Abstract
The specific activities and the mRNA expression levels associated with coenzyme A transferase, acetoacetate decarboxylase, and butyraldehyde dehydrogenase were elevated in hyper-solvent-producing Clostridium beijerinckii BA101 during the exponential growth phase. The increase in expression of the sol operon and associated enzyme activities may be responsible for enhanced solvent production by C. beijerinckii BA101.
Interest in the acetone-butanol fermentation in which the solventogenic clostridia are used has been renewed due to advances in our understanding of solvent production by these microorganisms at the physiological and genetic levels, as well as for economic and environmental reasons (4, 19).
Clostridium beijerinckii BA101 was generated as described previously (3). Pilot-scale (20-liter) fermentations in which semidefined P2 medium containing either 6% glucose or 6% STAR-DRI 5 maltodextrin was used demonstrated that C. beijerinckii BA101 produces up to 100% more butanol and acetone than the C. beijerinckii NCIMB 8052 parental strain (11). In addition, C. beijerinckii BA101 exhibited reduced acid production and increased carbohydrate utilization compared to C. beijerinckii NCIMB 8052 (11).
Alteration of the enzyme activity in either the solventogenic pathways or the acidogenic pathways has been found to affect solvent and acid production profiles in the solventogenic clostridia (5, 8, 12, 14, 18). mRNA expression levels of fermentative genes and activities of the associated enzymes in C. beijerinckii BA101 and NCIMB 8052 were examined in order to identify alterations in gene expression and enzyme activities that may be responsible for enhanced solvent production by C. beijerinckii BA101.
Escherichia coli DH5α was used as a host for pJT297 (16) and pBUT23 (13). pJT297 contains complete ctfB and partial ctfA genes from C. beijerinckii NRRL B593, and pBUT23 contains buk and ptb genes from C. beijerinckii NCIMB 8052. C. beijerinckii and E. coli strains were maintained and E. coli strains were grown as previously described (7). C. beijerinckii strains were grown in Trypticase-glucose-yeast extract medium (TGY medium) or P2 medium (2) containing 0.1% yeast extract and 6% glucose.
The acid and solvent contents of culture supernatants were measured by gas chromatography (7). For enzyme activity assays, C. beijerinckii crude cell extracts were prepared by using a French pressure cell (7). Protein contents were determined by using the dye-binding assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as the standard. Coenzyme A (CoA) transferase (CoAT) activity was assayed by monitoring the disappearance of acetoacetyl-CoA at 310 nm as described by Clark et al. (8); 1 U of enzyme activity was defined as the amount of enzyme which resulted in the disappearance of 1 μmol of acetoacetyl-CoA per min. Acetoacetate decarboxylase (AADC) activity was determined manometrically (9); activities were expressed in microliters of CO2 per minute per milligram of protein. Butyraldehyde dehydrogenase (BADH) and butanol dehydrogenase (BDH) activities were assayed by monitoring the oxidation of NAD(P)H at 340 nm (8, 10); 1 U of enzyme activity was defined as the amount of enzyme which resulted in oxidation of 1 μmol of NAD(P)H per min. The acetate kinase (AK) and butyrate kinase (BK) activity assays were carried out by using the hydroxamate methods of Rose (15). Phosphotransbutyrylase (PTB) activity was measured by monitoring the liberation of CoA after the addition of butyryl-CoA to the reaction mixture (1). Phosphotransacetylase (PTA) activity was determined as described by Brown et al. (6); 1 U of activity was defined as the amount of enzyme which resulted in the formation of 1 μmol of NADH per min.
Plasmid DNA was isolated from E. coli by using a Qiagen Midi kit (Qiagen Inc., Chatsworth, Calif.) according to the manufacturer’s instructions. RNA isolation, Northern hybridization, preparation of ctfAB and ptb probes, and gel documentation were carried out as previously described (7).
Enzyme activity assays.
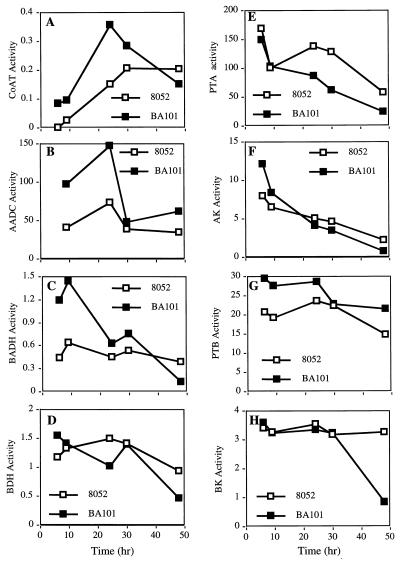
The in vitro specific activities of solventogenic and acidogenic enzymes associated with C. beijerinckii NCIMB 8052 and BA101 were assayed. Samples were collected at five different times from 1-liter batch fermentations in order to obtain samples that represented all of the growth phases; the 6- and 9-h samples corresponded to the early exponential growth phase, the 24-h samples corresponded to the late exponential growth phase, the 30-h samples corresponded to the early stationary growth phase, and the 48-h samples corresponded to the late stationary growth phase (Fig. 1). During the exponential growth phase, the specific activities of three solventogenic enzymes, CoAT, AADC, and NADH-dependent BADH, were substantially higher in C. beijerinckii BA101 than in NCIMB 8052 (Fig. 1A through C). Table 1 summarizes the increases in the specific activities of CoAT, AADC, and BADH observed at 9 and 24 h. C. beijerinckii BA101 and NCIMB 8052 exhibited similar NADH-dependent BDH specific activities over the course of the fermentation (Fig. 1D). The specific activities of NADPH-dependent BDH and BADH were less than 1/10th the specific activities of the NADH-dependent dehydrogenases and were not significantly different in C. beijerinckii BA101 and NCIMB 8052 (data not shown). Differences were also observed in the specific activities of acidogenic enzymes associated with C. beijerinckii BA101 and NCIMB 8052. C. beijerinckii BA101 exhibited lower PTA specific activities during the late exponential and stationary growth phases (Fig. 1E) and slightly higher AK specific activities during the early exponential growth phase than C. beijerinckii NCIMB 8052 exhibited (Fig. 1F). The PTB specific activities of C. beijerinckii BA101 were higher during the early exponential and stationary growth phases than the PTB specific activities of C. beijerinckii NCIMB 8052 were (Fig. 1G), whereas the BK specific activity of C. beijerinckii BA101 was similar to the BK specific activity of C. beijerinckii NCIMB 8052 except during the stationary growth phase (Fig. 1H).
FIG. 1.
Specific activities of solventogenic and acidogenic enzymes of C. beijerinckii NCIMB 8052 and BA101 following growth in 1 liter of semidefined P2 medium containing 6% glucose. (A) CoAT (acetoacetyl CoA:acetate-butyrate CoAT). (B) AADC. (C) BADH (NAD+ dependent). (D) BDH (NAD+ dependent). (E) PTA. (F) AK. (G) PTB. (H) BK. Symbols: □, NCIMB 8052; ■, BA101. The samples were collected from fermentations. Data are averages of data from duplicate experiments in which each sample was assayed three times; the standard deviations were within 7%.
TABLE 1.
Increases in specific enzyme activities of solventogenic enzymes associated with C. beijerinckii BA101 compared to NCIMB 8052 activities following 9 and 24 h of growth
| Time (h) | Fold increase in activity ofa:
|
||
|---|---|---|---|
| CoAT | AADC | BADH | |
| 9 | 3.6 | 2.4 | 2.3 |
| 24 | 2.4 | 2.0 | 1.4 |
Enzyme specific activity of C. beijerinckii BA101 divided by enzyme specific activity of NCIMB 8052.
Northern hybridization analyses.
Northern hybridization analyses were carried out in order to determine whether the observed differences in enzyme specific activities associated with C. beijerinckii BA101 and NCIMB 8052 were related to changes in the mRNA expression levels of the corresponding genes. In a previous study of C. beijerinckii NCIMB 8052, we demonstrated that this strain has a sol operon, which contains genes for aldehyde dehydrogenase (ald), CoAT (ctfA and ctfB), and AADC (adc), similar to the sol operon associated with C. beijerinckii NRRL B593 (7, 17). We also confirmed that there is a ptb-buk operon in C. beijerinckii NCIMB 8052 (7). The mRNA expression levels of the sol operon containing the ctfA and ctfB genes were consistently higher for C. beijerinckii BA101 than for strain NCIMB 8052 during the exponential and early stationary growth phases (Fig. 2A). In addition, the sol operon was expressed at high levels during the early exponential growth phase in both strains. However, during the late stationary growth phase (48 h), the sol operon was expressed at a high level in C. beijerinckii NCIMB 8052, but essentially no message was observed in C. beijerinckii BA101 (Fig. 2A). The mRNA expression levels of the ptb-buk genes were the highest during the early exponential growth phase and then gradually decreased over time for C. beijerinckii BA101, whereas in C. beijerinckii NCIMB 8052 there was strong expression of the ptb-buk transcript during the early exponential and late stationary growth phases and weak expression of the operon during the late exponential growth phase (Fig. 2B). The mRNA expression levels of the ptb-buk operon were higher in C. beijerinckii BA101 than in C. beijerinckii NCIMB 8052 for all of the growth phases except the late stationary growth phase, in which the transcript of the operon could be detected only in the C. beijerinckii NCIMB 8052 sample (Fig. 2B). The multiple bands produced during Northern hybridization may correspond to the polycistronic messages for the sol or ptb-buk operon (Fig. 2).
FIG. 2.
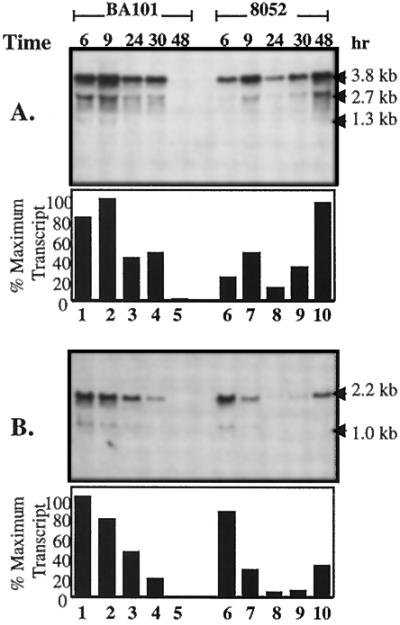
Northern hybridization analysis of RNA isolated at various times from C. beijerinckii NCIMB 8052 and BA101 following growth in 1 liter of semidefined P2 medium containing 6% glucose and probed with the ctfAB probe (A) or the ptb probe (B). The upper portion of each panel is the X-ray film picture of a Northern blot, and the lower portion of each panel is a densitometric analysis of the X-ray film. The relative intensities of transcripts are presented as percentages of the maximum transcript intensity, and the sample with the highest density was considered the sample with the maximum intensity. The arrows indicate the sizes of the transcripts.
During the exponential growth phase of C. beijerinckii BA101, there were dramatic increases in the specific activities of CoAT, AADC, and BADH and at least a twofold increase in the mRNA expression levels of the sol operon compared to the parent strain C. beijerinckii NCIMB 8052 when the organisms were grown in P2 medium (Table 1 and Fig. 2A). These results suggest that the increases in the CoAT, AADC, and BADH activities in C. beijerinckii BA101 may have been the result of an increase in sol operon expression in C. beijerinckii BA101 compared to the parent strain. The higher level of PTB activity in C. beijerinckii BA101 than in NCIMB 8052 is consistent with the results of the Northern hybridization analysis in which the ptb probe was used (Fig. 2B).
C. beijerinckii BA101 not only produced more solvents at a faster rate than C. beijerinckii NCIMB 8052 produced but also utilized glucose and reassimilated acids more completely (11). Consistent with the results of a previous study (7), an increase in acid reassimilation due to elevated CoAT and AADC activities may contribute to enhanced solvent production by C. beijerinckii BA101, since acetate uptake may enhance the glycolytic rate and, consequently, may increase glucose utilization. Since BADH catalyzes butyraldehyde formation by using butyryl-CoA as the substrate during butanol production, an increase in BADH activity may direct butyryl-CoA to butanol production instead of butyrate formation. Therefore, the increases in solvent production, glucose utilization, and acid uptake by C. beijerinckii BA101 compared to C. beijerinckii NCIMB 8052 may be attributed to the increased CoAT, AADC, and BADH activities observed in C. beijerinckii BA101.
In addition to enhanced acid reassimilation due to increases in CoAT and AADC activities, the decrease in PTA activity observed following the late exponential growth phase of C. beijerinckii BA101 compared to NCIMB 8052 may be responsible for the C. beijerinckii BA101 culture having a much lower acetate concentration in the fermentation broth than the C. beijerinckii NCIMB 8052 culture. The higher initial PTB activity associated with C. beijerinckii BA101 than with NCIMB 8052 is consistent with the finding that more butyrate is produced by C. beijerinckii BA101 than by C. beijerinckii NCIMB 8052 during the early exponential growth phase. However, the observation that the butyrate concentration was much lower in C. beijerinckii BA101 than in NCIMB 8052 following the early exponential phase indicates that the elevated acid reassimilation resulting from increases in the CoAT and AADC activities is able to offset the higher initial butyrate production in C. beijerinckii BA101. At 48 h, metabolic activities may have halted in C. beijerinckii BA101, as indicated by an absence of transcripts for the sol and ptb-buk operons. This may have been due to exhaustion of nutrients in the growth medium due to the elevated metabolic rate of C. beijerinckii BA101.
To further investigate and identify the nature and locations of the mutation(s) responsible for the increases in the mRNA expression levels of the sol operon and in the CoAT, AADC, and BADH activities associated with C. beijerinckii BA101, the sol operon should be cloned from both strains and the sequences obtained, including the sequence of the regulatory region, should be compared. Nevertheless, the results of this study clearly suggest that C. beijerinckii NCIMB 8052 can be genetically manipulated to produce more solvent and to increase its carbohydrate utilization efficiency by increasing the expression of genes associated with the sol operon.
Acknowledgments
This work was supported in part by the Illinois Corn Marketing Board and the USDA National Research Initiative Value-Added Program, grant AG96-35500-3247.
We thank J.-S. Chen (Virginia Polytechnic Institute and State University) for providing plasmid pJT297, as well as for stimulating discussions, and N. P. Minton for providing plasmid pBUT23.
REFERENCES
- 1.Andersch W, Bahl H, Gottschalk G. Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol. 1983;18:327–332. [Google Scholar]
- 2.Annous B A, Blaschek H P. Regulation and localization of amylolytic enzymes in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1990;56:2559–2561. doi: 10.1128/aem.56.8.2559-2561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annous B A, Blaschek H P. Isolation and characterization of Clostridium acetobutylicum mutants with enhanced amylolytic activity. Appl Environ Microbiol. 1991;57:2544–2548. doi: 10.1128/aem.57.9.2544-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaschek H P. Approaches to making the food processing industry more environmentally friendly. Trends Food Sci Technol. 1991;3:107–110. [Google Scholar]
- 5.Boynton Z L, Bennett G N, Rudolph F B. Cloning, sequencing, and expression of genes encoding phosphotransacetylase and acetate kinase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1996;62:2758–2766. doi: 10.1128/aem.62.8.2758-2766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown T D K, Jones-Mortimer M C, Kornberg H L. The enzymatic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1997;102:327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-K, Blaschek H P. Effect of acetate on molecular and physiological aspects of solvent production and strain degeneration in Clostridium beijerinckii NCIMB 8052. Appl Environ Microbiol. 1999;65:499–505. doi: 10.1128/aem.65.2.499-505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark S W, Bennett G N, Rudolph F B. Isolation and characterization of mutants of Clostridium acetobutylicum ATCC 824 deficient in acetoacetyl-coenzyme A:acetate/butyrate:coenzyme A-transferase (EC 2.8.3.9) and in other solvent pathway enzymes. Appl Environ Microbiol. 1989;55:970–976. doi: 10.1128/aem.55.4.970-976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies R. Studies on the acetone-butanol fermentation. 4. Acetoacetate decarboxylase of C. acetobutylicum (BY) Biochem J. 1943;37:230–238. doi: 10.1042/bj0370230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dürre P, Kuhn A, Gottwald M, Gottschalk G. Enzymatic investigations on butanol dehydrogenase and butyraldehyde dehydrogenase in extracts of Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1987;26:268–272. [Google Scholar]
- 11.Formanek J, Mackie R, Blaschek H P. Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl Environ Microbiol. 1997;63:2306–2310. doi: 10.1128/aem.63.6.2306-2310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green E M, Boynton Z L, Harris L M, Rudolph F B, Papoutsakis E T, Bennett G N. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology. 1996;142:2079–2086. doi: 10.1099/13500872-142-8-2079. [DOI] [PubMed] [Google Scholar]
- 13.Oultram J D, Burr I D, Elmore M J, Minton N P. Cloning and sequence analysis of genes encoding phosphotransbutyrylase and butyrate kinase from Clostridium acetobutylicum NCIMB 8052. Gene. 1993;131:107–112. doi: 10.1016/0378-1119(93)90677-u. [DOI] [PubMed] [Google Scholar]
- 14.Rogers P, Palosaari N. Clostridium acetobutylicum mutants that produce butyraldehyde and altered quantities of solvents. Appl Environ Microbiol. 1987;53:2761–2766. doi: 10.1128/aem.53.12.2761-2766.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose I A. Acetate kinase of bacteria (acetokinase) Methods Enzymol. 1955;1:591–593. [Google Scholar]
- 16.Toth, J., and J.-S. Chen. 1997. Unpublished data.
- 17.Toth, J., and J.-S. Chen. 1998. Personal communication.
- 18.Walter K A, Mermelstein L D, Papoutsakis E T. Studies of recombinant Clostridium acetobutylicum with increased dosages of butyrate formation genes. Ann N Y Acad Sci. 1994;721:69–72. doi: 10.1111/j.1749-6632.1994.tb47377.x. [DOI] [PubMed] [Google Scholar]
- 19.Woods D R. The genetic engineering of microbial solvent production. Trends Biotechnol. 1995;13:259–264. doi: 10.1016/S0167-7799(00)88960-X. [DOI] [PubMed] [Google Scholar]