Abstract
Background
Previous studies have demonstrated that high levels of estradiol (E2) impair blastocyst implantation through effects on the endometrium; however, whether high E2 directly affects blastocysts is not well established. The present study sought to clarify the direct impacts of high E2 levels on blastocysts in vitro.
Methods
ICR virgin albino mice were used. Using an in-vitro 8-day blastocyst culture model, immunofluorescence staining for the estrogen receptor (ER), blastocyst outgrowth assays, differential staining and TUNEL assays of blastocysts, and embryo transfer, we investigated the main outcomes of exposure to different E2 concentrations (10−7 to 10−4 M) in vitro and in vivo.
Results
ERα and ERβ expression were detected in pre-implantation stage embryos. In vitro exposure of blastocysts to 10−4 M E2 for 24 h followed by 7 days culture in the absence of E2 caused severe inhibition of implantation and post-implantation development. The late adverse effects of E2 on post-implantation development still occurred at concentrations of 10−7 to 10−5 M. In addition, blastocyst proliferation was reduced and apoptotic cells were increased following exposure to 10−4 M E2. Using an in vivo embryo-transfer model, we also showed that treatment with high E2 resulted in fewer implantation sites (38% vs. 72% in control) and greater resorption of implanted blastocysts (81% vs. 38% in control).
Conclusion
Exposure to high E2 concentrations in vitro is deleterious to blastocyst implantation and early post-implantation development, mainly owing to direct impacts of E2 on implanting blastocysts. In clinical assisted reproductive technique (ART), high serum E2 concentrations not only affects the endometrium, but also affects blastocysts directly at the period of implantation.
Keywords: Estradiol, Embryo, Blastocyst, Implantation
At a glance of commentary
Scientific background on the subject
In previous studies, most of data have been demonstrated that high levels of estradiol (E2) impair blastocyst implantation through direct effects on the endometrium; but few information is available regarding direct-impacts high E2 on the blastocysts. The present study sought to clarify the direct impacts of high E2 in vitro.
What this study adds to the field
Direct-impacted blastocysts with high E2 concentration in vitro are deleterious to blastocyst implantation and early post-implantation development in mice. In clinical ART, high serum E2 concentrations not only affects the endometrium, but also affects blastocysts directly at the period of implantation.
Estradiol (E2), the primary form of estrogen in women of reproductive age, acts as a growth hormone/regulator for a variety of female reproductive organs, including the vaginal lining, cervical glands, lining of the fallopian tubes, the endometrium, and the myometrium [1,2].
E2 has a physiological role in embryonic development and implantation, as demonstrated by a number of in vitro studies. Smith et al. demonstrated that mouse embryos, especially blastocysts, may develop an estrogen-sensitive mechanism at different concentrations of E2 (10−10, 10−9, and 10−5 M) immediately prior to implantation [3]. Effects of E2 on the metabolism of glucose in the mouse morula and early blastocyst in vitro have been investigated by Khurana et al. [4]. It has been hypothesized that during development or implantation of rabbit blastocysts, E2 acts as a local signal from the blastocyst to the uterus [5].Consistent with this, tamoxifen has been shown to inhibit E2-induced changes in the surface coating of mouse blastocysts in a concentration-dependent manner [6]. E2 produced in the rabbit morula and blastocyst could trigger embryonic differentiation and metabolic functions [7]. The role of E2 in the uterine and endometrial cells for pregnancy has also been well studied [[8], [9], [10], [11]]. However, the physiological concentrations of E2 and its direct role in blastocyst and post-implantation development has not been extensively studied.
It has been proposed that high serum E2 concentrations that may occur during controlled ovarian stimulation (COS) adversely affect the outcome of in vitro fertilization (IVF) [[12], [13], [14]]. However, some investigators have reported the controversial finding that high serum E2 concentrations are not detrimental to the outcome of IVF [[15], [16], [17]]. Most studies have shown that high E2 directly impacts the endometrium or indirectly affects blastocysts during the implantation period [[18], [19], [20], [21]]. Accumulated lines of evidence indicated that E2 does directly impair the embryonic development at cleavage-stage in vitro, resulting in a reduction in the number of blastocysts formed and their quality. Furthermore, embryos formed from these in vitro-developed blastocysts exhibit decreased adhesion to endometrial cells in vitro [22]. Nevertheless, the direct impacts of E2 on blastocysts have not been comprehensively researched.
A number of studies have investigated changes in E2 levels in the peri-ovulation period. In the natural cycle, serum E2 levels decrease between days 1 and 3 post-ovulation. However, E2 levels on post-ovulation days 4–6 (107–260 pg/mL) are still maintained at approximately 80% of the peak levels (133–329 pg/mL) observed before ovulation [23,24]. In ovarian hyper-stimulation syndrome (OHSS), after COS, peak E2 serum levels can reach levels (1590–13,484 pg/mL) 10–400 folds higher than those in the natural cycle [[25], [26], [27], [28]]. After ovulation in COS cycles, serum E2 levels on post-ovulation day5 (∼1000–3000 pg/mL) were maintained at ∼36%–41% of E2 levels on the day ovulation was triggered [29,30]. This means that serum E2 levels after ovulation in the COS cycle are still higher than those after ovulation in the natural cycle. A few studies have demonstrated a correlation between serum E2 levels and those in the uterine cavity during the menstrual cycle. In Zavy el al. study, the E2 level in uterine cavity increases as the E2 level in serum increases on post-ovulation days in the estrous cycle in mares [31], indicating that, if a high serum E2 level is noted on post-ovulation days, a higher E2 level would occur in the uterine cavity. The interaction between blastocysts and the endometrium during implantation process is affected by high E2 levels in the uterine cavity. The impacts of high E2 concentrations on the endometrium, and possibly on the embryos, were demonstrated in a study by Valbuena et al. [22], which showed that an in vitro E2 concentration of 10−7 M is equivalent to 27.24 pg/mL of E2 in serum. Compared with the natural cycles, we all know serum estradiol level could be more than 10,000 pg/mL [[26], [27], [28],32]. Therefore, a very high E2 level could possibly occur in the uterine cavity during the implantation period in OHSS.
Accordingly, we sought to investigate the direct effects of E2 on blastocysts, determine tolerable levels of E2 for blastocysts, and establish the mechanisms underlying the associated adverse effects during the peri-implantation period. In the present study, the direct impacts of E2 on blastocysts were investigated by using both in vitro and in vivo models.
Materials and Methods
Animal and blastocyst collection
ICR virgin albino mice were maintained under a 12-h/12-h cycle, with food and water provided ad libitum. All animals received humane animal care according the Guidelines for Care and Use of Experimental Animals. ICR mice (6–8 weeks old) were super-ovulated by injecting 5 IU Pregnant Mare Serum Gonadotropin (Sigma, St. Louis, MO, USA or PMSG; ProSpec, Ness-Ziona, Israel), followed by injection of human chorionic gonadotropin (HCG, 5 IU) 48 h later. Females were then mated overnight with a single fertile male of the same strain. Pregnancy was confirmed by the presence of a vaginal plug next morning. Females mice with vaginal plugs were selected immediately (as the day 1 of pregnancy) for the subsequent experiments. Blastocysts were obtained by flushing the fallopian tubes on the morning of day 4 of pregnancy with pre-warmed Earle's Balanced Salt Solution (EBSS; Sigma) containing 0.3% bovine serum albumin (BSA; Sigma), 1 mM pyruvate sodium, 1 mM glutamine and 2% (v/v) of an antibiotics preparation (100units penicillin and 100g streptomycin per ml; Gibco, Grand Island, NY, USA). Blastocysts were collected in uncoated 4-well culture dishes and washed a minimum of three times. At least 100 mice were used to collect more than 1000 blastocysts for subsequent experiments.
Blastocyst culture and definition of developmental stages
Embryos were acquired according to a previously described protocol [33,34]. Briefly, blastocysts acquired from different female mice by flushing the uterine horn on day 4 were expanded, pooled, and randomly selected for experiments. Embryos were cultured in EBSS medium containing 0.3% BSA during the pre-implantation stage, and in CMRL 1066 medium (Sigma) during the post-implantation stage. Both media contained 1 mM glutamine, 1 mM sodium pyruvate, and 50 IU/ml penicillin (Gibco) plus 50 mg/ml streptomycin (Gibco). CMRL 1066 medium containing 20% fetal bovine serum (FBS; Gibco) was used for culturing blastocysts during the first 4 days and then was replaced with medium containing 20% human placental cord serum. Embryos were inspected daily under a dissecting microscope and classified, as described previously [33,34]. Briefly, blastocysts possessing a zona pellucida were defined as Witschi stage 6. Free blastocysts that have shed the zona pellucida and attached lightly to the surface of the culture dish were defined as Witschi stage 7. Implanted blastocysts that had left an inner cell mass on trophoblast outgrowths were defined as Witschi stage 8. Those with an inner cell mass protruding from trophoblast outgrowths forming a small cavity in the center (gastrula) were defined as Witschi stage 9. Embryos in which the gastrula was increased in mass and elongated were defined as Witschi stage 10. Late egg cylinders with two distinguishable parts—the proximal part of the extra-embryonic region and the distal part of the embryonic region (snowman shape)—were defined as Witschi stage 11. Late egg cylinders with a primitive streak or neurula were defined as Witschi stage 12–13. Somite-stage embryos with a yolk sac enclosing the embryonic shield were defined as Witschi stage 14–15. The well-defined pictures of different developmental stages has been shown in the previous study [34]. In this study, early egg cylinder embryos were defined as embryos that had reached stage 9 or 10 by day 4; late egg cylinder embryos were defined as embryos that hadreached stages 11, 12 or 13 by day 6 of culture; and early somite embryos were defined as embryos that had reached stage 14 or 15 by day 8.
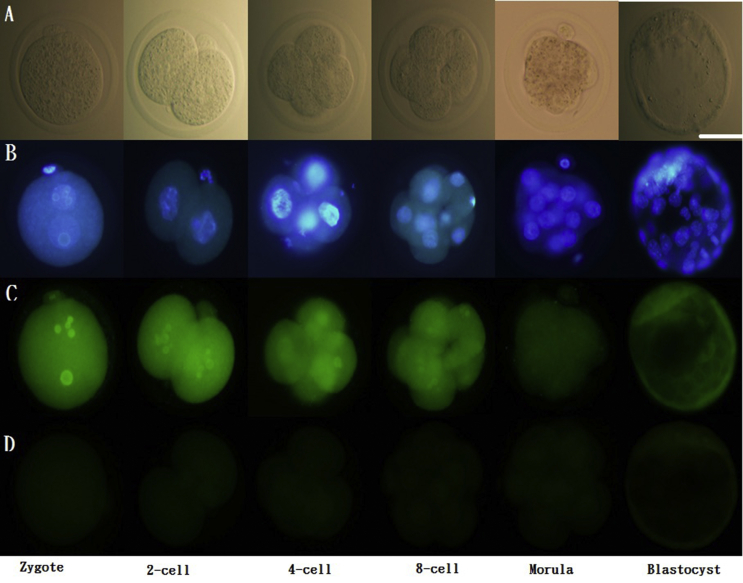
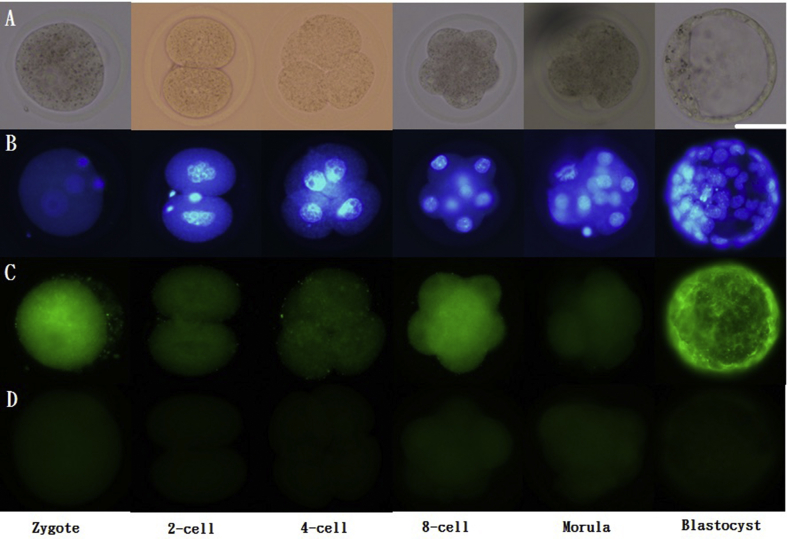
Immunofluorescence staining for the ER
Embryos at zygote stage or 2-cell/4-cell/8-cell/morula satge were collected from the oviducts following different timing (20 h, 48 h, 54 h, 65 h and 72 h respectively) after HCG injection. Blastocysts were collected from the uterine cavity following 96 h after HCG injection. Embryos collected at different stages were fixed immediately by incubating in 4% paraformaldehyde for 30 min at room temperature. Samples were permeabilized by incubating with 0.5% Triton X-100 and 1% BSA in PBS, and then were washed three times with PBS containing 0.2% Triton X-100 and 0.3% BSA. Therefore, samples were incubated in blocking buffer containing 4% BSA and 0.2% Triton X-100 in PBS at room temperature for 1 h, followed by incubation overnight at 4 °C with rabbit anti-mouse ERα (PA1-309) and ERβ (PA1-311) primary antibodies (Thermo Scientific, Waltham, MA, USA), diluted 1:100. Samples were then incubated at room temperature for 1 h with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG secondary antibody (31635; Thermo Scientific), diluted 1:100, following by counterstaining with bisbenzimide (BIS) to label nuclei.
Blastocyst outgrowth assays
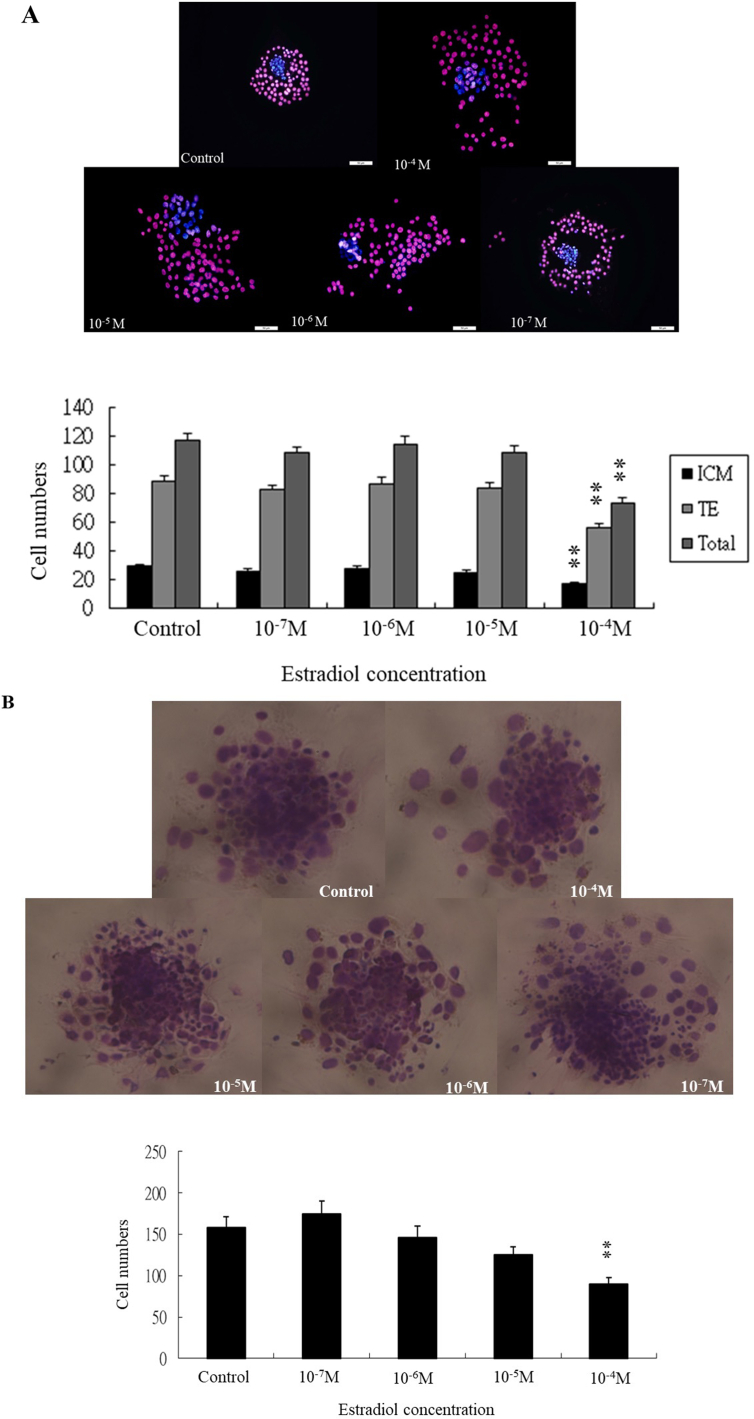
Blastocysts were cultured in the absence or presence of E2 (10−7, 10−6, 10−5, or 10−4 M) for 24 h and then cultured for 3 days without E2. The number of blastocysts was shown in the legends of [Fig. 3, B]. Thereafter, the culture medium was carefully removed and replaced with 5% hypotonic sodium citrate (30 μl/well) and blastocysts were incubated at room temperature for 5 min. After evaporating this solution under partial vacuum (200 bar) at 50 °C for 60 min, the expanded cells were fixed using FixDenat fixative at 50 °C for 60 min. The total number of nuclei in outgrowths was assessed after staining with a 4% Giemsa solution (Sigma) at room temperature for 15 min.
Fig. 3.
Effects of E2 on growth of blastocyst cells. (A) Proliferation of blastocyst cells following 24-h exposure to different concentrations of E2, determined by cell counting using a differential staining method. The sample size shown in each group (control:37; 10−7 M:38; 10−6 M:32; 10−5 M:31; 10−4 M:38 respectively). (B) Blastocyst cell outgrowth in vitro following a 24-h exposure to different concentrations of E2 and culturing for 3days. Total number of nuclei in outgrowths was assessed after staining with a 4% Giemsa solution. The sample size shown in each group (control:32; 10−7 M:34; 10−6 M:30; 10−5 M:32; 10−4 M:31 respectively). Data were recruited from four experiments and analyzed by one-way analysis of variance (ANOVA), ∗∗p < 0.01 vs. control.
Differential staining of blastocysts
Blastocysts with a normal appearance were selected and cultured in the absence or presence of E2 (10−7, 10−6, 10−5, or 10−4 M) for 24 h. The morphology of blastocysts was recorded. The proliferation of blastocysts was evaluated by separately counting the number of inner cell mass (ICM) and trophectoderm (TE) cells identified by dual differential staining, which also allows examination of dead cells with fragmented nuclei. This method is based on the impermeability of the TE layer, which protects ICM cells from exposure to the antibody and the complement reaction. The two cell lineages can be distinguished following fluorochrome staining [35].
The zona pellucida was removed by incubating blastocysts in a solution of 0.4% pronase in EBSS (Earle's Balanced Salt Solution, Sigma)-BSA medium containing 0.3% bovine serum albumin (BSA) at 37 °C for 5 min. Denuded blastocysts were exposed to 15% rabbit anti-embryonic cell membrane serum at 37 °C for 30 min and washed with EBSS-BSA medium. Blastocysts were further treated with 10% bovine serum,2 mg/ml bisbenzimide, and 1 mg/ml propidium iodide at 37 °C for 30 min.
The resulting immunolysed blastocysts were gently transferred onto slides, protected from light. Under appropriate UV light excitation, ICM cells that take up bisbenzimide but exclude propidium iodide are stained blue, whereas TE cells, which are labeled with both fluorochromes, are stained orange-red. Because it has been shown that multinucleated cells are infrequent in pre-implantation embryos, the number of nuclei was considered an accurate reflection of the number of cells.
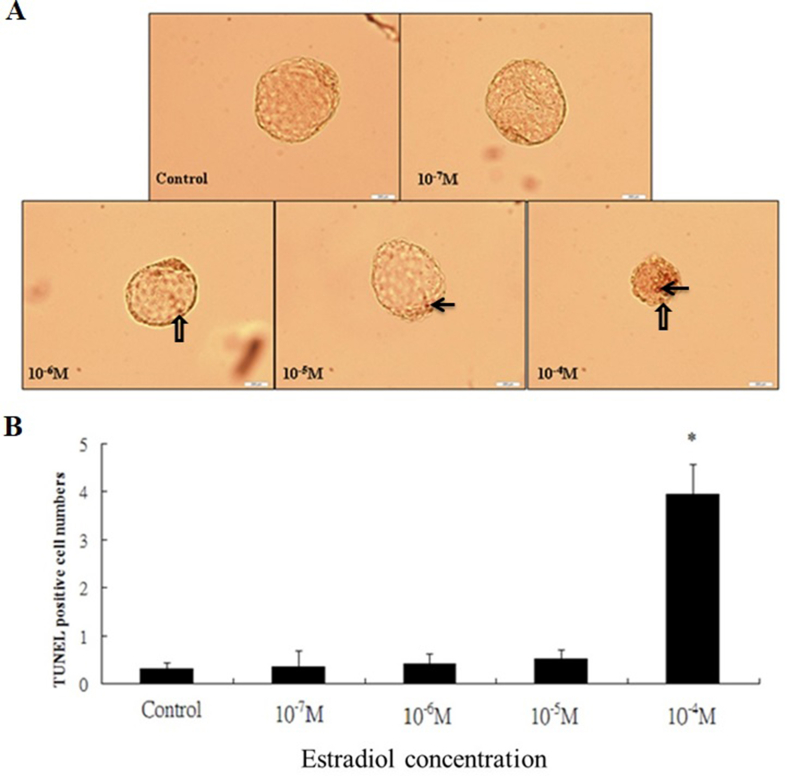
TUNEL assay of blastocysts
Blastocysts cultured with different concentrations of E2 (10−7, 10−6, 10−5, and 10−4 M) or vehicle for 24 h were collected and subjected to terminal deoxynucleotidy transferased UTP nick-end labeling (TUNEL) staining. The embryos were washed in puerarin-free medium, fixed, permeabilized and labeled using an In Situ Cell Death Detection kit (Cat.No.11684817910; Roche, Berlin, Germany), according to the manufacturer's protocol. Bright-field images were obtained using a fluorescence microscope (Olympus BX70, Tokyo, Japan).
Embryo transfer
Blastocysts with a normal appearance were selected and cultured in the absence or presence of E2 (10−4 M) for 24 h. Eight untreated control embryos and eight E2-treated embryos were transferred to the left and right uterine horns of each surrogate mice respectively, on day 4 of positive vaginal plug. Ten surrogate mice were analyzed and sacrificed on day 14 post-transfer (18-day fetuses); the frequency of implantation was calculated as the number of implantation sites per number of embryos transferred. The incidence rates of surviving fetuses (number of surviving fetuses/number of implantations) and resorbed fetuses ([number of implantations – number of surviving fetuses]/number of implantations) were also calculated. The implantation percentage represents the number of implantations per number of transferred embryos × 100. The percentage of resorbed or surviving fetuses represents the number of resorptions or surviving fetuses per number of implantations × 100. The weights of surviving fetuses were measured immediately after dissection.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) and Student's t-test using SPSS software (IBM19). The results are presented as means ± SEMs. p-values < 0.05 were considered statistically significant.
Results
ERα and ERβ protein expression in pre-implantation embryos
In order to investiage the role of E2 in the embryos, the ERα and ERβ would be identified in the embryos firstly. During the pre-implantation stage, both ERα [Fig. 1] and ERβ [Fig. 2] were expressed from the 1-cell stage to blastocysts. Strong expression of ERα was found before the morula stage. ERβ was strongly expressed in zygotes, 8-cell embryos, and blastocysts.
Fig. 1.
Immunofluorescence of ERα in pre-implantation embryos. Scattered ERα expression was detected in the cytoplasm as well as the nucleus (co-stained with DAPI) of 1-cell stage embryos to blastocysts. ERα: green; DAPI: blue. Scale bar = 50 μm (A) Unstained; (B) stained with DAPI; (C) stained with primary and secondary antibodies for ERα; (D) stained with only secondary antibodies for ER α. Representative images from three experiments using biologically different samples (n ≥ 15) are shown.
Fig. 2.
Immunofluorescence of ERβ in pre-implantation embryos. Scattered ERβ expression was detected in the cytoplasm as well as the nucleus (co-stained with DAPI) of 1-cell stage embryos to blastocysts. ERβ: green; DAPI: blue. Scale bar = 50 μm (A) Unstained; (B) stained with DAPI; (C) stained with primary and secondary antibodies for ERβ; (D) stained with only secondary antibodies for ERβ.Representative images from three experiments using biologically different samples (n ≥ 15) are shown.
Effects of E2 on blastocyst implantation and post-implantation in vitro
Having confirmed the differential expression of ERα and ERβ in blastocysts, we further examined the concentration-dependent effects of E2 on blastocyst implantation and post-implantation development in vitro for 8 days. In the experiments of 8-day culutre, all blastocysts were exposed to different concentrations of E2 (10−9, 10−8, 10−7, 10−6, 10−5, and 10−4 M) on first day, and then the blastocysts continued to be cultured without E2 exposure for anotehr 7 days. First, we determined the toxic concentrations of E2 in vitro, testing concentrations over a range from 10−9 to 10−6 M. E2 concentrations of 10−9 to 10−6 M had no apparent impact on blastocyst implantation or post-implantation embryo development. However, the development of late post-implantation embryos (late egg cylinder stage and early somite stage) was adversely affected at E2 concentrations of 10−7 and 10−6 M [Table 1]. To further assess concentration-dependent impacts of E2, we performed additional experiments using E2 concentrations of 10−7 to 10−4 M [Table 2]. The higher E2 concentration (10−4 M) exerted a more rapid impact on early-stage embryos, reducing the blastocyst implantation rate nearly in half (57%) compared with other groups, which showed implantation rates of 93% (control) to 100% (10−7, 10−6, and 10−5) [Table 2].
Table 1.
Eight-day development in vitro of mouse blastocysts exposed to moderate to high concentration of E2 at the blastocyst stage for 24 h.
| Developmental stage | Control group | E2 group |
|||
|---|---|---|---|---|---|
| 10−9M | 10−8M | 10−7M | 10−6M | ||
| Blastocysts | 72 | 56 | 56 | 54 | 58 |
| Hatched/implanted blastocysts (7–8) | 69 (96%) | 53 (95%) | 54 (96%) | 53 (98%) | 57 (98%) |
| Early egg cylinder stage (9–10) | 43 (60%) | 32 (57%) | 30 (54%) | 31 (57%) | 33 (57%) |
| Late egg cylinder stage (11–13) | 36 (50%) | 24 (43%) | 22 (39%) | 14 (26%)∗ | 16 (28%)∗ |
| Early somite stage (14–15) | 18 (25%) | 13 (23%) | 13 (23%) | 7 (13%)∗ | 4 (7%)∗ |
Data collected from 4 experiments and analyzed by one-way analysis of variance (ANOVA), ∗p < 0.05 vs. control.
10−6 M equivalent to 272.4 pg/mL.
Table 2.
Eight-day development in vitro of mouse blastocysts exposed to moderate to high concentration of E2 at the blastocyst stage for 24 h.
| Developmental stage | Control group | E2 group |
|||
|---|---|---|---|---|---|
| 10−7M | 10−6M | 10−5M | 10−4M | ||
| Blastocysts | 44 | 26 | 28 | 26 | 28 |
| Hatched/implanted blastocysts (7–8) | 41 (93%) | 26 (100%) | 28 (100%) | 26 (100%) | 16 (57%)∗∗∗ |
| Early egg cylinder stage (9–10) | 24 (55%) | 11 (42%) | 15 (54%) | 11 (42%) | 0∗∗∗ |
| Late egg cylinder stage (11–13) | 21 (48%) | 7 (27%∗) | 8 (29%) | 7 (27%)∗ | 0∗∗∗ |
| Early somite stage (14–15) | 12 (27%) | 2 (8%)∗ | 0∗∗ | 1 (4%)∗ | 0∗∗ |
Data collected from 3 experiments, and analyzed by one-way analysis of variance (ANOVA), ∗p < 0.05 vs. control, ∗∗p < 0.01 vs. control, ∗∗∗p < 0.001 vs. control.
10−4 M equivalent to 27,240 pg/mL.
Effects of E2 on blastocyst proliferation and outgrowth ability
In order to investigate the impacts of high E2 on cell proliferation of blastocyst in vitro, the differential staining and outgrowth assays were used. The numbers of inner cell mass (ICM) and trophectoderm (TE) cells were both decreased following treatment with 10−4 M E2 for 24 h [Fig. 3A].
The cell number of blastocyst outgrowth in vitro was significantly decreased at the group of 10−4 M E2, in comparison with other groups [Fig. 3B].
Apoptotic effects of 24-h exposure to E2 at the blastocyst stage
In order to investigate apoptotic effects of high E2 on the blastocyst in vitro, the TUNEL assay was used. The total number of apoptotic cells, including inner cell mass (ICM) and trophectoderm (TE) cells was significantly decreased at the group of 10−4 M E2, in comparison with other groups [Fig. 4].
Fig. 4.
Apoptotic effects of 24-h exposure to E2 at the blastocyst stage. (A) TUNEL-positive cell shown as arrow in the ICM cells of blastocysts. TUNEL-positive cell shown as blank arrow in the trophectoderm of blastocysts. (B) Apoptosis of blastocyst cells occurred at E2 levels above a threshold of 10−4 M. ∗P < 0.05 versus the control group. The sample size shown in each group (control:32; 10−7 M:30; 10−6 M:32; 10−5 M:31; 10−4 M:30 respectively). The apoptotic cells were noted using the TUNEL assays. Data were recruited from three experiments and analyzed by one-way analysis of variance (ANOVA).
E2 disruption of blastocysts in vitroin an embryo transfer model
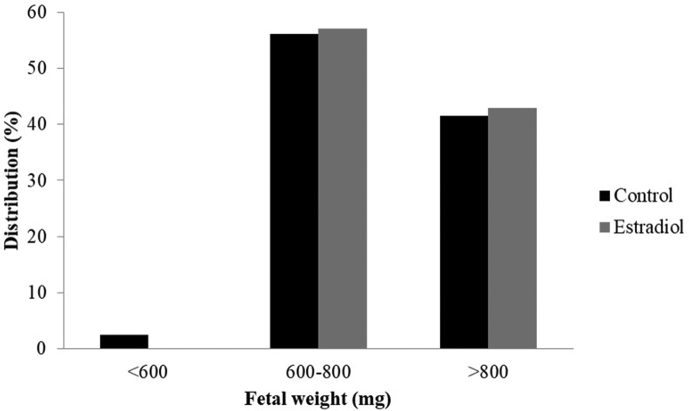
In order to investigate the pregnancy outome of E2-treated blastoycsts in the female uterus, the method of embryo transfer was used. The implantation rate in the E2 group was lower than that in the control group. Specifically, control blastocysts implanted at a rate of ∼72% (49 of 68 embryos), whereas E2-pretreated blastocysts implanted at a rate of ∼38% (14 of 37 embryos)—a decrease of ∼47% [Table 3]. Moreover, 81% (30 of 37 implants) of implanted embryos failed to develop normally (i.e., were resorbed) in the E2 group compared with 38% (26 of 68 implants) in the control group. The fetal survival rate in the E2 group was also significantly lower than that in the control group. Despite above differences, the body weight of surviving fetuses was similarly distributed between the two groups: ∼55% of fetuses in both groups weighed 600–800 mg, and ∼45% of fetuses in both groups weighed over 800 mg [Fig. 5].
Table 3.
Pregnancy outcomes following E2-treated blastocyst transferred into uterus.
| Group | Transferred embryo No. | Implantation rate (%) | Implanted embryos |
|
|---|---|---|---|---|
| Resorption | Surviving fetuses | |||
| Control | 94 | 68(72%) | 27/68(40%) | 41/68(60%) |
| E2-treated | 94 | 37(39%)∗∗∗ | 30/37(81%)∗∗∗ | 7/68(19%)∗∗∗ |
Data were analyzed by one-way analysis of variance (ANOVA), ∗∗∗p < 0.001 vs. control.
Fig. 5.
Neonatal birth weight distribution following E2-treated blastocysts transferred into uterus. E2 exposure and no-exposure at the blastocyst stage for 24 h in vitro followed by embryo transfer. 18-day fetuses were collected from the surrogate mice on day 14 post-transfer. Weight distribution of surviving fetuses was analyzed by three subgroup of birth weight. Data were collected from 41 surviving fetuses in the control group and 7 surviving fetuses in the treatment group.
Discussion
In the study, we have demonstrated that ERα and ERβ protein in the pre-implanted embryos, especially the blastocysts. At most, the mRNA expression of ER is noted in the previous studies. We also find the direct impacts of high E2 on the blastoycsts firstly, which is not well known in the previous studies.
The detection of ER protein in blastocysts lends further support to possible direct effects of E2 on blastocysts during the pre-implantation period. In one previous study, Hou et al. demonstrated that ER protein is detectable in implanting blastocysts of mouse and early egg cylinder stage embryos developed in culture [36]. In another study, Saito et al. showed that ERα expression was down-regulated in activated blastocysts upon completion of blastocyst implantation in mice [37]. In the current study, we have extended these observations, demonstrating ERα and ERβ protein expression in different-stage embryos. Focusing on the blastocyst, we found that ERβ was more strongly expressed than ERα, suggesting that the actions of E2 on blastocyst implantation and post-implantation development are mediated by ERβ. Thus, ERα may be a housekeeping receptor, whereas ERβ may be the major receptor responsible for the actions of E2. However, the actual differences of signal inensity of ERα and ERβ protein did not be investigated in the study. Blastocyst implantation and development studies designed to confirm this hypothesis using antagonists selective for ERα or ERβ subtypes are ongoing in our laboratory.
Despite our findings and previous reports of ER expression in blastocysts, the actual roles of E2 in blastocysts are still not well investigated. In a study by Valbuena et al. [22], the effects of E2 in vitro were investigated at concentrations of10−7 M (27.24 pg/mL), 10−6 M (272.4 pg/mL), 10−5 M (2724 pg/mL), which could be very close to the serum E2 concentrations routinely achieved in controlled ovarian stimulation (COS) used for human assisted ART. And the concentration of 10−4 M (27240 pg/mL) could occur possibly, but not usually at the condition of OHSS [22,[26], [27], [28],32]. To determine the toxic concentrations of E2 on the mouse blastocysts in vitro, a range of E2 concentration (10−9 M to 10−4 M) was investigated in the study. Immediate cytotoxic concentration of E2 is 10−4 M, which has been shown that cell proliferation and blastocyst outgrowth in vitro were significantly inhibited, and revealed an increase in the number of apoptotic cells. The adverse effects of E2 on post-implantation development were most prominent at a concentration of 10−4 M, which completely inhibited development from the early egg cylinder stage to the early somite stage. Although E2 concentration of 10−7 M to 10−5 M did not induce the immediate toxic effects, they still showed the delayed impacts on post-implantation embryonic development (late egg cylinder stage and early somite stage). These observations suggest that E2 levels moderately greater than those observed physiologically may have carry-on effects on the blastocyst. To prevent the direct impacts of high E2 on the blastocyst, control of the serum E2 level (not above 10000 pg/mL) or freezing embryos when E2 reaching 10000 pg/mL would be a sound strategy in clinical ART.
Most previous studies addressing the pharmacological effects of E2 on blastocyst implantation and early post-implantation development have stressed endometrial effects [[18], [19], [20], [21]]. Although one study showed direct effects of a high E2 concentration on pre-implantation embryo development [22], it did not provide clear evidence for an effect of high E2 on blastocyst for implantation. According to another previous study [38], administration of 17β-estradiol at a concentration of 10−6 M had negative effects on human trophoblast cell lines, derived from the TE of blastocysts following implantation. Notably, the ERis also detected in the TE of blastocysts [36]. These findings raise concerns about the potential effects of E2 on the blastocyst, especially on cells of the TE. Human embryonic stem cells are derived from the ICM of the blastocyst, and their differentiation occurs through an intermediate step involving the formation of embryoid bodies (EBs), which are aggregates of embryonic stem cells. EBs mimic the initial stages of early post-implanted blastocyst development. E2 administration at a concentration of 10−7 M exerts effects on endodermal and mesodermal differentiation of human EBs [39]. In our study, we demonstrated a decrease in cell proliferation and an increase in the number of apoptotic cells in whole blastocysts at the E2 concentration of 10−4 M, especially in the ICM area, which is the major part of the fetus in post-implantation development. The adverse effects of high E2 on the ICM are compatible with their effects on EBs reported by Kim et al. [39]. These findings demonstrate that E2 has a direct action in the blastocyst for implantation and post-implantation development.
Similar findings were obtained in Valbuena's study [22], which reported that a very high E2 level (10−4 M) has a direct impact on pre-implantation embryo development, decreasing the number and quality of blastocysts formed in vitro. These findings provide evidence of ERs in the pre-implantation embryo, a result compatible with our findings of the presence of ERα and ERβ in mouse embryos. This previous study also showed that the poor quality of in vitro blastocysts resulted in diminished embryo adhesion to endometrial cells in vitro. A number of studies have concluded that high levels of E2 reduce the receptivity of the endometrium, but direct effects of E2 on blastocysts were largely unaddressed, possibly owing to limitations of the experimental models used in these studies. Using our in vitro model, we have provided evidence that high E2 may directly affect blastocysts, resulting in subsequent adverse effects. In clinical ART, we have found that pregnancy outcomes are higher for thawed embryo transfer cycles than for fresh embryo transfer cycles [40]. The explanation for this improvement does not seem to lie only in avoiding the poor receptivity of endometrium, but also in preventing damage of embryos by a high E2 environment following COS.
In conclusion, we have provided the evidence of direct effects of E2 on the mouse blastocyst. Direct-impacted blastocysts with high E2 concentration (≧10−7 M) may affect the implantation and post-implantation embryonic development in vitro and in vivo. In clinical ART, high serum E2 concentrations not only affects the endometrium, but also affects blastocysts directly at the period of implantation.
Conflicts of interest
The authors declare no competing financial or non-financial interests.
Acknowledgement
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST-101-2314-B-182A-110, MOST-102-2314-B-182A-085, MOST-103-2314-B-182A-108) and Chang Gung Memorial Hospital, Taiwan (CMRPG8G0091 and CMRPG8G0092).
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Wen-Hsiung Chan, Email: whchan@cycu.edu.tw.
Fu-Jen Huang, Email: huangfj1885@gmail.com.
References
- 1.Stillwell W. Elsevier Science; Netherlands: 2016. An introduction to biological membranes: composition, Structure and Function; pp. 453–478. [Google Scholar]
- 2.Dahlman-Wright K., Cavailles V., Fuqua S.A., Jordan V.C., Katzenellenbogen J.A., Korach K.S., et al. International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 3.Smith D.M., Smith A.E.S. Uptake and incorporation of amino acids by cultured mouse embryos: estrogen stimulation. Biol Reprod. 1971;4:66–73. doi: 10.1093/biolreprod/4.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Khurana N.K., Wales R.G. Effects of oestradiol and progesterone on the metabolism of (U-14C)glucose by mouse morula and early blastocysts in vitro. J Reprod Fertil. 1987;79:267–273. doi: 10.1530/jrf.0.0790267. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt B.M., Bullock D.W. Binding of oestradiol to rabbit blastocysts and its possible role in implantation. J Reprod Fertil. 1974;39:65–70. doi: 10.1530/jrf.0.0390065. [DOI] [PubMed] [Google Scholar]
- 6.Bloxham P.A., Pugh D.M. Tamoxifen inhibition of an in vitro oestradiol-induced surface coat change on mouse blastocysts. Br J Pharmacol. 1977;60:517–519. doi: 10.1111/j.1476-5381.1977.tb07529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Para B.C., Sengupta J., Manchanda S.K. Role of embryonic oestrogen in rabbit blastocyst development and metabolism. J Reprod Fertil. 1984;70:429–436. doi: 10.1530/jrf.0.0700429. [DOI] [PubMed] [Google Scholar]
- 8.Bai J., Qi Q.R., Li Y., Day R., Makhoul J., Magness R.R., et al. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int J Mol Sci. 2020;21:4349. doi: 10.3390/ijms21124349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., Cui D., Kong Y. FoxM1 influences embryo implantation and is regulated by 17 beta-estradiol and progesterone in mouse uteri and endometrium cells. Int J Mol Sci. 2014;7:6585–6595. [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R., Yadav A., Pakrasi P.L. Expression of ER-α and ER-β during peri-implantation period in uterus is essential for implantation and decidualization in golden hamster. Life Sci. 2017;170:115–122. doi: 10.1016/j.lfs.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Joshi A., Mahfooz S., Maurya V.K., Kumar V., Basanna C.S., Kaur G., et al. PARP1 during embryo implantation and its upregulation by estradiol in mice. Reproduction. 2014;147:765–780. doi: 10.1530/REP-13-0588. [DOI] [PubMed] [Google Scholar]
- 12.Pereira N., Elias R.T., Christos P.J., Petrini A.C., Hancock K., Lekovich J.P., et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32:1410–1417. doi: 10.1093/humrep/dex095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Zeng C., Shang J., Wang S., Gao X.L., Xue Q. Association between serum estradiol level on the human chorionic gonadotropin administration day and clinical outcome. Chin Med J. 2019;132:1194–1201. doi: 10.1097/CM9.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W., Tian Y., Xie D., Miao Y., Liu J., Wang X. The impact of peak estradiol during controlled ovarian on the cumulative live birth rate of IVF/ICSI in non-PCOS patients. J Assist Reprod Genet. 2019;36:2333–2344. doi: 10.1007/s10815-019-01568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharara F.I., Abdo G. High estradiol levels and high oocyte yield are not detrimental to IVF outcome in blastocyst cycles. Fertil Steril. 2012;98:S52–S53. [PubMed] [Google Scholar]
- 16.Dunne C., Cho K., Shan A., Hutcheon J., Durland U.S., Seethram K., et al. Peak serum estradiol level during controlled ovarian stimulation is not associated with lower level of pregnancy-associated plasma protein-A or small for gestational age infants: a cohort study. J Obstet Gynaecol Can. 2017;39:870–879. doi: 10.1016/j.jogc.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Kailasam C., Griffith H., Wilson P., Gordon U. The effect of early coasting on blastocyst development and outcome following blastocyst transfer in IVF/ICSI programme. JBRA Assist Reprod. 2018;22:301–306. doi: 10.5935/1518-0557.20180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W.G., Song H., Das S.K., Paria B.C., Dey S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon C., Cano F., Valbuena D., Remohí J., Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum estradiol levels in high and normal responder patients. Hum Reprod. 1995;10:2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 20.Simon C., Domínguez F., Valbuena D., Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metabol. 2003;14:197–199. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 21.Ullah K., Rahman T.U., Pan H.T., Guo M.X., Dong X.Y., Liu J., et al. Serum estradiol levels in controlled ovarian stimulation directly affect the endometrium. J Mol Endocrinol. 2017;59:105–119. doi: 10.1530/JME-17-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valbuena D., Martin J., de Pablo J.L., Remohí J., Pellicer A., Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–968. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 23.Pauerstein C.J., Eddy C.A., Croxatto H.D., Hess R., Siler-Khodr T.M., Croxatto H.B. Temporal relationships of estrogen, progesterone, and luteinizing hormone levels to ovulation in women and infrahuman primates. Am J Obstet Gynecol. 1978;130:876–886. doi: 10.1016/0002-9378(78)90264-8. [DOI] [PubMed] [Google Scholar]
- 24.Roos J., Johnson S., Weddell S., Godehardt E., Schiffner J., Freundl G., et al. Monitoring the menstrual cycle: comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept Reprod Health Care. 2015;20:438–450. doi: 10.3109/13625187.2015.1048331. [DOI] [PubMed] [Google Scholar]
- 25.Simon C., Garcia Velasco J.J., Valbuena D., Peinado J.A., Moreno C., Remohí J., et al. Increasing uterine receptivity by decreasing estradiol levels during the periimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;70:234–239. doi: 10.1016/s0015-0282(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee K.H., Kim S.H., Jee B.C., Kim Y.J., Suh C.S., Kim K.C., et al. Comparison of clinical characteristics between early and late patterns in hospitalized patients with ovarian hyperstimulation syndrome. Fertil Steril. 2010;73:2274–2280. doi: 10.1016/j.fertnstert.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z., Li R., Ma Y., Deng B., Zhang X., Meng Y., et al. Effect of HCG-day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRh agonist cycles. RBM online. 2012;24:511–520. doi: 10.1016/j.rbmo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Ma T., Niu Y., Wei B., Xu L., Zou L., Che X., et al. Moderate-to-severe ovarian hyperstimulation syndrome: a retrospective multivariate logistic regression analysis. Adv Clin Exp Med. 2020;29:85–90. doi: 10.17219/acem/92916. [DOI] [PubMed] [Google Scholar]
- 29.Sealey J.E., Itskovitz-Eldor J., Rubattu S., James G.D., August P., Thaler I., et al. Estradiol- and progesterone-related increases in the renin-aldosterone system: studies during ovarian stimulation and early pregnancy. J Clin Endocrinol Metab. 1994;79:258–264. doi: 10.1210/jcem.79.1.8027239. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y.Q., Luo J., Xu W.M., Xie Q.Z., Yan W.J., Wu G.X., et al. Can steroidal ovarian suppression during the luteal phase after oocyte retrieval reduce the risk of severe OHSS? J Ovarian Res. 2015;8:63. doi: 10.1186/s13048-015-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavy M.T., Vernon M.W., Sharp D.C., III, et al. Endocrine aspects of early pregnancy in pony mares: a comparison of uterine luminal and peripheral plasma levels of steroids during the estrous cycle and early pregnancy. Endocrinology. 1984;115:214–219. doi: 10.1210/endo-115-1-214. [DOI] [PubMed] [Google Scholar]
- 32.Deng L., Li X.L., Ye D.S., Blockeel C., Zhou X.Y., Chen C.L., et al. A second dosed of GnRHa in combination with luteal GnRH antagonist may eliminate ovarian hyperstimulation syndrome in women with >30 follicles measuring >11 mm in diameter on trigger day and/or pre-trigger estradiol exceeding 10000 pg/mL. Current med Science. 2019;39:278–284. doi: 10.1007/s11596-019-2031-5. [DOI] [PubMed] [Google Scholar]
- 33.Witschi E. 2nd ed. Federation of American Societies for Experimental Biologies; Washington DC: 1972. Characterization of developmental stages. Part II; pp. 178–180. (Biology data book). [Google Scholar]
- 34.Huang F.J., Wu T.C., Tsai M.Y. Effects of retinoic acid on implantation and post-implantation development of mouse embryo in vitro. Hum Reprod. 2001;16:2171–2176. doi: 10.1093/humrep/16.10.2171. [DOI] [PubMed] [Google Scholar]
- 35.Huang F.J., Shen C.C., Chang S.Y., Wu T.C., Hsuuw Y.D. Retinoic acid decreases the viability of mouse blastocysts in vitro. Hum Reprod. 2003;18:130–136. doi: 10.1093/humrep/deg018. [DOI] [PubMed] [Google Scholar]
- 36.Hou Q., Paria B.C., Mui C., Dey S.K., Gorski J. Immunolocalization of estrogen receptor protein in the mouse blastocyst during normal and delayed implantation. Proc Natl Acad Sci U S A. 1996;93:2376–2381. doi: 10.1073/pnas.93.6.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito K., Furukawa E., Kobayashi M., Fukui E., Yoshizawa M., Matsumoto H. Degradation of estrogen receptor α in activated blastocysts is associated with implantation in the delayed implantation mouse model. Mol Hum Reprod. 2014;20:384–391. doi: 10.1093/molehr/gau004. [DOI] [PubMed] [Google Scholar]
- 38.Bechi N., Sorda G., Spagnoletti A., Bhattacharjee J., Vieira Ferro E.A., de Freitas Barbosa B., et al. Toxicity assessment on trophoblast cells for some environment polluting chemicals and 17β-estradiol. Toxicol Vitro. 2013;27:995–1000. doi: 10.1016/j.tiv.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Kim H., Kim Y.Y., Ku S.Y., Kim S.H., Choi Y.M., Moon S.Y. The effect of estrogen compounds on human embryoid bodies. Reprod Sci. 2013;20:661–669. doi: 10.1177/1933719112462630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W., Xiao X., Zhang J., Wang W., Wu J., Peng L., et al. Clinical outcomes of frozen embryo versus fresh embryo transfer following in vitro fertilization: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2018;298:259–272. doi: 10.1007/s00404-018-4786-5. [DOI] [PubMed] [Google Scholar]