Abstract
Purpose.
Pediatric obesity is a growing public health concern. Previous work has observed diet to impact nucleus accumbens (NAcc) inflammation in rodents, measured by the reactive proliferation of glial cells. Recent work in humans has demonstrated a relationship between NAcc cell density—a proxy for neuroinflammation—and weight gain in youth; however, the directionality of this relationship in the developing brain and association with diet remain unknown.
Methods.
Waist circumference (WC) and NAcc cell density were collected in a large cohort of children (n > 2,000) participating in the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (release 3.0) at baseline (9–10 y) and at a Year 2 follow-up (11–12 y). Latent change score modeling (LCSM) was used to disentangle contributions of baseline measures to two-year changes in WC percentile and NAcc cellularity. In addition, the role of NAcc cellularity in mediating the relationship between diet and WC percentile was assessed using dietary intake data collected at Year 2.
Results.
LCSM indicates that baseline WC percentile influences change in NAcc cellularity and that baseline NAcc cell density influences change in WC percentile. NAcc cellularity was significantly associated with WC percentile at Year 2 and mediated the relationship between dietary fat consumption and WC percentile.
Conclusions.
These results implicate a vicious cycle whereby NAcc cell density biases longitudinal changes in WC percentile, and vice versa. Moreover, NAcc cell density may mediate the relationship between diet and weight gain in youth. These findings suggest that diet-induced inflammation of reward circuitry may lead to behavioral changes that further contribute to weight gain.
More than 18% of youth and 39% of adults are overweight or obese worldwide [1]. Obesity increases the risk of several physical illnesses such as hypertension, type 2 diabetes, coronary heart disease, stroke, cancer, and mortality [2]. Childhood overweight and obesity is a strong predictor of obesity later in life [3] and has been linked to anxiety, depression, lower self-esteem, and lower self-reported quality of life [4]. Given the high prevalence and serious physical and mental health consequences, studies have sought to understand the biological risk factors underlying the development of obesity; however, the neurobiological mechanisms leading to excessive weight gain and obesity in youth remain less well understood.
The mesolimbic dopaminergic pathway, which includes the nucleus accumbens (NAcc) [5], is necessary for motivated behavior [6], such as eating behavior. Previous work has observed varying relationships between obesity-related metrics and NAcc structure and function. For example, NAcc volume is positively associated with genetic risk for obesity in children [7] as well as with body mass index (BMI) and percent body fat, although this relationship may be age-dependent and specific to youth [8,9]. Moreover, NAcc blood oxygen level-dependent (BOLD) responses to reward cues have demonstrated associations with obesity-related behaviors and outcomes (i.e., eating behavior, weight gain) in adolescents and adults [10–13], although findings are mixed and may be dependent on additional factors [14], such as the type of reward cue (e.g., monetary reward; food reward) [15,16], stimulus type (e.g., visual gustatory) [17], and metabolic factors (e.g., insulin sensitivity) [18]. Despite a clear role of the NAcc in reward motivation more generally, the associations between this region and obesity-related behaviors and outcomes appear to be complex and dependent on a variety of factors.
Animal models of diet-induced obesity have revealed microstructural differences in the NAcc indicative of neuroinflammation [19–21]. NAcc inflammation—measured by an increase in glial cells responding to proinflammatory factors and upregulation of pro-inflammatory genes—has been associated with highly palatable, caloric diets in rodents [20,21], and more specifically, has been linked to a high saturated fat diet and visceral fat accumulation in mice [19]. Critically, diet-induced NAcc inflammation has been shown to modify subsequent eating behavior in rodents, and reducing this inflammation through intervention has been shown to revert increased consumption of highly palatable foods, consequently reverting diet-induced weight gain [19,20]. Taken together, this work suggests that diet, and the consumption of highly palatable foods in particular, plays a crucial role in driving NAcc inflammation, which may, in turn, promote further unhealthy eating behavior and subsequent weight gain.
Detecting neuroinflammation via reactive gliosis in vivo presents a challenge in the human brain. However, recent methods allow researchers to provide an index of cell density in the brain using magnetic resonance imaging (MRI). More specifically, restriction spectrum imaging (RSI) is a non-invasive imaging technique based on diffusion MRI that separates signal contributions from intracellular (restricted) diffusion and extracellular (hindered) diffusion [22,23]. RSI provides histologically validated measures of cell density in subcortical brain structures [23]. Coupled with behavioral and cellular-level evidence from experiments conducted in animals, RSI may offer complementary insight into microscale properties in the human NAcc associated with diet and weight gain in youth. For example, RSI has been used to identify a relationship between individual differences in NAcc cell density and weight gain after one year in children [24], which may indicate diet-induced variability in glial cell density akin to those observed in animal models of obesity. Although this study demonstrates an association between NAcc cell density and weight gain across individuals, the extent to which weight gain contributes to—or reciprocally influences—changes in NAcc cellularity within an individual remains an open question. Moreover, the influence of diet on NAcc cellularity in humans remains untested.
Here we leverage longitudinal modeling in a large cohort of youth to examine directional relationships between two-year changes in NAcc cellularity and weight-related anthropometrics. BMI is a widely used index of body size and provides a clinical standard for classifying individuals according to physical thresholds (i.e., underweight, healthy weight, overweight, or obese) and according to normed growth charts in youth (e.g., BMI percentile). Although BMI percentile provides an important clinical metric for evaluating an individual’s body size relative to the general population, recent discussions have highlighted potential limitations of relying on this technique [25]. Relative to BMI, waist circumference (WC) may be more informative for estimating body fat and fat gain in youth [26] and may provide a better indicator of early risk for negative health outcomes such as cardiovascular disease and metabolic dysfunction [27,28], particularly when normed according to age and sex [28].
Longitudinal changes in WC percentile and RSI were evaluated using latent change score modeling (LCSM). LCSM takes advantage of the strengths of structural equation modeling (SEM) to estimate cross-domain coupling, or the contributions of baseline measurements on changes in longitudinal data [29,30]. By explicitly specifying change scores as latent variables, LCSMs allow for evaluating individual differences in intra-individual change across timepoints [29] and have been proposed to provide a powerful and flexible framework for understanding dynamic processes between brain and behavior underlying development [30].
Based on animal models demonstrating a vicious cycle of diet-induced inflammation of the NAcc followed by further unhealthy eating and weight gain [19,20], we hypothesized that NAcc cellularity and WC percentile would mutually influence each other. In other words, we expected NAcc cell density at baseline to predict two-year change in WC percentile and WC percentile at baseline to predict two-year change in NAcc cell density. Given the role of diet in driving neuroinflammation in animal models of obesity, we additionally hypothesized that NAcc cellularity would mediate the relationship between fatty diet and WC percentile in youth.
Methods
Data source
The Adolescent Brain Cognitive DevelopmentSM (ABCD) Study is an ongoing longitudinal study of brain development and child health in the United States, following over 11,000 9–10-year-olds through adolescence [31]. The baseline cohort was recruited from 21 sites using a rigorous epidemiologically informed school-based sampling and recruitment strategy, with the objective of approximating the demographic and socio-economic diversity of the U.S. population [32]. Data is collected from consenting parents and assenting children through yearly multimodal assessments including environmental, behavioral, physical health, and neurocognitive measures—as well as biennial structural and functional MRI scans. ABCD Study® recruitment, sample selection, complete battery of assessments, study design, and data collection are detailed elsewhere [33]. Study-wide exclusion criteria for enrollment included a diagnosis of moderate to severe autism spectrum disorder, schizophrenia, moderate to severe intellectual disability, major neurological disorders, or a substance use disorder at recruitment. Children with non-correctable vision, hearing, or sensorimotor impairments, gestational age less than 28 weeks, birth weight less than 1.2 kg, birth complications requiring more than a 1-month hospitalization, history of traumatic brain injury, and standard MRI contraindications (e.g., implanted metals, claustrophobia, orthodonture) were also excluded. All study procedures were approved by the participating study site Institutional Review Boards and by the ABCD Study centralized Institutional Review Board.
Participants
Analyses were conducted on data from the ABCD Study 3.0 release, which includes baseline data from 11,875 participants and 6,571 participants at a 2-year follow-up. In addition to study-wide exclusionary criteria, the current analysis excluded participants reporting a history of neurological disorders (e.g., cerebral palsy, seizures), concussion, diabetes, lead poisoning, muscular dystrophy, multiple sclerosis, and substance abuse, as well as participants presently or previously meeting diagnostic criteria for an eating disorder (anorexia nervosa, bulimia nervosa, binge eating disorder), schizophrenia, or psychosis (assessed using the K-SADS-PL) at baseline and/or at Year 2. Moreover, participants identified as having low quality anatomical images (ABCD NDA name: fsqc_qc) were further excluded from respective analyses at baseline and Year 2. For consistency within the data, only subjects whose data was acquired using MRI scanners from a single vendor (Siemens Healthineers AG, Erlangen, Germany) were included in our analysis (n=14 of 21 sites). To avoid extreme values due to potential measurement error, participants whose waist circumference, BMI, or NAcc cellularity fell outside of four standard deviations from the group mean at either time point (n=35) were excluded from further analysis. Participants with missing data for any variables of interest or covariates were further excluded, resulting in 2,378 participants with complete data at Year 2, and 2,333 participants with complete data at both baseline and Year 2 (44.6% female; mean [s.d.] age at baseline: 9.97 [0.61] years; mean [s.d.] at Year 2: 11.96 [0.63] years) (see Table 1 for complete participant demographics).
Table 1. Participant demographics.
Descriptive statistics for all variables of interest and covariates collected during baseline and/or Year 2. Values represent mean (s.d.) unless specified otherwise. Genetic ancestry scores were utilized for all analyses, but self-report race and ethnicity data are included here for ease of interpretability.
| Baseline | Year 2 | |||
|---|---|---|---|---|
| Anthropometries | Waist circumference (%ile) | 59.56 (28.88) | 62.04 (28.05) | |
| Body Mass Index (%ile) | 58.44 (30.52) | 60.95 (30.60) | ||
| Underweight (%) | 4.03 | 3.78 | ||
| Healthy weight | 68.71 | 65.14 | ||
| Overweight | 13.46 | 15.35 | ||
| Obese | 13.80 | 15.73 | ||
|
| ||||
| Brain imaging variables | NAcc cellularity | 0.21 (0.02) | 0.22 (0.02) | |
| NAcc volume (cm3) | 5.80 (0.88) | 5.76 (0.88) | ||
| Head motion (mm) | 1.29 (0.52) | 1.16 (0.44) | ||
|
| ||||
| Participant demographics | Age (yrs) | 9.97 (0.61) | 11.96 (0.63) | |
| Sex (%F) | 44.62 | – | ||
| Puberty (stage) | ||||
| Prepuberty | 54.35% | 22.54% | ||
| Early puberty | 23.23% | 26.07% | ||
| Mid puberty | 21.69% | 33.64% | ||
| Late puberty | 0.73% | 17.75% | ||
| Race/ethnicity (%) | ||||
| White | 64.63 | |||
| Black | 10.89 | – | ||
| Hispanic | 16.12 | – | ||
| Asian | 0.69 | – | ||
| Other | 7.67 | – | ||
| Parent marital status (%M) | 73.89 | – | ||
| Parent income (%) | – | |||
| < $50,000 | 24.13 | – | ||
| $50,000 to $100,000 | 32.23 | – | ||
| > $100,000 | 43.63 | – | ||
| Parent education (%) | – | |||
| No high school diploma | 2.10 | – | ||
| High school diploma or GED | 5.14 | – | ||
| Some college | 25.42 | – | ||
| Bachelor’s degree | 30.78 | – | ||
| Post-graduate degree | 36.56 | – | ||
|
|
|
|||
| Dietary intake | Dietary fat (g) | – | 49.90 (21.80) | |
| Dietary carbohydrates (g) | – | 142.08 (55.14) | ||
| Dietary protein (g) | – | 50.76 (21.48) | ||
| Dietary fiber (g) | – | 10.36 (4.58) | ||
| Dietary caloric intake (kcal) | – | 120.32 (46.85) | ||
Data acquisition and preprocessing
Waist circumference.
WC was measured on each participant at baseline and Year 2. Measurements were taken by placing a tape measure along the highest point of the pelvic bone and rounded to the nearest 0.1 inch. Measurements were collected twice and averaged to maximize accuracy. To account for differences in age- and sex-specific growth curves, resulting values were converted to percentiles based on data from the US National Health and Nutrition Survey (NHANES III) [28] using the R package childsds.
Body mass index.
Standing height and weight were measured using a stadiometer and digital scale, respectively. Measurements were collected twice and averaged to maximize accuracy. BMI was calculated for each participant using the following formula: weight (kg) / (height [cm] /100)2. Resulting BMI values were converted to CDC-standard percentiles and stratified according to BMI class using the R package PAutilities.
Diet.
Dietary fat consumption was estimated using the Block Kids Food Screener (BKFS). The BKFS is a parent-reported, youth-confirmed assessment measuring food intake over the previous week. Quantity and frequency for each of 39 food items are collected and immediately analyzed by NutritionQuest database for average daily intake of predetermined dietary variables based on the participant’s age-sex group. Dietary intake data was only collected at the Year 2 follow-up and was not collected at baseline.
Pubertal status.
Child pubertal status was assessed by self- and parent-report of physical development, yielding a categorical maturation score similar to that of Tanner staging.
Parent marital status, income, and education.
Parent-reported demographic covariates included total combined family income (less than $50,000; between $50,000 and $100,000; greater than $100,000), marital status (married; single); and parental years of education (No high school diploma; high school diploma or GED; Some college; Bachelor’s degree; Post-graduate degree).
Genetic ancestry.
Saliva samples were collected at baseline and immediately shipped to Rutgers University Cell and DNA Repository (RUCDR), where the sample was prepared for genotyping. Genotyping data for 733,293 single nucleotide polymorphisms (SNPs) was generated using the Affymetrix NIDA Smokescreen™ array.
Restriction spectrum imaging.
Diffusion images were acquired at baseline and Year 2 using a spin-echo EPI acquisition with TE/TR=88/4100 ms, multiband acceleration factor of 3, phase partial Fourier factor of 0.75, matrix size of 140x140, 81 slices, and an axial acquisition with 1.7-mm isotropic resolution. Diffusion-weighted data were acquired with 6 directions at b=500 s/mm2, 15 directions at b=1000 s/mm2, 15 directions at b=2000 s/mm2, and 60 directions at b=3000 s/mm2. The RSI model was fitted on a voxelwise basis at baseline and Year 2 using a linear estimation approach [22]. The NAcc was anatomically defined using automated atlas-based segmentation and used to extract NAcc-specific cellularity estimates. Subject-specific estimates of head motion (mean framewise displacement) during diffusion scans were included in all RSI analyses.
Longitudinal changes
Linear mixed-effects models (lme4) were used to quantify two-year changes in body measurements. Fixed effect covariates included age, sex, pubertal stage, genetic ancestry, parental education, income, and marital status. Random effects included subject ID and family ID nested within site. Two-year change in NAcc cellularity was similarly assessed with NAcc volume as an additional fixed covariate and family ID nested with scanner ID (rather than site) as a random effect.
Latent Change Score Modeling
Bivariate LCS modeling was performed using Lavaan in R and utilized publicly available code provided by Kievit et al. [30] (https://osf.io/4bpmq/). Two-year changes in WC percentile and NAcc cellularity were modeled as latent change scores to identify contributions of baseline measurements on respective outcomes. To provide a comparison to a clinical standard, two-year changes in BMI percentile were additionally modeled using an identical statistical framework (see supplemental materials). Models were computed using maximum likelihood estimation with robust standard errors and a Yuan-Bentler correction for non-normality.
Time-dependent covariates included in the model were age (mean-centered), pubertal stage, head motion (framewise-displacement) during respective RSI scans, and NAcc volume at baseline and Year 2. Time-independent covariates included sex, parental education, household income, parent marital status, and continuous genetic estimates of African, American, and Asian ancestry. Time-independent covariates were included for baseline WC percentile and NAcc cellularity. Time-dependent covariates were modeled as separate regressions with both WC percentile and NAcc cellularity for respective timepoints and were allowed to covary between timepoints. Variables were allowed to covary based on known associations (e.g., age and puberty) and as observed in the current dataset (see Figure S1). The LCSM was additionally computed without covariates to rule out the possibility that covariate adjustment led to false or misleading findings [34], without the inclusion of siblings (n = 301; randomly selected from each sibling pair) to ensure effects were not influenced by family structure, and with site-wise regressors (dummy coded as 0 or 1) to account for potential differences across sites.
Based on a two-index presentation strategy recommended by Hu & Bentler [35], LCSM fit was assessed using the Comparative Fit Index (CFI) and Root Mean Square Error of Approximation (RMSEA). CFI is a relative measure of model fit, comparing the hypothesized model to an unstructured baseline model and adjusting for sample size. CFI ranges from 0 to 1 with scores greater than 0.95 indicating a good fit. RMSEA is an absolute measure of model fit that compares the hypothesized model to the population covariance matrix, with values ranging from 0 to 1 and scores less than 0.06 indicating a close fit. Effects of interest were bootstrapped (5,000 iterations) to estimate 95% confidence intervals, and path coefficients were standardized to allow for interpretability across variables included in the model.
Mediation analysis
To further probe the relationship between NAcc cellularity and obesity-related outcomes, a mediation analysis was performed. Diet information obtained at the Year 2 follow-up allowed for a secondary analysis of the relationship between diet and WC percentile, mediated by NAcc cell density. Dietary fat was used to assess this relationship based on animal literature demonstrating the role of a high-fat diet in promoting NAcc inflammation [19,20] and subsequent weight gain. Dietary fat was normalized by total caloric consumption to obtain an estimate of relative dietary fat consumed. Non-fat macronutrients (dietary carbohydrates, protein, and fiber—each normalized by total caloric consumption) and total caloric intake were used for comparison to demonstrate specificity to dietary fat.
For consistency across analyses, Lavaan was used to perform the mediation analysis within an SEM that included all time-independent covariates used in the LCSM (i.e., sex, genetic ancestry, parental income, marital status, and education) as well as time-dependent covariates at Year 2 (i.e., age, puberty, head motion, and NAcc volume). Non-imaging covariates were regressed with dietary fat.
Results
Longitudinal change
WC percentile was significantly correlated with BMI percentile at baseline (Pearson’s r = 0.69; 95% CI: [0.67, 0.71]; p < 0.0001) and at Year 2 (Pearson’s r = 0.76; 95% CI: [0.74, 77]; p < 0.0001). Two-year change in WC percentile was significantly correlated with two-year change in BMI percentile (Pearson’s r = 0.34; 95% CI: [0.30, 0.37]). Baseline WC percentile (Figure 1A) and BMI percentile (Figure 1B) significantly increased at the Year 2 follow-up visit (WC: β = 2.46; SE = 0.47; t = 5.26; p < 0.0001; BMI: β = 2.24; SE = 0.34; t = 6.68; p < 0.0001). Likewise, NAcc cell density significantly increased after two years (β = 0.011; SE = 0.0003; t = 38.50; p < 0.0001) (Figure 1C).
Figure 1.
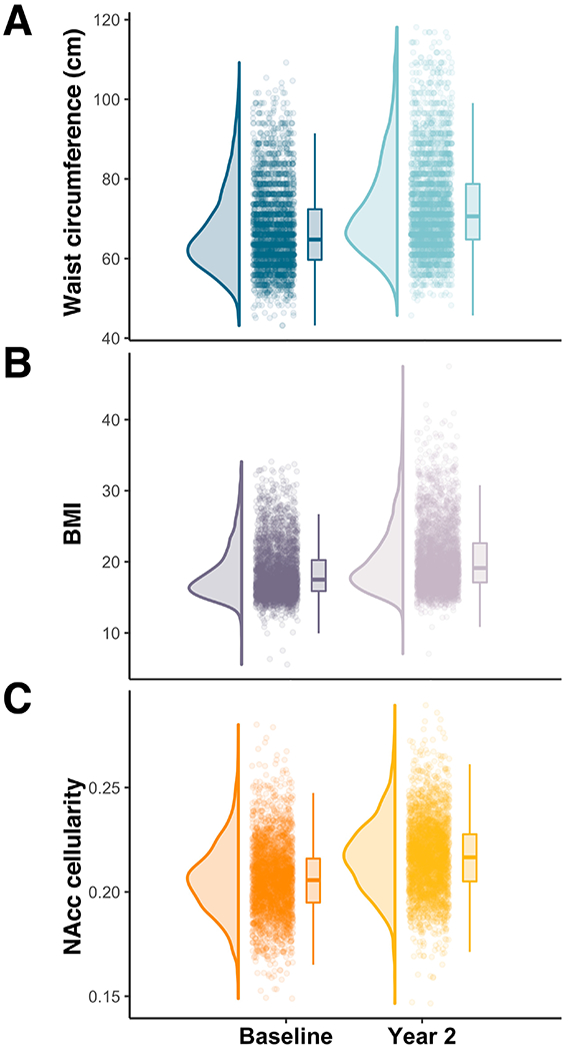
Two-year change in waist circumference and NAcc cellularity. Raincloud plots of waist circumference (A), BMI (B), and RSI-based NAcc cellularity (C) at baseline and at Year 2 follow-up. Sex-specific WC and BMI distributions are visualized in Figure S2.
Latent Change Score Model
The LCSM demonstrated a good model fit (CFI = 0.98; Root Mean Square Error of Approximation [RMSEA] = 0.039; 90% CI: [0.036, 0.041]; p <= 0.05 = 1.0) (Figure 2). Baseline measures of both WC percentile and NAcc cellularity negatively predicted two-year changes in respective measures (WC: β = −0.49; p < 0.0001; NAcc cellularity: β = −0.43; p < 0.0001), such that higher values at baseline corresponded with smaller change scores. Cross-domain paths demonstrated that baseline WC percentile predicted two-year change in NAcc cellularity (β = 0.10; 95% CI = [0.06, 0.14]; p < 0.0001) and that NAcc cellularity predicted two-year change in WC percentile (β = 0.08; 95% CI = [0.04, 0.12]; p = 0.001). Although the effect size for the relationship between WC percentile and change in NAcc cellularity was stronger, bootstrapped confidence intervals demonstrate no difference in strength of cross-domain parameter estimates. Using the likelihood ratio test, model fit significantly decreased when either cross-domain path was constrained to zero (baseline WC percentile: Δχ2(1) = 24.19; p < 0.0001; baseline NAcc cellularity: Δχ2(1) = 17.24; p < 0.0001).
Figure 2.
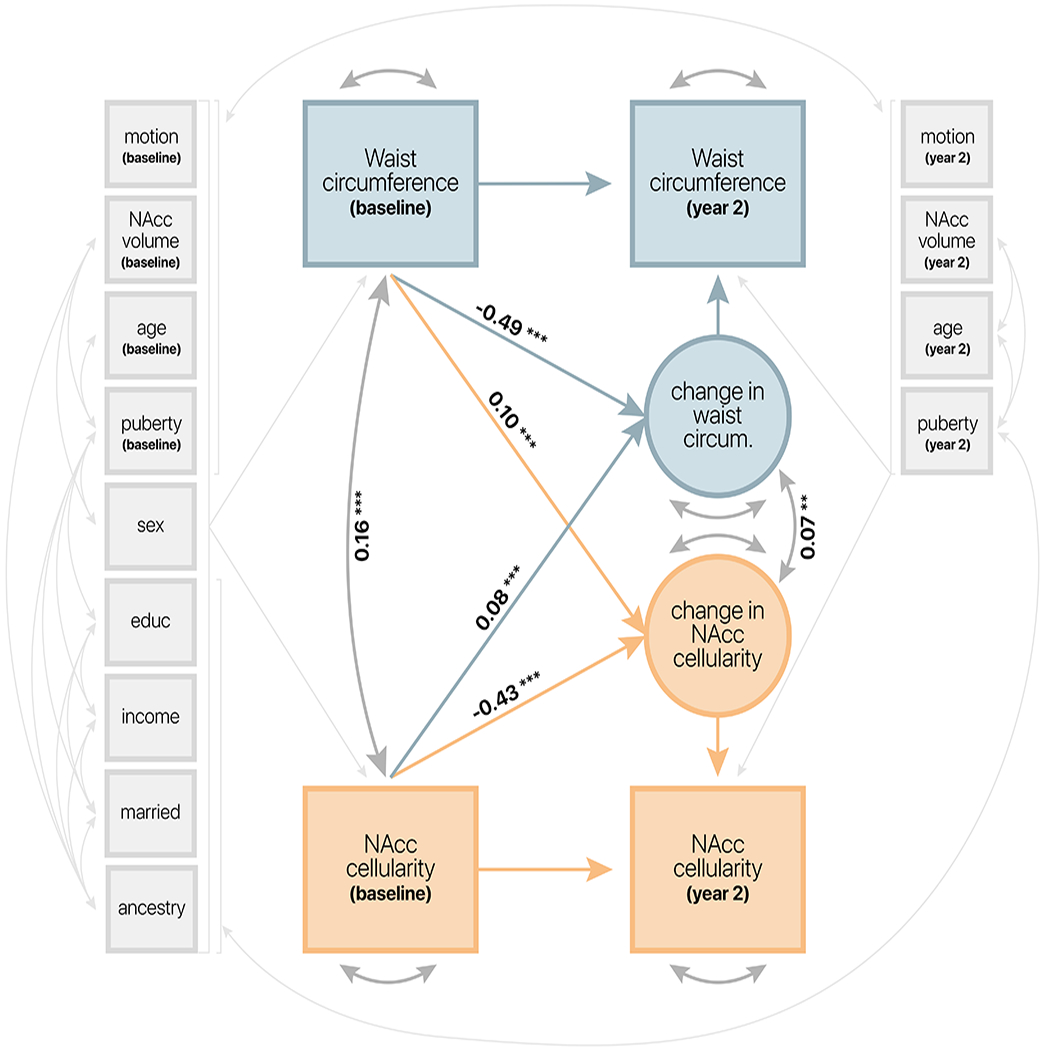
Latent change score model demonstrating longitudinal associations between waist circumference percentile and NAcc cellularity. Standardized coefficients plotted for paths of interest. Significance represented as p < 0.0001 (***); p < 0.001 (**). Thin light gray arrows represent covariate paths of no interest.
Consistent with previous work [24], WC percentile and NAcc cellularity were significantly associated at baseline (β = 0.16; p < 0.0001). Moreover, there was a significant association between latent change scores for WC percentile and NAcc cellularity (β = 0.07; p < 0.001). Excluding all covariates from the LCSM (Figure S3), excluding siblings (Figure S4), or including site-wise regressors (Figure S5) did not affect the interpretation of results, suggesting that the effects observed here are robust to the inclusion of covariates, siblings, and potential site differences. Similar effects were also observed when considering change in BMI percentile (see supplemental materials) regardless of whether covariates were included (Figure S6) or excluded (Figure S7).
Association with diet at Year 2
Anthropometrics such as waist circumference and BMI may serve as a proxy for the cumulative impact of diet; however, directly testing the contribution of diet on NAcc cellularity is needed to understand the potential role of diet-induced neuroinflammation on childhood weight gain. Food intake data collected at Year 2 allowed for a more explicit evaluation of the associations between diet, NAcc cellularity, and anthropometrics. An analysis of NAcc cellularity mediating the relationship between diet and WC percentile demonstrated good model fit (CFI = 0.97; RMSEA = 0.035; 90% CI: [0.031, 0.039]; p <= 0.05 = 1.0).
Dietary fat was significantly associated with WC percentile at Year 2 (total effect [path c]: β = 0.07; 95% CI = [0.03, 0.11]; p < 0.001) and was fully mediated by NAcc cellularity (direct effect [path c’]: β = 0.036; 95% CI = [−0.002, 0.07]; p = 0.06). Dietary fat was associated with NAcc cellularity (path a: β = 0.049; 95% CI = [0.01, 0.08]; p = 0.008), and consistent with previous findings at baseline and Year 1 [24], NAcc cellularity was associated with WC percentile at Year 2 (path b: β = 0.18; 95% CI = [0.15, 0.22]; p < 0.0001). The indirect effect of NAcc cellularity on the relationship between dietary fat and WC percentile was significant (a*b: β = 0.01; 95% CI = [0.003, 0.02]; p < 0.01; proportion mediated = 0.20), suggesting a role of NAcc cell density in mediating diet-induced weight gain in youth. Similar results were observed using BMI percentile (see supplemental results; Figure S8).
As a comparison, additional mediation models were tested using non-fat macronutrients (dietary carbohydrates, protein, fiber) and total caloric intake as independent variables in identical models. Among non-fat macronutrients and caloric intake, only dietary fat showed a significant positive relationship with WC percentile that was mediated by NAcc cellularity (see Supplemental material). Dietary carbohydrates demonstrated an inverse association with NAcc cellularity and waist circumference (Figure S9), and no significant mediations were observed with dietary fiber, protein, or total caloric intake.
Discussion
Consistent with the “vicious cycle” hypothesis of diet-induced brain changes promoting unhealthy eating and weight gain [36], the current study observed reciprocal influences of baseline waist circumference percentile (as well as BMI percentile) and NAcc cellularity on two-year changes in the converse measures. In addition, NAcc cell density was found to mediate the relationship between diet and WC percentile at the Year 2 follow-up. These findings replicate and extend prior work linking NAcc cellularity and weight gain in youth [24] by further demonstrating the longitudinal associations between obesity-related metrics (e.g., WC) and the microstructural properties of the developing brain. Moreover, these findings mirror rodent models of obesity that have demonstrated diet-induced neuroinflammation of the NAcc, marked by an increase in glial cell proliferation [19,20]. Taken together with previous human and animal literature [19,24], the current study suggests that diet influences NAcc inflammation, which may in turn contribute to further unhealthy eating and weight gain (Figure 4). Future work is needed to disentangle potential mechanisms underlying this cycle and to more explicitly test relationships within this framework that were not directly assessed in the current study.
Figure 4.
Cartoon schematic illustrating the proposed cycle of a highly palatable diet contributing to neuroinflammation, particularly within brain regions associated with reward (e.g., the NAcc), subsequently influencing behavioral changes in eating behaviors and increases in weight gain.
Despite the evidence supporting weight-related changes in NAcc inflammation observed here, the mechanisms underlying these proposed inflammatory changes remain unclear. The production of proinflammatory cytokines by white adipose tissue is a major source of obesity-related inflammation [37]. Diets that are high in fat and sugar promote abdominal fat accumulation, and thus greater waist circumference, to favor local immune responses that can propagate to the brain [38]. Previous studies have found that prolonged exposure to a highly palatable (i.e., high-fat, high-sugar) diet additionally produces neuroplastic and functional changes in the NAcc that influence behavior. For example, Gutiérrez-Martos et al. [20] examined the NAcc of mice fed a “cafeteria” diet and observed an increase in the expression of inflammatory cytokines (IL-1β, IFN-γ) as well as a morphological change in microglia characteristic of a reactive state. This diet-induced neuroinflammation was associated with increased consumption of calories and a corresponding increase in body weight. However, these behavioral changes reversed when inflammation and microglia activation was reduced via systemic administration of minocycline, a broad inhibitor of peripheral and central inflammation. Importantly, inflammatory responses were accompanied by structural changes in dendritic cell density, which may contribute to functional differences in the rewarding effects of food and food-motivated behaviors.
A number of studies have begun to explore the possibility that the impact of palatable foods on neural plasticity [39], as well as on neuroinflammatory responses [40], may contribute to altered emotional and cognitive processing [41], ultimately giving rise to dysfunctions in learning and memory, mood regulation, and compulsive behaviors. The direct contribution of NAcc inflammation to heightened food-seeking in obesity is underscored by observations of reduced compulsivity for sugar in diet-induced obese mice with targeted genetic inhibition of inflammation in the NAcc [19]. In another study, increased dendritic spine density within the NAcc was associated with the consumption of palatable foods—independent of caloric content—and occurred specifically in mice demonstrating enhanced food-seeking behaviors [42]. Although the consumption of high palatable foods appears to drive NAcc inflammation, different classes of macronutrients have been shown to differentially impact inflammatory responses. For example, several studies have found that a high-fat diet leads to increased neuroinflammation [20,43–45], and NAcc inflammation has been linked specifically to saturated fats [19]. Moreover, the current study observed an inverse association between WC percentile and dietary carbohydrates—an association that was negatively mediated by NAcc cell density (Figure S9). Future work should aim to further disentangle the contributions of various macronutrients on neuroinflammation, as well as consider a possible protective mechanism whereby certain macronutrients buffer against neuroinflammation.
Another potential mechanism by which this cycle may occur is in interactions with neuroinflammation in the hypothalamus and subsequent differences in hypothalamic neuroendocrine and metabolic regulation. Hypothalamic inflammation accelerates energy imbalance [46], interferes with the ability to regulate food intake [44], and has been suggested to modify hypothalamic circuitry and interfere with outputs to other brain regions—including regions involved in reward-processing [45] and eating behavior [47]. As a result, the hypothalamus has been a key target for investigations of diet-induced neuroinflammation in rodents, which suggest that prolonged exposure to high-fat diets and associated metabolic dysfunction are drivers that sustain neuroinflammation in subcortical structures [43,48]. Methodological challenges have precluded replicable segmentation of the hypothalamus in human neuroimaging, and thus hypothalamus-specific cellularity estimates are not available in the ABCD Study release 3.0 data. However, recent advances in machine learning have allowed for the development of new segmentation algorithms that are capable of automated hypothalamic segmentation using a deep convolutional neural network [49]. As tools such as these become more readily available, future work will be able to examine potential relationships between diet and neuroinflammation of the hypothalamus and NAcc in humans.
Several limitations of the current study warrant further consideration. First, although the study motivation and interpretation are based on prior work conducted in animal models of obesity and neuroinflammation [19], the ABCD Study release 3.0 dataset does not include direct markers of neuroinflammation. Recent work has used diffusion-based spectrum imaging (DBSI) to relate imaging markers of striatal neuroinflammation to self-report emotional eating and obesity in adults [50]. DBSI provides biomarkers of inflammation by characterizing water diffusion properties associated with axon/myelin injury and inflammation [51] and may provide convergent information alongside RSI-based measures of cell density. However, additional work is needed to directly quantify and assess the relationship between eating behavior, obesity, and in vivo neuroinflammation in humans. Human neuroimaging has demonstrated relationships between food reward sensitivity in the NAcc and genetic risk for obesity [7], eating behavior [52], and weight gain [53] in youth, yet it remains unclear how these findings might relate to potential diet-induced inflammatory factors. Future studies are needed to integrate prior work in animals with human studies examining reward-related brain function and behavior.
Second, waist circumference measurements are prone to measurement error. Variation in WC measurements may be due to inconsistencies across experimenters, differences in lean muscle and bone mass, and difficulty locating anatomical landmarks in overweight participants [54]. The current study sought to identify potential measurement errors by excluding individuals with waist circumference values (and BMIs) outside of four standard deviations; however, this exclusion criteria does not identify inaccurate values within four standard deviations. Moreover, waist circumference and BMI—even when accurately measured—may not adequately capture adiposity levels relative to more direct measures (e.g., dual-energy X-ray absorptiometry) [55]. Future work should consider additional anthropometrics that more directly and accurately measure fat mass associated with negative health outcomes.
Third, to test a specific hypothesis regarding dietary fat based on prior work in animal models [19], the current study utilized self-report dietary intake data to estimate the relative amount of dietary fat an individual consumes daily. However, individuals tend to poorly estimate their energy intake and commonly underestimate the number of calories they consume [56]. Although unavailable in the current ABCD Study data release (3.0), future work should consider assessing dietary metabolites and corresponding blood markers directly (e.g., LDL cholesterol levels increased by the consumption of saturated and trans fats) as an alternative to self-report intake data.
Fourth, the current study examined relationships between NAcc cellularity and non-fat macronutrients (carbohydrates, fiber, protein) to consider the specificity of the observed relationship to dietary fat. We found that NAcc cellularity fully mediated the relationship between WC percentile and dietary fat intake only, and did not mediate relationships with dietary fiber, protein, or overall caloric intake. However, we additionally observed a partial negative mediation with dietary carbohydrates (supplemental results) such that carbohydrate intake was inversely associated with NAcc cellularity and WC percentile at Year 2 (Figure S9). Although these findings suggest that dietary fat intake may increase neuroinflammation and weight gain—and that dietary carbohydrates may buffer against these effects—additional work is needed to further examine these relationships. Macronutrients are not consumed in isolation and combinations of different food groups can have synergistic effects on an individual’s health. Thus, caution is necessary in interpreting these single-nutrient results. In addition to measuring blood markers of diet and health, future work should consider evaluating an index of the overall pattern of diet, which may provide a more informative indicator of diet quality.
Finally, the interpretation of our results is constrained by the selection of variables and inclusion criteria of participants. For example, the current study does not incorporate other factors that may influence dietary intake—such as physical activity, access to healthy foods, and medications that affect appetite. In addition, the current study exclusively examines data from participants scanned using a single MRI manufacturer (Siemens). Although excluding MRI data from other scanner manufacturers (Philips and GE) reduces potential confounds due to scanner platform, doing so may inadvertently bias the sample demographics such that participants from underrepresented groups or with limited access to resources are disproportionately excluded. Future work should take into consideration additional covariates, inclusion criteria (e.g., non-Siemens data), and exclusionary criteria (e.g., medications) that may impact individual differences in diet and how these relate to neuroinflammation in youth.
Taken together, the current study builds on previous literature in animals and in humans to understand the relationship between neuroinflammation of the NAcc and diet-induced weight gain. We observed longitudinal changes in NAcc cellularity to be reciprocally influenced by changes in WC percentile, and further demonstrated a role of NAcc cell density in mediating the relationship between dietary fat intake and WC percentile. These findings extend prior work linking NAcc cell density with weight gain in youth [24] and suggest that diet plays an important role in neurodevelopmental changes that may influence eating behavior. Given that adolescence is characterized by neurodevelopmental changes as well as a heightened sensitivity to learning [57] and the formation of habits [58] that can affect later health outcomes, it is crucial to understand the impact of diet on the developing brain as well as the behavioral consequences. Understanding the role of diet-induced neuroinflammation on the developing brain may provide key insight into interventions that can mitigate pediatric obesity.
Supplementary Material
Figure 3.
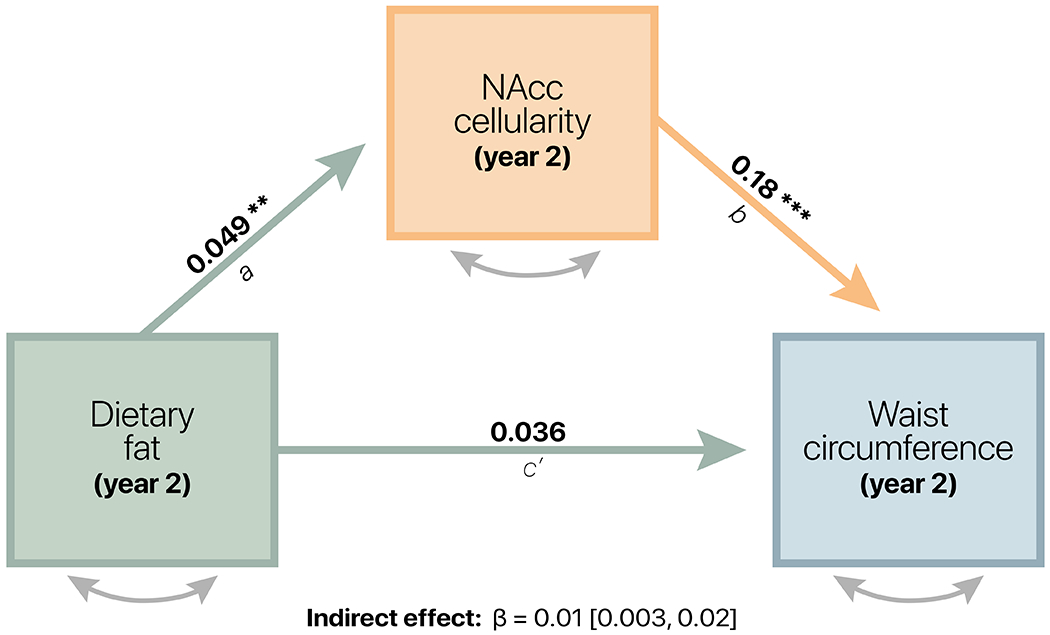
Mediation model. NAcc cellularity significantly mediated the relationship between dietary fat and waist circumference percentile at Year 2. Path coefficients are standardized, and significance is represented as p < 0.0001 (***); p < 0.01 (**). Covariates were consistent with those included in the LCSM at Year 2.
Implications and Contribution summary statement.
Neuroinflammation of reward circuitry both predicts and is predicted by weight gain in youth—a vicious cycle that may be associated with a high-fat diet. Identifying early predictors and consequences of diet-induced neuroinflammation may provide insight into interventions that have the potential to interrupt this cycle prior to obesity onset.
Acknowledgments
Data used in the preparation of this article were obtained from the ABCD study (https://abcdstudy.org), held in the National Institute of Mental Health (NIMH) Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children ages 9 to 10 and follow them over 10 y into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NIMH Data Archive Digital Object Identifier DOI 10.15154/1519007. DOIs can be found at https://nda.nih.gov/study.html?id=901. This work was supported in part by U01DA041174 (to B.J.C.) and R01DK097399-01 (to K.M.R.). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements
All authors declare no conflicts of interest.
Data sharing
ABCD data are publicly available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The ABCD data used in this report came from the ABCD Data Release 3.0 (DOI: 10.15154/1519007, November 2020).
References
- [1].Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. AccessedApril 21, 2021.
- [2].National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Institutes of Health; 1998. [Google Scholar]
- [3].Simmonds M, Llewellyn A, Owen CG, et al. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 2016;17:95–107. [DOI] [PubMed] [Google Scholar]
- [4].Puder JJ, Munsch S. Psychological correlates of childhood obesity. Int J Obes 2010;34 Suppl 2:S37–43. [Google Scholar]
- [5].Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 2007;191:391–431. [DOI] [PubMed] [Google Scholar]
- [7].Rapuano KM, Zieselman AL, Kelley WM, et al. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci U S A 2017;114:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kakoschke N, Lorenzetti V, Caeyenberghs K, et al. Impulsivity and body fat accumulation are linked to cortical and subcortical brain volumes among adolescents and adults. Sci Rep 2019;9:2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].García-García I, Morys F, Dagher A. Nucleus accumbens volume is related to obesity measures in an age- dependent fashion. J Neuroendocrinol 2019;2019:1. [Google Scholar]
- [10].Rapuano KM, Huckins JF, Sargent JD, et al. Individual Differences in Reward and Somatosensory-Motor Brain Regions Correlate with Adiposity in Adolescents. Cereb Cortex 2015. [Google Scholar]
- [11].Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 2012;32:5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lawrence NS, Hinton EC, Parkinson JA, et al. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 2012;63:415–22. [DOI] [PubMed] [Google Scholar]
- [13].Lopez RB, Hofmann W, Wagner DD, et al. Neural Predictors of Giving in to Temptation in Daily Life. Psychol Sci 2014:0956797614531492 –. [Google Scholar]
- [14].Morys F, García-García I, Dagher A. Is obesity related to enhanced neural reactivity to visual food cues? A review and meta-analysis. Social Cognitive and Affective Neuroscience 2020. [Google Scholar]
- [15].Adise S, Allgaier N, Laurent J, et al. Multimodal brain predictors of current weight and weight gain in children enrolled in the ABCD study ®. Developmental Cognitive Neuroscience 2021;49:100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verdejo-Román J, Vilar-López R, Navas JF, et al. Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Hum Brain Mapp 2017;38:666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kroemer NB, Sun X, Veldhuizen MG, et al. Weighing the evidence: Variance in brain responses to milkshake receipt is predictive of eating behavior. Neuroimage 2016;128:273–83. [DOI] [PubMed] [Google Scholar]
- [19].Décarie-Spain L, Sharma S, Hryhorczuk C, et al. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol Metab 2018;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gutiérrez-Martos M, Girard B, Mendonça-Netto S, et al. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict Biol 2018;23:735–49. [DOI] [PubMed] [Google Scholar]
- [21].Molina J, Joaquim A, Bonamin LV, et al. Reduced astrocytic expression of GFAP in the offspring of female rats that received hypercaloric diet. Nutr Neurosci 2018:1–11. [Google Scholar]
- [22].White NS, McDonald CR, Farid N, et al. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol 2013;34:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].White NS, Leergaard TB, D’Arceuil H, et al. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum Brain Mapp 2013;34:327–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rapuano KM, Laurent JS, Hagler DJ, et al. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proceedings of the National Academy of Sciences 2020;117:26977–84. [Google Scholar]
- [25].Hendrickson MA, Pitt MB. Three Areas Where Our Growth Chart Conversations Fall Short—Room to Grow. JAMA Pediatr 2021. [Google Scholar]
- [26].Brambilla P, Bedogni G, Heo M, et al. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes 2013;37:943–6. [Google Scholar]
- [27].Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020;16:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharma AK, Metzger DL, Daymont C, et al. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardiometabolic risks. Pediatr Res 2015;78:723–9. [DOI] [PubMed] [Google Scholar]
- [29].McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol 2009;60:577–605. [DOI] [PubMed] [Google Scholar]
- [30].Kievit RA, Brandmaier AM, Ziegler G, et al. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Dev Cogn Neurosci 2018;33:99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Compton WM, Dowling GJ, Garavan H. Ensuring the Best Use of Data: The Adolescent Brain Cognitive Development Study. JAMA Pediatr 2019. [Google Scholar]
- [33].Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience 2018;32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kraemer HC. A Source of False Findings in Published Research Studies. JAMA Psychiatry 2015;72:961. [DOI] [PubMed] [Google Scholar]
- [35].Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 1999;6:1–55. [Google Scholar]
- [36].Hargrave SL, Jones S, Davidson TL. The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci 2016;9:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bastard J-P, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006;17:4–12. [PubMed] [Google Scholar]
- [38].Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morin J-P, Rodríguez-Durán LF, Guzmán-Ramos K, et al. Palatable Hyper-Caloric Foods Impact on Neuronal Plasticity. Front Behav Neurosci 2017;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Castanon N, Luheshi G, Layé S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Frontiers in Neuroscience 2015;9. [Google Scholar]
- [41].Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes 2013;37:382–9. [Google Scholar]
- [42].Guegan T, Cutando L, Ayuso E, et al. Operant behavior to obtain palatable food modifies neuronal plasticity in the brain reward circuit. European Neuropsychopharmacology 2013;23:146–59. [DOI] [PubMed] [Google Scholar]
- [43].Thaler JP, Yi C-X, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 2009;296:E1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun 2014;42:10–21. [DOI] [PubMed] [Google Scholar]
- [46].Zhang X, Zhang G, Zhang H, et al. Hypothalamic IKKβ/NF-κB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell 2008;135:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cazettes F, Cohen JI, Yau PL, et al. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res 2011;1373:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Waise TMZ, Toshinai K, Naznin F, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 2015;464:1157–62. [DOI] [PubMed] [Google Scholar]
- [49].Billot B, Bocchetta M, Todd E, et al. Automated segmentation of the hypothalamus and associated subunits in brain MRI. Neuroimage 2020;223:117287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Samara A, Li Z, Rutlin J, et al. Nucleus accumbens microstructure mediates the relationship between obesity and eating behavior in adults. Obesity 2021;29:1328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain 2011;134:3590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gearhardt AN, Yokum S, Harris JL, et al. Neural response to fast food commercials in adolescents predicts intake. Am J Clin Nutr 2020;111:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yokum S, Gearhardt AN, Harris JL, et al. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity 2014;22:2544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Verweij LM, Terwee CB, Proper KI, et al. Measurement error of waist circumference: gaps in knowledge. Public Health Nutr 2013;16:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009;89:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schoeller DA. Limitations in the assessment of dietary energy intake by self-report. Metabolism 1995;44:18–22. [Google Scholar]
- [57].Davidow JY, Foerde K, Galván A, et al. An Upside to Reward Sensitivity: The Hippocampus Supports Enhanced Reinforcement Learning in Adolescence. Neuron 2016;92:93–9. [DOI] [PubMed] [Google Scholar]
- [58].Decker JH, Otto AR, Daw ND, et al. From Creatures of Habit to Goal-Directed Learners: Tracking the Developmental Emergence of Model-Based Reinforcement Learning. Psychol Sci 2016;27:848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ABCD data are publicly available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The ABCD data used in this report came from the ABCD Data Release 3.0 (DOI: 10.15154/1519007, November 2020).


