Abstract
The ketogenic diet (KD) is a high-fat, low-carbohydrate diet, in which fat, instead of glucose, acts as a major energy source through the production of ketone bodies. The KD was formally introduced in 1921 to mimic the biochemical changes associated with fasting and gained recognition as a potent treatment for pediatric epilepsy in the mid-1990s. Recent clinical and scientific knowledge supports the use of the KD in drug-resistant epilepsy patients for its anti-seizure efficacy, safety, and tolerability. The KD is also receiving growing attention as a potential treatment option for other neurological disorders. This article will review on the recent updates on the KD, focusing on its mechanisms of action, its alternatives, expansion on its use in terms of age groups and different regions in the world, and future issues.
Keywords: Ketogenic diet, Epilepsy, Pediatric
History of the ketogenic diet
The ketogenic diet (KD) is a high-fat, low-carbohydrate diet, in which fat, instead of glucose, acts as a major energy source through the production of ketone bodies. Before advent of the KD, fasting was recognized for centuries as a potential therapeutic modality for epilepsy; indeed, it was described as the only anti-epileptic treatment in the Hippocratic Collection [1]. The first documented modern use of fasting for epilepsy appeared in 1911, when fasting successfully improved the seizures of 20 children and adults with epilepsy [2]. Subsequently, it was discovered that the benefits of fasting could be replicated by inducing ketosis through a high-fat diet, which led to implementation of a KD in epilepsy patients, with the distinct advantage that this treatment could be maintained for a prolonged period of time [3]. KDs were widely applied throughout the 1920s and 1930s, but their use dramatically decreased with the introduction of new anti-epileptic drugs (AEDs) [1].
The modern era of KDs for epilepsy began in 1994 with the appearance of a story on television of a boy named Charlie whose drug-resistant seizures were controlled by a KD. This gained nationwide attention in the United States. Subsequent clinical trials confirmed the efficacy of KDs for seizure control, especially in childhood drug-resistant epilepsy [4].
Current practices of KDs for epilepsy are well outlined in guidelines published by the International Ketogenic Diet Study Group in 2009 and 2018. These guidelines provide practical information regarding patient selection, pre-KD counseling, diet selection and implementation, dietary supplementation, follow-up evaluation, adverse effects, and discontinuation of the diet [5,6]. KDs have been established as one of four main treatments for drug-resistant epilepsy, together with newer AEDs, epilepsy surgery, and neuromodulation. In contrast to surgical treatments, use of a KD is reversible, inexpensive, and easily accessible.
Proposed mechanisms of the ketogenic diet
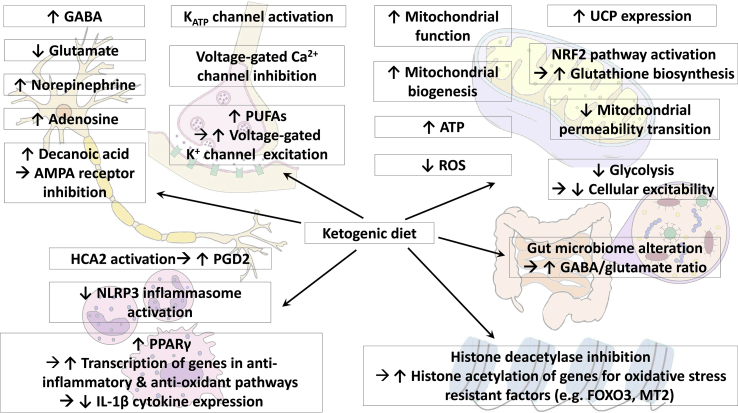
Despite a steady increase over the past decade in our knowledge of the underlying mechanisms of the KD, it is still not completely understood how the diet is clinically effective in seizure disorders [7]. Multiple mechanisms likely participate in interconnected ways to produce anti-seizure effects, as well as neuroprotective effects. These mechanisms probably act jointly and in parallel with one another. Several main proposed mechanisms of action of KDs are discussed in the following paragraphs (Fig. 1).
-
(1)
Alteration of neurotransmitters and regulation of ion channels
Fig. 1.
Proposed mechanisms of action of the KD. KD alters neurotransmitter levels: GABA, norepinephrine, and adenosine levels are increased, while glutamate level is decreased. Increased decanoic acid levels in MCT KD directly inhibits AMPA receptors. KD induces changes in regulation in ion channels: ATP-sensitive potassium channels are activated and voltage-gated calcium channels are inhibited. Increase in polyunsaturated fatty acids also results in increased excitation of voltage-gated potassium channels. KD enhances mitochondrial function and biogenesis, inducing increased ATP production and decreased ROS production. KD enhances expression of uncoupling proteins and increased glutathione biosynthesis via NRF2 pathway activation, both of which lead to decreased ROS production as well. KD inhibits mitochondria permeability transition, and thus inhibits apoptotic and necrotic cell death. Decreased glycolysis during KD decreases cellular excitability. KD activates hydroxy-carboxylic acid receptor 2, which in turn stimulates the synthesis of prostaglandin D2, inducing a neuroprotective phenotype in monocytes and/or macrophages. KD also suppresses NLRP3 inflammasome activation. KD increased PPARγ expression, which in turn diminishes IL-1β levels. KD increases histone acetylation by inhibiting histone deacetylases, leading to increased transcription of genes encoding for oxidative stress resistant factors (FOXO3 and MT2). KD induces gut microbiome alteration, which in turn leads to increased GABA/glutamate ratio. AMPA, alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid; ATP, adenosine triphosphate; FOXO3, forkhead box O3; GABA, gamma-aminobutyric acid; HCA2, hydroxy-carboxylic acid receptor 2; IL-1β, interleukin 1β; KD, ketogenic diet, MCT, medium-chain triglyceride; MT2, metallothionein 2; NRF2, nuclear factor E2-related factor 2; PGD2, prostaglandin D2; PPARγ, peroxisome proliferator activated receptor gamma; PUFAs, polyunsaturated fatty acids; ROS, reactive oxygen species; UCP, uncoupling protein.
KDs may produce anti-seizure effects via altering neurotransmitter levels [8]. Increases in gamma-aminobutyric acid (GABA), adenosine, and norepinephrine levels and decreases in glutamate levels have been observed during KDs [9]. In astrocytes, glutamate is converted to glutamine, which is then exported to neurons where glutamine is converted back to glutamate. This glutamate is then converted to either GABA or aspartate. Conversion of glutamate to aspartate requires oxaloacetate, which is also an important component of the tricarboxylic acid (TCA) cycle. During a KD, metabolism of ketone bodies leads to increased production of oxaloacetate, but the oxaloacetate is diverted into the TCA cycle to provide cellular energy. This reduces the amount of oxaloacetate available for conversion of glutamate to aspartate, thereby increasing conversion of glutamate to GABA [10].
Glutamate release through vesicular glutamate transporters is regulated by chloride ions, which act as an allosteric activator [11]. The ketone bodies beta-hydroxybutyrate and acetoacetate compete with chloride ions at the site of allosteric regulation and thus inhibit glutamate release, resulting in decreased presynaptic glutamate release [11]. KDs also decrease levels of adenosine kinase, a major adenosine-metabolizing enzyme, and thus increase extracellular adenosine levels [12]. These diets also increase activation of adenosine A1 receptors, resulting in increased inhibitory effects mediated by these receptors. KDs have also been shown to increase extracellular norepinephrine levels [13].
KDs may further exhibit anti-seizure effects by inducing changes in regulation of ion channels. These diets decrease neuronal firing rates through activation of adenosine triphosphate (ATP)-sensitive potassium channels and GABAB receptors [14]. Acetoacetate also inhibits voltage-dependent calcium channels, leading to reduced excitatory postsynaptic currents [15].
The medium chain-triglyceride (MCT) KD induces increased plasma levels of decanoic acid and ketones [16]. The decanoic acid directly and selectively inhibits alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, acting as a non-competitive antagonist in a voltage- and subunit-dependent manner [17,18].
-
(2)
Enhancement of mitochondrial function and oxidative stress reduction
Aberrant mitochondrial function and production of reactive oxygen species (ROS), both of which are known to contribute to epileptogenesis, may be influenced by KDs [19]. These diets not only increase ATP production by mitochondria, but they also directly increase mitochondrial biogenesis [20,21]. KDs enhance expression of uncoupling proteins, which in turn decrease the mitochondrial membrane potential, leading to decreased ROS levels [22]. Activation of the nuclear factor E2-related factor 2 pathway also occurs with KDs, resulting in diverse changes, including increased biosynthesis of glutathione, which has antioxidant activity [23]. KDs also inhibit mitochondrial permeability transition, a process linked to apoptotic and necrotic cell death [24]. This inhibition has anti-seizure effects, as well as presumed neuroprotective effects [24].
-
(3)
Mediation of inflammatory and immune function
Anti-inflammatory activity and improved immune function may contribute to the neuroprotective effects of KDs. Beta-hydroxybutyrate activates hydroxy-carboxylic acid receptor 2, which in turn stimulates the synthesis of prostaglandin D2, inducing a neuroprotective phenotype in monocytes and/or macrophages [25]. Beta-hydroxybutyrate also suppresses mitochondrial translocation of dynamin-related protein 1 to inhibit nucleotide-binding domain-like receptor protein 3 inflammasome activation, resulting in neuroprotective effects [26,27].
-
(4)
Inhibition of histone deacetylases
KDs may exhibit antioxidant activity through inhibition of histone deacetylases. Beta-hydroxybutyrate increases histone acetylation by inhibiting class I histone deacetylases, leading to changes in the transcription of various genes, including those encoding two oxidative stress–resistant factors (forkhead box O3 and metallothionein 2) [28,29].
-
(5)
Alteration of gut microbiota
Alteration of the gut microbiota is another proposed anti-seizure mechanism of KDs [30]. Dysbiosis of the gut microbiota has been observed in patients with epilepsy, which can lead to reduced production of short-chain fatty acids and neurotransmitters such as GABA and serotonin [31,32]. Reduction of anti-inflammatory short-chain fatty acids results in alterations of the blood–brain barrier [33]. KDs have been shown to alter the gut microbiota, resulting in an elevated GABA/glutamate ratio and thus anti-seizure effects [34]. Moreover, KDs do not exhibit anti-seizure activity in mice treated with antibiotics or reared in a germ-free environment [34].
-
(6)
Decreased glycolysis and increased fatty acids
Reduced glycolysis induced by KDs may lower cellular excitability [35]. Polyunsaturated fatty acids (PUFAs) modulate voltage sensitivity of voltage-gated potassium channels and induce channel opening [36]. Increased PUFA intake with KDs decrease excitation by opening voltage-gated potassium channels, thereby improving seizures [36,37]. Increased fatty acid levels during KDs also lead to increased expression of the transcription factor peroxisome proliferator activated receptor gamma (PPARγ), which regulates genes involved in anti-inflammatory and antioxidant pathways, which in turn caused diminished expression of interleukin-1β (IL-1β) cytokine [38]. IL-1β is linked to hyperexcitability and seizure generation, and decreased levels of IL-1β were associated reduction in seizures [38].
Modifications and alternatives to the classic ketogenic diet
Although its efficacy is proven, KD is not an easy and convenient method of treatment to both patients and caregivers. Maintaining high-fat diet can be unpalatable and result in various adverse effects. Preparing each meal with calculation and measurement of food composition and ingredients can be impossible to some patients and caregivers. Therefore, alternatives to the classic KD have been developed and studied.
-
(1)
Medium-chain triglyceride diet, modified Atkins diet, and low glycemic index treatment
The term “ketogenic diet” refers to any diet that results in the ketogenic stage of metabolism. Following the development of the classic KD, which consists of long-chain triglycerides, usually supplied as a 4:1 or 3:1 ratio of fats to nonfats (proteins and carbohydrates), alternative diets have been proposed in attempts to increase compliance and palatability, while mimicking the metabolic effects of the original diet. Currently, four main KDs are used in clinical practice: the classic KD, the MCT diet, the modified Atkins diet (MAD), and the low glycemic index treatment (LGIT).
As the name suggests, the main component of the MCT diet is MCT oils, which yield more ketones per kilocalorie than long-chain triglycerides [16,39]. This increased ketogenic potential reduces the quantity of total fat that must be consumed, allowing for more carbohydrate and protein intake and potentially better food choices and less food refusal. MAD typically consists of 1:1 to 1.5:1 ketogenic ratio, with no limitation on proteins, fluids, or calories, which makes meal planning and preparation easier and increases tolerability of the diet [40]. LGIT involves exchanging high glycemic index foods with low glycemic index alternatives [41]. The glycemic index reflects the tendency of a food to increase the blood glucose, compared to the increase induced by an equivalent amount of a reference carbohydrate [42]. Refined carbohydrates are examples of high glycemic index foods, whereas meat, dairy, vegetables, and unprocessed whole-grain foods are examples of low glycemic index foods [42]. While LGIT does not necessarily produce ketosis, both decreased glucose metabolism and stable glucose levels contribute to the mechanism of KDs [41]. Both MAD and LGIT are suitable for adolescents and adults and can be easily used in outpatient settings or in settings with limited resources (e.g., lack of trained dietitians) [40].
The efficacy of the three KD alternatives—MCT diet, MAD, and LGIT—has been compared to that of classic KD in various studies, including randomized controlled trials. While the efficacy of each depends on the patient's age, specific epilepsy syndrome, and etiology, these alternatives are not inferior to the classic KD [[43], [44], [45], [46], [47]]. When KDs must be maintained for several years because of seizure recurrence or clinical course of the disease, it is reasonable to consider switching to MAD or LGIT from the classic KD when considering the risks of long-term complications [48].
-
(2)
Triheptanoin
Triheptanoin is a synthetic MCT composed of three heptanoate fatty acids [49]. It is a tasteless oil that dissolves easily in food and was first approved in the United States for use in patients with long-chain fatty acid oxidation disorders [49,50]. Because of its anaplerotic ability to replenish TCA cycle intermediates, which are present in reduced amounts in patients with epilepsy, triheptanoin has been studied for seizure control in these patients [50]. Triheptanoin is administered at mealtimes or with snacks, with a recommended target daily dosage of up to 35% of the total prescribed daily caloric intake, which is divided into at least 4 doses [49]. The few studies evaluating triheptanoin usage in patients with epilepsy have shown promising effects for seizure control, as well as good tolerability [[51], [52], [53]]. A clear advantage of triheptanoin is its simple preparation, in contrast to the difficult meal preparation with KDs, which require calculations of food composition and special recipes. Further studies are warranted to assess the anti-seizure efficacy, safety, and tolerability of triheptanoin.
Expansion of KD applications
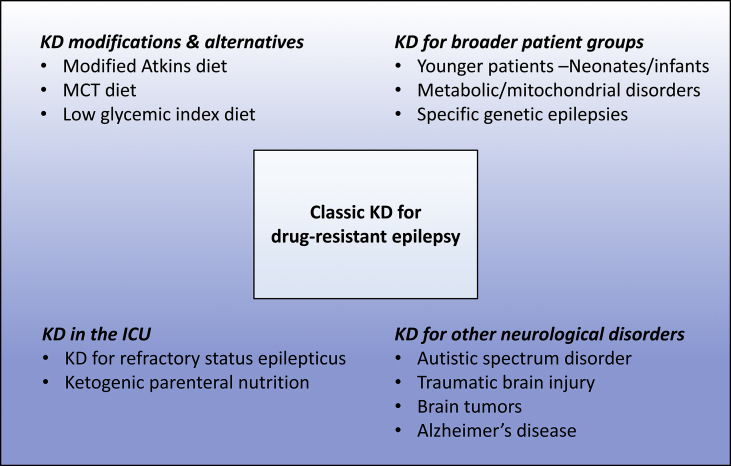
The indications for the KD are expanding, and broader patient groups are being subject to the dietary therapy with accumulation of experience with the KD (Fig. 2).
-
(1)
KD in infants/neonates
Fig. 2.
Applications of the KD in the past and present. With accumulation of experience with the KD, indications for the KD and application methods have expanded (bigger box) from the narrow application of the classic KD in the past (smaller box).
The first two years of life are critical for rapid brain growth and psychosocial development related to synapse formation and myelination [54]. Patients at this age are most at risk of neurodevelopmental impairment. Additionally, the incidence of epilepsy is highest during this period. Until recently, KDs were not used in infants based on assumptions that this age group could not maintain a state of ketosis and the risk of adverse events was too high. However, it has been shown that ketone bodies constitute a significant fuel source for brain development in utero and during infancy, and the clinical efficacy and safety of KDs in this age group have now been documented [[55], [56], [57]]. In a case series of patients younger than 3 months of age in a neonatal intensive care unit, a KD was well tolerated and improved seizures [58]. KDs can also be safely and effectively initiated in infants who are simultaneously receiving human breast milk [59]. Moreover, an interesting case of an exclusively breastfed infant was reported recently, whose seizures were controlled after achieving ketosis through altering the composition of the mother's own milk by placing the healthy mother on the KD [60].
-
(2)
KD for refractory status epilepticus
Status epilepticus is one of the major causes for pediatric neurocritical care admissions, along with traumatic brain injury and infection/inflammation such as meningitis and encephalitis [61]. The incidence of refractory status epilepticus is unknown, and adult data suggest that about 1/3 of patients with convulsive status epilepticus progress to refractory status epilepticus [62]. There is an increasing report on efficacy of the KD in refractory status epilepticus patients in pediatric intensive care units, and the existing reports consistently favor early applications of the KD in refractory status epilepticus based on their experience on seizure reduction in majority of the patients [[63], [64], [65], [66]]. Although more evidences are needed for ascertainment, there was a trend that patients with febrile infection-related epilepsy syndrome showed more response to the KD than refractory status epilepticus patients unrelated to the febrile infection-related epilepsy syndrome [66].
-
(3)
Ketogenic parenteral nutrition
Application of the KD in the intensive care settings is facilitated by use of the ketogenic parenteral nutrition administration. Patients with refractory status epilepticus are usually under heavy sedation from effects of anesthetics and anti-epileptic medications, and commonly experience gastro-intestinal dysmotility causing poor absorption of orally fed nutrition. Using the ketogenic parenteral nutrition can therefore prevent the delay on KD implementation, since its efficacy on seizure reduction and safety are supported by several reports [63,[67], [68], [69], [70]].
-
(4)
KD in specific genetic epileptic encephalopathy
In general, KDs improve seizure outcomes in 50% of patients but offer no benefits in the remaining patients. Predicting KD efficacy before implementation therefore remains an important question for treating epileptologists. With recent advances in gene discovery and genetics, specific causative genetic variants are identified in about 1/3 of the patients previously classified as developmental and epileptic encephalopathy of unknown etiology [71]. In this context, KDs were found to be effective for seizure control in patients with SCN1A, KCNQ2, STXBP1, and SCN2A mutations but less effective in patients with CDKL5 mutations in a small pilot study of patients with identified causative genetic variants responsible for developmental and epileptic encephalopathy [72]. However, another study suggested beneficial effect of the KD in patients with CDKL5 mutations although poor long-term efficacy was noted [73]. More studies with larger patient cohorts are warranted to draw conclusions on efficacy of KD according to epilepsies caused by specific genetic variants.
-
(5)
KD in metabolic/mitochondrial disorders
Metabolic epilepsies arise in the context of inborn errors of metabolism. Drug-resistant epilepsy is one of the characteristic clinical manifestations of certain neurologic disorders, including inborn errors of metabolism or genetic diseases in infancy. Although disorders caused by inborn errors of metabolism are rare, their collective prevalence is 1 in 1000, and most have no cure. Given that KDs are a fundamentally metabolism-based treatment that induce a broad range of biochemical, hormonal, and physiologic effects, they may be considered a treatment option for these patients [9].
The effects of KDs have been reported for metabolic disorders involving mitochondria. Clinical results have indicated that the KD may effectively treat children with drug-resistant epilepsy associated with mitochondrial respiratory chain complex defects and mitochondrial encephalopathy with lactic acidosis and stroke-like episodes [[74], [75], [76]]. Since KDs enhance mitochondrial function and biogenesis, they are now indicated and confirmed to be effective for treating mitochondrial disorders, especially complex 1 deficiency syndrome. Of note, before publication of these clinical reports, KDs were generally considered contraindicated in patients with mitochondrial disorders. Thus, continued efforts to expand the indications for KDs are warranted.
-
(6)
KD for other neurological disorders
As KDs can elicit a broad spectrum of systemic effects, there has been considerable interest in using the diets for neurologic disorders other than epilepsy. Although the data are preliminary, KDs have been used for many disorders, including autism spectrum disorder (ASD), Alzheimer's disease, brain tumors, and traumatic brain injury [77,78]. Interest in neurodegenerative disorders arose from studies of neuroprotection and energy metabolism related to the mechanisms of KDs [79].
In patients with ASD and concomitant seizures, the classic KD is the most effective non-AED treatment for improving seizures [80]. KDs also favorably affect other relevant clinical factors related to ASD, such as improving autism rating scale scores [80,81]. Recent studies have focused on specific types of KDs for targeted disorders: MCTs as a potential supplemental treatment for mild Alzheimer's disease and the classic KD for Down syndrome [82,83]. Ketone bodies are considered primary mediators for preventing aging and neurodegeneration in Alzheimer's disease, acting through enhancement of mitochondrial function [78]. Impaired brain glucose metabolism and amyloid-β plaques may lead to the development of Alzheimer's disease, and KDs likely reduce formation of these plaques and replace glucose with alternative energy sources in the brain [84]. The potential anti-tumor effects of KDs are also of growing interest. Previous studies have suggested that KD inhibits tumor cell growth by altering cellular metabolism, thereby enhancing the effectiveness of other anti-tumor treatments [85,86].
Making the KD available to more patients worldwide
Despite widespread use of KDs, there are still vast regions of the world that do not offer this treatment [[87], [88], [89]]. Many of these regions are in the Caribbean, Central America, Africa, Eastern Europe, and Southeast Asia [89]. Thus, encouraging use of KDs in more countries and regions is necessary. While drug-resistant epilepsy patients may benefit from newer drugs, surgery, and neuromodulation, these options are almost impossible to implement because of the high cost and lack of supplies in resource-deficient regions. KDs are much more achievable in these regions and have become even easier with the development of KD alternatives. To promote wider use of the KD globally, the International League Against Epilepsy published a special report listing the minimum requirements for KD services in resource-limited regions and has provided KD workshops for physicians and dietitians in various areas, including Indonesia, Mongolia, and Uzbekistan [87].
Conclusion
KDs, which were initially established as a nonpharmacologic but powerful treatment for patients with epilepsy, reemerged in the mid-1990s in a variety of forms. Depending on the individual situation and extent of disease, KDs can be a good option for patients with drug-resistant epilepsy. Numerous mechanisms of action of these diets have been proposed, which has led to exploration of their use for various other neurologic disorders. In the future, it is hoped that the effectiveness of ketogenic therapies will be demonstrated in more disease groups and that KDs will become more widely used on a global basis.
Conflicts of interest
There is no competing interest among the authors.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wheless J.W. History of the ketogenic diet. Epilepsia. 2008;49 Suppl 8:3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 2.Guelpa G., Marie A. La lutte contre l'epilepsie par la desintoxication et par la reeducation alimentaire. Rev Ther Med Chir. 1911;78:8–13. [Google Scholar]
- 3.Wilder R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin Proc. 1921;2:307–308. [Google Scholar]
- 4.Martin K., Jackson C.F., Levy R.G., Cooper P.N. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD001903.pub3. Update in: Cochrane Database Syst Rev 2018;11:CD001903. [DOI] [PubMed] [Google Scholar]
- 5.Kossoff E.H., Zupec-Kania B.A., Amark P.E., Ballaban-Gil K.R., Christina Bergqvist A.G., Blackford R., et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 6.Kossoff E.H., Zupec-Kania B.A., Auvin S., Ballaban-Gil K.R., Christina Bergqvist A.G., Blackford R., et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175–192. doi: 10.1002/epi4.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youngson N.A., Morris M.J., Ballard J.W.O. The mechanisms mediating the antiepileptic effects of the ketogenic diet, and potential opportunities for improvement with metabolism-altering drugs. Seizure. 2017;52:15–19. doi: 10.1016/j.seizure.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Rho J.M. How does the ketogenic diet induce anti-seizure effects? Neurosci Lett. 2017;637:4–10. doi: 10.1016/j.neulet.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Gavrilovici C., Rho J.M. Metabolic epilepsies amenable to ketogenic therapies: indications, contraindications, and underlying mechanisms. J Inherit Metab Dis. 2021;44:42–53. doi: 10.1002/jimd.12283. [DOI] [PubMed] [Google Scholar]
- 10.Yudkoff M., Daikhin Y., Melø T.M., Nissim I., Sonnewald U., Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juge N., Gray J.A., Omote H., Miyaji T., Inoue T., Hara C., et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masino S.A., Li T., Theofilas P., Sandau U.S., Ruskin D.N., Fredholm B.B., et al. A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors. J Clin Investig. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martillotti J., Weinshenker D., Liles L.C., Eagles D.A. A ketogenic diet and knockout of the norepinephrine transporter both reduce seizure severity in mice. Epilepsy Res. 2006;68:207–211. doi: 10.1016/j.eplepsyres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma W., Berg J., Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki A., Sada N., Juge N., Wakasa A., Moriyama Y., Inoue T. Neuronal inhibition and seizure suppression by acetoacetate and its analog, 2-phenylbutyrate. Epilepsia. 2017;58:845–857. doi: 10.1111/epi.13718. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y.M., Wang H.S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J. 2013;36:9–15. doi: 10.4103/2319-4170.107154. [DOI] [PubMed] [Google Scholar]
- 17.Chang P., Augustin K., Boddum K., Williams S., Sun M., Terschak J.A., et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139:431–443. doi: 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augustin K., Khabbush A., Williams S., Eaton S., Orford M., Cross J.H., et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17:84–93. doi: 10.1016/S1474-4422(17)30408-8. [DOI] [PubMed] [Google Scholar]
- 19.Rowley S., Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121–131. doi: 10.1016/j.freeradbiomed.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bough K.J., Wetherington J., Hassel B., Pare J.F., Gawryluk J.W., Greene J.G., et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 21.Kim D.Y., Davis L.M., Sullivan P.G., Maalouf M., Simeone T.A., van Brederode J., et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101:1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan P.G., Dubé C., Dorenbos K., Steward O., Baram T.Z. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milder J.B., Liang L.P., Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–244. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D.Y., Simeone K.A., Simeone T.A., Pandya J.D., Wilke J.C., Ahn Y., et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M., Muhammad S., Khan M.A., Chen H., Ridder D.A., Müller-Fielitz H., et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 26.Guo M., Wang X., Zhao Y., Yang Q., Ding H., Dong Q., et al. Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci. 2018;11:86. doi: 10.3389/fnmol.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Wu X., Liu Q., Kong G., Zhou J., Jiang J., et al. Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats. Neuroscience. 2017;366:36–43. doi: 10.1016/j.neuroscience.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Amlerova J., Šroubek J., Angelucci F., Hort J. Evidences for a role of gut microbiota in pathogenesis and management of epilepsy. Int J Mol Sci. 2021;22:5576. doi: 10.3390/ijms22115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K., Kim N., Shim J.O., Kim G.H. Gut bacterial dysbiosis in children with intractable epilepsy. J Clin Med. 2020;10:5. doi: 10.3390/jcm10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Caro C., Leo A., Nesci V., Ghelardini C., di Cesare Mannelli L., Striano P., et al. Intestinal inflammation increases convulsant activity and reduces antiepileptic drug efficacy in a mouse model of epilepsy. Sci Rep. 2019;9:13983. doi: 10.1038/s41598-019-50542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173 doi: 10.1016/j.cell.2018.04.027. 1728–41.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutas A., Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazdi S., Stein M., Elinder F., Andersson M., Lindahl E. The molecular basis of polyunsaturated fatty acid interactions with the shaker voltage-gated potassium channel. PLoS Comput Biol. 2016;12 doi: 10.1371/journal.pcbi.1004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan J., Bailur S., Datay K., Sharma S., Khedekar Kale D. A switch to polyunsaturated fatty acid based ketogenic diet improves seizure control in patients with drug-resistant epilepsy on the mixed fat ketogenic diet: a retrospective open label trial. Cureus. 2019;11:e6399. doi: 10.7759/cureus.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simeone T.A., Matthews S.A., Samson K.K., Simeone K.A. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol. 2017;287:54–64. doi: 10.1016/j.expneurol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huttenlocher P.R., Wilbourn A.J., Signore J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology. 1971;21:1097–1103. doi: 10.1212/wnl.21.11.1097. [DOI] [PubMed] [Google Scholar]
- 40.Kossoff E.H., McGrogan J.R., Bluml R.M., Pillas D.J., Rubenstein J.E., Vining E.P. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–424. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer H.H., Thiele E.A. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65:1810–1812. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson F.S., Foster-Powell K., Brand-Miller J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neal E.G., Chaffe H., Schwartz R.H., Lawson M.S., Edwards N., Fitzsimmons G., et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.A., Yoon J.R., Lee E.J., Lee J.S., Kim J.T., Kim H.D., et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016;57:51–58. doi: 10.1111/epi.13256. [DOI] [PubMed] [Google Scholar]
- 45.Kverneland M., Molteberg E., Iversen P.O., Veierød M.B., Taubøll E., Selmer K.K., et al. Effect of modified Atkins diet in adults with drug-resistant focal epilepsy: a randomized clinical trial. Epilepsia. 2018;59:1567–1576. doi: 10.1111/epi.14457. [DOI] [PubMed] [Google Scholar]
- 46.Sharma S., Sankhyan N., Gulati S., Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: a randomized controlled trial. Epilepsia. 2013;54:481–486. doi: 10.1111/epi.12069. [DOI] [PubMed] [Google Scholar]
- 47.Sondhi V., Agarwala A., Pandey R.M., Chakrabarty B., Jauhari P., Lodha R., et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr. 2020;174:944–951. doi: 10.1001/jamapediatrics.2020.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang H.C., Chung D.E., Kim D.W., Kim H.D. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45:1116–1123. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 49.Shirley M. Triheptanoin: first approval. Drugs. 2020;80:1595–1600. doi: 10.1007/s40265-020-01399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enkhtuy B., Kwon H.E., Kim H.D. Advances in ketogenic diet therapies in pediatric epilepsy. Ann Child Neurol. 2019;27:105–112. [Google Scholar]
- 51.Borges K., Kaul N., Germaine J., Carrasco-Pozo C., Kwan P., O'Brien T.J. Open-label long-term treatment of add-on triheptanoin in adults with drug-resistant epilepsy. Epilepsia Open. 2020;5:230–239. doi: 10.1002/epi4.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calvert S., Barwick K., Par M., Ni Tan K., Borges K. A pilot study of add-on oral triheptanoin treatment for children with medically refractory epilepsy. Eur J Paediatr Neurol. 2018;22:1074–1080. doi: 10.1016/j.ejpn.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Borges K., Kaul N., Germaine J., Kwan P., O'Brien T.J. Randomized trial of add-on triheptanoin vs medium chain triglycerides in adults with refractory epilepsy. Epilepsia Open. 2019;4:153–163. doi: 10.1002/epi4.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobbing J., Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotter D.G., d'Avignon D.A., Wentz A.E., Weber M.L., Crawford P.A. Obligate role for ketone body oxidation in neonatal metabolic homeostasis. J Biol Chem. 2011;286:6902–6910. doi: 10.1074/jbc.M110.192369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyons L., Schoeler N.E., Langan D., Cross J.H. Use of ketogenic diet therapy in infants with epilepsy: a systematic review and meta-analysis. Epilepsia. 2020;61:1261–1281. doi: 10.1111/epi.16543. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-Herrero J., Cañedo-Villarroya E., Pérez-Sebastián I., Bernardino-Cuesta B., Pedrón-Giner C. Efficacy and safety of ketogenic dietary theraphies in infancy. A single-center experience in 42 infants less than two years of age. Seizure. 2021;92:106–111. doi: 10.1016/j.seizure.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Thompson L., Fecske E., Salim M., Hall A. Use of the ketogenic diet in the neonatal intensive care unit-safety and tolerability. Epilepsia. 2017;58:e36–e39. doi: 10.1111/epi.13650. [DOI] [PubMed] [Google Scholar]
- 59.Le Pichon J.B., Thompson L., Gustafson M., Abdelmoity A. Initiating the ketogenic diet in infants with treatment refractory epilepsy while maintaining a breast milk diet. Seizure. 2019;69:41–43. doi: 10.1016/j.seizure.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Tan-Smith C., Little H., Fabe J., Dickson C., Shillito P. Increase of human milk fat inducing nutritional ketosis in exclusively breastfed infant, brought about by treating the mother with ketogenic dietary therapy. J Hum Lact. 2021;5 doi: 10.1177/08903344211048422. 8903344211048422. [DOI] [PubMed] [Google Scholar]
- 61.Williams C.N., Piantino J., McEvoy C., Fino N., Eriksson C.O. The burden of pediatric neurocritical care in the United States. Pediatr Neurol. 2018;89:31–38. doi: 10.1016/j.pediatrneurol.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagoda A., Riggio S. Refractory status epilepticus in adults. Ann Emerg Med. 1993;22:1337–1348. doi: 10.1016/s0196-0644(05)80120-9. [DOI] [PubMed] [Google Scholar]
- 63.Lin K.L., Lin J.J., Wang H.S. Application of ketogenic diets for pediatric neurocritical care. Biomed J. 2020;43:218–225. doi: 10.1016/j.bj.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nabbout R., Mazzuca M., Hubert P., Peudennier S., Allaire C., Flurin V., et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 65.Arya R., Peariso K., Gaínza-Lein M., Harvey J., Bergin A., Brenton J.N., et al. Efficacy and safety of ketogenic diet for treatment of pediatric convulsive refractory status epilepticus. Epilepsy Res. 2018;144:1–6. doi: 10.1016/j.eplepsyres.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Park E.G., Lee J., Lee J. The ketogenic diet for super-refractory status epilepticus patients in intensive care units. Brain Dev. 2019;41:420–427. doi: 10.1016/j.braindev.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Jung D.E., Kang H.-C., Lee J.S., Lee E.J., Kim H.D. Safety and role of ketogenic parenteral nutrition for intractable childhood epilepsy. Brain Dev. 2012;34:620–624. doi: 10.1016/j.braindev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Strzelczyk A., Reif P.S., Bauer S., Belke M., Oertel W.H., Knake S., et al. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure. 2013;22:581–583. doi: 10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Lin J.-J., Lin K.-L., Chan O.-W., Hsia S.-H., Wang H.-S. Intravenous ketogenic diet therapy for treatment of the acute stage of super-refractory status epilepticus in a pediatric patient. Pediatr Neurol. 2015;52:442–445. doi: 10.1016/j.pediatrneurol.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Farias-Moeller R., Bartolini L., Pasupuleti A., Brittany Cines R.D., Kao A., Carpenter J.L. A practical approach to ketogenic diet in the pediatric intensive care unit for super-refractory status epilepticus. Neurocritical Care. 2017;26:267–272. doi: 10.1007/s12028-016-0312-4. [DOI] [PubMed] [Google Scholar]
- 71.Ko A., Youn S.E., Kim S.H., Lee J.S., Kim S., Choi J.R., et al. Targeted gene panel and genotype-phenotype correlation in children with developmental and epileptic encephalopathy. Epilepsy Res. 2018;141:48–55. doi: 10.1016/j.eplepsyres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Ko A., Jung D.E., Kim S.H., Kang H.C., Lee J.S., Lee S.T., et al. The efficacy of ketogenic diet for specific genetic mutation in developmental and epileptic encephalopathy. Front Neurol. 2018;9:530. doi: 10.3389/fneur.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim Z., Wong K., Olson H.E., Bergin A.M., Downs J., Leonard H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: experience of >100 patients. Epilepsia. 2017;58:1415–1422. doi: 10.1111/epi.13813. [DOI] [PubMed] [Google Scholar]
- 74.Lee Y.M., Kang H.C., Lee J.S., Kim S.H., Kim E.Y., Lee S.K., et al. Mitochondrial respiratory chain defects: underlying etiology in various epileptic conditions. Epilepsia. 2008;49:685–690. doi: 10.1111/j.1528-1167.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 75.Kang H.C., Lee Y.M., Kim H.D., Lee J.S., Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–88. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 76.Steriade C., Andrade D.M., Faghfoury H., Tarnopolsky M.A., Tai P. Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) may respond to adjunctive ketogenic diet. Pediatr Neurol. 2014;50:498–502. doi: 10.1016/j.pediatrneurol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Barañano K.W., Hartman A.L. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. 2008;10:410–419. doi: 10.1007/s11940-008-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stafstrom C.E., Rho J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gasior M., Rogawski M.A., Hartman A.L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castro K., Faccioli L.S., Baronio D., Gottfried C., Perry I.S., dos Santos Riesgo R. Effect of a ketogenic diet on autism spectrum disorder: a systematic review. Res Autism Spectr Disord. 2015;20:31–38. [Google Scholar]
- 81.Evangeliou A., Vlachonikolis I., Mihailidou H., Spilioti M., Skarpalezou A., Makaronas N., et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18:113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 82.Taylor M.K., Sullivan D.K., Mahnken J.D., Burns J.M., Swerdlow R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimers Dement. 2018;4:28–36. doi: 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaneko K., Wong M., Corley M., Lee R.W., Honolulu H.I., et al. The ketogenic diet as a potential therapy in Down syndrome. J Pediatr Pediatr Med. 2018;2:11–17. [Google Scholar]
- 84.Broom G.M., Shaw I.C., Rucklidge J.J. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer's disease. Nutrition. 2019;60:118–121. doi: 10.1016/j.nut.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Klement R.J., Champ C.E., Otto C., Kämmerer U. Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz K., Chang H.T., Nikolai M., Pernicone J., Rhee S., Olson K., et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3. doi: 10.1186/s40170-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kossoff E.H., Al-Macki N., Cervenka M.C., Kim H.D., Liao J., Megaw K., et al. What are the minimum requirements for ketogenic diet services in resource-limited regions? Recommendations from the International League Against Epilepsy Task Force for Dietary Therapy. Epilepsia. 2015;56:1337–1342. doi: 10.1111/epi.13039. [DOI] [PubMed] [Google Scholar]
- 88.Seo J.H., Kim H.D. Cultural challenges in using the ketogenic diet in Asian countries. Epilepsia. 2008;49:50–52. doi: 10.1111/j.1528-1167.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- 89.Kossoff E.H., McGrogan J.R. Worldwide use of the ketogenic diet. Epilepsia. 2005;46:280–289. doi: 10.1111/j.0013-9580.2005.42704.x. [DOI] [PubMed] [Google Scholar]