Abstract
Drug-resistant epilepsy, characterized by ongoing seizures despite appropriate trials of anti-seizure medications, affects approximately one-third of people with epilepsy. Brain stimulation has recently become available as an alternative treatment option to reduce symptomatic seizures in short and long-term follow-up studies. Several questions remain on how to optimally develop patient-specific treatments and manage therapy over the long term. This review aims to discuss the clinical use and mechanisms of action of Responsive Neural Stimulation and Deep Brain Stimulation in the treatment of epilepsy and highlight recent advances that may both improve outcomes and present new challenges. Finally, a rational approach to device selection is presented based on current mechanistic understanding, clinical evidence, and device features.
Keywords: Neurostimulation, Epilepsy, Seizure, Deep brain stimulation, Responsive neural stimulation
Approximately 50 million people worldwide have active epilepsy [1]. Despite numerous advancements in the available medical treatments, about 35% of treated individuals continue to develop drug-resistant epilepsy (DRE), characterized by intractable seizures [2]. Ongoing seizures can significantly reduce quality of life, disrupt cognitive function, and increase the risk of death [3]. Traditional pharmacologic treatments lack regional specificity and are often associated with adverse side effects [4]. The proportion of patients that develop DRE has remained relatively stable for more than three decades [2,5] despite the release of over a dozen new anti-seizure medications. Conventional surgical options involve removing or disconnecting the epileptic brain tissue, which can alleviate seizures in some patients with DRE [6]. However, these destructive techniques place patients at risk for permanent surgical complications and other adverse outcomes [7]. Furthermore, many patients are not eligible for these procedures, because the suspected seizure focus is diffuse, poorly-localized, or in regions associated with a high risk for neurologic deficits [8]. Thus, despite the availability of both medical and surgical therapies for epilepsy, there is an urgent need for additional treatments.
Brain stimulation provides an alternative, reversible, and adjustable treatment option for patients with DRE. Deep Brain Stimulation (DBS) [9,10], Responsive Neural Stimulation (RNS) [11], and Vagus Nerve Stimulation (VNS) are safe and effective neurostimulation therapies used to treat refractory epilepsy. This review is intended to focus on the two currently available direct brain stimulation devices (RNS and DBS) and does not cover other neurostimulation devices that do not electrically stimulate the brain directly (e.g., VNS and transcranial magnetic stimulation). DBS is an open-loop neurostimulator that provides continuous periodic electrical stimulation (Fig. 1B), which is used to suppress seizures [12]. RNS is a closed-loop neurostimulator (Fig. 1C) that automatically analyzes electrocortical activity to detect and rapidly deliver electrical stimulation to terminate seizures. Despite significant design differences, both RNS and DBS require a prolonged programming period lasting several months to years to achieve an acceptable response [13] and demonstrate progressive improvements over years of treatment [9,11]. Clinical and technical aspects of these devices will be discussed in more detail later in this review, after first discussing more general properties of brain stimulation.
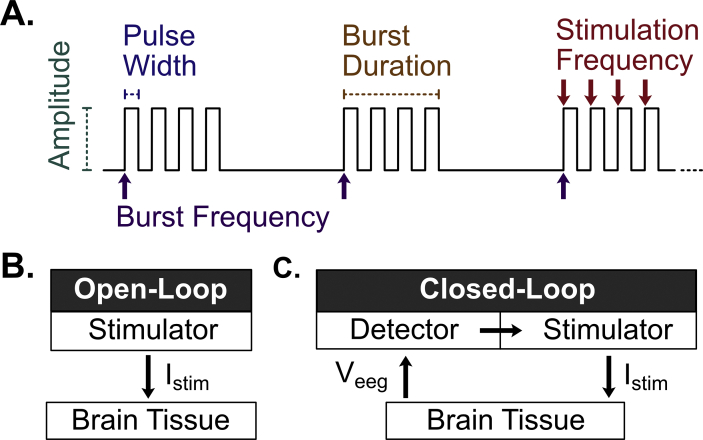
Fig. 1.
Neurostimulation overview. (A) The stimulation waveform is primarily defined by the amplitude of stimulation (μA-mA), stimulation pulse width (μs-ms), burst duration (ms-s), stimulation frequency (Hz), and burst frequency (<1 Hz) (B) Open-loop stimulation devices (e.g., Deep Brain Stimulation). Deliver continuous periodic stimulation (Istim) to the brain (C) Closed-loop stimulation devices (e.g., Responsive Neural Stimulation). Deliver stimulation only when seizure activity is detected from the recorded EEG signal (Veeg).
During brain stimulation, electrical current is generated by an implanted pulse generator and delivered directly to the brain via intracranial electrodes. These electrodes are surgically implanted either cortically on the surface of the target structure using strip electrodes or penetrating deep into the brain tissue using depth electrodes. Electrical current is conducted either as (1) monopolar between one electrode and the pulse generator, (2) bipolar between two electrodes, or (3) multipolar between several electrode contacts. The shape of the stimulus current is defined by several parameters, including current amplitude, pulse-width, burst duration, and frequency (Fig. 1A). Initial stimulation settings are generally common to all patients and are adjusted empirically in a trial-and-error fashion until an acceptable response is achieved.
The effects of brain stimulation may depend on several factors, including the stimulation settings, electrode locations, seizure onset location, and triggering strategy (open-loop versus closed-loop). A greater understanding of the underlying effects and mechanisms of brain stimulation is needed to develop improved patient-specific treatment plans and make optimal adjustments throughout long-term therapy.
Anti-seizure effects and mechanisms of brain stimulation
The electrical current delivered by brain stimulation can modulate several neurophysiologic mechanisms spanning the molecular, cellular, and network levels. The effects on seizure activity can be broadly classified into one of three putative categories: seizure suppression, seizure termination, and disease modification. Specific device selection, electrode placement, and programming strategy may be required to elicit each effect and outcome, which have not yet been thoroughly evaluated.
Seizure suppression
Most treatments for epilepsy, including anti-seizure medications, are used to suppress seizures, typically by reducing neuronal excitability and preventing seizure occurrence and propagation only during the time when the treatment is applied (Fig. 2A). Anti-seizure medications are grouped by their principal mechanisms of action, such as modulating ion channels, neurotransmitter (e.g., GABA, glutamate) receptors, or other mechanisms. These effects are dependent on the serum concentration and wear off when below the therapeutic window, without any residual permanent benefit. Similarly, electrical brain stimulation has been shown to have seizure-suppressive effects. While electrical stimulation was traditionally used in the nervous system to excite neurons [14,15], further studies have shown that neuronal activity can be suppressed with specific stimulation locations and parameters [16,17].
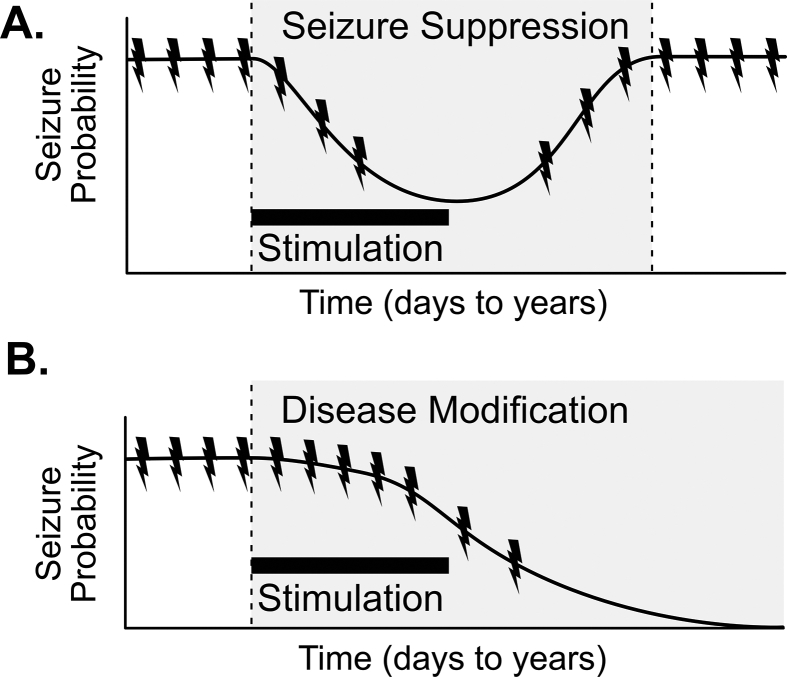
Fig. 2.
Representation of chronic effects of neurostimulation (A) In established epilepsy, seizure suppression (shaded region) temporarily reduces the probability of seizures, with no permanent effect on disease state of epilepsy. (B) In established epilepsy, disease modification (shaded region) results in permanent reduction in seizure frequency, ideally resulting in seizure freedom or “cure”, even after stimulation is stopped. The black bar represent the time period of stimulation.
High-frequency stimulation (HFS) has been shown to have desynchronizing effects on focal and large-scale networks [18]. Whether HFS acts via excitatory or inhibitory mechanisms has been actively debated for several decades [[19], [20], [21], [22]]. Defining the effect of electrical stimulation by recording nearby neuronal activity has been challenging due to the electrical artifact produced during stimulation. One approach has been to approximate activity by using very low amplitude stimulation settings, or to infer the effects by evaluating neuronal activity recorded immediately post-stimulation. Using this technique, HFS was shown to reduce the firing activity of nearby neurons [[23], [24], [25]], an effect that can persist post-stimulation [24,26,27]. HFS has demonstrated both suppressive [28] and excitatory effects [[29], [30], [31]] on stimulated network activity, depending on the location of stimulation and recording. Intracellular studies evaluating the effects of neurostimulation on subthalamic neuron activity demonstrated mixed effects, first producing an increased firing rate, followed by a switch to bursting mode and then electrographic silence [32]. Several interpretations for these findings have been proposed, including direct inhibition of neuronal firing [33] and activation of inhibitory GABAergic afferents [24,26].
Low-frequency stimulation (LFS) has also demonstrated several effects on neuronal firing activity. LFS has been shown to interfere with synchronization between brain regions [34], and can suppress epileptiform activity when targeting the entorhinal cortex [35], amygdalo-hippocampus [36], and the seizure onset zone [34]. LFS can produce network stabilizing effects [37] and causes long-term depression with a long-lasting decrease in synaptic efficacy [38,39]. Each of these effects may reduce the excitability of the epileptic network and lead to a suppression of spontaneous seizure activity.
Seizure termination
Most seizures spontaneously terminate within a few minutes after onset through neurophysiologic mechanisms intrinsic to the brain [40]. During a seizure, ictal activity at the level of an individual neuron can result in a loss of normal potassium and other ionic gradients, thereby blocking depolarization and stopping the seizure [41,42]. Excessive neuronal activity can deplete energy substrates [43] and produce a depressant effect on neuronal activity [44]. Additional mechanisms act at a network level to oppose the amplification and spread of ictal activity, including the depletion of neurotransmitter-containing synaptic vesicles [45], glial buffering of glutamate [46], and GABA-ergic inhibition [47]. These mechanisms represent promising targets for anti-seizure treatments that enhance their anticonvulsant effects.
Seizure termination may also occur when a treatment is applied acutely during an ongoing seizure (Fig. 3). Cortical electrical stimulation is a standard clinical tool used to map brain function, which occasionally generates unwanted epileptiform after-discharges that interfere with clinical testing. Brief bursts of electrical stimulation were found to be effective at aborting these after-discharges in DRE patients [48,49], which supported the concept of using electrical stimulation to terminate seizures. However, since after-discharges are provoked, their underlying physiology may differ, with questionable pertinence to spontaneous seizures. Validation of seizure terminating effects has been challenging, as spontaneous seizures occur randomly and are difficult to predict. Early work using implanted brain stimulation devices triggered by patients at the onset of seizures provided some evidence for possible termination of seizures [50]; however, this effect has yet to be thoroughly evaluated in human subjects. Likewise, animal studies have primarily focused on seizure suppressive effects [51,52] on provoked epileptiform activity [53,54], with more limited studies on termination effects [55,56]. Several potential mechanisms have been proposed to explain how electrical stimulation can acutely terminate seizures. At a molecular level, HFS can lead to excessive extracellular potassium accumulation [17], enhancing the intrinsic anticonvulsant blockade of depolarization and conduction. Neurostimulation may induce short-term synaptic depression of excitatory synapses [57]. At the cellular and network level, neurostimulation may modulate the activity of inhibitory neurons and either synchronize [58] or desynchronize focal and large-scale networks [18]. These immediate anti-seizure effects can act to terminate or inhibit the propagation of seizures.
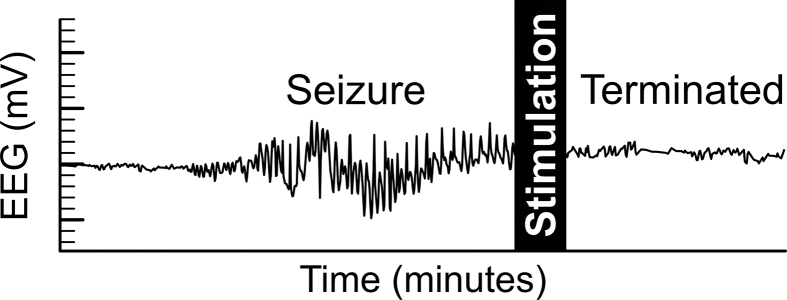
Fig. 3.
Representation of acute seizure-termination effects of neurostimulation. In this simulated EEG tracing, seizure activity is interrupted by a brief pulse of electrical neurostimulation.
Disease-modification
Disease modification effects typically involve a change in the underlying pathophysiology of epilepsy that results in permanent seizure reductions, optimally resulting in seizure freedom, that persist even after the treatment has been discontinued (Fig. 2B). Long-term neurostimulation across several different device designs has been shown to result in a progressive reduction of seizure occurrences [9,11], suggesting the possibility of a disease-modifying effect. In the long-term SANTÉ trial of DBS for epilepsy, increasing seizure reductions were seen throughout the study. Initial treatment effects were −41% at year one, which progressively improved to −75% at year seven [9]. Similar trends have been seen in VNS [59] and RNS [11]. The mechanistic basis of this empirical observation is unclear but may be due to inherent disease remission, optimization of the terminating or suppressive factors, or an underlying disease-modifying effect.
Whether brain stimulation treatments of epilepsy have true disease-modifying effects remains unproven, as controlled trials have not assessed whether beneficial effects of neurostimulation persist permanently after stimulation is stopped. Disease-modifying effects have had limited evaluation in preclinical models, though are suggested by at least one publication of LFS [51]. Nevertheless, there are several potential avenues by which neurostimulation may trigger chronic disease-modifying changes. Neurostimulation in a rat model of temporal lobe epilepsy was shown to induce differential expression of mRNA in ion channel and signaling pathways [60], as well as partially block pathologic changes in genetic expression [61]. Neurostimulation was also shown to reduce the excitability of epileptic networks [62]. This effect has recently been shown to have clinical relevance, as patients who demonstrated early reorganization of functional network connectivity achieved improved clinical outcomes [63], suggesting that neurostimulation may involve plasticity of the underlying epileptic network. These alterations of gene expression and network activity may, at least in theory, underlie putative disease-modifying effects of chronic neurostimulation in epilepsy [64].
Spatial aspects of brain stimulation
The effects of brain stimulation on seizures are highly dependent on the anatomy of the epileptic network and the anatomic target(s) used for stimulation. Epilepsies can be classified as focal (single or multifocal), generalized, combined (focal & generalized), or unknown [65]. This classification is determined by clinicians based on the imaging, EEG, and other diagnostic tests and can have significant implications on treatment options. A roughly spherical electric field is generated during stimulation, modulating a finite volume of tissue [66], with variable effects on different neuronal and cellular elements [21,67,68]. The location, size, and shape of this volume of modulated tissue depend on the parameter and electrode configurations [66], as well as the location of active electrodes. RNS typically uses electrodes implanted within or adjacent to an epileptic focus in the cortex or hippocampus. DBS typically uses electrodes implanted into the anterior nucleus of the thalamus (ANT). However, several recent studies demonstrate anti-seizure effects in other locations, with some overlapping regions between the two different therapies, such as RNS targeting the ANT [69] and DBS targeting the hippocampus [10]. Several additional locations have been investigated, including cerebellum, basal ganglia (subthalamic nucleus, caudate), deep brain nuclei (nucleus accumbens), and white matter targets (corpus callosum, intra-hippocampal commissure), though there is currently insufficient evidence to support their general clinical use. The most commonly used targets for brain stimulation are the neocortex, hippocampus, and thalamus.
Neocortex
Cortical electrodes are often used to treat focal neocortical epilepsy, especially when eloquent regions are involved. The human neocortex is structured as six layers of neurons and has a columnar organization. Generally speaking, there are two primary neuron classes in the cerebral cortex: (a) projection or principal neurons that are primarily excitatory, and (b) interneurons that are primarily inhibitory. Typically, projection neurons send information to remote brain regions, whereas interneurons influence nearby neurons [70]. Interneurons can have extensive axonal projections, providing synchronization or pacemaker activity to isolated networks [71]. Cortical seizures are thought to develop when hypersynchronous discharges occur in a discrete region [72,73]. Seizures can then propagate if surround inhibition from neighboring interneurons is overcome by sufficient excitation and recruitment [21,74]. Clinicians use electrocorticography, imaging, and additional studies to identify the site of seizure initiation or seizure-onset zone. Cortical stimulation of the seizure-onset zone has demonstrated long-term seizure suppression effects [13,75] and has become a widely used method for RNS.
Hippocampus
The hippocampus plays a significant role in developing mesial temporal lobe epilepsy (MTLE), one of the most common forms of drug-refractory focal epilepsy. The hippocampus usually functions as part of the limbic system for memory and navigation functions. It is located in the medial aspect of the temporal lobe and is phylogenetically the oldest part of the cortex, with three neuronal layers. Within the hippocampus, the dentate gyrus has afferent connections to a network that directly activates the projection neurons (granule cells) and local interneurons (bipolar and basket cells). During the development of MTLE, excitatory axon sprouting [76,77], or a selective loss of inhibitory neurons [78,79], can lead to an increased tendency towards seizures, progressive injury, and hippocampal sclerosis [80]. Direct hippocampal stimulation has demonstrated seizure suppression effects [10,81]. This effect may have a regional dependence, with possible increased efficacy near the subiculum [82]. Hippocampal stimulation in MTLE has been evaluated using both RNS [83,84] and DBS [81,85].
Thalamus
The thalamus is part of the corticothalamic relay network associated with absence [86] and temporal lobe epilepsies [87]. The thalamus is a paired gray matter structure composed of multiple subcortical nuclei and acts as a relay station to transfer information between subcortical structures and the cortex via widespread thalamocortical connections. The thalamus projects to the hippocampus via the mammillothalamic tract, which has been shown to play a role in propagating temporal lobe seizures [88,89]. DBS targeting the ANT has been effective [9], which may involve connections between the ANT and the circuit of Papez [90]. The centro-median nucleus of the thalamus (CMN) has widespread cortical projections, making it an attractive alternative target for stimulation. CMN-DBS has been evaluated in both neocortical [91] and generalized epilepsies [92,93].
Open-loop: Deep Brain Stimulation
DBS devices have an open-loop stimulation design, meaning that stimulation is continuous and periodic (Fig. 1B). DBS was first widely adopted to treat Parkinson's disease and other movement disorders by targeting subcortical basal ganglia nuclei [94]. Early studies also evaluated cerebellar DBS as a possible treatment for epilepsy [95,96]; however, results were underwhelming, and DBS for epilepsy was largely abandoned. Fortunately, DBS was subsequently evaluated in several additional deep brain structures [97].
DBS received European CE-Mark approval in 2010 and approval by the US Food and Drug Administration (FDA) in 2018 as an adjunctive treatment for adults with medically refractory focal-onset seizures in adult patients who are 18 or older [98]. This approval was based on the multicenter, double-blind, and randomized trial of ANT stimulation for localization-related epilepsy (the SANTÉ trial), wherein a statistically significant median seizure reduction was seen at the end of a three-month blinded period (40.4% in stimulated vs. 14.5% in controls) [12]. In the long-term follow-up study, participants demonstrated a gradual and sustained improvement in seizure reduction over time with a 69% median seizure reduction and 68% responder rate at 5-years [9]. At the combined visit at year seven or time of discontinuation, 9% of patients (10/110) reported an ongoing 6-month or longer seizure-free interval. Patients had a low risk of sudden unexplained death (SUDEP) and overall mortality (2.0 and 6.9 deaths per 1000 person-years, respectively). This SUDEP rate is lower than the historical control (6.9 per 1000 patient-years) in patients with medically intractable epilepsy who were enrolled in the placebo arm of randomized controlled trials [99]. There was a 34% rate of serious adverse effects at five years, most of which occurred within the first few months after implantation. Adverse events were primarily infection (10%) and electrodes placed outside the planned target (8.2%). In addition to the seizure reduction, patients reported an overall improvement in their quality of life, with 43% reporting a 5-point or more improvement on the QOLIE-31.
Though ANT stimulation has proven effective, ongoing research has continued to evaluate additional stimulation targets, particularly DBS targeting the CMN. While CMN-DBS in patients with localization-related epilepsy showed no statistically significant improvement [100,101], results are more promising for generalized epilepsy [92,102]. In a recent cohort of 20 consecutive patients who received CMN-DBS, with 2.5 years follow-up, patients achieved a mean reduction of 66% (tonic), 83% (atonic), 89% (bilateral generalized tonic-clonic), and 78% (atypical absence seizures) [103]. Only one patient (5%, 1/20) reported seizure freedom.
CMN-DBS has also been shown to reduce seizures in Lennox-Gastaut syndrome (LGS), one of the most debilitating forms of epilepsy. LGS is characterized by several seizure types, including tonic, atonic and atypical absence seizures. Seizures are often refractory to medications [104], and the prognosis is generally poor with cognitive deterioration over time. In patients with idiopathic LGS, VNS therapy has provided modest seizure reductions [105], with a recent meta-analysis reporting an approximate 54% response rate [106] (>50% reduction in seizures). CMN-DBS in LGS has demonstrated possible improved efficacy over VNS, resulting in 53–100% seizure reduction at 18 months of follow-up (response rate not reported), with two patients (11%, 2/18) achieving seizure freedom [93]. More recently, in a mixed cohort including four patients with LGS, CMN-DBS resulted in a 68% mean seizure reduction and a 9% seizure freedom rate (1/11). All four patients with LGS demonstrated a >50% reduction in seizure frequency (100% response rate), though no patient with LGS achieved seizure freedom [92]. While these results are encouraging, additional work is needed to define if CMN-DBS in patients with LGS outperforms other stimulation targets.
The hippocampus is an additional DBS target that has been used to treat temporal lobe epilepsy. In a recent prospective, randomized, controlled, double-blind study of hippocampal DBS, 16 patients with refractory temporal lobe epilepsy (including MTLE) were randomized to active stimulation or a sham-control arm. During a six-month blinded phase, 50% (4/8) of patients receiving active stimulation became seizure-free from focal impaired-awareness seizures, and 88% (7/8) were considered responders, compared to 0% seizure freedom and 38% (3/8) responder rate in the control group [107]. In addition, a retrospective review was performed on 25 patients with refractory temporal lobe epilepsy who received open-loop hippocampal stimulation, in whom focal seizures with and without impaired awareness were reduced by 91% and 66%, with reported seizure-freedom rates of 21% and 32%, respectively [10]. These studies support the efficacy of hippocampal DBS in the treatment of refractory temporal lobe epilepsy.
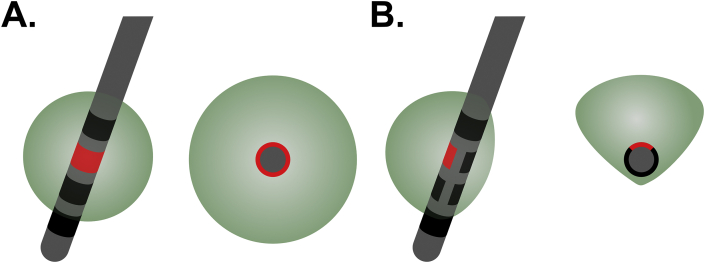
Higher stimulus currents may be associated with unwanted side effects, such as sleep disruption [108]. Reshaping the electric field to avoid regions that cause unwanted side effects can be achieved by parameter adjustments and using different combinations of contacts. However, conventional DBS electrodes use an omnidirectional ring electrode with axial symmetry, which may cause issues if only one side of the electrode is causing side effects (Fig. 4A). Recently, directional electrodes (Fig. 4B) have been developed to allow clinicians to reshape the volume of tissue modulated by stimulation asymmetrically. These directional electrode leads were approved in 2020 to treat refractory epilepsy by both the European CE-Mark and US FDA. These new electrodes have limitations, including a smaller electrode contact area with higher impedances requiring higher voltages to generate equivalent stimulus current amplitudes. They also increase the complexity of electrode planning, placement, and device programming. Further work is needed to assess the clinical utility of this new technology.
Fig. 4.
Schematic representation of stimulation fields from conventional and directional electrodes. (A) A single active symmetric ring contact (red) creates a roughly spherical volume of modulated tissue (green sphere), as seen from oblique (left) and axial (right) directions. (B) A single active directional contact creates an asymmetric volume of modulated tissue (green), as seen from oblique (left) and axial (right) directions, which could be designed to avoid brain regions associated with undesirable side effects.
Closed-loop: responsive neural stimulation
The responsive neural stimulation system (RNS, Neuropace, Mountain View, CA) was designed to detect and terminate seizures shortly after they occur (Fig. 1C). In contrast to DBS, RNS has a closed-loop design that uses real-time analysis of multi-channel electrocorticography recordings to trigger a stimulus pulse when seizure-like activity is detected. However, the underlying mechanism that drives the therapeutic effect of responsive neural stimulation remains unclear. While the device was designed to acutely terminate detected seizures, current methodology typically involves tuning the detection algorithm to have a high sensitivity, such that between 600 and 2000 stimulations are typically triggered per day [84], even though patients in the cohort typically had less than one clinical seizure per day [11]. This means that the majority of stimulations may be triggered by false positive detections, including interictal spikes, non-epileptiform EEG, and artifacts. Therefore, RNS effectively may be using a combination of both open-loop (false detections) and closed-loop stimulations to produce a combination of seizure suppressive and seizure termination effects; how each of these effects contributes to the outcome remains unknown.
Closed-loop stimulation has the potential to reduce unwanted side effects by stimulating only on an as-needed basis, with potential battery-saving benefits. The newest RNS device (RNS-320) weighs 17-g and has a projected lifespan of 5.1–9.4 years with typical use, comparable if not longer than the 61-g Medtronic Percept PC device for DBS which has a 5-year projected lifespan [109].
The FDA approved RNS in 2013 as an adjunctive treatment for adults with medically refractory focal-onset seizures arising from one or two sites of seizure onset in patients who are 18 or older [110]. Approval was based on a multicenter, double-blind, randomized, and controlled trial, wherein RNS was shown to reduce the frequency of patient-reported disabling focal seizures, with improvements in quality of life, and without significant mood or cognitive effects [110]. Based on the available data and study design, it remains unclear if this effect is primarily due to (1) seizure suppression indicated by a reduction in seizure onsets, or (2) termination after seizure onset prior to the seizures becoming clinically apparent, without reducing the rate of seizure onsets. Regardless, participants reported a gradual and sustained improvement in seizure reduction over time, with a 44% median reduction at year one, 48% reduction at year three [111], and 75% at year nine [11]. In the 9-year prospective open-label follow-up study, seizure-freedom during the most recent six months was reported in 21% of subjects at final follow-up, and the rate of SUDEP was 2.8 per 1000 patient-stimulation years [11]. In the initial pivotal trial, adverse events occurred in 2.5% or more patients over the 2-year initial follow-up period, most frequently implant site infection (2.6%) and intracranial hemorrhage (2.1%) [111]. In the 9-year prospective follow-up study, the infection rate per procedure was 4.1%, and non-seizure-related hemorrhage occurred in 2.7% of patients [11]. In addition, RNS of the hippocampal region was shown to be effective in a cohort of 111 subjects with mesial temporal lobe DRE, providing a median seizure reduction of 66.5% at six years, and seizure-freedom of the most recent three months in 20.8% of subjects at final follow-up [84].
While DBS is conventionally used to target subcortical structures, RNS electrodes are typically implanted in or adjacent to the presumed seizure-onset zone(s). One benefit of this placement is that RNS devices provide clinicians with frequent samples of chronic electrocorticography recordings in the implanted regions and a limited set of quantitative measures; however, this does require frequent data uploads by patients. These data can be accessed online, providing long-term insights to assist clinicians in making data-driven adjustments to the RNS device settings and medications. In patients with two potential foci of suspected seizure onset, RNS may help determine which focus is the most active. In a recent study of suspected bilateral MTLE, chronic RNS recordings were used to identify and resect the more active side, resulting in a mean seizure reduction of 94% and seizure-freedom in 71% of patients [112].
RNS has conventionally been used to stimulate a small localized area of seizure onset. However, some patients have a spatially extensive (“regional”) seizure-onset zone that a single electrode may not cover well. Recently, a retrospective review was published, including 30 patients with a regional neocortical seizure onset zone, who received RNS with the electrode leads spaced at least 1-cm apart over the onset zone. Of these patients, 21 received stimulation between the two electrodes, seven received bipolar (single electrode), and two received monopolar stimulation. Across the entire cohort, the median clinical seizure frequency reduction was 75.5% at a mean follow-up of 21 months. This finding is encouraging for patients with a regional seizure-onset zone. However, it also raises several questions for treating regional epilepsy, such as the importance of localizing the seizure onset zone and whether lead-to-lead stimulation is better than bipolar or monopolar stimulation.
Patients with DRE often require additional brain MRI studies after implantation, which previously was complicated as the original RNS devices were not deemed safe for MRI. Fortunately, the RNS-320 neurostimulator received MRI-conditional status in 2020, allowing patients to undergo up to 1.5 T MRI scans under appropriate conditions. This change is especially pertinent to patients with epilepsy due to conditions requiring MRI surveillance, such as tuberous sclerosis, brain tumors, or multiple sclerosis.
Discussion and future directions
Brain stimulation devices provide a therapeutic option for people with DRE, including patients where the seizure-onset zone is multifocal, non-resectable, or poorly localized. RNS and DBS fill an essential gap for patients with DRE that either may not be eligible or be ready to commit to irreversible neurosurgical treatments. RNS and DBS brain stimulation devices have achieved similar efficacy in long-term (>5 years) clinical trials, providing seizure reductions of 75% [13] and 69% [9], respectively, with low rates of adverse outcomes and few reported side effects. However, these results may be artificially elevated due to the lack of an appropriate long-term control group, or when accounting for the natural progression of epilepsy. The rates for patients achieving seizure-freedom, a much more meaningful clinical indicator, were much lower, reported to be 21% for RNS [11] and 6% for DBS [9]. While these devices have become more widely available over the past decade, there continue to be significant questions regarding how they should best be implemented in clinical practice, and which underlying mechanisms produce their therapeutic effect.
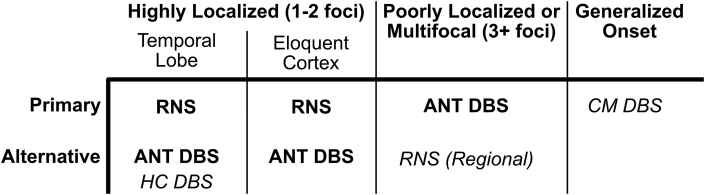
There have been no randomized head-to-head studies comparing RNS and DBS; therefore, deciding which device to implant is based primarily on clinical experience and expert opinion (Fig. 5). Several factors can help guide this selection, including the patient's epilepsy classification, device characteristics, and patient preference. In patients with generalized, poorly-localized, or multifocal onset (i.e., more than two sites), DBS may be the most appropriate option since RNS can only target two locations. RNS may be preferred if long-term recording capabilities will be helpful with management (e.g., lateralizing seizure onset). Patient preferences about the device design should be discussed, both regarding the placement of the pulse generators (scalp for RNS, chest for DBS) and device management (data uploads with RNS). A proposed clinical approach to neuromodulation selection is presented in Fig. 5.
Fig. 5.
A proposed clinical approach to neuromodulation selection. Highly localized focal epilepsy arising from 1 to 2 foci are preferentially treated with RNS to benefit from continuous monitoring. ANT DBS is an approved alternative. Hippocampal DBS for temporal lobe epilepsy is a reasonable alternative. Multifocal (3+ foci) or poorly-localized focal epilepsy are preferentially treated with ANT DBS, though regional RNS is a reasonable alternative. Brain stimulation is not currently approved for generalized epilepsies, though CMN stimulation is reasonable based on recent studies. ANT: Anterior nucleus of the thalamus. CM: Centro-median nucleus of the thalamus. DBS: Deep Brain Stimulation. HC: Hippocampus. RNS: Responsive Neurostimulation. US FDA approved indications are in bold. Off-label investigational treatments are in italics.
The underlying mechanisms that drive the therapeutic effects of neurostimulation continue to be debated [22,113]. Both excitation [19] and inhibition [35] of neuronal activity have been demonstrated with neurostimulation, and these effects may have significant parameter dependence [114]. While closed-loop stimulation has demonstrated the capability to interrupt provoked seizure-like activity [48,49,115], it remains unclear if long-term reductions in seizures are primarily due to the termination or chronic suppression of spontaneous seizures. Since false-positive detections may be the primary trigger for RNS, this therapy may have a significant seizure suppressive component, similar to DBS. In addition, the possibilities of disease-modifying effects exist, but are difficult to prove in standard controlled clinical trials. There is a great need to understand which mechanisms of actions should be targeted by stimulation, as this may guide the selection of the device, placement of electrodes and adjustments to stimulation parameters. Recent studies have demonstrated that RNS may be effective even if stimulation does not occur immediately over the ictal onset region [116]. RNS treatment is typically adjusted to trigger stimulation when seizures are detected; however, if open-loop stimulation is most effective, then RNS may perform better if programmed to provide essentially open-loop stimulation. Studies designed to compare combined open-and-closed-loop stimulation, compared to open-loop stimulation alone, could help define the adjunctive benefit of closed-loop stimulation. In addition, there is a significant opportunity to improve the usual empiric trial-and-error method for device programming which is prone to produce suboptimal results. Global optimization strategies such as Bayesian methods have been used to optimize DBS treatment of Parkinson's disease [117], and have the potential to improve device programming and outcomes in neurostimulation for treating epilepsy.
Current RNS and DBS programming strategies primarily target seizure termination or suppression over the short-term. However, chronic stimulation may modify the course of disease, which is a relatively unexplored approach to neurostimulation. The progressive improvements over time seen with both DBS [9] and RNS [11] suggest a potential underlying change in the brain's excitability. Neurostimulation of the ANT using conventional stimulation settings in animal studies has been associated with reduced mossy fiber sprouting [118] and decreased neurodegeneration with altered gene expression and cytokines [61]. However, disease modification effects may be optimized with different parameter settings than those typically used to terminate or suppress seizures [119]. While current methods in neurostimulation produce significant reductions in seizure frequency, adjusting neurostimulation to promote disease modification has the potential to provide meaningful improvements in seizure freedom, but may require novel clinical trial designs.
Further work is needed to define the effects of neurostimulation in epilepsy, optimize the patient-specific use of RNS and DBS, and guide the long-term management of brain stimulation therapies. Understanding the chronic cellular and molecular effects of neurostimulation in the brain and determining more rigorously whether brain stimulation has true disease-modifying effects may help optimize current stimulation therapies and also lead to the development of novel pharmacological or surgical therapies for DRE.
Conflicts of interest
All authors declared that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Institutes of Health (R01 NS056872).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.GBD 2016 Epilepsy Collaborators Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:357–375. doi: 10.1016/S1474-4422(18)30454-X. Erratum in: Lancet Neurol 2019;18:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z., Brodie M.J., Liew D., Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75:279–286. doi: 10.1001/jamaneurol.2017.3949. Erratum in: JAMA Neurol 2018;75:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Mannan O., Sutcliffe A.G. A national surveillance study of childhood epilepsy mortality in the UK and Ireland. Eur J Neurol. 2020;27:327–333. doi: 10.1111/ene.14081. [DOI] [PubMed] [Google Scholar]
- 4.Perucca P., Gilliam F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11:792–802. doi: 10.1016/S1474-4422(12)70153-9. Erratum in: Lancet Neurol 2012;11:746. [DOI] [PubMed] [Google Scholar]
- 5.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 6.Al-Kaylani M., Konrad P., Lazenby B., Blumenkopf B., Abou-Khalil B. Seizure freedom off antiepileptic drugs after temporal lobe epilepsy surgery. Seizure. 2007;16:95–98. doi: 10.1016/j.seizure.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Duchowny M., Bhatia S. Epilepsy: preserving memory in temporal lobectomy-are networks the key? Nat Rev Neurol. 2014;10:245–246. doi: 10.1038/nrneurol.2014.67. [DOI] [PubMed] [Google Scholar]
- 8.Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 9.Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M., et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cukiert A., Cukiert C.M., Burattini J.A., Mariani P.P. Long-term seizure outcome during continuous bipolar hippocampal deep brain stimulation in patients with temporal lobe epilepsy with or without mesial temporal sclerosis: an observational, open-label study. Epilepsia. 2021;62:190–197. doi: 10.1111/epi.16776. [DOI] [PubMed] [Google Scholar]
- 11.Nair D.R., Laxer K.D., Weber P.B., Murro A.M., Park Y.D., Barkley G.L., et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95:e1244–e1256. doi: 10.1212/WNL.0000000000010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 13.Nair D., Morrell M. Nine-year prospective safety and effectiveness outcomes from the long-term treatment trial of the RNS® system (S36.005) Neurology. 2019;92:S36.005. [Google Scholar]
- 14.Hambrecht F.T. In: Neural Prostheses: Fundamental Studies, Englewood Cliffs, NJ: Prentice Hall. Agnew W.F., McCreery D.B., editors. 1990. The history of neural stimulation and its relevance to future neural prostheses; pp. 1–24. [Google Scholar]
- 15.Grill W.M., Kirsch R.F. Neuroprosthetic applications of electrical stimulation. Assist Technol. 2000;12:6–20. doi: 10.1080/10400435.2000.10132006. [DOI] [PubMed] [Google Scholar]
- 16.Lian J., Bikson M., Sciortino C., Stacey W.C., Durand D.M. Local suppression of epileptiform activity by electrical stimulation in rat hippocampus in vitro. J Physiol. 2003;547:427–434. doi: 10.1113/jphysiol.2002.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand D.M., Jensen A., Bikson M. Suppression of neural activity with high frequency stimulation. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:1624–1625. doi: 10.1109/IEMBS.2006.259396. [DOI] [PubMed] [Google Scholar]
- 18.Yu T., Wang X., Li Y., Zhang G., Worrell G., Chauvel P., et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain. 2018;141:2631–2643. doi: 10.1093/brain/awy187. [DOI] [PubMed] [Google Scholar]
- 19.Bartoli A., Tyrand R., Vargas M.I., Momjian S., Boëx C. Low frequency microstimulation is locally excitatory in patients with epilepsy. Front Neural Circ. 2018;12:22. doi: 10.3389/fncir.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Postupna N., Falkenberg J., Anderson M.E. High frequency deep brain stimulation: what are the therapeutic mechanisms? Neurosci Biobehav Rev. 2008;32:343–351. doi: 10.1016/j.neubiorev.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre C.C., Grill W.M., Sherman D.L., Thakor N.V. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre C.C., Savasta M., Kerkerian-Le Goff L., Vitek J.L. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Dostrovsky J.O., Levy R., Wu J.P., Hutchison W.D., Tasker R.R., Lozano A.M. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–574. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y.R., Levy R., Ashby P., Tasker R.R., Dostrovsky J.O. Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Mov Disord. 2001;16:208–216. doi: 10.1002/mds.1046. [DOI] [PubMed] [Google Scholar]
- 25.Tai C.H., Boraud T., Bezard E., Bioulac B., Gross C., Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. Faseb J. 2003;17:1820–1830. doi: 10.1096/fj.03-0163com. [DOI] [PubMed] [Google Scholar]
- 26.Boraud T., Bezard E., Bioulac B., Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- 27.Filali M., Hutchison W.D., Palter V.N., Lozano A.M., Dostrovsky J.O. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274–281. doi: 10.1007/s00221-003-1784-y. [DOI] [PubMed] [Google Scholar]
- 28.Ledonne A., Mango D., Bernardi G., Berretta N., Mercuri N.B. A continuous high frequency stimulation of the subthalamic nucleus determines a suppression of excitatory synaptic transmission in nigral dopaminergic neurons recorded in vitro. Exp Neurol. 2012;233:292–302. doi: 10.1016/j.expneurol.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Anderson M.E., Postupna N., Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto T., Elder C.M., Okun M.S., Patrick S.K., Vitek J.L. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galati S., Mazzone P., Fedele E., Pisani A., Peppe A., Pierantozzi M., et al. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson's disease. Eur J Neurosci. 2006;23:2923–2928. doi: 10.1111/j.1460-9568.2006.04816.x. [DOI] [PubMed] [Google Scholar]
- 32.Magariños-Ascone C., Pazo J.H., Macadar O., Buño W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 33.Bikson M., Lian J., Hahn P.J., Stacey W.C., Sciortino C., Durand D.M. Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J Physiol. 2001;531:181–191. doi: 10.1111/j.1469-7793.2001.0181j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler K., Elger C.E., Lehnertz K. Changes of EEG synchronization during low-frequency electric stimulation of the seizure onset zone. Epilepsy Res. 2007;77:108–119. doi: 10.1016/j.eplepsyres.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 35.D'Arcangelo G., Panuccio G., Tancredi V., Avoli M. Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol Dis. 2005;19:119–128. doi: 10.1016/j.nbd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Vonck K., Boon P., Achten E., De Reuck J., Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556–565. doi: 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- 37.Ghasemi Z., Naderi N., Shojaei A., Ahmadirad N., Raoufy M.R., Mirnajafi-Zadeh J. Low frequency electrical stimulation attenuated the epileptiform activity-induced changes in action potential features in hippocampal CA1 pyramidal neurons. Cell J. 2018;20:355–360. doi: 10.22074/cellj.2018.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christie B.R., Kerr D.S., Abraham W.C. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–135. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- 39.Paschen E., Elgueta C., Heining K., Vieira D.M., Kleis P., Orcinha C., et al. Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. Elife. 2020;9:e54518. doi: 10.7554/eLife.54518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lado F.A., Moshé S.L. How do seizures stop? Epilepsia. 2008;49:1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timofeev I., Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 42.Fröhlich F., Bazhenov M., Iragui-Madoz V., Sejnowski T.J. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist. 2008;14:422–433. doi: 10.1177/1073858408317955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovac S., Domijan A.-M., Walker M.C., Abramov A.Y. Prolonged seizure activity impairs mitochondrial bioenergetics and induces cell death. J Cell Sci. 2012;125:1796–1806. doi: 10.1242/jcs.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada K., Ji J.J., Yuan H., Miki T., Sato S., Horimoto N., et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 45.Staley K.J., Longacher M., Bains J.S., Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- 46.Tian G.F., Azmi H., Takano T., Xu Q., Peng W., Lin J., et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stell B.M., Brickley S.G., Tang C.Y., Farrant M., Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motamedi G.K., Lesser R.P., Miglioretti D.L., Mizuno-Matsumoto Y., Gordon B., Webber W.R.S., et al. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia. 2002;43:836–846. doi: 10.1046/j.1528-1157.2002.24901.x. [DOI] [PubMed] [Google Scholar]
- 49.Lesser R.P., Kim S.H., Beyderman L., Miglioretti D.L., Webber W.R., Bare M., et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53:2073–2081. doi: 10.1212/wnl.53.9.2073. [DOI] [PubMed] [Google Scholar]
- 50.Chkhenkeli S.A., Sramka M., Lortkipanidze G.S., Rakviashvili T.N., Bregvadze E.S., Magalashvili G.E., et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–329. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Rashid S., Pho G., Czigler M., Werz M.A., Durand D.M. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia. 2012;53:147–156. doi: 10.1111/j.1528-1167.2011.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salam M.T., Velazquez J.L.P., Genov R. Seizure suppression efficacy of closed-loop versus open-loop deep brain stimulation in a rodent model of epilepsy. IEEE Trans Neural Syst Rehabil Eng. 2016;24:710–719. doi: 10.1109/TNSRE.2015.2498973. [DOI] [PubMed] [Google Scholar]
- 53.Wyckhuys T., Raedt R., Vonck K., Wadman W., Boon P. Comparison of hippocampal Deep Brain Stimulation with high (130Hz) and low frequency (5Hz) on afterdischarges in kindled rats. Epilepsy Res. 2010;88:239–246. doi: 10.1016/j.eplepsyres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Shigeto H., Boongird A., Baker K., Kellinghaus C., Najm I., Lüders H. Systematic study of the effects of stimulus parameters and stimulus location on afterdischarges elicited by electrical stimulation in the rat. Epilepsy Res. 2012;104:17–25. doi: 10.1016/j.eplepsyres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi Y., Harangozó M., Pedraza L., Földi T., Kozák G., Li Q., et al. Closed-loop stimulation of the medial septum terminates epileptic seizures. Brain. 2021;144:885–908. doi: 10.1093/brain/awaa450. [DOI] [PubMed] [Google Scholar]
- 56.Sobayo T., Mogul D.J. Should stimulation parameters be individualized to stop seizures: evidence in support of this approach. Epilepsia. 2016;57:131–140. doi: 10.1111/epi.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiller Y., Bankirer Y. Cellular mechanisms underlying antiepileptic effects of low- and high-frequency electrical stimulation in acute epilepsy in neocortical brain slices in vitro. J Neurophysiol. 2007;97:1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- 58.Park E.H., Barreto E., Gluckman B.J., Schiff S.J., So P. A model of the effects of applied electric fields on neuronal synchronization. J Comput Neurosci. 2005;19:53–70. doi: 10.1007/s10827-005-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain P., Arya R. Vagus nerve stimulation and seizure outcomes in pediatric refractory epilepsy. Neurology. 2021 doi: 10.1212/WNL.0000000000012030. Apr:10.1212/WNL.0000000000012030. [DOI] [PubMed] [Google Scholar]
- 60.Liu D.F., Chen Y.C., Zhu G.Y., Wang X., Jiang Y., Liu H.G., et al. Effects of anterior thalamic nuclei stimulation on gene expression in a rat model of temporal lobe epilepsy. Acta Neurol Belg. 2019:1–10. doi: 10.1007/s13760-019-01240-1. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y.C., Zhu G.Y., Wang X., Shi L., Jiang Y., Zhang X., et al. Deep brain stimulation of the anterior nucleus of the thalamus reverses the gene expression of cytokines and their receptors as well as neuronal degeneration in epileptic rats. Brain Res. 2017;1657:304–311. doi: 10.1016/j.brainres.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Li D., Luo D., Wang J., Wang W., Yuan Z., Xing Y., et al. Electrical stimulation of the endopiriform nucleus attenuates epilepsy in rats by network modulation. Ann Clin Transl Neurol. 2020;7:2356–2369. doi: 10.1002/acn3.51214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khambhati A.N., Shafi A., Rao V.R., Chang E.F. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med. 2021;13:eabf6588. doi: 10.1126/scitranslmed.abf6588. [DOI] [PubMed] [Google Scholar]
- 64.Pitkänen A., Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 65.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butson C.R., Cooper S.E., Henderson J.M., McIntyre C.C. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McIntyre C.C., Grill W.M. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol. 2002;88:1592–1604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- 68.Mcintyre C.C., Foutz T.J. Handbook of clinical neurology. Handb Clin Neurol. 2013;116:55–61. doi: 10.1016/B978-0-444-53497-2.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elder C., Friedman D., Devinsky O., Doyle W., Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open. 2019;4:187–192. doi: 10.1002/epi4.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris K.D., Shepherd G.M.G. The neocortical circuit: themes and variations. Nat Neurosci. 2015;18:170–181. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Bon-Jego M., Yuste R. Persistently active, pacemaker-like neurons in neocortex. Front Neurosci. 2007;1:123–129. doi: 10.3389/neuro.01.1.1.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiruska P., Curtis M de, Jefferys J.G.R., Schevon C.A., Schiff S.J., Schindler K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol. 2013;591:787–797. doi: 10.1113/jphysiol.2012.239590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fine A.S., Nicholls D.P., Mogul D.J. Assessing instantaneous synchrony of nonlinear nonstationary oscillators in the brain. J Neurosci Methods. 2010;186:42–51. doi: 10.1016/j.jneumeth.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grill W.M., Snyder A.N., Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 75.Alcala-Zermeno J.L., Gregg N.M., Van Gompel J.J., Stead M., Worrell G.A., Lundstrom B.N. Cortical and thalamic electrode implant followed by temporary continuous subthreshold stimulation yields long-term seizure freedom: a case report. Epilepsy Behav Rep. 2020;14:100390. doi: 10.1016/j.ebr.2020.100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tauck D.L., Nadler J.V. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buckmaster P.S. In: Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. National Center for Biotechnology Information (US); Bethesda (MD): 2012. Mossy fiber sprouting in the dentate gyrus; pp. 416–431. [PubMed] [Google Scholar]
- 78.Sloviter R.S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 79.Shao L.R., Dudek F.E. Detection of increased local excitatory circuits in the hippocampus during epileptogenesis using focal flash photolysis of caged glutamate. Epilepsia. 2005;46:100–106. doi: 10.1111/j.1528-1167.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 80.Mathern G.W., Babb T.L., Leite J.P., Pretorius K., Yeoman K.M., Kuhlman P.A. The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res. 1996;26:151–161. doi: 10.1016/s0920-1211(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 81.Velasco A.L., Velasco F., Velasco M., Jiménez F., Carrillo-Ruiz J.D., Castro G. The role of neuromodulation of the hippocampus in the treatment of intractable complex partial seizures of the temporal lobe. Acta Neurochir Suppl. 2007;97:329–332. doi: 10.1007/978-3-211-33081-4_36. [DOI] [PubMed] [Google Scholar]
- 82.Bondallaz P., Boëx C., Rossetti A.O., Foletti G., Spinelli L., Vulliemoz S., et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure. 2013;22:390–395. doi: 10.1016/j.seizure.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Nunna R.S., Borghei A., Brahimaj B.C., Lynn F., Garibay-Pulido D., Byrne R.W., et al. Responsive neurostimulation of the mesial temporal white matter in bilateral temporal lobe epilepsy. Neurosurgery. 2021;88:261–267. doi: 10.1093/neuros/nyaa381. [DOI] [PubMed] [Google Scholar]
- 84.Geller E.B., Skarpaas T.L., Gross R.E., Goodman R.R., Barkley G.L., Bazil C.W., et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58:994–1004. doi: 10.1111/epi.13740. [DOI] [PubMed] [Google Scholar]
- 85.Jin H., Li W., Dong C., Wu J., Zhao W., Zhao Z., et al. Hippocampal deep brain stimulation in nonlesional refractory mesial temporal lobe epilepsy. Seizure. 2016;37:1–7. doi: 10.1016/j.seizure.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 86.Sorokin J.M., Davidson T.J., Frechette E., Abramian A.M., Deisseroth K., Huguenard J.R., et al. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. 2017;93:194–210. doi: 10.1016/j.neuron.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guye M., Régis J., Tamura M., Wendling F., McGonigal A., Chauvel P., et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- 88.Norden A.D., Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 89.Bertram E.H., Mangan P.S., Zhang D., Scott C.A., Williamson J.M. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- 90.Piacentino M., Durisotti C., Garofalo P.G., Bonanni P., Volzone A., Ranzato F., et al. Anterior thalamic nucleus deep brain Stimulation (DBS) for drug-resistant complex partial seizures (CPS) with or without generalization: long-term evaluation and predictive outcome. Acta Neurochir. 2015;157:1525–1532. doi: 10.1007/s00701-015-2498-1. discussion 1532. [DOI] [PubMed] [Google Scholar]
- 91.Burdette D.E., Haykal M.A., Jarosiewicz B., Fabris R.R., Heredia G., Elisevich K., et al. Brain-responsive corticothalamic stimulation in the centromedian nucleus for the treatment of regional neocortical epilepsy. Epilepsy Behav. 2020;112:107354. doi: 10.1016/j.yebeh.2020.107354. [DOI] [PubMed] [Google Scholar]
- 92.Son B.C., Shon Y.M., Choi J.G., Kim J., Ha S.W., Kim S.H., et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. 2016;94:187–197. doi: 10.1159/000446611. [DOI] [PubMed] [Google Scholar]
- 93.Velasco A.L., Velasco F., Jiménez F., Velasco M., Castro G., Carrillo-Ruiz J.D., et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 94.Okun M.S., Foote K.D. Parkinson's disease DBS: what, when, who and why? The time has come to tailor DBS targets. Expert Rev Neurother. 2010;10:1847–1857. doi: 10.1586/ern.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Buren J.M., Wood J.H., Oakley J., Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48:407–416. doi: 10.3171/jns.1978.48.3.0407. [DOI] [PubMed] [Google Scholar]
- 96.Cooper I.S., Amin I., Upton A., Riklan M., Watkins S., McLellan L. Safety and efficacy of chronic cerebellar stimulation. Appl Neurophysiol. 1977-8;40:124–134. doi: 10.1159/000102438. [DOI] [PubMed] [Google Scholar]
- 97.Sprengers M., Vonck K., Carrette E., Marson A.G., Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD008497.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salanova V., Sperling M.R., Gross R.E., Irwin C.P., Vollhaber J.A., Giftakis J.E., et al. The SANTÉ study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021;62:1306–1317. doi: 10.1111/epi.16895. [DOI] [PubMed] [Google Scholar]
- 99.Ryvlin P., Cucherat M., Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol. 2011;10:961–968. doi: 10.1016/S1474-4422(11)70193-4. [DOI] [PubMed] [Google Scholar]
- 100.Fisher R.S., Uematsu S., Krauss G.L., Cysyk B.J., McPherson R., Lesser R.P., et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 101.Velasco F., Velasco M., Jiménez F., Velasco A.L., Brito F., Rise M., et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47:295–304. doi: 10.1097/00006123-200008000-00007. discussion 304-5. [DOI] [PubMed] [Google Scholar]
- 102.Cukiert A., Burattini J.A., Cukiert C.M., Argentoni-Baldochi M., Baise-Zung C., Forster C.R., et al. Centro-median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure. 2009;18:588–592. doi: 10.1016/j.seizure.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Cukiert A., Cukiert C.M., Burattini J.A., Mariani P.P. Seizure outcome during bilateral, continuous, thalamic centromedian nuclei deep brain stimulation in patients with generalized epilepsy: a prospective, open-label study. Seizure - Eur J Epilepsy. 2020;81:304–309. doi: 10.1016/j.seizure.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 104.Oguni H., Hayashi K., Osawa M. Long-term prognosis of Lennox-Gastaut syndrome. Epilepsia. 1996;37 Suppl 3:44–47. doi: 10.1111/j.1528-1157.1996.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 105.Kang B.S., Woo Y.S., Lee J., Yi Y.Y., Koo B.S., Kang J.W. Treatment outcomes of vagus nerve stimulation in Lennox-gastaut syndrome. Ann Child Neurol. 2019;27:63–70. [Google Scholar]
- 106.Dibué M., Greco T., Spoor J.K.H., Tahir Z., Specchio N., Hänggi D., et al. Vagus nerve stimulation in patients with Lennox-Gastaut syndrome: a meta-analysis. Acta Neurol Scand. 2021;143:497–508. doi: 10.1111/ane.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cukiert A., Cukiert C.M., Burattini J.A., Mariani P.P., Bezerra D.F. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: a prospective, controlled, randomized, double-blind study. Epilepsia. 2017;58:1728–1733. doi: 10.1111/epi.13860. [DOI] [PubMed] [Google Scholar]
- 108.Voges B.R., Schmitt F.C., Hamel W., House P.M., Kluge C., Moll C.K., et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia. 2015;56:e99–e103. doi: 10.1111/epi.13045. [DOI] [PubMed] [Google Scholar]
- 109.Paff M., Loh A., Sarica C., Lozano A.M., Fasano A. Update on current technologies for deep brain stimulation in Parkinson's disease. J Mov Disord. 2020;13:185–198. doi: 10.14802/jmd.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morrell M.J., RNS System in Epilepsy Study Group Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 111.Heck C.N., King-Stephens D., Massey A.D., Nair D.R., Jobst B.C., Barkley G.L., et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirsch L.J., Mirro E.A., Salanova V., Witt T.C., Drees C.N., Brown M., et al. Mesial temporal resection following long-term ambulatory intracranial EEG monitoring with a direct brain-responsive neurostimulation system. Epilepsia. 2020;61:408–420. doi: 10.1111/epi.16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.King-Stephens D. Neuromodulation: the search is on for the most effective parameters. Epilepsy Current. 2020;20:359–361. doi: 10.1177/1535759720954227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mina F., Benquet P., Pasnicu A., Biraben A., Wendling F. Modulation of epileptic activity by deep brain stimulation: a model-based study of frequency-dependent effects. Front Comput Neurosci. 2013;7:94. doi: 10.3389/fncom.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osorio I., Frei M.G., Sunderam S., Giftakis J., Bhavaraju N.C., Schaffner S.F., et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258–268. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- 116.Ma B.B., Fields M.C., Knowlton R.C., Chang E.F., Szaflarski J.P., Marcuse L.V., et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia. 2020;61:96–106. doi: 10.1111/epi.16409. [DOI] [PubMed] [Google Scholar]
- 117.Louie K.H., Petrucci M.N., Grado L.L., Lu C., Tuite P.J., Lamperski A.G., et al. Semi-automated approaches to optimize deep brain stimulation parameters in Parkinson's disease. J NeuroEng Rehabil. 2021;18:83. doi: 10.1186/s12984-021-00873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu G., Meng D., Chen Y., Du T., Liu Y., Liu D., et al. Anterior nucleus of thalamus stimulation inhibited abnormal mossy fiber sprouting in kainic acid-induced epileptic rats. Brain Res. 2018;1701:28–35. doi: 10.1016/j.brainres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 119.Lee D.J., Lozano C.S., Dallapiazza R.F., Lozano A.M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 2019;131:333–342. doi: 10.3171/2019.4.JNS181761. [DOI] [PubMed] [Google Scholar]