Abstract
The objective of this study was to develop a non-fermented probiotic milk that maintains its physicochemical properties, microbial properties, antioxidant activity, and sensory properties during storage (0, 7, and 14 days). During storage, pH and viable cell counts decreased; however, titratable acidity increased. In addition, the composition and sensory characteristics of the non-fermented probiotic milk showed no significant differences between samples (MLN; milk with Lactobacillus plantarum Ln1, MGG; milk with Lactobacillus rhamnosus GG, and milk control). The antioxidant activities of MLN determined using 2,2-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, ABTS+ radical scavenging, and reducing power assay were higher during the examined storage periods when compared with those of the other samples. Overall, the physicochemical properties, microbial properties, and sensory factors of MLN showed no significant differences. However, high antioxidant activity was observed. Thus, we present a new functional dairy product with antioxidant activity.
Keywords: Probiotics, Dairy product, Non-fermented probiotic milk, Viability, Sensory evaluation
Introduction
Lactic acid bacteria (LAB) are generally classified as “generally regarded as safe” (GRAS) and are the most important group of bacteria. LAB are classified as Lactobacillus sp., Leuconostoc sp., Lactococcus sp., Weissella sp. and Pediococcus sp. among several species (Jang et al., 2019). LAB have reported properties including non-pathogenicity, tolerance to acid and bile, antimicrobial activity, and the possibility of being suitable for industrial processes. LAB have gained increasing attention and are used as probiotics in various ways in the food industry, such as in starter cultures, food supplements, and food preservation. (Aarti et al., 2017; Lee et al., 2014; Son et al., 2018).
Probiotics are defined as live microorganisms that, when administered in adequate amounts, can confer various health benefits to the host, such as antioxidant, anticancer, diarrhea prevention, and immune system modulation activities (Han et al., 2020; Jang et al., 2020; Jeon et al., 2016; Kimoto-Nira et al., 2015; Vitali et al., 2012 WHO). Moreover, probiotics are used as starter cultures or additives to develop functional products. Probiotic foods are developed through fermentation and have been utilized in various products, including yogurt, meat, cereals, fruit, and beverages (Aspri et al, 2020). Dairy products are classified as probiotic foods (Kumar et al., 2015). Dairy products are the most commonly consumed probiotic foods because of their convenience (Oliveira et al., 2017). Dairy products have been reported to have effects on cholesterol reduction, modulation of the immune system, diabetes, alleviation of lactose intolerance, irritable bowel syndrome, treatment of diarrhea, and inflammatory bowel diseases (Shafi et al., 2018; Aspri et al., 2020). Especially, non-fermented probiotic milk is research that could be used as a convenient food that helps consumers take probiotics, and could be an alternative product that could be developed into various non-fermented probiotic milk with added sugar or additives in the future (Oliveira et al., 2017).
Among them, dairy-based beverages have rapidly increased in the food market, and their market value is expected to reach 13.9 billion USD by 2021, with the exception of some kinds of dairy beverage products (Turkmen et al., 2019). Recently, non-fermented probiotic milk, which does not contain sugar or any other additives, has become interestingly popular as a substitute for various types of probiotic foods for consumers concerned about their health (Oliveira et al., 2017). In particular, milk is widely known as a dairy product and has nutrient components with recognized health benefits (Gaucheron, 2013; Kaur et al., 2021). Therefore, non-fermented probiotic milk is expected to be a product with functional probiotic effects and fast manufacturing process.
When selecting probiotics for non-fermented probiotic milk, the safety and metabolism during production and storage periods need to be taken into account because these conditions affect the sensory qualities (Bayarri et al., 2011; Tripathi and Giri, 2014). To manufacture products containing probiotics, their sensory characteristics should be considered when selecting suitable strains to improve or maintain product quality (Cruz et al., 2010).
In a previous study, Lactobacillus plantarum Ln1 was reported to have desirable characteristics, including tolerance to artificial gastric conditions, enzyme production, production of β-galactosidase and β-glucosidase, adhesion to HT-29 cells, and immune-enhancing activities. In addition, the antioxidant activities (DPPH free radical scavenging and β-carotene bleaching assay) of L. plantarum Ln1 were reported to be 21.08% and 31.92%, respectively (Jang et al., 2018b). Thus, the aim of this study was to develop a non-fermented probiotic milk and evaluate the physicochemical and microbiological properties, sensory qualities, and antioxidant activities at 0, 7, and 14 days.
Materials and methods
Materials and cultures of bacterial strains
Milk was purchased from Seoul Milk Co. (Korea) and used as a drink. L. plantarum Ln1 (Jang et al., 2018b) was isolated from the traditional Korean fermented food, kimchi and Lactobacillus rhamnosus GG (LGG) were obtained from the Korean Collection for Type Cultures (KCTC, Daejeon, Korea) and add as a probiotic. These strains were used as the probiotic strains. They were cultivated in Man, Rogosa, and Sharpe broth (MRS; Becton–Dickinson, Sparks, MD, USA) at 37 °C for 24 h. To obtain pellets of LAB strains, the strains were centrifuged (14,240×g, 5 min, 4 °C), removed the supernatants, and washed three times with PBS (HyClone, Logan, UT, USA). The pellets were re-suspended in sterilized milk to a final concentration of 109 CFU/mL.
Preparation of non-fermented probiotic milk
Milk was placed in a sterilized container and sterilized at 90 °C for 10 min. Next, the milk was refrigerated at 4 °C after adding a probiotics strain (L. plantarum Ln1 and L. rhamnosus GG) (109 CFU/mL). Milk samples with LGG and L. plantarum Ln1 (MGG and MLN) were used as experimental groups, and milk (without probiotics) was used as a control. The milk was mixed and stored under refrigeration for 14 days at 4 °C. The milk samples were freeze-dried and stored at – 20 °C prior to the measurement of functional activities.
Composition, pH, titratable acidity, and viable cell counts
The physicochemical composition including protein, fat, moisture, total solids, and ash, of the non-fermented probiotic milk was measured following the protocol of the Association of Official Analytical Chemists (AOAC, 2012) using a Milcoscan™ (Type 78,110; FOSS Analytical A/S, Denmark). In addition, the pH, titratable acidity, and viable cell count of the non-fermented probiotic milk samples were measured. The pH was determined using a pH meter following the modified method by Jang et al. (2019). The titratable acidity was determined by mixing 10 mL of the milk sample with 10 mL of distilled water. The acidity was measured by titrating the mixture with NaOH (0.1 N) and confirming that the pH changed to 8.1 (Jung et al., 2016). Viable cells were cultivated on bromocresol purple agar plate (BCP; KisanBio, Seoul, Korea) at 37 °C for 48 h. And then, the viable cells counted and confirmed.
Preparation of non-fermented probiotic milk for antioxidant activity
To analyze antioxidant activity, freeze-dried non-fermented probiotic milk samples were resuspended in distilled water. The supernatant was obtained by centrifugation at 8000×g at 4 °C for 30 min, and the solid was removed.
DPPH radical scavenging activity assay
2,2-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was evaluated according to a previously described method (Jang et al., 2018b). The sample (400 μL) was mixed with 1 mL of 100 μM DPPH solution. The mixture was shaken and left for 20 min in a dark. Absorbance was measured at 517 nm using a spectrophotometer. The percentage inhibition of DPPH radicals was calculated using the following formula:
ABTS+ radical scavenging assay
Antioxidant activity was evaluated by the ABTS+ radical scavenging assay (Ji et al., 2015) with minor method. The ABTS+ solution was mixed with 14 mM 2′2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) with 5 mM potassium persulfate in the dark at room temperature for 16 h. The ABTS+ solution was mixed with distilled water until it had an absorbance of 0.7 at 734 nm. Fifty microliters of each sample was mixed with 1 mL of ABTS+ solution. The mixture was allowed to react at room temperature for 15 min in the dark. The absorbance was measured at 734 nm. The percentage inhibition of ABTS+ radicals was calculated using the following formula:
Reducing power assay
The reducing power assay was performed according to a modified method (Das and Goyal, 2015). Twenty microliters of sample (25 mg/mL) was added to 0.2 M sodium phosphate buffer (pH 6.6) (250 μL) and 1% potassium ferricyanide (250 μL), and the mixture was incubated at 50 °C for 20 min for reaction. Next, 10% trichloroacetic acid (250 μL) was added, and the mixture was centrifuged at 12,000 rpm for 5 min. Five hundred microliters of the supernatant was mixed with 0.1% ferric chloride (100 μL) and distilled water (500 μL). The mixture was allowed to react for 10 min, and the absorbance was measured at 700 nm. Reducing power was evaluated by using L-Cysteine standard curve as a control.
Sensory analysis
A panel of 30 individuals (untrained; females and males; aged 20 to 60 years, free of lactose intolerance, except for specific allergy patients), who were students and staff of the Konkuk university and Sangmyung university, participated and were trained on how to perform the sensory evaluation of non-fermented probiotic milk before executing the sensory test. The panels measured nine samples in a cup by analyzing color, flavor, texture, appearance, and overall acceptability using a 5-point hedonic scale (strongly dislike = 1 and strongly like = 5). The hedonic scale was as follows: strongly like = 5, moderately like = 4, neither like nor dis like = 3, moderately dislike = 2, and strongly dislike = 1 (Chavan et al., 2018).
Statistical analysis
All data were statistically analyzed by one-way analysis of variance (ANOVA) using the SPSS statistical software program version 19 (SPSS Inc., USA). ANOVA and Duncan’s multiple range tests were used to determine differences among results (p < 0.05).
Results and discussion
Physicochemical properties of non-fermented probiotic milk
The composition and physicochemical properties of non-fermented probiotic milk were evaluated during the storage period (0, 7, and 14 days). The protein, fat, ash, moisture, and total solid contents are presented in Table 1. Milk was used as a control (without probiotics) to compare to the experimental groups. There were no significant differences between the composition of non-fermented probiotic milk with LGG and L. plantarum Ln1 compared to the control (p < 0.05). In addition, when compared by storage period, the composition of the samples (control, MLN, and MGG) showed no differences. The pH and titratable acidity are shown in Table 2. The pH and titratable acidity were related to each other during storage. The initial titratable acidity of the control, MGG, and MLN was 6.709–6.625. The acidity was slightly lower in the experimental group in which probiotics were added. During storage period, the pH value decreased from 6.267 to 6.056. On the other hand, titratable acidity increased from 18.93 to 21.43. Accordingly, a general relationship was confirmed between the pH and titratable acidity. One study reported that pH and titratable acidity were related to each other, and these results were correlated with the quality of the samples (Jang et al., 2018a).
Table 1.
The composition of non-fermented probiotic milk with probiotics during storage periods at 4 °C (0, 7, and 14 days)
| Composition (%) | Storage time (day) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MS | MGG | MLN | |||||||
| 0 | 7 | 14 | 0 | 7 | 14 | 0 | 7 | 14 | |
| Moisture | 87.91 ± 0.57a | 87.50 ± 0.54a | 88.95 ± 1.34a | 87.75 ± 0.54a | 87.75 ± 0.54a | 88.45 ± 0.57a | 89.15 ± 2.60a | 87.79 ± 0.57a | 88.60 ± 0.54a |
| Ash | 99.50 ± 0.01a | 99.51 ± 0.00a | 99.51 ± 0.00a | 99.35 ± 0.28a | 99.51 ± 0.00a | 99.34 ± 0.29a | 99.68 ± 0.28a | 99.66 ± 0.26a | 99.34 ± 0.28a |
| Fat | 3.68 ± 0.01b | 3.62 ± 0.05b | 3.67 ± 0.05ab | 3.61 ± 0.02c | 3.62 ± 0.01b | 3.64 ± 0.01b | 3.72 ± 0.00a | 3.74 ± 0.01a | 3.72 ± 0.01a |
| Protein | 2.95 ± 0.01a | 2.88 ± 0.05a | 2.94 ± 0.04a | 2.93 ± 0.01b | 2.91 ± 0.01a | 2.93 ± 0.01a | 2.93 ± 0.00b | 2.92 ± 0.01a | 2.90 ± 0.03a |
| Lactose | 4.96 ± 0.01a | 4.94 ± 0.02a | 4.92 ± 0.01a | 4.92 ± 0.02a | 4.88 ± 0.02b | 4.88 ± 0.03ab | 4.94 ± 0.01ab | 4.90 ± 0.00b | 4.88 ± 0.01b |
| Total Solid | 12.27 ± 0.06a | 12.29 ± 0.07a | 12.26 ± 0.04a | 12.30 ± 0.01a | 12.27 ± 0.02a | 12.27 ± 0.07a | 12.26 ± 0.01a | 12.30 ± 0.00a | 12.27 ± 0.01a |
All values are mean ± standard deviation
MS milk, MGG milk with L. rhamnosus GG, MLN milk with L. plantarum Ln1
a,bThe superscript lowercase letters in the same row indicate statistical differences by ANOVA (p < 0.05)
Table 2.
Average pH and titratable acidity values for non-fermented probiotic milk samples with probiotics during storage periods at 4 °C (0, 7, and 14 days)
| Sample | pH | Titratable acidity | ||||
|---|---|---|---|---|---|---|
| Storage period (day) | Storage period (day) | |||||
| 0 | 7 | 14 | 0 | 7 | 14 | |
| MS | 6.84 ± 0.01a | 6.34 ± 0.00a | 6.27 ± 0.02a | 11.97 ± 0.06b | 16.00 ± 0.04c | 18.93 ± 0.31b |
| MGG | 6.77 ± 0.00c | 6.15 ± 0.00c | 6.07 ± 0.01b | 14.90 ± 0.10a | 19.93 ± 0.15a | 21.43 ± 0.32a |
| MLN | 6.78 ± 0.00b | 6.16 ± 0.00b | 6.06 ± 0.01b | 14.60 ± 0.35a | 19.33 ± 0.25b | 21.10 ± 0.36a |
All values are mean ± standard deviation
MS milk, MGG milk with L. rhamnosus GG, MLN milk with L. plantarum Ln1
a−cThe superscript lowercase letters in the same column indicate statistical differences by ANOVA (p < 0.05)
Microbial properties of non-fermented probiotic milk
The probiotic strains were added to milk with an initial microbial content higher than 106 CFU/mL in milk. The viable cell counts of LGG and L. plantarum Ln1 in non-fermented probiotic milk during the storage period are shown in Fig. 1. The number of viable cells was confirmed, and at least 8 Log CFU/mL was maintained in all experimental groups during the storage period. Viable cell counts decreased from 8.52 to 8.39 CFU/mL and 8.55 to 8.50 Log CFU/mL, respectively. These results are similar to those of the other groups. There were no significant differences in viable cell counts between LGG and L. plantarum Ln1 (p < 0.05). Additionally, a previous study showed that the number of viable cells in non-fermented probiotic milk with Bifidobacterium animalis and Lactobacillus sp. increased, however, Bifidobacterium longum significantly decreased during the storage period (Oliveira et al., 2017). Compared with our study and another study, there were no significant differences between probiotic beverages and non-fermented probiotic beverages according to the data about 8.1 to 10.55 Log CFU/mL (Chavan et al., 2018).
Fig. 1.
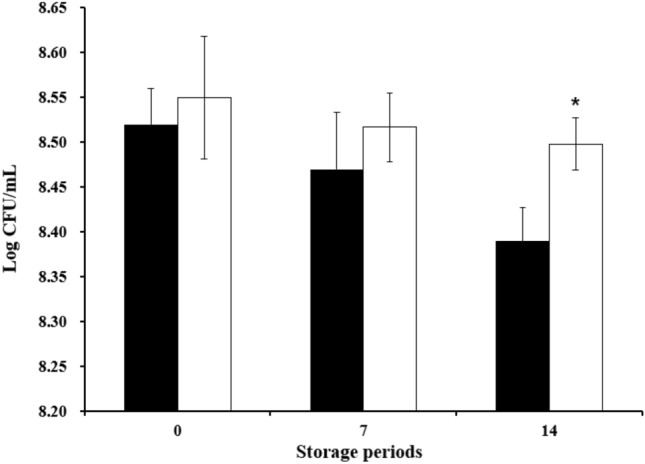
The viable cell counts of L. rhamnosus GG and Lactobacillus plantarum Ln1 in non-fermented probiotic milk during storage periods at 4 °C (0, 7, and 14 days). ■, milk with L. rhamnosus GG; □, milk with Lactobacillus plantarum Ln1. Each value represents the mean ± standard deviation, and different letters on each bar represent a significant difference between values (*p < 0.05)
Antioxidant activities of non-fermented probiotic milk
Recent studies have reported that antioxidant substances should be present to prevent oxidative stress in foods (Khan et al., 2017). In addition, probiotics have been used for a long time for their health benefits (Yang et al., 2019). In particular, the oxidation of milk causes strong off-flavors and reduces the quality of the milk (Khan et al., 2019). The antioxidant activities of the control, MGG, and MLN were measured using three modified methods (DPPH radical scavenging, ABTS+ radical scavenging, and reducing power assay) during the storage period. The antioxidant activities are shown in Table 3. Overall, the antioxidant activity decreased during the storage period in all experimental groups. The DPPH radical scavenging activity of MLN showed the lowest decrease (47.98% to 41.50%) from day 0 to 14. Although the initial activity of MGG was the highest among these groups, the activity value was lower than that of MLN at the end of storage. The control showed the lowest antioxidant activity compared with the initial value as well as the lowest antioxidant activity on day14, and showed a tendency to decrease the most during the storage period compared to the other experimental groups (38.12% to 22.44%). Consequently, MGG and MLN showed significant values (p < 0.05) compared to the control. The ABTS+ radical scavenging activities of the control, MGG, and MLN were not significantly different (p < 0.05). However, compared to DPPH radical scavenging, a decrease was confirmed during the storage period. In the case of ABTS+, the antioxidant activity of ascorbic acid as a positive control was slightly higher than that of all the experimental groups. Lastly, the reducing power of MLN was significantly different on day 0 when compared to the other samples (p < 0.05). Although the activity value of MLN decreased and the highest decrease was measured during the storage period, the activity value of MLN was the highest on day 14 (63.61 μM/L). The antioxidant activities of non-fermented probiotic milk were demonstrated through these results. Non-fermented soy milk showed DPPH, ABTS, and FRAP values of 27.32%, 58.91%, and 0.32 mmol Fe2+/L, respectively (Ahsan et al., 2020). The antioxidant activity was affected, but the composition or property of soy milk and milk are different. In addition, compared with non-fermented soy milk, our study showed much higher antioxidant activity. Yilmaz-Ersan et al. (2018) reported that the DPPH and ABTS+ values of cow and ewe milk were 3.14%, 8.70%, 21.48%, and 33.18%, respectively. On the other hand, it was confirmed that the antioxidant activity of MLN and MGG depends on the antioxidant activity of the probiotics (L. plantarum Ln1 and LGG). Therefore, our findings seem to show that non-fermented probiotic milk could be a healthy product with antioxidant activities.
Table 3.
Antioxidant activities of non-fermented probiotic milk sample with probiotic during storage at 4 °C (0, 7, and 14 days)
| Sample | Day | Antioxidant activity | ||
|---|---|---|---|---|
| DPPH radical scavenging activity (%) | ABTS+ radical scavenging activity (%) |
Reducing power assay (mM) | ||
| MS | 0 | 38.12 ± 2.58d | 90.45 ± 0.30a | 82.70 ± 4.79c |
| 7 | 34.14 ± 1.40e | 87.95 ± 0.30b | 62.81 ± 3.97e | |
| 14 | 22.44 ± 0.67f | 86.10 ± 0.35d | 52.67 ± 1.38f | |
| MGG | 0 | 48.45 ± 0.19a | 90.20 ± 0.17a | 88.07 ± 4.82b |
| 7 | 47.39 ± 0.22ab | 86.00 ± 0.15d | 76.53 ± 3.01d | |
| 14 | 38.15 ± 0.41d | 85.05 ± 0.10e | 60.82 ± 1.36e | |
| MLN | 0 | 47.98 ± 0.37a | 90.20 ± 0.25a | 106.76 ± 2.64a |
| 7 | 45.73 ± 0.26b | 87.40 ± 0.05c | 80.11 ± 3.79 cd | |
| 14 | 41.50 ± 1.40c | 86.05 ± 0.10d | 63.61 ± 0.40e | |
All values are mean ± standard deviation
MS milk, MGG milk with L. rhamnosus GG, MLN milk with L. plantarum Ln1
a−fThe superscript lowercase letters in the same column indicate statistical differences by ANOVA (p < 0.05)
Sensory analysis
The sensory property has the greatest impact on consumer buying patterns for selecting high- quality products (Lim et al., 2020). The quality factors of non-fermented probiotic milk included flavor, color, mouth feel, and taste. Subsequently, overall acceptance was assessed based on these values. Sensory evaluation was conducted to compare the difference between the control (milk) and non-fermented probiotic milk (with L. plantarum Ln1 and LGG). The sensory evaluation of the non-fermented probiotic milk is presented in Table 4. All samples showed no significant differences between the control and probiotic strains (p < 0.05). Generally, these two samples, as well as control, showed a better flavor and taste. In particular, MLN showed the highest sensory values on day 0 when compared with other samples. All the values of all the non-fermented probiotic milk were acceptable. Many studies on the sensory evaluation of probiotic drinks generally indicate flavor, taste, appearance, color, and overall acceptability (Ahsan et al., 2020; Chavan et al., 2018). One study reported that non-fermented milk with L. acidophilus LA-5 (LA) showed better sensory values than other samples [with Bifidobacterium animalis subsp. lactis (BA)] during storage periods (Oliveira et al., 2017). Therefore, our research results showed no sensory problem and confirmed the potential for further development of the non-fermented probiotic milk.
Table 4.
Sensory properties of non-fermented probiotic milk sample with probiotic during storage at 4 °C (0, 7, and 14 days)
| Sample | Day | Sensory properties | ||||
|---|---|---|---|---|---|---|
| Flavor | Color | Mouth feel | Taste | Overall acceptance | ||
| MS | 0 | 3.25 ± 0.70a | 3.67 ± 0.73a | 3.64 ± 0.62a | 3.46 ± 0.79a | 3.54 ± 0.79a |
| 7 | 3.27 ± 0.63a | 3.74 ± 0.76a | 3.75 ± 0.65a | 3.64 ± 0.91a | 3.61 ± 0.99a | |
| 14 | 3.39 ± 0.63a | 3.63 ± 0.79a | 3.75 ± 0.89a | 3.57 ± 1.00a | 3.44 ± 1.09a | |
| MGG | 0 | 3.50 ± 0.79a | 3.70 ± 0.72a | 3.54 ± 0.69a | 3.50 ± 0.88a | 3.64 ± 0.83a |
| 7 | 3.39 ± 0.83a | 3.56 ± 0.85a | 3.50 ± 1.07a | 3.46 ± 1.07a | 3.43 ± 1.17a | |
| 14 | 3.39 ± 1.13a | 3.63 ± 0.88a | 3.36 ± 0.83a | 3.46 ± 1.17a | 3.39 ± 1.07a | |
| MLN | 0 | 3.21 ± 0.92a | 3.41 ± 0.80a | 3.50 ± 0.88a | 3.29 ± 0.90a | 3.25 ± 1.04a |
| 7 | 3.43 ± 1.03a | 3.41 ± 1.01a | 3.39 ± 0.99a | 3.68 ± 1.12a | 3.68 ± 1.12a | |
| 14 | 3.54 ± 0.69a | 3.56 ± 0.70a | 3.54 ± 0.92a | 3.75 ± 0.89a | 3.68 ± 0.72a | |
All values are mean ± standard deviation
MS milk, MGG milk with L. rhamnosus GG, MLN milk with L. plantarum Ln1
aThe superscript lowercase letters in the same column indicate statistical differences by ANOVA (p < 0.05)
Acknowledgements
This study do not receive any fund.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The study was assessed in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (Approval Number: 7001355-202010-HR-403) of Konkuk University, Korea.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hye Ji Jang, Email: gpwljjj@naver.com.
Jong Ha Kim, Email: gkelsrnr@naver.com.
Hyun-Sook Lee, Email: leehs9292@smu.ac.kr.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Aarti C, Khusro A, Varghese R, Varghese R, Arasu MV, Agastian P, Al-Dhabi NA, Ilavenil S, Choi KC. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT-Food Sciencde and Technology. 2017;86:438–446. doi: 10.1016/j.lwt.2017.07.055. [DOI] [Google Scholar]
- Ahsan S, Khaliq A, Chughtai MFJ, Nadeem M, Din AA, Hlebova M, Rebezov M, Khayrullin M, Mikolaychik I, Morozova L, Shariati MA. Functional exploration of bioactive moieties of fermented and non-fermented soy milk with reference to nutritional attibutes. Journal of Microbiology Biotechnology and Food Sciences. 2020;10:145–149. doi: 10.15414/jmbfs.2020.10.1.145-149. [DOI] [Google Scholar]
- AOAC. AOAC International. 19th ed. Official Methods of Analysis. Gaithersburg, MD, USA (2012)
- Aspri M, Papademas P, Tsaltas D. Review on non-dairy probiotics and their use in non-dairy based on products. Fermentation. 2020;6:30. doi: 10.3390/fermentation6010030. [DOI] [Google Scholar]
- Bayarri S, Carbonell I, Barrios EX, Costell E. Impact of sensory differences on consumer acceptability of yoghurt and yoghurt-like products. International Dairy Journal. 2011;21:111–118. doi: 10.1016/j.idairyj.2010.09.002. [DOI] [Google Scholar]
- Chavan M, Gat Y, Harmalkar M, Waghmare R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT-Food Sciencde and Technology. 2018;91:339–344. doi: 10.1016/j.lwt.2018.01.070. [DOI] [Google Scholar]
- Cruz AG, Cadena RS, Walter EHM, Mortazavian AM, Granato D, Faria JAF, Bolini HMA. Sensory Analysis: Relevance for prebiotic, probiotic, and symbiotic product development. Comprehensive Reviews in Food Science and Food Safety. 2010;9:358–373. doi: 10.1111/j.1541-4337.2010.00115.x. [DOI] [PubMed] [Google Scholar]
- Das D, Goyal A. Antioxidant activity and c-aminobutyl acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sciencde and Technology. 2015;61:263–268. doi: 10.1016/j.lwt.2014.11.013. [DOI] [Google Scholar]
- Gaucheron F. Milk and dairy products: a unique micronutrient combination. Journal of the American College of Nutrition. 2013;30:400S–409S. doi: 10.1080/07315724.2011.10719983. [DOI] [PubMed] [Google Scholar]
- Han KJ, Lee JE, Lee NK, Paik HD. Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. Journal of Microbioloy and Biotechnology. 2020;30:591–598. doi: 10.4014/jmb.2002.02052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Jung J, Yu HS, Lee NK, Paik HD. Evaluation of the quality of yogurt using ginseng extract powder and probiotic Lactobacillus plantarum NK181. Korean Journal for Food Science of Animal Resources. 2018;38:1160–1167. doi: 10.5851/kosfa.2018.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. Journal of Food Science and Technology. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Lee NK, Paik HD. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Science and Biotechnology. 2019;28:1521–1528. doi: 10.1007/s10068-019-00576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Yu HS, Lee NK, Paik HD. Immune-stimulating effect of Lactobacillus plantarum Ln1 isolated from the traditional Korean fermented food, kimchi. Journal of Microbioloy and Biotechnology. 2020;30:926–929. doi: 10.4014/jmb.2001.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Science and Biotechnology. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Jang NY, Kim YT. Isolation of Lactic acid bacteria showing antioxidative and probiotic activities from kimchi and infant feces. Journal of Microbiology and Biotechnology. 2015;25:1568–1577. doi: 10.4014/jmb.1501.01077. [DOI] [PubMed] [Google Scholar]
- Jung J, Paik HD, Yoon HJ, Jang HJ, Jeewanthi RKC, Jee HS, Li X, Lee NK, Lee SK. Physicochemical chararcteristics and antioxidant capacity in yogurt fortifired with red ginseng extract. Korean Journal for Food Science of Animal Resources. 2016;36:412–420. doi: 10.5851/kosfa.2016.36.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Bedi JS, Dhaka P, Vijay D, Aulakh RS. Exposure assessment and risk characterization of aflatoxin M1 through consumption of market milk and milk products in Ludhiana, Punjab. Food Control. 2021;126:107991. doi: 10.1016/j.foodcont.2021.107991. [DOI] [Google Scholar]
- Khan IT, Nadeem M, Imran M, Ayaz M, Ajaml M, Ellahi MY, Khalique A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids in Health and Disease. 2017;16:163. doi: 10.1186/s12944-017-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IT, Nadeem M, Imran M, Ullah R, Ajaml M, Jaspal MH. Antioxidant properties of milk and dairy products: a comprehensive review of the current knowledge. Lipids in Health and Disease. 2019;18:41. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto-Nira H, Suzuki S, Suganuma H, Moriya N, Suzuki C. Growth characteristics of Lactobacillus brevis KB290 in the presence of bile. Anaerobe. 2015;35:96–101. doi: 10.1016/j.anaerobe.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Kumar BV, Vijayendra SVN, Reddy OVS. Trends in dairy and non-dairy probiotic products – a Review. Journal of Food Science and Technology. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sciencde and Technology. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- Lim Sung-Min, Lee Na-Kyoung, Kim Kee-Tae, Paik Hyun-Dong. Probiotic Lactobacillus fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microbial Pathogenesis. 2020;147:104430. doi: 10.1016/j.micpath.2020.104430. [DOI] [PubMed] [Google Scholar]
- Oliveira D, Vidal L, Ares G, Walter EHM, Rosenthal A, Deliza R. Sensory, microbiological and physicochemical screening of probiotic cultures for the development of non-fermented probiotic milk. LWT-Food Sciencde and Technology. 2017;79:234–241. doi: 10.1016/j.lwt.2017.01.020. [DOI] [Google Scholar]
- Shafi A, Raja HN, Farooq U, Akram K, Hayat Z, Naz A, Nadeem HR. Antimicrobial and antidiabetic potential of symbiotic fermented milk: A functional dairy product. International Journal of Dairy Technology. 2018;72:15–22. doi: 10.1111/1471-0307.12555. [DOI] [Google Scholar]
- Son SH, Jeon HL, Yang SJ, Sim MH, Kim YJ, Lee NK, Paik HD. Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Science and Biotechnology. 2018;27:123–129. doi: 10.1007/s10068-017-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi MK, Giri SK. Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods. 2014;9:225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- Turkmen N, Akal C, Ozer B. Probiotic dairy – based beverages: A review. Journal of Functional Foods. 2019;53:62–75. doi: 10.1016/j.jff.2018.12.004. [DOI] [Google Scholar]
- Vitali B, Minervini G, Rizzello CG, Spisni E, Maccaferri S, Brigidi P, Gobbetti M, Cagno RD. Novel probiotic candidates for humans isolated from raw fruits and vegetables. Food Microbiology. 2012;31:116–125. doi: 10.1016/j.fm.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Science and Biotechnology. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz-Ersan L, Ozcan T, Akpinar-Bayizit A, Sahin S. Comparison of antioxidant capacity of cow and ewe milk kefirs. Journal of Dairy Science. 2018;101:3788–3798. doi: 10.3168/jds.2017-13871. [DOI] [PubMed] [Google Scholar]


