgery, peripheral artery surgery, and unstable angina requiring hospitalization, consistent with the atherosclerotic mechanisms underlying the intervention hypotheses, especially for the cocoa flavanols. This expansion of the total CVD outcome would provide us with a similar number of outcomes as used in our initial power calculations. However, this mix of cardiovascular outcomes may be less rigorous and biologically relevant than a stricter composite cardiovascular outcome limited to MI, stroke, and CVD death. For the multivitamin intervention, the primary outcome was invasive cancer, excluding non-melanoma skin cancer.
Sample Size and Power Calculations
Power calculations for the primary outcomes were initially based upon a 2×2 factorial trial in 18,000 participants, including 12,000 women aged ≥65 years and 6,000 men aged ≥60 years, and then were revised upward to 22,000 participants, including 13,000 women and 9,000 men, based on initial WHI and BWH recruitment patterns. Each primary outcome comparison (cocoa extract versus placebo and multivitamins versus placebo) was based on an independent, marginal test at α=0.05. Estimates of study power using the log-rank tables by Freedman49 and the expected numbers of confirmed events were based on the following assumptions: (1) independent and equal allocation of participants to each treatment; (2) an age distribution based on that observed among WHI women ≥65 years and men aged ≥60 years responding to the VITAL mailings; (3) annualized age-specific event rates based on observed rates during WHI and VITAL follow-up, adjusted for aging; (4) equivalent event rates for men and women given that women are approximately 5 years older than men; (5) expected mean trial follow-up of 4 years, with minimal loss to follow-up as seen previously in WHI and VITAL; and (6) an average of 80% compliance with study pills. These assumptions remained identical for our power calculations for 18,000, then 22,000, participants. For 22,000 randomized participants, we had at least 80% power to detect an 11% relative hazard reduction in total CVD with the expected number of events. Similarly, there would be at least 90% power to detect a 14% reduction in total cancer.
Secondary outcomes
For the cocoa extract intervention, secondary outcomes included the original composite CVD outcome of MI, stroke, cardiovascular mortality and coronary revascularization; a combined outcome of CVD events and all-cause mortality; individual cardiovascular events comprising the expanded composite outcome; invasive cancer (excluding non-melanoma skin cancer); and key site-specific cancers, including breast, colorectal, and lung cancer. For the multivitamin intervention, secondary outcomes again included key site-specific cancers in older individuals, including breast, colorectal, and lung cancer; the risk of total cancer among those with a baseline history of cancer (given previous evidence for a significant 27% reduction in total cancer among men aged 50 years or older in PHS II41); the expanded total CVD outcome; a combined outcome of CVD and all-cause mortality; and individual cardiovascular events comprising the expanded composite total cardiovascular outcome.
Medical record collection
COSMOS participants reporting a primary or secondary study outcome signed a medical record release form allowing WHI and BWH to request related medical records that were evaluated and processed according to standardized procedures.50 COSMOS participants from the WHI Medical Records Cohort (MRC) had medical record retrieval and outcome adjudication using established WHI procedures; events reported by other WHI participants enrolled in COSMOS were identified and adjudicated using the same procedures as the WHI MRC participants. COSMOS participants from BWH followed the same medical record retrieval and adjudication procedures, which were conducted by BWH staff.
Outcome adjudication
Self-reported primary and secondary outcomes were confirmed or disconfirmed through medical record review by a committee of physicians and investigators blinded to treatment assignment. To adjudicate CVD events, discharge summaries, electrocardiograms (ECGs), laboratory values, and test reports were sought. MI was defined by standardized criteria including cardiac pain, cardiac enzymes including troponin levels, and ECG findings. Coronary revascularization included documented coronary artery bypass graft (CABG) surgery, percutaneous coronary intervention (PCI), coronary stent, or artherectomy.50 Unstable angina requiring hospitalization included reports of increased pain, use of medications to alleviate pain, no evidence of MI, plus other related factors. Because unstable angina requiring hospitalization had not previously been adjudicated for WHI participants in COSMOS, these medical records were obtained by BWH staff and self-reports adjudicated by BWH adjudicators. Carotid artery surgery and peripheral artery surgery included review and identification from surgical and radiology reports. Stroke was classified according to the Trial of Org 10172 in Acute Stroke Therapy (TOAST)51 and Oxfordshire subtype.52 Coronary heart disease and stroke deaths included deaths consistent with either outcome as an underlying cause. For incident cancers, discharge summaries, pathology reports, operative reports, and diagnostic or treatment procedure reports, including both inpatient and outpatient diagnoses, were requested. Invasive cancer was confirmed by a biopsy or surgical pathology report that substantiated a malignant invasive cancer at any location other than non-melanoma skin cancer.50 All histologic types and anatomic subsites were included. Noncancerous colorectal polyps, atypical benign breast disease, in situ cancers, and other premalignant benign conditions were excluded. Finally, second primaries at a site and recurrences of cancer were also ascertained. For participants determined to be deceased, BWH or WHI Regional Center staff, as appropriate, reached out to the family requesting permission to obtain medical records and a copy of the death certificate. Death certificates were alternatively requested from the participant’s next of kin and periodic National Death Index Plus searches were also performed.
Biospecimen collection and clinic subcohort
COSMOS is a hybrid trial that combines large-scale remote interventions with in-person biospecimen collections and clinic-based assessments for in-depth mechanistic studies on how the proposed interventions may lead to study outcomes.53 COSMOS participants were asked to provide optional baseline biospecimens, and we invited an equal number of women and men to provide sufficient power to evaluate effect modification by sex. A total of 6,867 randomized participants returned blood and spot urine samples collected either (a) on their own through mailed biospecimen kits that were returned by the participant or (b) by home-based visits by Examination Management Services, Inc. that also included seated BP and anthropometrics measurements. A third option of visiting a Quest clinic for a biospecimen collection was added during the study to assist with follow-up biospecimen collection and shipments. At least one follow-up biospecimen was collected at 1-, 2-, and/or 3-, year follow-up in 2,155 participants using similar procedures.
The COSMOS clinic subcohort consisted of 603 participants in the greater Boston area willing to complete a baseline in-person clinic visit toward the end of the run-in phase and just before their final eligibility assessment and randomization. The clinic visits consisted of in-depth phenotyping with measurements of anthropometrics, seated BP, 24-hour ambulatory BP, physical performance, biospecimen collection, dual-energy x-ray absorptiometry testing for spine and hip bone mineral density, pulse wave analysis and velocity, and structured cognitive assessments.45 A total of 535 (89% of those completing a baseline assessment) participants had repeated follow-up clinic assessments at 2 years follow-up.
Cognitive ancillary studies
Two separately funded COSMOS substudies were integrated into the initial COSMOS trial design to examine cognitive outcomes. First, COSMOS Mind [clinicaltrials.gov NCT03035201] examined a global composite measure of cognitive function across 3 years of telephone-based assessments in 2,142 non-demented participants aged ≥65 years.54 Second, COSMOS Web [clinicaltrials.gov NCT04582617] examined the interventions on a range of aging-related cognitive measures using a novel online-administered test battery initially in nearly 4,000 COSMOS participants with 3 years of follow-up assessments. COSMOS Web was built upon earlier55 and more recent findings56 on the effect of cocoa flavanol supplementation over 12 weeks on changes in cognition in healthy older adults. The primary outcome for COSMOS Web will be change in ModRey immediate recall performance56,57 over 1 year.
Data analyses
The primary analyses of this 2×2 factorial trial will be based on the intention-to-treat principle58 and use Cox proportional hazards models for time-to event data59 to estimate the hazard ratio for each intervention using indicators for the specific treatment and stratifying the baseline hazard function by the second intervention, sex, age group, and recruitment center. Kaplan-Meier cumulative incidence curves, interactions between randomization groups with time, and analyses that exclude the first 1 and 2 years of follow-up will assess whether treatment effects vary over time.60 In secondary analyses, we will examine effect modification by the other randomized intervention, age, sex, other key risk factors, concomitant medications, baseline dietary intake of chocolate (for the cocoa extract analyses) or fruit and vegetable intake (for the multivitamin analyses), and baseline history of disease (CVD or cancer). Interactions will be interpreted cautiously as hypothesis generating. We will also examine compliance analyses that estimate the effects on our outcomes when weighted by the inverse probability of dependent-censoring for non-compliance with study procedures, with compliance defined as missing ≤8 days of study pills per month and abstaining from use of non-study cocoa extract or multivitamins.61 Main results from the trial are expected to be published in 2022.
For the present study, to ascertain the comparability of treatment groups at baseline, we calculated the mean or median values of continuous variables and percentages in each category of discrete variables. Differences for continuous variables were tested by two-sample t-test or analysis of variance for more than two groups for normally distributed data and the nonparametric two-sample Wilcoxon rank–sum or Kruskal–Wallis test comparing multiple groups for data that were not normally distributed. Percentages were compared by chi-square tests. Statistical significance was assessed with two-sided p-values.
Results
Table 1 presents the baseline characteristics of the COSMOS trial cohort overall and stratified by randomized treatment assignment. Of the 21,442 randomized participants, 59.1% are women and 40.9% are men. The mean age of the cohort is 72.1 years (range 60–102.7 years). The majority of the cohort reported their race as white (90.0%) while 5.3% are Black 2.3% are Asian/Pacific Islander, and 0.3% are American Indian/Alaskan Native. 2.6% of the cohort reported their ethnicity as Hispanic/Latino. All four geographic regions of the United States are well-represented. There were no clinically important or statistically significant differences between treatment groups comparing these demographic factors. For major cancer and/or CVD risk factors, 27.2% of participants were obese (body mass index ≥30 kg/m2), 58.1% were hypertensive, 13.4% had diabetes, 44.2% current use cholesterol-lowering medication, and 4.0% were current smokers. Of the 3,550 individuals (16.6% of the entire COSMOS trial cohort) who reported a diagnosis of cancer more than two years prior to study enrollment, 679 (3.2%) reported melanoma and 2,740 (12.8%) reported non-skin cancer. Of those reporting a non-skin cancer, 32.0% had a history of breast cancer and 21.0% had a history of prostate cancer. There were no clinically important or statistically significant differences between the treatment groups for these factors or for any other health history or behavioral characteristics listed below. At the initial screening, 0.4% of the cohort reported using cocoa flavanol supplements and 41.0% of individuals reported using multivitamins. However, these individuals agreed to stop taking cocoa flavanol or multivitamin supplements for the trial duration and thus were randomized into COSMOS.
Table 1.
Baseline demographic, health, and behavioral characteristics among 21,442 COSMOS participants, according to randomized treatment assignment.
| Baseline Characteristic | Total cohorta
(N=21,442 ) |
Cocoa extract active and Multivitami n active (N=5360) | Cocoa extract active and Multivitami n placebo (N=5359) | Cocoa extract placebo and Multivitami n active (N=5360) | Cocoa extract placebo and Multivitami n placebo (N=5363) |
|---|---|---|---|---|---|
| Sex, % Male Female |
8776 (40.9) 12666 (59.1) |
2189 (40.8) 3171 (59.2) |
2193 (40.9) 3166 (59.1) |
2193 (40.9) 3167 (59.1) |
2201 (41.0) 3162 (59.0) |
| Mean age (SD), years | 72.1 (6.6) | 72.1 (6.6) | 72.1 (6.6) | 72.1 (6.5) | 72.1 (6.6) |
| Age group, years, % 60–69 70–74 75–79 80–84 ≥85 |
9224 (43.0) 5774 (26.9) 3751 (17.5) 1761 (8.2) 932 (4.4) |
2305 (43.0) 1443 (26.9) 938 (17.5) 441 (8.2) 233 (4.4) |
2305 (43.0) 1443 (26.9) 938 (17.5) 441 (8.2) 232 (4.3) |
2306 (43.0) 1443 (26.9) 937 (17.5) 448 (8.4) 226 (4.2) |
2308 (43.0) 1445 (26.9) 938 (17.5) 431 (8.0) 241 (4.5) |
| Hispanic/Latino, % | 544 (2.6) | 134 (2.6) | 118 (2.3) | 150 (2.9) | 142 (2.8) |
| Race, % White Black Asian/Pacific Islander American Indian/Alaskan Native Multiracial/other/unknown or not reported |
19294 (90.0) 1131 (5.3) 499 (2.3) 59 (0.3) 459 (2.1) |
4796 (89.5) 290 (5.4) 138 (2.6) 17 (0.3) 119 (2.2) |
4828 (90.1) 268 (5.0) 136 (2.5) 14 (0.3) 113 (2.1) |
4832 (90.1) 278 (5.2) 120 (2.2) 20 (0.4) 110 (2.1) |
4838 (90.2) 295 (5.5) 105 (2.0) 8 (0.1) 117 (2.2) |
| Geographic region, % Northeastb Midwest/Mountain South West/Southwest |
5941 (27.7) 5071 (23.6) 6151 (28.7) 4279 (20.0) |
1495(27.9) 1295 (24.2) 1497 (27.9) 1073 (20.0) |
1450 (27.1) 1241 (23.2) 1567 (29.2) 1101 (20.5) |
1504 (28.1) 1266 (23.6) 1547 (28.9) 1043 (19.5) |
1492 (27.8) 1269 (23.7) 1540 (28.7) 1062 (19.8) |
| Education, % High school diploma/GED or less Attended or graduated from college Post-college |
2296 (10.8) 8685 (40.9) 10241 (48.3) |
588 (11.1) 2117 (39.9) 2596 (49.0) |
553 (10.4) 2211 (41.6) 2551 (48.0) |
592 (11.2) 2198 (41.5) 2508 (47.3) |
563 (10.6) 2159 (40.7) 2586 (48.7) |
| Health History | |||||
| Mean body mass index (SD), kg/m2 | 27.7 (5.4) | 27.6 (5.3) | 27.7 (5.4) | 27.7 (5.4) | 27.8 (5.5) |
| Obesityc, % | 5718 (27.2) | 1402 (26.7) | 1457 (27.7) | 1398 (26.6) | 1461 (27.8) |
| Hypertension, % | 12423 (58.1) | 3048 (57.0) | 3142 (58.8) | 3105 (58.1) | 3128 (58.5) |
| Ever use of anti-hypertensive medication, % | 11217 (53.0) | 2758 (52.3) | 2817 (53.1) | 2814 (53.2) | 2828 (53.3) |
| Current use of cholesterol-lowering medication, % | 9405 (44.2) | 2366 (44.4) | 2346 (44.0) | 2348 (44.2) | 2345 (44.0) |
| Diabetes, % | 2864 (13.4) | 702 (13.1) | 715 (13.3) | 713 (13.3) | 734 (13.7) |
| Current use of antidiabetic medication, % | 2233 (10.4) | 541 (10.1) | 558 (10.4) | 557 (10.4) | 577 (10.8) |
| Parental history of premature myocardial infarctiond, % | 4112 (21.6) | 1044 (22.1) | 1027 (21.5) | 1054 (22.1) | 987 (20.7) |
| Personal history of revascularization procedure, % | 862 (4.0) | 225 (4.2) | 205 (3.8) | 201 (3.8) | 231 (4.3) |
| Personal history of cancer, % Only melanoma Melanoma plus non-skin cancer Only non-skin cancer |
3550 (16.6) 679 (3.2) 131 (0.6) 2740 (12.8) |
917 (17.1) 168 (3.1) 36 (0.7) 713 (13.3) |
858 (16.0) 163 (3.0) 34 (0.6) 661 (12.3) |
896 (16.7) 183 (3.4) 25 (0.5) 688 (12.8) |
879 (16.4) 165 (3.1) 36 (0.7) 678 (12.6) |
| Family history of cancer in first degree relative Colorectal, % Breast (women only, %) Prostate (men only, %) Lung, % |
3748 (18.5) 2522 (21.1) 1548 (18.7) 3569 (17.7) |
929 (18.3) 648 (21.5) 389 (18.9) 873 (17.3) |
920 (18.2) 605 (20.2) 385 (18.4) 911 (18.0) |
938 (18.6) 638 (21.6) 390 (18.8) 896 (18.0) |
961 (19.0) 631 (21.2) 384 (18.5) 889 (17.6) |
| Behavioral characteristics and medication use | |||||
| Smoking, % Current Past Never |
835 (4.0) 8731 (41.3) 11565 (54.7) |
199 (3.8) 2173 (41.2) 2904 (55.0) |
199 (3.8) 2223 (42.1) 2862 (54.2) |
218 (4.1) 2172 (41.0) 2904 (54.9) |
219 (4.2) 2163 (41.0) 2895 (54.9) |
| Leisure-time physical activity and stair climbing, total MET-hours/week, median (IQR) | 17.6 (5.7–33.5) | 18.0 (5.7–34.1) | 17.3 (5.5–33.5) | 17.8 (6.0–33.5) | 17.1 (5.6–33.4) |
| Aspirin use in past month, % | 10379 (48.9) | 2617 (49.3) | 2594 (48.9) | 2551 (48.1) | 2617 (49.3) |
| Postmenopausal hormone use, % (women only) Current Past Never |
1021 (8.2) 6158 (49.7) 5199 (42.0) |
256 (8.3) 1548 (49.9) 1298 (41.8) |
246 (7.9) 1561 (50.4) 1290 (41.7) |
247 (8.0) 1521 (49.0) 1333 (43.0) |
272 (8.8) 1528 (49.6) 1278 (41.5) |
| Screening behaviors in past 10 years Colonoscopy/sigmoidoscop y, % Prostate-specific antigen test, % (men only) Mammogram, % (women only) |
17572 (83.4) 6958 (81.3) 11650 (93.4) |
4393 (83.3) 1715(80.4) 2906 (93.0) |
4373 (83.0) 1764 (82.2) 2918 (93.6) |
4422 (83.8) 1718 (80.5) 2919 (93.7) |
4384 (83.4) 1761 (82.0) 2907 (93.4) |
| Dietary factors at initial screening | |||||
| Calcium intake from supplementse, % None ≤1200 mg/day >1200 mg/day |
10917 (51.5) 9200 (43.4) 1066 (5.0) |
2708 (51.0) 2342 (44.1) 255 (4.8) |
2731 (51.7) 2279 (43.1) 273 (5.2) |
2775 (52.5) 2254 (42.6) 261 (4.9) |
2703 (51.0) 2325 (43.8) 277 (5.2) |
| Vitamin D intake from supplementse, % None ≤1000 IU/day >1000 IU/day |
7960 (37.6) 8670 (41.0) 4536 (21.4) |
1971 (37.2) 2218 (41.9) 1109 (20.9) |
1962 (37.1) 2133 (40.3) 1193 (22.6) |
2041 (38.6) 2113 (40.0) 1129 (21.4) |
1986 (37.5) 2206 (41.6) 1105 (20.9) |
| Current use of multivitaminsf, % | 8795 (41.2) | 2237 (41.9) | 2201 (41.2) | 2176 (40.8) | 2181 (40.8) |
| Current use of cocoa extract supplementsf, % | 91 (0.4) | 22 (0.4) | 23 (0.4) | 20 (0.4) | 26 (0.5) |
| Chocolate intakeg, % Rarely Monthly Weekly Daily or more often |
3352 (17.0) 2923 (14.8) 11129 (56.4) 2317 (11.8) |
850 (17.2) 716 (14.5) 2800 (56.8) 568 (11.5) |
790 (16.1) 739 (15.1) 2806 (57.3) 566 (11.5) |
850 (17.1) 751 (15.1) 2775 (55.9) 586 (11.8) |
862 (17.5) 717 (14.6) 2748 (55.8) 597 (12.1) |
| Alcohol useg, % Rarely Monthly Weekly Daily or more often |
5867 (29.7) 1462 (7.4) 7136 (36.2) 5276 (26.7) |
1408 (28.5) 376 (7.6) 1821 (36.9) 1331 (27.0) |
1489 (30.4) 349 (7.1) 1779 (36.3) 1289 (26.3) |
1483 (29.9) 375 (7.6) 1736 (35.0) 1366 (27.5) |
1487 (30.1) 362 (7.3) 1800 (36.4) 1290 (26.1) |
For categorical variables, this column contains # participants in category/% of nonmissing responses. For continuous variables, this column contains mean (standard deviation) or median (interquartile range) for nonmissing responses. The prevalence of missing responses for all variables ranged from 0 to <5%, except for the following: parental history of premature myocardial infarction (11.2%), history of cancer in first-degree relative (colorectal, 5.6%; prostate, 5.5%; lung, 6.0%; breast 5.7%), chocolate intake (8.0%), alcohol consumption (7.9%),
Includes one individual living on a US Army Base in Europe.
Height and weight were self-reported
Defined as a heart attack prior to 65 years of age
Participants had to agree to limit vitamin D to ≤1000 IU/day and calcium to ≤1200 mg/day from all supplemental sources during the trial
Participants had to agree to forgo supplements of cocoa extract and multivitamins (chocolate intake was not restricted) during the trial.
Includes consumption of milk chocolate, dark chocolate, white chocolate and candy bars
Includes beer, liquor, red wine, and white wine.
Table 2 provides the baseline demographic, health, and behavioral characteristics of study participants stratified by sex. As expected given the sex-specific age cut points for trial entry (age ≥60 years for men and age ≥65 years for women), the average age of female participants was older than male participants (mean age 74.2 versus 69.0 years; P < 0.001). There were significant differences between men and women in nearly all baseline characteristics examined. For example, men were more likely to report a lower prevalence of obesity, parental history of MI, personal history of non-skin cancer, family history of colorectal or lung cancer, multivitamin use, vitamin D or calcium supplement use at initial screening; had higher levels of physical activity; and had a higher prevalence of cholesterol-lowering medication use, diabetes and antidiabetic medications, personal history of a revascularization procedure, personal history of melanoma only, current smoking, aspirin use, consumption of alcohol daily or more often, and colonoscopy/sigmoidoscopy in the past 10 years.
Table 2.
Baseline demographic, health, and behavioral characteristics among 21,442 COSMOS participants, according to sex.
| Baseline Characteristica | Men (N=8776)b | Women (N=12666)b |
|---|---|---|
| Mean age (SD), years | 69.0 (6.1) | 74.2 (6.0) |
| Age group, years, % 60–69 70–74 75–79 80–84 ≥85 |
5580 (63.6) 1753 (20.0) 898 (10.2) 382 (4.4) 163 (1.9) |
3644 (28.8) 4021 (31.7) 2853 (22.5) 1379 (10.9) 769 (6.1) |
| Hispanic/Latino, % | 233 (2.8) | 311 (2.6) |
| Race, % White Black Asian/Pacific Islander American Indian/Alaskan Native Multiracial/other/unknown or not reported |
7940 (90.5) 392 (4.5) 254 (2.9) 19 (0.2) 171 (1.9) |
11354 (89.6) 739 (5.8) 245 (1.9) 40 (0.3) 288 (2.3) |
| Geographic region, % Northeast Midwest/Mountain South West/Southwest |
2721 (31.0) 2063 (23.5) 2596 (29.6) 1396 (15.9) |
3220 (25.4) 3008 (23.7) 3555 (28.1) 2883 (22.8) |
| Education, % High school diploma/GED or less Attended or graduated from college Post-college |
540 (6.2) 3348 (38.5) 4808 (55.3) |
1756 (14.0) 5337 (42.6) 5433 (43.4) |
| Health History | ||
| Mean body mass index (SD), kg/m2 | 27.9 (4.7) | 27.5 (5.8) |
| Obesity, % | 2291 (26.3) | 3427 (27.8) |
| Hypertension, % | 5074 (57.9) | 7349 (58.2) |
| Ever use of anti-hypertensive medication, % | 4530 (52.3) | 6687 (53.4) |
| Current use of cholesterol-lowering medication, % | 4302 (49.2) | 5103 (40.7) |
| Diabetes, % | 1273 (14.5) | 1591 (12.6) |
| Current use of antidiabetic medication, % | 1014 (11.6) | 1219 (9.6) |
| Parental history of premature myocardial infarction, % | 1625 (20.4) | 2487 (22.4) |
| Personal history of revascularization procedure, % | 564 (6.4) | 298 (2.4) |
| Personal history of cancer, % Only melanoma Melanoma plus non-skin cancer Only non-skin cancer |
1346 (15.3) 342 (3.9) 59 (0.7) 945 (10.8) |
2204 (17.4) 337 (2.7) 72 (0.6) 1795 (14.2) |
| Family history of cancer in first degree relative Colorectal, % Breast (women only, %) Prostate (men only, %) Lung, % |
1384 (16.7) NA 1548 (18.7) 1378 (16.7) |
2364 (19.7) 2522 (21.1) NA 2191 (18.4) |
| Behavioral characteristics and medication use | ||
| Smoking, % Current Past Never |
415 (4.8) 3622 (41.8) 4620 (53.4) |
420 (3.4) 5109 (41.0) 6945 (55.7) |
| Leisure-time physical activity and stair climbing, total MET hours/week, median (IQR) | 20.3 (7.5, 36.9) | 15.9 (4.7, 31.3) |
| Aspirin use in past month, % | 4700 (53.8) | 5679 (45.4) |
| Postmenopausal hormone use, % (women only) Current Past Never |
NA NA NA |
1021 (8.2) 6158 (49.7) 5199 (42.0) |
| Screening behaviors in past 10 years Colonoscopy/sigmoidoscopy, % Prostate-specific antigen test, % (men only) Mammogram, % (women only) |
7441 (85.8) 6958 (81.3) NA |
10131 (81.7) NA 11650 (93.4) |
| Dietary factors at initial screening | ||
| Calcium intake from supplements, % None ≤1200 mg/day >1200 mg/day |
5920 (67.9) 2668 (30.6) 126 (1.5) |
4997 (40.1) 6532 (52.4) 940 (7.5) |
| Vitamin D intake from supplements, % None ≤1000 IU/day >1000 IU/day |
4519 (52.0) 3008 (34.6) 1166 (13.4) |
3441 (27.6) 5662 (45.4) 3370 (27.0) |
| Current use of multivitamins, % | 3208 (36.7) | 5587 (44.3) |
| Current use of cocoa extract supplements, % | 28 (0.3) | 63 (0.5) |
| Chocolate intake Rarely Monthly Weekly Daily or more often |
1442 (18.5) 1108 (14.2) 4378 (56.2) 862 (11.1) |
1910 (16.0) 1815 (15.2) 6751 (56.6) 1455 (12.2) |
| Alcohol use, % Rarely Monthly Weekly Daily or more often |
7810 (89.0) 1814 (23.2) 441 (5.6) 2762 (35.4) 2793 (35.8) |
11931 (94.2) 4053 (34.0) 1021 (8.6) 4374 (36.7) 2483 (20.8) |
Variable definitions are provided in footnotes to Table 1.
For categorical variables, this column contains # participants in category/% of nonmissing responses. For continuous variables, this column contains mean (standard deviation) or median (interquartile range) for nonmissing responses.
Table 3 provides the baseline characteristics of the 6,867 participants who provided an optional blood sample (with nearly all additionally providing a spot urine) at baseline (the “blood subcohort”), the 603 individuals who had a baseline health examination at the Center for Clinical Investigation (CCI) at BWH (the “clinic subcohort”), and the 3,550 individuals who reported a history of cancer at study enrollment. Overall, the blood subcohorts was similar to the overall COSMOS cohort, although there was a higher percentage of non-Hispanic white individuals and of men. This difference in sex distribution is expected given the design decision to enroll equal numbers of men and women into the blood subcohort. Due to the higher percentage of men and the lower age eligibility in men compared to women, we also observed a slightly higher percentage of individuals in the youngest age group (60 to 69 years) in the blood subcohort than in the total cohort. To participate in the clinic subcohort, individuals had to be willing to travel to the CCI clinic and complete a 5- to 7-hour assessment. As a result, the clinic subcohort is younger and somewhat healthier than the overall cohort, with a lower prevalence of hypertension, cholesterol-lowering medication use, diabetes, and obesity, a higher level of physical activity, and a higher prevalence of compliance with cancer screening recommendations (colonoscopy/sigmoidoscopy and mammography). Further, the majority of COSMOS clinic subcohort participants were non-Hispanic white. Of the 603 individuals in the clinic subcohort, 236 individuals also participated in the blood subcohort and provided remotely collected blood and urine samples. Finally, those with a history of cancer at study enrollment were slightly older than the overall cohort (mean age 73.9 years) and were slightly more likely to report a family history of cancer in their first-degree relatives.
Table 3.
Baseline demographic, health, and behavioral characteristics among 21,442 COSMOS participants, in the overall cohort and according to participation in (a) the baseline blood subcohort, (b) the baseline clinic subcohort, and (c) those with a baseline history of cancer.
| Baseline Characteristica | Total cohortb (N=21,442) | Blood subcohort (N=6867) | Clinic subcohortc (N=603) | Baseline History of Cancer (N=3550) |
|---|---|---|---|---|
| Sex, % Male Female |
8776 (40.9) 12666 (59.1) |
3369 (49.1) 3498 (50.9) |
306 (50.7) 297 (49.3) |
1346 (37.9) 2204 (62.1) |
| Mean age (SD), years | 72.1 (6.6) | 71.1 (6.3) | 69.7 (5.5) | 73.9 (6.5) |
| Age group, years, % 60–69 70–74 75–79 80–84 ≥85 |
9224 (43.0) 5774 (26.9) 3751 (17.5) 1761 (8.2) 932 (4.4) |
3363 (49.0) 1785 (26.0) 1078 (15.7) 421 (6.1) 220 (3.2) |
347 (57.5) 165 (27.4) 63 (10.4) 25 (4.1) 3 (0.5) |
1043 (29.4) 1110 (31.3) 776 (21.9) 398 (11.2) 223 (6.3) |
| Hispanic/Latino, % | 544 (2.6) | 160 (2.4) | 6 (1.0) | 65 (1.9) |
| White race, % | 19294 (90.0) | 6427 (93.6) | 586 (97.2) | 3246 (91.4) |
| Geographic region, % Northeast Midwest/Mountain South West/Southwest |
5941 (27.7) 5071 (23.7) 6151 (28.7) 4279 (20.0) |
1969 (28.7) 1696 (24.7) 1887 (27.5) 1315 (19.1) |
603 (100) |
955 (26.9) 876 (24.7) 961 (27.1) 758 (21.4) |
| Education, % High school diploma/GED or less Attended or graduated from college Post-college |
2296 (10.8) 8685 (40.9) 10241 (48.3) |
588 (8.7) 2773 (40.9) 3426 (50.5) |
37 (6.2) 236 (39.8) 320 (54.0) |
363 (10.4) 1405 (40.2) 1731 (49.5) |
| Health History | ||||
| Mean body mass index (SD), kg/m2 | 27.7 (5.4) | 27.6 (5.3) | 27.1 (4.9) | 27.6 (5.4) |
| Obesity, % | 5718 (27.2) | 1753 (25.9) | 136 (22.8) | 923 (26.5) |
| Hypertension, % | 12423 (58.1) | 3865 (56.4) | 301 (49.9) | 2190 (61.9) |
| Ever use of anti-hypertensive medication, % | 11217 (53.0) | 3499 (51.6) | 261 (44.0) | 1989 (56.8) |
| Current use of cholesterol-lowering medication, % | 9405 (44.2) | 3118 (45.6) | 254 (42.2) | 1672 (47.5) |
| Diabetes, % | 2864 (13.4) | 871 (12.7) | 66 (11.0) | 484 (13.6) |
| Current use of antidiabetic medication, % | 2233 (10.4) | 660 (9.6) | 53 (8.8) | 377 (10.6) |
| Parental history of premature myocardial infarction, % | 4112 (21.6) | 1386 (22.3) | 124 (22.8) | 692 (22.1) |
| Personal history of revascularization procedure, % | 862 (4.0) | 291 (4.2) | 23 (3.8) | 159 (4.5) |
| Personal history of cancer, % Only melanoma Melanoma plus non-skin cancer Only non-skin cancer |
3550 (16.6) 679 (3.2) 131 (0.6) 2740 (12.8) |
1149 (16.7) 205 (3.0) 43 (0.6) 901 (13.1) |
94 (15.6) 20 (3.3) 1 (0.2) 73 (12.1) |
3550 (100.0) 679 (19.1) 131 (3.7) 2740 (77.2) |
| Family history of cancer in first degree relative Colorectal, % Breast (women only, %) Prostate (men only, %) Lung, % |
3748 (18.5) 2522 (21.1) 1547 (18.7) 3569 (17.7) |
1220 (18.7) 717 (21.5) 602 (18.8) 1160 (17.8) |
98 (17.2) 56 (19.6) 54 (19.1) 107 (18.9) |
677 (20.3) 509 (24.6) 321 (25.3) 629 (18.9) |
| Behavioral characteristics and medication use | ||||
| Smoking, % Current Past Never |
835 (4.0) 8731 (41.3) 11565 (54.7) |
254 (3.7) 2829 (41.7) 3701(54.6) |
14 (2.3) 260 (43.6) 323 (54.1) |
117 (3.3) 1562 (44.7) 1817 (52.0) |
| Leisure-time physical activity and stair climbing, total METhours/week, median (IQR) | 17.6 (5.7–33.5) | 20.0 (7.3, 35.3) | 20.0 (8.1, 36.6) | 16.6 (5.4, 31.4) |
| Aspirin use in past month, % | 10379 (48.9) | 3383 (49.6) | 274 (45.8) | 1681 (47.9) |
| Postmenopausal hormone use, % (women only) Current Past Never |
1021 (8.2) 6158 (49.7) 5199 (42.0) |
337 (9.8) 1717 (50.2) 1368 (40.0) |
29 (9.8) 121 (41.0) 145 (49.2) |
128 (6.0) 1096 (51.1) 921 (42.9) |
| Screening behaviors in past 10 years Colonoscopy/sigmoidoscopy, % Prostate-specific antigen test, % (men only) Mammogram, % (women only) |
17572 (83.4) 6958 (81.3) 11650 (93.4) |
5862 (86.5) 2748 (83.2) 3280 (94.9) |
557 (93.5) 234 (78.3) 290 (98.3) |
3047 (87.3) 1159 (88.1) 2007 (92.8) |
| Dietary factors at initial screening | ||||
| Calcium intake from supplements, % None ≤1200 mg/day >1200 mg/day |
10917 (51.5) 9200 (43.4) 1066 (5.0) |
3511 (51.7) 2938 (43.3) 343 (5.1) |
328 (54.9) 246 (41.2) 23 (3.9) |
1639 (46.8) 1663 (47.5) 202 (5.8) |
| Vitamin D intake from supplements, % None ≤1000 IU/day >1000 IU/day |
7960 (37.6) 8670 (41.0) 4536 (21.4) |
2535 (37.3) 2718 (40.0) 1545 (22.7) |
239 (40.0) 249 (41.7) 109 (18.3) |
1123 (32.0) 1524 (43.4) 863 (24.6) |
| Current use of multivitamins, % | 8795 (41.2) | 2904 (42.4) | 231 (38.4) | 1594 (45.1) |
| Current use of cocoa extract supplements, % | 91 (0.4) | 19 (0.3) | 2 (0.3) | 17 (0.5) |
| Chocolate intake Rarely Monthly Weekly Daily or more often |
3352 (17.0) 2923 (14.8) 11129 (56.4) 2317 (11.8) |
1054 (16.2) 967 (14.9) 3712 (57.2) 757 (11.7) |
96 (16.7) 82 (14.2) 330 (57.5) 66 (11.5) |
562 (17.3) 462 (14.2) 1846 (56.8) 382 (11.7) |
| Alcohol use, % Rarely Monthly Weekly Daily or more often |
5867 (29.7) 1462 (7.4) 7136 (36.2) 5276 (26.7) |
1745 (26.9) 431 (6.6) 2387 (36.7) 1936 (29.8) |
131 (22.9) 33 (5.8) 226 (39.6) 181 (31.7) |
970 (29.8) 238 (7.3) 1167 (35.9) 877 (27.0) |
Variable definitions are provided in footnotes to Table 1.
For categorical variables, this column contains # participants in category/% of nonmissing responses. For continuous variables, this column contains mean (standard deviation) or median (interquartile range) for nonmissing responses.
Participants in the greater Boston area willing to complete a baseline clinic visit toward the end of the run-in phase and just before their final eligibility assessment and randomization
Discussion
We observed balance in the distribution of demographic, health history, and behavioral characteristics across the four randomized treatments groups in COSMOS. This implies that unknown or unmeasured confounders are also likely to be equally distributed across the treatment groups. As a result, we expect that any observed differences in our outcomes will be attributable to the interventions themselves rather than to uncontrolled confounding.
For efficiency, COSMOS recruited participants from the WHI and non-randomized participants in VITAL. Among WHI and VITAL participants invited to participate in COSMOS, randomization rates were 6.4% and 2.9%, respectively, figures which are substantially higher than the randomization rate of 0.36% which we observed from our nationwide mass mailings to age-eligible adults.53 This demonstrates that leveraging other ongoing epidemiologic studies may help to improve and expedite recruitment in clinical trials, which typically involves major logistical, budgetary, and time challenges. In addition, this approach successfully recruited an older population of individuals for a trial that will be conducted almost entirely through remote assessments. Notably, over the course of the trial to date, more than 75% of the study population was willing to complete questionnaires through REDCap (electronic data capture). Although men aged ≥60 years and women aged ≥65 years were eligible to participate in the trial, the average age of the trial cohort was 72.1 years. The ability to collect not only baseline questionnaire data, but also biospecimens, demonstrates the feasibility of using pragmatic and cost-effective recruitment and data collection methods in older adults.
Another important feature of COSMOS is the hybrid design, which combines a large-scale remotely-conducted trial to complement primary and secondary clinical outcomes with detailed phenotyping on a subset of individuals to answer mechanistic questions as well as address additional clinically meaningful outcomes such as cognitive function. As described earlier, the COSMOS blood subcohort was designed to have equal numbers of men and women. As a result, the blood subcohort has a higher percentage of males than the overall cohort, but is otherwise similar to the overall COSMOS study population. This suggests that analyses utilizing the data from the blood subcohort will be generalizable to the full trial population. Further, the availability of multiple follow-up biospecimen collections will allow us to examine changes in compliance-based biomarkers of both interventions, as well as changes in other markers related to the study outcomes. Although the clinic subcohort was slightly younger and healthier than the total cohort, the results from the clinic subcohort will be internally valid and should provide important in-depth, in-person phenotyping of participants in the context of the results from the main trial. However, generalizability may be limited given the modest racial and ethnic diversity of the cohort as well as the potential for healthy volunteer bias for participants willing and eligible to enroll in a largely mail-based clinical trial.
In summary, COSMOS is a large-scale, innovative, and pragmatic randomized trial that will provide important and relevant information about the balance of benefits and risks of a cocoa extract supplement for the prevention of CVD and of a multivitamin for the prevention of cancer among older adults. The unique hybrid design of COSMOS, which includes biospecimen collections and clinic-based assessments, will also allow for in-depth mechanistic studies on how the proposed interventions may lead to study outcomes.
Supplementary Material
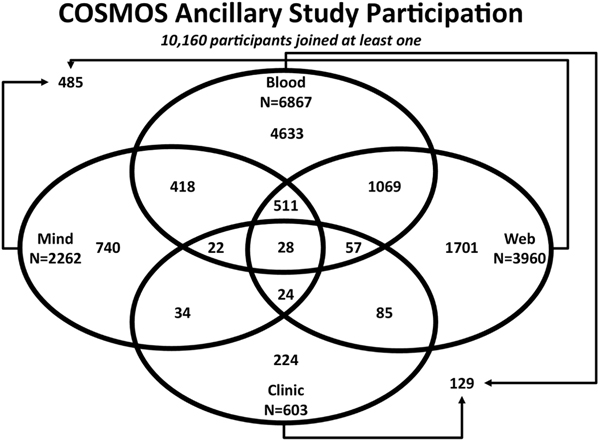
Figure 2.
Summary of COSMOS ancillary study participation.
Acknowledgements
COSMOS has been approved and overseen by the Institutional Review Board of Brigham and Women’s Hospital/Mass General Brigham. COSMOS is registered at clinicaltrials.gov (NCT02422745). The COSMOS website is www.cosmostrial.org. We are deeply indebted to the 21,442 COSMOS participants for their steadfast and conscientious collaboration and to our COSMOS Research Group of trial investigators and staff for their commitment and perseverance to the trial despite the challenges of the COVID-19 pandemic. We would like to specifically acknowledge the following individuals from the COSMOS Research Group for their scientific (Brigham and Women’s Hospital [BWH]), Fred Hutchinson Cancer Research Center [FHCRC], Women’s Health Initiative [WHI], Data Safety and Monitoring Board [DSMB], Mars Edge) and logistical (BWH, FHCRC, DSMB, Mars Edge, Contract Pharmacal Corp, Pfizer Consumer Healthcare [now GSK Consumer Healthcare]) contributions:
The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) is supported by an investigator-initiated grant from Mars Edge, a segment of Mars dedicated to nutrition research and products, which included infrastructure support and the donation of study pills and packaging. Pfizer Consumer Healthcare (now part of GSK Consumer Healthcare) provided support through the partial provision of study pills and packaging. COSMOS is also supported in part by grants AG050657, AG071611, EY025623, and HL157665 from the National Institutes of Health, Bethesda, MD. The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. Neither company had a role in the trial design or conduct, data collection (other than blinded assays supported by Mars Edge and completed independently), data analysis, or manuscript preparation or review.
Sources of Funding: The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) is supported by an investigator-initiated grant from Mars Edge (HDS, JEM), a segment of Mars dedicated to nutrition research and products, which included infrastructure support and the donation of study pills and packaging. Pfizer Consumer Healthcare (now part of GSK Consumer Healthcare) provided support through the partial provision of study pills and packaging (HDS, JEM). COSMOS is also supported in part by grants AG050657, AG071611, EY025623, and HL157665 from the National Institutes of Health, Bethesda, MD. The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005 (GLA). Neither company had a role in the trial design or conduct, data collection (other than blinded assays supported by Mars Edge and completed independently), data analysis, or manuscript preparation or review.
Footnotes
Brigham and Women’s Hospital: Principal Investigators, JoAnn E. Manson, Howard D. Sesso; Co-Investigators, Pamela M. Rist, Susanne Rautiainen Lagerstrom, Shari Bassuk, Lu Wang, Aditi Hazra, Heike Gibson, Meryl LeBoff, Samia, Mora, Olivia Okereke, Deirdre K. Tobias, Nancy R. Cook, Paulette Chandler, William C. Christen; Study Managers/Coordinators, Georgina Friedenberg, Trisha Copeland, Jasmah Hanna, Allison Clar, Denise D’Agostino; Statisticians/Analysts, Manickavasagar Vinayagamoorthy, Heike Gibson, Eunjung Kim, Marty Van Denburgh, Gregory Kotler, Chunying Li, Vadim Bubes; Programmers, Ara Sarkissian, Douglas C. Smith, Eduardo Pereira, Melvyn Okeke, Elise Roche, David Bates, Claire Ridge; Research Assistants, Alexandra Phillips, Brielle Salvo, Annalee Wilson, Leah Hall, Laurel Meyer, Jenessa Vogt, Jimaldy Baez, Young-Hwan Sim, Alyssa Lawrence, Hayara Cardoso, Gabriel Senor, Connor Rudnicki, Lauren Lee, Hanh Huynh, Viviane Nguyen, Montgomery Rich, Tessa Pliakas, Patrick Petrossian, Madison Hardee, Elena McCarthy, Maria Revilla, Corey Theodore, Nicholas Terrell; Outcomes, Beth Holman, Joseph Walter, Lisa Fields-Johnson, Amy Casarella, Julia O’Connell, Bonnie Church; Outcome Adjudicators, William C. Christen, Susanne Rautiainen Lagerstrom, Luc Djoussé, Paulette D. Chandler, Aditi Hazra, Deidre K. Tobias, Zareen M. Farukhi, Lu Wang, Xuehong Zhang; Mailings/Processing, Kenneth Breen, George Menjin, Henry Ouellette, Rolando Rodriguez, Tony Montgomery, Keith Morse, Stanley Lamothe, Annie Murray, Shamikhah Curry, Diana Walrond-Williams, Angela Zhang; Safety Oversight, Samia Mora; Clinic Research Assistants, Leah Arsenault, Olubunmi Solano, Alison Weinberg, Jennifer Coates, Matthew Kilroe, Lincoln Zernicke, Katelyn Hasson, Karen Matthew; Lab, Samia Mora, Christopher Pfeffer, Julie Duszlak, David Bates, Vincent Guzman, Eric Lee, Katherine Bush, Josue Falcon; Information Technology, Alex Romero, Henry Kupets, Frank Cortez, James LeSuer; Administration, Andrea Hrbek, Harriet Samuelson, Eileen Bowes, Mary-Anne Ryan, Philomena Quinn, Megan Mele, Joseph Niola, Ellen Connors
Fred Hutchinson Cancer Research Center/Women’s Health Initiative: Principal Investigator, Garnet L. Anderson; Co-Investigators, Lisa Johnson, Leslie F. Tinker, Aaron K. Aragaki; Study Managers/Coordinators, Megan Skinner Herndon, Sue Mann; Statisticians/Analysts, Mary Pettinger, Rebecca Hunt; Programmers, Bill Carrick, Nancy Bonnington, Mike Tennyson; Outcomes, Emily Wion, Kate Szyperski, Lori Proulx-Burns, Beth Burrows, Karol Namatame; Outcome Adjudicators, Marian Limacher, Karol Namatame, Judith Hsia, Ganesh Asaithambi, Muhib Khan, Nandakumar Nagaraja, Lenore Ocava, Jana Wold, Brian Silver, Stephanie Connelly; Mailings/Processing, Gretchen Van Lom, Cris Garvida, Kathy Hightower, Patti Spaulding, Sally Rosario, Ernesto Rosario, Henry Beauchamp, Doris Nodtvedt; Information Technology, Wei Lin; Administration, Jenny Schoenberg, Patti Olee
Data Safety and Monitoring Board: Lawrence S. Cohen (Chair), Theodore Colton, I. Craig Henderson, Stephen B. Hulley, Alice H. Lichtenstein, Eugene Passamani, Rebecca A. Silliman, Nanette K. Wenger, Shari E. Ludlam (NIH Observer)
Mars Edge: Hagen Schroeter, Michael Fare, Javier Ottawani, Catherine Kwik-Uribe
Contract Pharmacal Corp: Cassandra Arnaiz, Ann Costanza, John Greene, Paul Hennessey
Pfizer Consumer Healthcare (now GSK Consumer Healthcare): Sarma Vadlamani, Mallik Karmsetty, Paul Martini, Jan-Willem van Klinken, Alpa Shah, Lori Stern
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest Disclosures: Drs. Sesso and Manson reported receiving investigator-initiated grants from Mars Edge, a segment of Mars Incorporated dedicated to nutrition research and products, for infrastructure support and donation of COSMOS study pills and packaging, Pfizer Consumer Healthcare (now part of GSK Consumer Healthcare) for donation of COSMOS study pills and packaging during the conduct of the study. Dr. Sesso additionally reported receiving investigator-initiated grants from Pure Encapsulations and Pfizer Inc. and honoraria and/or travel for lectures from the Council for Responsible Nutrition, BASF, NIH, and American Society of Nutrition during the conduct of the study. Dr. Schroeter is employed by Mars Edge, a segment of Mars, Incorporated, a company engaged in flavanol research and flavanol-related commercial activities. No other disclosures were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: clinicaltrials.gov NCT02422745
References
- 1.Dillinger TL, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr 2000;130:2057S–72S. [DOI] [PubMed] [Google Scholar]
- 2.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Molecular nutrition & food research 2008;52:79–104. [DOI] [PubMed] [Google Scholar]
- 3.Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. European heart journal 2010;31:2583–92. [DOI] [PubMed] [Google Scholar]
- 4.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–42S. [DOI] [PubMed] [Google Scholar]
- 5.Raman G, Avendano EE, Chen S, et al. Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies. Am J Clin Nutr 2019;110:1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ottaviani JI, Heiss C, Spencer JPE, Kelm M, Schroeter H. Recommending flavanols and procyanidins for cardiovascular health: Revisited. Mol Aspects Med 2018;61:63–75. [DOI] [PubMed] [Google Scholar]
- 7.Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 8.Lin X, Zhang I, Li A, et al. Cocoa Flavanol Intake and Biomarkers for Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Nutr 2016;146:2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansone R, Rodriguez-Mateos A, Heuel J, et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study. Br J Nutr 2015;114:1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Mateos A, Weber T, Skene SS, et al. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial. Am J Clin Nutr 2018;108:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Zimmermann D, De Castro CA, Actis-Goretta L. Dose-response relationship between cocoa flavanols and human endothelial function: a systematic review and meta-analysis of randomized trials. Food Funct 2019;10:6322–30. [DOI] [PubMed] [Google Scholar]
- 12.Schroeter H, Heiss C, Balzer J, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A 2006;103:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison K, Berry NM, Misan G, Coates AM, Buckley JD, Howe PR. Dose-related effects of flavanolrich cocoa on blood pressure. J Hum Hypertens 2010;24:568–76. [DOI] [PubMed] [Google Scholar]
- 15.Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005;46:398–405. [DOI] [PubMed] [Google Scholar]
- 16.Kuebler U, Arpagaus A, Meister RE, et al. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: A randomized controlled trial. Brain Behav Immun 2016;57:200–8. [DOI] [PubMed] [Google Scholar]
- 17.Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr 2015. [DOI] [PubMed] [Google Scholar]
- 18.Rein D, Paglieroni TG, Pearson DA, et al. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr 2000;130:2120S–6S. [DOI] [PubMed] [Google Scholar]
- 19.Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr 2000;72:30–5. [DOI] [PubMed] [Google Scholar]
- 20.Actis-Goretta L, Leveques A, Rein M, et al. Intestinal absorption, metabolism, and excretion of (−)epicatechin in healthy humans assessed by using an intestinal perfusion technique. Am J Clin Nutr 2013;98:924–33. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Mateos A, Vauzour D, Krueger CG, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 2014;88:1803–53. [DOI] [PubMed] [Google Scholar]
- 22.Ottaviani JI, Borges G, Momma TY, et al. The metabolome of [2–14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep 2016;6:29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr 2008;88:1018–25. [DOI] [PubMed] [Google Scholar]
- 24.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief 2011:1–8. [PubMed] [Google Scholar]
- 26.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. Jama 2016;316:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use was very high among older adults in the United States in 2011–2014. J Nutr 2017;147:1968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med 2001;161:805–10. [DOI] [PubMed] [Google Scholar]
- 29.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. Washington DC: National Center for Health Statistics; 1999. Report No.: Vital Health Stat 11(244):i–14. [PubMed] [Google Scholar]
- 30.Li K, Kaaks R, Linseisen J, Rohrmann S. Vitamin/mineral supplementation and cancer, cardiovascular, and all-cause mortality in a German prospective cohort (EPIC-Heidelberg). Eur J Nutr 2012;51:40713. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011;173:906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson SC, Akesson A, Bergkvist L, Wolk A. Multivitamin use and breast cancer incidence in a prospective cohort of Swedish women. Am J Clin Nutr 2011;91:1268–72. [DOI] [PubMed] [Google Scholar]
- 33.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch Intern Med 2009;169:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens VL, McCullough ML, Diver WR, et al. Use of multivitamins and prostate cancer mortality in a large cohort of US men. Cancer Causes Control 2005;16:643–50. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs EJ, Connell CJ, Patel AV, et al. Multivitamin use and colon cancer mortality in the Cancer Prevention Study II cohort (United States). Cancer Causes Control 2001;12:927–34. [DOI] [PubMed] [Google Scholar]
- 36.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med 1998;129:517–24. [DOI] [PubMed] [Google Scholar]
- 37.Bailey RL, Fakhouri TH, Park Y, et al. Multivitamin-mineral use is associated with reduced risk of cardiovascular disease mortality among women in the United States. J Nutr 2015;145:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pocobelli G, Peters U, Kristal AR, White E. Use of supplements of multivitamins, vitamin C, and vitamin E in relation to mortality. Am J Epidemiol 2009;170:472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rautiainen S, Lee IM, Rist PM, et al. Multivitamin use and cardiovascular disease in a prospective study of women. Am J Clin Nutr 2015;101:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rautiainen S, Rist PM, Glynn RJ, Buring JE, Gaziano JM, Sesso HD. Multivitamin use and the risk of cardiovascular disease in men. J Nutr 2016;146:1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. Jama 2012;308:1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. Jama 2012;308:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauer MS, Gordon D, Wei G, Pearson G. Efficient design of clinical trials and epidemiological research: is it possible? Nature Reviews Cardiology 2017;14:493–501. [DOI] [PubMed] [Google Scholar]
- 44.Cauley JA, Crandall C. The Women’s Health Initiative: a landmark resource for skeletal research since 1992. J Bone Mineral Res 2020;35:845–60. [DOI] [PubMed] [Google Scholar]
- 45.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemporary clinical trials 2016;47:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 47.Bussy U, Hewitt G, Olanrewaju Y, et al. Single-Laboratory Validation for the Determination of Cocoa Flavanols and Procyanidins (by Degree of Polymerization DP1–7) in Cocoa-Based Products by Hydrophilic Interaction Chromatography Coupled with Fluorescence Detection: First Action 2020.05. Journal of AOAC INTERNATIONAL 2021;104:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run-in on the power of the Physicians’ Health Study. Stat Med 1991;10:1585–93. [DOI] [PubMed] [Google Scholar]
- 49.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1982;1:121–9. [DOI] [PubMed] [Google Scholar]
- 50.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 2003;13:S122–8. [DOI] [PubMed] [Google Scholar]
- 51.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 52.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–6. [DOI] [PubMed] [Google Scholar]
- 53.Rist PM, Sesso HD, Manson JE. Innovation in the design of large-scale hybrid randomized clinical trials. Contemporary clinical trials 2020;99:106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker LD, Rapp SR, Shumaker SA, et al. Design and baseline characteristics of the cocoa supplement and multivitamin outcomes study for the Mind: COSMOS-Mind. Contemporary clinical trials 2019;83:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brickman AM, Khan UA, Provenzano FA, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 2014;17:1798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sloan RP, Wall M, Yeung LK, et al. Insights into the role of diet and dietary flavanols in cognitive aging: results of a randomized controlled trial. Sci Rep 2021;11:3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hale C, Last BS, Meier IB, et al. The ModRey: An Episodic Memory Test for Nonclinical and Preclinical Populations. Assessment 2019;26:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peto R. Clinical trial methodology. Biomedicine 1978;28 Spec No:24–36. [PubMed] [Google Scholar]
- 59.Cox DR. Regression models and life-tables. J Royal Statist Soc B 1972;34:187–220. [Google Scholar]
- 60.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 61.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56:779–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.