Abstract
Background
Factors associated with stroke mortality are understudied in sub-Saharan Africa but have implications for designing interventions that improve stroke outcomes. We investigated predictors of in-hospital and 90-day post-discharge stroke mortality in Lusaka, Zambia.
Methods
Data from consecutive adults admitted with stroke at University Teaching Hospital in Lusaka, Zambia between October 2018 and March 2019 were retrospectively reviewed for clinical in-hospital outcomes. Vital status at 90-days post-discharge was determined through phone calls. Factors associated with stroke mortality were included in multivariable logistic regression models utilizing multiple imputation analysis to determine independent predictors of in-hospital and 90-days post-discharge mortality.
Results
In-hospital mortality was 24%, and 90-day post-discharge mortality was 22% among those who survived hospitalization. Hemorrhagic and unknown strokes, ICU care, seizures, and aspiration pneumonia were significantly associated with in-hospital mortality. Among these, hemorrhagic stroke (OR 2.88, 95% CI 1.27–6.53, p=0.01) and seizures (OR 29.5, 95% CI 2.14–406, p=0.01) remained independent predictors of in-hospital mortality in multivariable analyses. Ninety-day post-discharge mortality was significantly associated with older age, previous stroke, atrial fibrillation, and aspiration pneumonia, but only older age (OR 1.04, 95% CI 1.01–1.06, p=0.007) and aspiration pneumonia (OR 3.93, 95% CI 1.30–11.88, p=0.02) remained independently associated with 90-day mortality in multivariable analyses.
Conclusion
This Zambian stroke cohort had high in-hospital and 90-day post-discharge mortality that were associated with several in-hospital complications. Our data indicate the need for improvement in both acute stroke care and post-stroke systems of care to improve stroke outcomes in Zambia.
Keywords: Predictors of stroke mortality, In-hospital mortality, Post-discharge mortality, Zambia, Stroke care delay
Introduction
Stroke is the second leading cause of mortality worldwide [1]. In high-income countries (HICs), a decreasing trend in both acute and post-discharge stroke-related mortality have resulted from a variety of interventions, including concerted efforts to control modifiable stroke risk factors such as hypertension and diabetes; development of stroke units that improve post-stroke functional outcome and reduce the occurrence of in-hospital complications such as aspiration pneumonia; enhanced acute endovascular interventions that lead to improved long-term outcomes; and optimization of post-discharge rehabilitations services [2]. On the contrary, stroke incidence and mortality are increasing in low- and middle-income countries (LMICs), including countries in sub-Saharan Africa (SSA) where in-hospital stroke mortality ranges from 20%- 40% [3]. Factors contributing to increased stroke mortality in SSA include limited diagnosis and sub-optimal control of common stroke risk factors such as hypertension and diabetes, limited access to acute stroke interventions such as thrombolysis and thrombectomy, limited resources to prevent and treat in-hospital complications of stroke, few available stroke units, and few post-stroke rehabilitation systems [4].
In addition to high rates of in-hospital mortality, individuals in SSA who survive their hospitalization for acute stroke continue to be at high risk of morbidity and mortality after their discharge. Potential post-discharge complications leading to significant morbidity include recurrent stroke, aspiration pneumonia, the development of decubitus ulcers or deep vein thromboses secondary to mobility difficulties, cognitive decline, and emotional or behavioral changes. However, the limited data on stroke epidemiology and outcomes from the SSA region hinders development of targeted interventions and therapies to improve post-discharge stroke outcomes [3].
Zambia is an SSA country with a high stroke burden and high stroke-related mortality [5]. We previously described the significant stroke burden at the University Teaching Hospital (UTH), the 1600 bed national referral hospital in the capital city of Lusaka and the only hospital in the country with a dedicated neurology service [6]. Adults with stroke account for 43% of all inpatient admissions to the neurology inpatient service at UTH. In-hospital post-stroke complications such as aspiration pneumonia are frequent (16%), and in-hospital mortality is high (24%) [6]. In this work, we investigated factors associated with in-hospital and 90-day post-discharge mortality at UTH.
Methods
Study Population and Setting.
We conducted a retrospective cohort study of adults (≥ 18 years old) admitted to the neurology service at UTH, Zambia’s national referral hospital, between October 2018 and March 2019. As a tertiary center, patients are referred to UTH for specialist care, including neurological care, from across Zambia. In this study, patients were included in the study if their stroke was confirmed on computed tomography (CT) imaging or if clinical suspicion for stroke was high by the evaluating neurologist in the absence of imaging. This cohort and detailed study procedures have been previously described in detail [6].
Study Procedures.
Data from individuals meeting inclusion criteria were retrospectively retrieved and included demographics, clinical presentation, medical co-morbidities, family history, social history, neurological examination findings on presentation, laboratory and imaging results, and final stroke subtype classification. Stroke was classified as ischemic or hemorrhagic stroke based on CT imaging and as unknown stroke if neuroimaging was not obtained during the admission. Intracerebral hemorrhage (ICH) score [7] was collected for participants with hemorrhagic stroke. Hospitalization details including length of stay, ICU care, and diagnoses of aspiration pneumonia were also extracted. Further details on the study protocol and variable definitions have been previously described [6]. Of note, measures of stroke severity, including the NIH Stroke Scale (NIHSS) and modified Rankin Scale (mRS), were not routinely collected at UTH during this study period, so these details were not available for analysis. At discharge, patients were given an appointment for a three-month follow-up in the hospital’s neurology outpatient clinic. Patients received a reminder phone call a few days before their scheduled appointment. If a patient was deceased at the time of the phone call, this was recorded in the clinic register. Patient follow-up dates, phone calls to patients, and attendance on clinic days are in hand-written registers available in the outpatient clinic. We matched individuals from our in-hospital stroke cohort to the clinic registers to ascertain vital status at 90-days after patients’ discharge from UTH. Mortality is reported as in-hospital mortality if death occurred during the participant’s initial hospitalization, 90-day post-discharge mortality if the individual survived their hospitalization but died in the first 90-days after discharge, and 90-day total mortality which includes both in-hospital and post-discharge mortality.
Statistical Analyses.
Means and standard deviations were used to describe parametric continuous variables while medians and interquartile ranges and proportions were used to describe non-parametric continuous variables and categorical variables, respectively. Potential predictors of in-hospital and 90-day post-discharge stroke mortality were initially analyzed using Student’s t-tests for parametric continuous variables, Wilcoxon rank-sum tests for non-parametric continuous variables, and Chi-square or Fisher’s exact tests for categorical variables. All variables with p<0.20 for their association with mortality were then included in multivariable logistic regression models using multiple imputation analysis to investigate independent associations between predictors and in-hospital and 90-day mortality. While vital status was missing for very few participants (Figure 1), multiple imputation analysis was used to overcome high proportions of missingness in several predictor variables that otherwise limited the development of multivariable regression models. Data were entered into REDCap [8,9] and all analyses were conducted with Stata 14 (College Station, Texas) [10].
FIGURE 1.
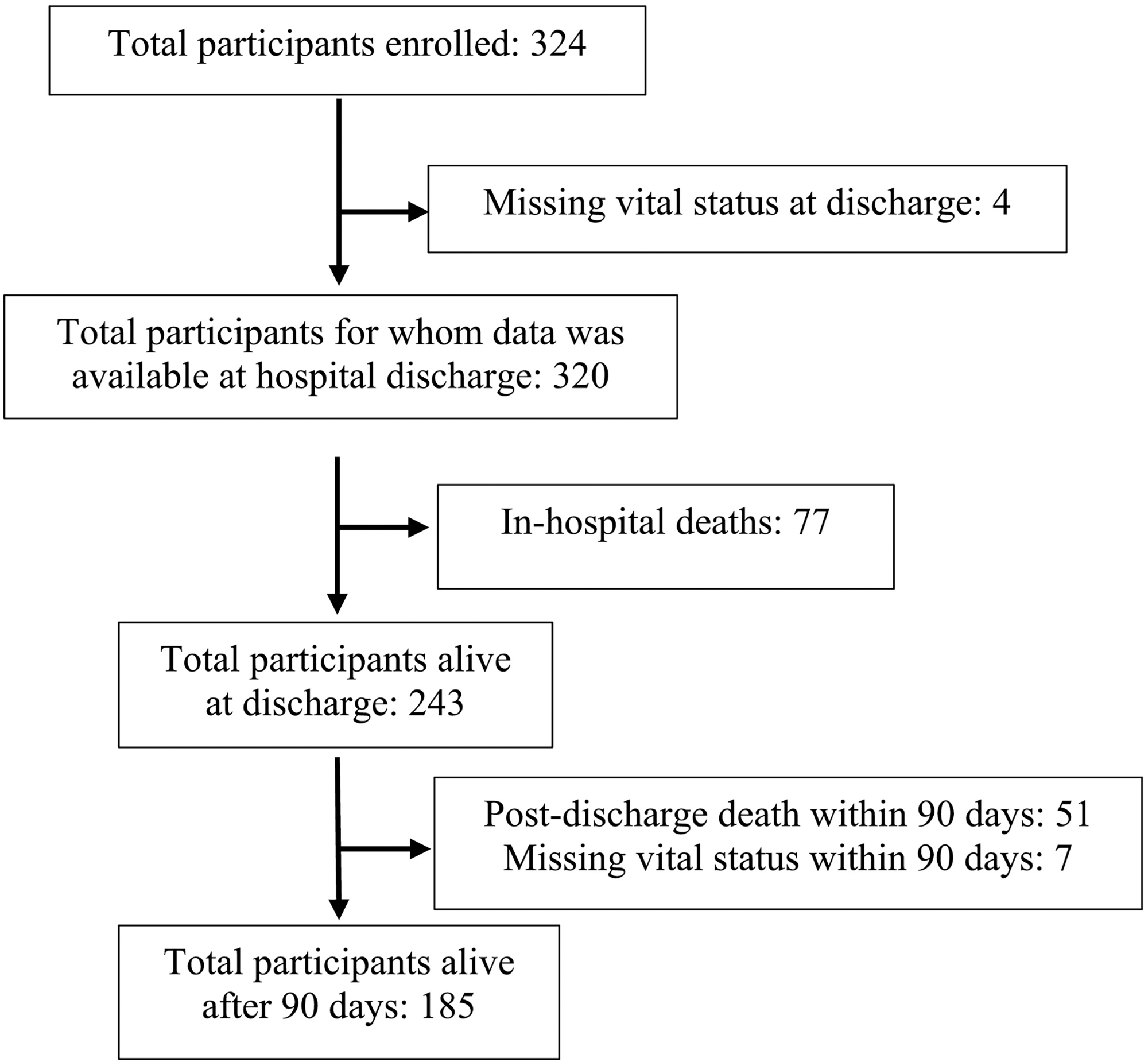
Participant retention from hospitalization to 90-days post-discharge.
Standard Registrations and Ethical Approvals.
Our data were acquired through retrospective analysis of clinical information collected from routine clinical practice. No identifying patient information was gathered or used for study purposes, and, as such, informed consent was not obtained from participants. This study was approved by the University of Zambia Biomedical Research Ethics Council, the Zambia National Health Research Authority, and the Johns Hopkins Institutional Review Board.
Results
Demographic and Medical Characteristics
This Zambian stroke cohort (n=324) had an average age of 60 ±18 years and consisted of 62% females. Ischemic strokes were the most common stroke subtype (n=188; 58%), followed by hemorrhagic stroke (n=91; 28%) and unknown stroke (n=45; 14%). Hypertension was the most common stroke risk factor (80%), followed by heart disease (34%), prior stroke (22%), HIV (18%), diabetes (16%), and atrial fibrillation (9%). Other demographic, clinical, and hospitalization characteristics are summarized in Table 1.
TABLE 1.
Demographics, clinical characteristics, and risk factors by in-hospital mortality and 90-day post-discharge mortality.
| In-Hospital Mortality | 90-day Post-Discharge Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall n=324 | Dead (n=77) | Alive (n=243) | p | Dead (n=51) | Alive (n=185) | p | |||
| Age, mean years (SD) | 60 (18) | 63 (22) | 59 (16) | 0.14 | 68 (15) | 56 (16) | <0.001 | ||
| Female, n (%) | 200 (62%) | 44 (57%) | 155 (64%) | 0.3 | 42 (82%) | 108 (58%) | 0.002 | ||
| Stroke type [n (%)] | |||||||||
| Ischemic | 188 (58%) | 33 (43%) | 155 (64%) | 0.005 | 33 (65%) | 117 (63%) | 0.47 | ||
| Hemorrhagic | 91 (28%) | 29 (38%) | 59 (24%) | 10 (20%) | 48 (26%) | ||||
| Unknown | 45 (14%) | 15 (19%) | 29 (12%) | 8 (16%) | 20 (11%) | ||||
| Stroke risk factors [n (%)] | |||||||||
| Hypertension | 259 (80%) | 65 (84%) | 192 (79%) | 0.3 | 43 (84%) | 142 (77%) | 0.34 | ||
| Diabetes | 51 (16%) | 14 (18%) | 36 (15%) | 0.48 | 12 (24%) | 23 (12%) | 0.048 | ||
| Hyperlipidemia | 44 (14%) | 7 (9%) | 37 (15%) | 0.19 | 12 (24%) | 24 (13%) | 0.06 | ||
| Heart Disease | 111 (34%) | 16 (21%) | 95 (39%) | 0.003 | 23 (45%) | 70 (38%) | 0.35 | ||
| Previous Stroke | 71 (22%) | 22 (29%) | 49 (20%) | 0.12 | 19 (37%) | 29 (16%) | 0.001 | ||
| Atrial Fibrillation | 30 (9%) | 5 (6%) | 25 (10%) | 0.38 | 10 (20%) | 14 (8%) | 0.01 | ||
| HIV | 58 (18%) | 12 (19%) | 46 (22%) | 0.65 | 8 (18%) | 38 (23%) | 0.54 | ||
| Current alcohol use | 47 (16%) | 13 (19%) | 33 (14%) | 0.58 | 1 (2%) | 30 (17%) | 0.02 | ||
| Current tobacco use | 26 (8%) | 5 (7%) | 19 (8%) | 0.97 | 17 (9%) | 0 (0%) | <0.001 | ||
| Family history of stroke | 48 (15%) | 10 (13%) | 38 (16%) | 0.57 | 7 (14%) | 30 (16%) | 0.66 | ||
| Family history of hypertension | 135 (42%) | 10 (13%) | 38 (16%) | 0.57 | 14 (27%) | 92 (50%) | 0.005 | ||
| Family history of diabetes | 49 (15%) | 26 (34%) | 108 (44%) | 0.1 | 8 (16%) | 28 (15%) | 1 | ||
| Stroke characteristics [n (%)] | |||||||||
| Altered mental status on admission (n=242) |
146 (60%) | 56 (93%) | 89 (49%) | <0.001 | 28 (72%) | 57 (41%) | 0.001 | ||
| In-hospital complications [n (%)] | |||||||||
| ICU admission | 4 (1%) | 3 (4%) | 1 (0.4%) | 0.04 | 1 (2%) | 0 (0%) | 0.22 | ||
| Fever | 50 (15%) | 21 (27%) | 29 (12%) | 0.001 | 11 (22%) | 16 (9%) | 0.01 | ||
| Aspiration pneumonia | 51 (16%) | 26 (34%) | 25 (10%) | <0.001 | 16 (31%) | 9 (5%) | <0.001 | ||
| Decubitus ulcers | 4 (1%) | 3 (4%) | 1 (0.4%) | 0.04 | 0 (0%) | 1 (0.5%) | 1 | ||
| Seizures | 4 (1%) | 3 (4%) | 1 (0.4%) | 0.04 | 0 (0%) | 1 (0.5%) | 1 | ||
Predictors of In-Hospital Mortality
Nearly a quarter of participants with stroke died during their hospitalization (n=77; 24%). Average age (63±22 years) was higher among participants who died than among those who survived their hospitalization (59±16 years), although this difference did not reach statistical significance (p=0.14) (Table 2). The median time to death was 5.5 days (IQR 3–10), and 21% of deaths occurred within the first 24 hours of admission (Figure 2).
TABLE 2:
Stroke characteristics, risk factors, and post-stroke in-hospital complications as predictors of in-hospital mortality.
| Univariable Regression | Multivariable Regression | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Age (years) [mean (SD)] | 1.01 | (1.0, 1.03) | 0.03 | 1.008 | (0.99, 1.03) | 0.36 | |
| Stroke characteristics | |||||||
| Stroke type [n (%)] | |||||||
| Ischemic | reference | reference | |||||
| Hemorrhagic | 2.31 | (1.29, 4.13) | 0.005 | 2.88 | (1.27, 6.53) | 0.01 | |
| Unknown | 2.42 | (1.17, 5.03) | 0.02 | 1.81 | (0.73, 4.47) | 0.2 | |
| Altered mental status on admission [n (%)] (n=242) | 14.6 | (5.09, 42.0) | <0.001 | 3.57 | (1.18, 10.8) | 0.02 | |
| Stroke risk factors | |||||||
| Hyperlipidemia | 0.56 | (0.24, 1.30) | 0.18 | 0.57 | (0.18, 1.74) | 0.32 | |
| Heart Disease | 0.41 | (0.22, 0.75) | 0.004 | 0.31 | (0.14, 0.68) | 0.004 | |
| Prior stroke | 1.58 | (0.88, 2.84) | 0.12 | 1.44 | (0.65, 3.20) | 0.37 | |
| Post-stroke in-hospital complications | |||||||
| ICU admission | 10.1 | (1.03, 98.6) | 0.047 | 4.25 | (0.16, 109) | 0.38 | |
| Fever | 2.77 | (1.47, 5.22) | 0.002 | 1.38 | (0.56, 3.36) | 0.48 | |
| Aspiration pneumonia | 4.44 | (2.37, 8.33) | <0.001 | 2.39 | (0.98, 5.80) | 0.055 | |
| Decubitus ulcers | 9.81 | (1.005, 95.7) | 0.049 | 4.46 | (0.36, 55.7) | 0.25 | |
| Seizures | 9.81 | (1.005, 95.7) | 0.049 | 29.5 | (2.14, 406) | 0.01 | |
FIGURE 2.
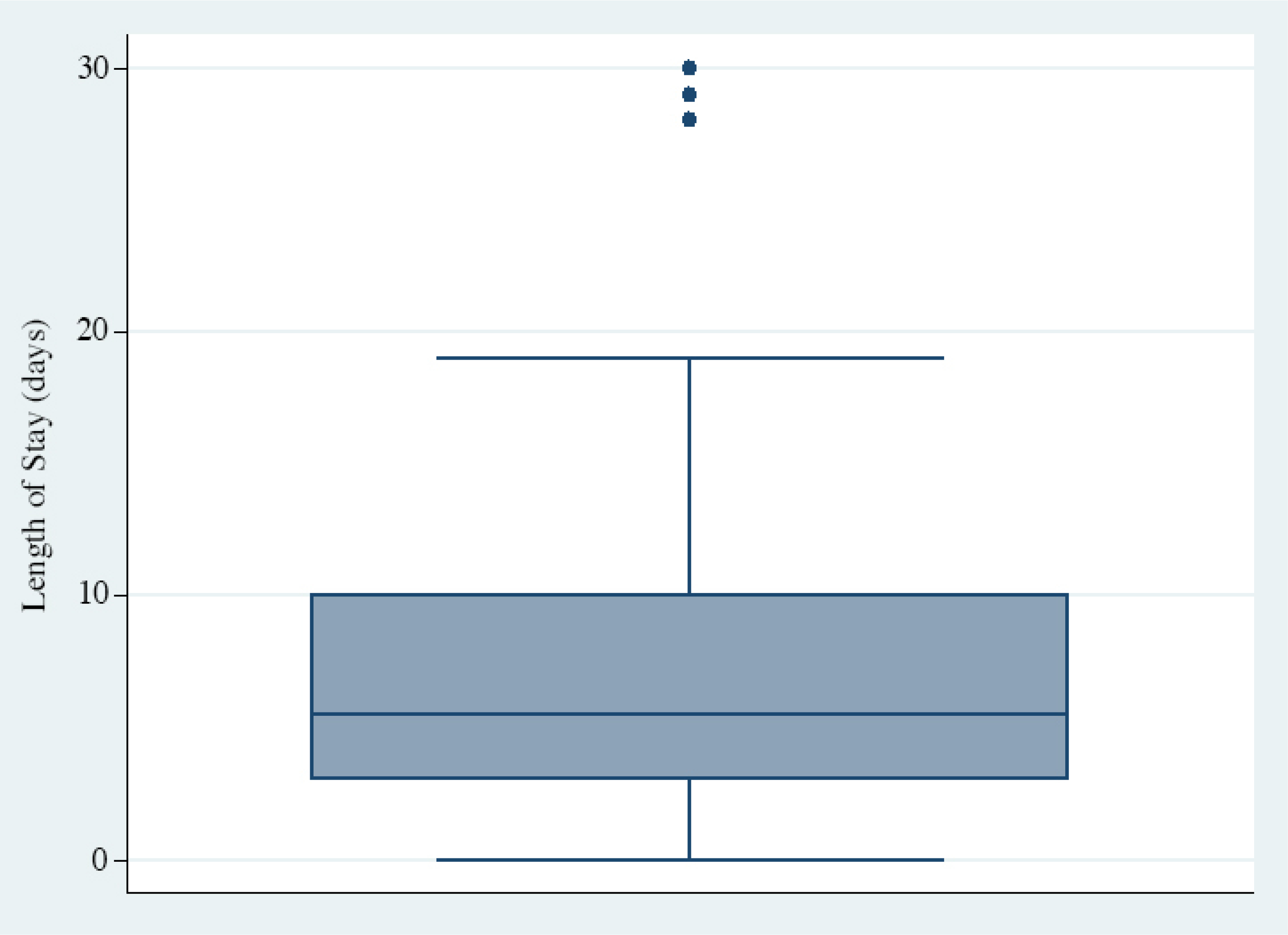
Distribution of in-hospital deaths showing median time of 5.5 days to death and 21% of in-hospital deaths occurring within 24 hours of admission.
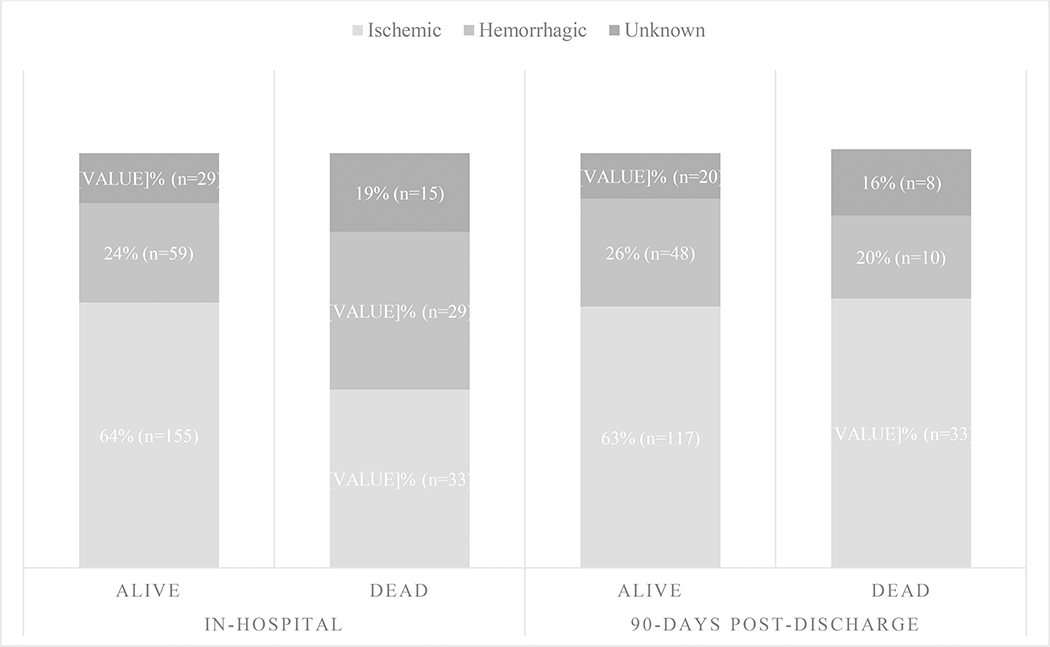
Individuals with hemorrhagic and unknown strokes were overrepresented among those who died in the hospital than among those who survived (Figure 3). Additionally, ICH Score was significantly higher among those with hemorrhagic stroke who died than those who survived [2.6 ± 1.2 vs 1.3 ±1.1, p<0.001). There were no significant differences in the prevalence of medical co-morbidities in univariable analyses, except for heart disease which was less prevalent among those who died than those who survived (21% vs 39%, p=0.003). Compared to those who survived their hospitalization, participants who died were more likely to have lower Glasgow Coma Scale (GCS) scores upon presentation [8.0±3 (dead) vs 11±3 (alive), p<0.001] to have altered mental status on hospital admission (93% (dead) vs. 49% (alive), p<0.001), and to be admitted to the ICU (4% (dead) vs. 0.4% (alive), p=0.04). In-hospital complications including aspiration pneumonia (35% (dead) vs. 10% (alive), p<0.001), fever (27% (dead) vs. 12% (alive), p=0.001), decubitus ulcers (4% (dead) vs. 0.4% (alive), p=0.04) and seizures (4% (dead) vs. 0.4% (alive), p=0.04) were significantly more prevalent in individuals who died during hospitalization compared to those who survived. In multivariable models, hemorrhagic stroke was associated with 2.88 increased odds of death (95% CI 1.27 – 6.54, p=0.01) while altered mental status and seizures were associated with 3.57 (95% CI 1.18 – 10 .8, p=0.02) and 29.5 (95% CI 2.14 – 406, p=0.01) increased odds of death, respectively (Table 2).
FIGURE 3.
In-hospital (p=0.005) and 90-day post-discharge (p=0.47) mortality breakdown by stroke subtype.
Predictors of 90-Day Post-Discharge Mortality
Ninety-day vital status was obtained for 97% (236/243) of participants who survived their initial hospitalization. At 90-days post-discharge, mortality among those individuals who survived their hospitalization and were successfully contacted was 22% (51/236).
Compared to those who survived to 90-days post-discharge, individuals who died in the post-discharge period were more likely to be older (68±15 vs. 56±16 years, p<0.001) and female (82% vs. 58%, p=0.002) (Table 3). As with in-hospital mortality, factors significantly higher in frequency among individuals who died within 90-days of hospital discharge included lower GCS upon initial presentation to the hospital (9.8±3.4 (dead) vs. 11.7±3.3(alive), p=0.02) and altered mental status on admission (72% (dead) vs. 41% (alive), p=0.001). Similarly, in-hospital complications, including fever (22% (dead) vs. 9% (alive), p=0.01) and aspiration pneumonia (31% (dead) vs. 5% (alive), p<0.001), were higher in frequency among individuals who died before 90-days post-discharge. No significant difference in 90-day post-discharge mortality was observed by stroke subtype (Figure 3). Compared to those who were alive at 90-days after hospital discharge, individuals who died were more likely to have diabetes (24% vs. 12%, p=0.048), prior stroke (37% vs. 16%, p=0.001) and atrial fibrillation (20% vs. 8%, p=0.01). Among all the risk factors associated with 90-day post-discharge mortality, only age (OR 1.04, 95% CI 1.01 – 1.06, p=0.007) and aspiration pneumonia during the inpatient hospitalization (OR 3.93, 95% CI 1.30 – 11.88, p=0.02) remained significant in the multivariable model (Table 3).
TABLE 3:
Stroke characteristics, risk factors, and post-stroke in-hospital complications as predictors of 90-day post-discharge stroke mortality.
| Univariable Regression | Multivariable Regression | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Age (years) [mean (SD)] | 1.06 | (1.03, 1.08) | <0.001 | 1.04 | (1.01, 1.06) | 0.007 | |
| Female [n (%)] | 3.33 | (1.53, 7.24) | 0.002 | 1.96 | (0.71, 5.43) | 0.19 | |
| Stroke characteristics | |||||||
| Altered mental status on admission [n (%)] (n=242) |
3.62 | (1.66, 785) | 0.001 | 1.49 | (0.44, 5.07) | 0.52 | |
| Stroke risk factors | |||||||
| Diabetes [n (%)] | 2.17 | (0.99, 4.73) | 0.052 | 1.5 | (0.54, 4.15) | 0.44 | |
| Hyperlipidemia [n (%)] | 2.06 | (0.95, 4.48) | 0.07 | 1.84 | (0.68, 4.98) | 0.23 | |
| Prior stroke | 3.19 | (1.24, 7.18) | 0.02 | 1.32 | (0.55, 3.20) | 0.53 | |
| Atrial fibrillation | 1.83 | (1.04, 3.22) | 0.04 | 1.55 | (0.46, 5.17) | 0.48 | |
| Post-stroke in-hospital complications | |||||||
| Fever during hospitalization | 2.9 | (1.25, 7.74) | 0.01 | 1.24 | (0.38, 3.99)0 | 0.72 | |
| Aspiration pneumonia during hospitalization | 8.94 | (3.66, 31.8) | <0.001 | 3.93 | (1.30, 11.88) | 0.02 | |
Total mortality at 90-days for the cohort of hospitalized adults with stroke at UTH was 40%.
Discussion
In this cohort of adults hospitalized for strokes at the national referral hospital in Zambia, in-hospital mortality was 24% and 90-day post-discharge mortality for those who survived their hospitalization was 22%, bringing total mortality by 90-days to 40%. Significant independent predictors of in-hospital mortality included hemorrhagic stroke and altered mental status on admission while significant independent predictors of 90-day post-discharge mortality were older age and post-stroke aspiration pneumonia during their hospitalization.
In-hospital mortality of 24% in this Zambian stroke cohort was similar to rates reported from other countries in SSA, including Zimbabwe (25%) [11], Kenya (22%) [12], Malawi (23%) [13], and Cameroon (27%) [14]. It is lower than rates reported from tertiary hospitals in West African countries such as Nigeria (35%) [15] and Ghana (43%) [16], likely due to higher hemorrhagic stroke frequency in these cohorts. Our reported in-hospital stroke mortality, as well as those reported in other SSA countries, was markedly higher than that reported from high-income countries (HICs), where stroke mortality has been significantly and steadily decreasing [2,17,18].
Our reported in-hospital mortality can be attributed, at least in part, to the higher proportion of hemorrhagic strokes compared to Western cohorts. Hemorrhagic stroke is associated with higher mortality than ischemic strokes, and hemorrhagic stroke was found to be an independent predictor of in-hospital mortality in our study, a finding similar to studies in Cameroon [14] and Burkina Faso [19]. In HICs, the combination of public health efforts that have enhanced both hypertension awareness and diagnosis [20], and advances in treatments [21, 22] have substantially reduced rates of uncontrolled hypertension and risk for hemorrhagic stroke, thereby resulting in lower rates of stroke-related mortality. However, uncontrolled hypertension and resultant hemorrhagic strokes continue to contribute to higher in-hospital stroke mortality in the SSA region [23]. In fact, our data likely underestimate the proportion of hemorrhagic stroke since individuals with “unknown” strokes comprised 14% of our cohort and had significantly higher mortality than individuals with ischemic stroke. Thus, they may have been disproportionately likely to have hemorrhagic strokes. As such, our data reinforce the urgent need for public health programs to enhance primary stroke prevention through stroke risk factor management, especially improved hypertension diagnosis and treatment, increased stroke risk factor awareness and education, and promotion of lifestyle modifications including low-salt diets and exercise.
However, we suspect the higher in-hospital mortality in our cohort is multifaceted and includes barriers to optimal stroke care. Challenges to optimal stroke care in Zambia, and the larger SSA region, include a limited range of available blood pressure medications, particularly intravenous formulations, limited neurosurgical interventions for post-stroke complications, and absence of acute interventions for stroke, including tissue plasminogen activator (tPA) and endovascular therapies, among others [4,24]. In addition, UTH does not currently have a dedicated stroke unit. As seen in Conakry, Guinea, an SSA country in which reported stroke mortality is similar to that of our cohort, the implementation of a three-bed stroke unit with continuous vital sign monitoring and dysphagia screening significantly reduced post-stroke in-hospital mortality [25]. As such, the development of stroke-specific systems of care would likely reduce the high stroke mortality observed in our cohort.
In addition, patient factors likely contribute to the higher in-hospital mortality as well. Patients in LMICs often face significant delays between stroke onset and accessing stroke care [24]. For example, in our cohort, 19% of stroke patients faced at least a one-day delay from stroke onset to initial presentation to care, and 62% waited more than 24 hours between admission and neuroimaging, when neuroimaging was obtained at all. Delays in accessing post-stroke care reduces the window for providing timely and appropriate treatment, such as treatment for high blood pressure and hyperglycemia, while increasing the risk of the development of post-stroke complications such as aspiration pneumonia and decubitus ulcers prior to hospital admission, all of which could further increase in-hospital stroke mortality. Delays in obtaining neuroimaging – the only validated method to differentiate ischemic and hemorrhagic strokes – once care is accessed also likely contribute to higher in-hospital mortality. Acute treatment of ischemic and hemorrhagic strokes differs, and the inability to definitively identify stroke subtype prevents initiation of subtype-specific treatment. Nearly one-fifth of participants in our cohort died within 24 hours of hospitalization, which likely can be attributed to two major factors. First, patients with higher stroke severity are less likely to be stabilized in the absence of acute interventions like tPA, endovascular therapy and neurosurgical interventions. Second, many individuals either delay seeking care altogether or are only referred from primary health care settings after developing complications such as aspiration pneumonia that leave them critically ill.
In comparison to a prior stroke cohort studied at UTH in 2010, our current cohort had a lower in-hospital mortality (40% vs. 24%, p<0.001), likely due at least in part to the decreased proportion of hemorrhagic strokes in our cohort (35% vs. 28%, p=0.07) [26]. The development of the first neurology inpatient service and post-graduate neurology training program at UTH likely also contributed to the lower mortality in the current cohort. However, our cohort also showed continued high prevalence of in-hospital complications, including aspiration pneumonia (11% vs. 16%, p=.12). The continued burden of modifiable stroke risk factors, hemorrhagic strokes, and continued high post-stroke complications at UTH suggests a crucial need for targeted interventions to improve stroke care within and outside the hospital.
The 90-day mortality of 22% in our stroke cohort highlights limitations in post-stroke acute care facilities. In our setting, patients are almost exclusively discharged from the hospital into their home setting, most under the care of an informal family caregiver with little training in caring for individuals with complex chronic medical needs [27]. As such, these high rates of early post-discharge mortality may be reflective of a lack of post-acute care facilities, limited medical follow-up, limited rehabilitation services in the hospital and community [28], and limited caregiver education prior to and in the days after discharge [27]. This highlights the need to improve stroke care not only in the hospital but also in the community setting with improved access to close medical follow-up and rehabilitation services such as physical, occupational and speech therapy, and provision of community programs for caregiver education and support.
In our setting, older age was an independent risk factor of 90-day post-stroke mortality, as seen in other stroke cohorts in SSA including Kenya [12] and Uganda [29]. It is likely that the higher rates of co-morbidities such as hypertension and diabetes that increase risk of recurrent stroke, reduced neural plasticity with age, and reduced mobility that impairs recovery from stroke [30] are major factors that play a role in increased mortality due to age.
Of note, female sex was associated with higher 90-day post-discharge mortality in our cohort, a finding that was also reported in other SSA stroke cohorts [12, 31]. In the SSA setting, nearly all post-stroke care is provided by family caregivers, who tend to be close female relatives who often reside in the same household as the patient [32, 33]. As such, we hypothesize that higher rates of post-discharge mortality among females in our cohort may reflect absence of a female caregiver in the house. Females, especially older females, are also less likely to be the primary income earners in our setting which may lead toother disparities in access to post-stroke care. However, this finding should be further clarified with future studies on differences in access to healthcare and care seeking behavior in Zambia by sex of both the patient and the primary caregiver, especially amongst older adults.
Our findings also showed atrial fibrillation detected on in-hospital electrocardiogram or echocardiogram was significantly associated with in-hospital and 90-day mortality. Previous studies conducted in HICs also shared this finding [34]. In SSA settings, atrial fibrillation is an under-reported risk factor of stroke due to limited continuous cardiac monitoring and limited access to electrocardiograms and echocardiograms in many healthcare settings [35]. In these settings, difficulty managing atrial fibrillation extends beyond its diagnosis to challenges with providing and monitoring anticoagulation. Novel oral anticoagulants are available on the private market in Zambia, but their expense precludes their use by the vast majority of individuals, leaving warfarin as the only viable option. Warfarin is not readily available in most public health facilities and must be purchased from private pharmacies. The regular long-term INR monitoring whilst on warfarin is also not readily available in public laboratory facilities but is available in private facilities where investigations tend to be more costly. Furthermore, the laboratory monitoring required is logistically complicated as laboratories capable of performing INR are largely limited to large metropolitan areas, and transportation is expensive and logistically complicated for stroke survivors with mobility limitations as most of the Zambian population does not own private vehicles.
High rates of post-stroke complications, including aspiration pneumonia, fever, seizures, and decubitus ulcers, were also seen in our cohort and were associated with in-hospital mortality in univariable analyses, although only seizures remained associated with mortality in multivariable models. Our findings are similar to studies from across SSA, including Nigeria [15], Sierra Leone [36], and Ethiopia [37], where post-stroke complications were high and associated with mortality. Higher rates of these complications in our stroke cohort and similar stroke cohorts in other LMICs likely reflect the limited availability of standardized post-stroke protocols for in-hospital stroke care in these settings, including limited access to stroke centers. As such, a strategy to develop standardized protocols, such as bedside swallowing evaluations, head of bed elevation, and modified diets in the setting of dysphagia, as part of a stroke unit using available resources in Zambia warrants further study in our setting to evaluate whether it can reduce post-stroke complications and mortality.
The major limitation to our study is its retrospective nature such that outcomes that may contribute significantly to in-hospital mortality, including decubitus ulcers, catheter-associated urinary tract infections, and deep vein thromboses, were likely not systematically captured. Furthermore, as this study was retrospectively conducted during the first six months of Zambia’s first neurology training program, stroke severity scales (mRS and NIHSS) were not routinely performed or collected, thus limiting our ability to quantify the severity of strokes in our cohort beyond admission GCS. Missing data for some potential predictors of stroke mortality required the use of multiple imputation analysis and limited our ability to analyze mortality by stroke subtype. However, given that most patients with stroke in SSA are cared for in hospital settings where neuroimaging is unavailable, this distinction is likely less important in our setting. Because this was an exploratory study, we did not correct for multiple comparisons so some of the associations found here may be due to chance alone. Finally, our data showing nearly 21% of in-hospital deaths happened within 24 hours of admission suggests a selection bias of the most severe strokes being referred to our hospital. Thus, our results may not be generalizable to all clinical settings in Zambia since this cohort was recruited from Zambia’s national referral hospital and cared for by neurology specialists. Still, the results of this study provide preliminary data that can be used to guide the development of interventions to reduce in-hospital and post-discharge mortality in Zambia.
In conclusion, our findings reflect the need for better systems of both in-hospital and post-stroke care in Zambia to prevent post-stroke complications, especially aspiration pneumonia, and reduce stroke-related mortality. Development of stroke units adapted to resource-limited settings that could reduce in-hospital stroke-related morbidity and mortality, just as they have done in HICs, is one potential solution. In addition, our results demonstrate the need for further post-discharge interventions to reduce early post-discharge mortality such as increasing access to close medical follow-up and rehabilitation services and caregiver education and community support services, especially for women with stroke.
Acknowledgements.
Funding:
This work was supported by the American Academy of Neurology Medical Student Research Scholarship; United States Department of State Fulbright Scholar Fellowship; and National Institutes of Health (grant number R21 NS118543–01, 1K01TW011771–01A1, D43TW009340).
Footnotes
Disclaimer: This article was partially prepared while Dr. Rebecca Gottesman was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donkor ES. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res Treat. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality a statement from the american heart association/american stroke association. Stroke. 2014. Jan;45(1):315–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owolabi MO, Akarolo-Anthony S, Akinyemi R, et al. The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2):S27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adoukonou TA, Vallat J, Joubert J, Macian F, Kabore R. ´ re ´ braux en Prise en charge des accidents vasculaires ce Afrique subsaharienne Management of stroke in sub-Saharan Africa : Current issues. Rev Neurol (Paris) [Internet]. 2010;166(11):882–93. [DOI] [PubMed] [Google Scholar]
- 5.CDC Global Health – Zambia. https://www.cdc.gov/globalhealth/countries/zambia/default.htm
- 6.Nutakki A, Chomba M, Chishimba L, et al. Risk factors and outcomes of hospitalized stroke patients in Lusaka, Zambia. J Neurol Sci [Internet]. 2021;424:117404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–7. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, JG. Conde, Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium, The REDCap consortium: Building an international community of software partners, J Biomed Inform. 2019. May 9 [doi: 10.1016/j.jbi.2019.103208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.StataCorp, 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 11.Kaseke F, Stewart A, Gwanzura L, et al. Clinical characteristics and outcomes of patients with stroke admitted in tertiary hospitals in Zimbabwe: A retrospective one-year study. Malawi Med J. 2017;29(2):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaduka L, Muniu E, Oduor C, et al. Stroke Mortality in Kenya’s Public Tertiary Hospitals: A Prospective Facility-Based Study. Cerebrovasc Dis Extra. 2018. May 1;8(2):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahawish KM, Heikinheimo T. Teaching Corner [Internet]. Vol. 22, Malawi Medical Journal. 2010. Available from: www.mmj.medcol.mw [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mapoure NY, Tchaleu Nguenkam CB, Mbatchou Ngahane HB, et al. Predictors of in-hospital mortality for stroke in Douala, Cameroon. Stroke Res Treat. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekeh B, Ogunniyi A, Isamade E, et al. Stroke mortality and its predictors in a Nigerian teaching hospital. Afr Health Sci. 2015;15(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarfo FS, Acheampong JW, Appiah LT, et al. The profile of risk factors and in-patient outcomes of stroke in Kumasi, Ghana. Ghana Med J. 2014;48(3):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seminog OO, Scarborough P, Wright FL, et al. Determinants of the decline in mortality from acute stroke in England: Linked national database study of 795 869 adults. BMJ. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Tong X, Schieb L, et al. Vital Signs : Recent Trends in Stroke Death Rates — United States, 2000 – 2015. 2017;66(35):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djingri Labodi L, Kadari C, Nobila Valentin Y, et al. Intra-hospital mortality of stroke and its predictive factors in a reference hospital in Ouagadougou, Burkina Faso. Brain and Nerves. 2017;1(3). [Google Scholar]
- 20.Yoon SS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. 2015;(220):1–8. [PubMed] [Google Scholar]
- 21.Dastur CK, Yu W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc Neurol. 2017. 24;2(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rymer MM. Hemorrhagic stroke: intracerebral hemorrhage. Mo Med. 2011;108(1):50–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Ataklte F, Erqou S, Kaptoge S, et al. Burden of undiagnosed hypertension in sub-saharan africa: A systematic review and meta-analysis. Hypertension. 2015;65(2):291–8. [DOI] [PubMed] [Google Scholar]
- 24.Urimubenshi G, Cadilhac DA, Kagwiza JN, Wu O, Langhorne P. Stroke care in Africa : A systematic review of the literature. Int J Stroke. 2018;13(8):797–805. [DOI] [PubMed] [Google Scholar]
- 25.Cisse FA, Damien C, Bah AK, Touré ML, Barry M, Djibo Hamani AB, et al. Minimal Setting Stroke Unit in a Sub-Saharan African Public Hospital [Internet]. Vol. 10, Frontiers in Neurology. 2019. p. 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atadzhanov M Stroke Characteristics and Outcomes of Adult Patients Admitted to the University Teaching Hospital, Lusaka, Zambia. Open Gen Intern Med J. 2012;5(1):3–8. [Google Scholar]
- 27.Khondowe O, Rhoda A, Mpofu R. Perceived needs of caregivers of stroke patients’ receiving out-patient physiotherapy treatment in Lusaka, Zambia. South African Journal of Physiotherapy. 2012;68(1):1–5. [Google Scholar]
- 28.Chimatiro GL, Rhoda AJ. The challenge to providing stroke care and rehabilitation in Malawi. Journal of Global Health Reports. 2019;3:e2019049. [Google Scholar]
- 29.Namale G, Kamacooko O, Makhoba A, et al. Predictors of 30-day and 90-day mortality among hemorrhagic and ischemic stroke patients in urban Uganda: A prospective hospital-based cohort study. BMC Cardiovasc Disord. 2020;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lui SK, Nguyen MH. Elderly Stroke Rehabilitation: Overcoming the Complications and Its Associated Challenges. Curr Gerontol Geriatr Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heikinheimo T, Chimbayo D, Kumwenda JJ, et al. Stroke Outcomes in Malawi, a Country with High Prevalence of HIV: A Prospective Follow-Up Study. PLOS ONE. (2012) 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalomo EN, Besthorn FH Caregiving in Sub-Saharan Africa and Older, Female Caregivers in the Era of HIV/AIDS: A Namibian Perspective. GrandFamilies: The Contemporary Journal of Research, Practice and Policy. (2018). 5 (1). [Google Scholar]
- 33.Schatz E, Seeley J. Gender, ageing and carework in East and Southern Africa: A review. Glob Public Health. 2015;10(10):1185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller K, Hobohm L, Wenzel P, et al. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Heart Rhythm. 2020. 1;17(3):383–90. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs MS, van Hulst M, Adeoye AM, et al. Atrial Fibrillation in Africa-An Under-Reported and Unrecognized Risk Factor for Stroke: A Systematic Review. Glob Heart. 2019;14(3):269–279. [DOI] [PubMed] [Google Scholar]
- 36.Russell JBW, Charles E, Conteh V, et al. Risk factors, clinical outcomes and predictors of stroke mortality in Sierra Leoneans: A retrospective hospital cohort study. Ann Med Surg [Internet]. 2020;60(October):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fekadu G, Chelkeba L, Kebede A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: prospective observational study. BMC Neurol. 2019;19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]