Abstract
Introduction
Over the years, many psychosocial interventions for individual having both a psychotic spectrum disorder and a substance use disorder diagnoses have been developed and studied. However, there is a high dropout rate among this clinical population.
Objectives
This meta-analysis aims to replicate a previous meta-analysis on the effects of psychosocial treatment for dual disorders, while including and determining the dropout rates in those type of interventions.
Method
Based on a Cochrane systematic review conducted in 2019, we conducted a meta-analysis including 40 randomized clinical trials on psychosocial treatment among persons suffering from schizophrenia spectrum disorder and substance use disorder.
Results
A dropout rate of 27,2% was obtained. Stimulants use significantly affected dropout rates. Age, gender, diagnosis, alcohol and cannabis abuse, and duration of treatment did not affect dropout rates.
Conclusion
The 27,2% rate of dropout from psychosocial treatment highlights the need to engage participants having a dual diagnosis from the start by focusing on therapeutic alliance and motivation for treatment.
Keywords: dropout, psychosocial interventions, severe mental illness, psychotic spectrum disorder, substance use disorder, dual diagnosis
Introduction
Severe mental disorders are defined by the nature of the diagnosis, the degree of disability and the duration of the disorder (1). As such, the following diagnoses are considered severe mental disorders: schizophrenia and related disorders, bipolar disorders, and severe depressive disorders (2–6). Approximately 40–60% of individuals with a serious mental disorder also present with a comorbid substance use disorder (7–9). For individuals with schizophrenia, the risk of comorbid alcohol misuse is three times more likely, whereas the risk for drugs misuse is six times more likely (10), when compared to people without a psychiatric disorder. Overall, people with schizophrenia are 5.3 times at greater risk to present with a substance use disorder than the general population (11). In fact, the proportion of individuals with schizophrenia who present with a substance use disorder is significantly higher than what is found in most other clinical or non-clinical populations (7, 9, 12–14).
It is important to note that severe mental disorders come with a variety of challenges (15), and these are exacerbated with substance misuse, namely isolation, anxiety, depression, suicidal thoughts, behavioral and emotional problems (2). Even mild substance abuse is associated with increased risk for suicide, AIDS, hepatitis, assault, incarceration, homelessness, and fewer social and financial resources (4, 16). Furthermore, substance abuse in severe mental disorders interferes with diagnostic and treatment and causes a multitude of difficulties in a clinical population already facing major difficulties (14, 17).
Over the years, many psychosocial interventions have been developed specifically for this dual diagnosis population. These include interventions and programs such as motivational interviewing (MI), cognitive behavioral therapy (CBT), contingency management (CM), psychoeducation, integrated treatments (IT), psychosocial treatment, and assertive community treatment (4, 18–20). However, people with comorbid severe mental disorders and substance misuse have been described as particularly vulnerable to treatment dropout (21). Ensuring treatment adherence is a major issue in psychiatry, as well as in general medical practice (22). Why are individuals with comorbid severe mental and substance use disorders at higher risk of treatment dropout? In their review on the subject, Kreyenbuhl et al. (23) reported that younger age, male gender, lack of insight, a tendency to minimize symptoms and their impact, and low social functioning as well as a low socioeconomic status was linked to drop out rates. Of the most cited reasons for disengaging is the desire to solve problems on their own (23), dissatisfaction with the treatment or the impression that it wouldn't help, feeling that they already had improved, feeling that they were too unwell, and medication and its side-effects. Other reasons mentioned were having forgotten the appointment and a fear of the mental health system due to previous negative experiences (23). Treatment willingness and engagement can also be influenced by the therapeutic alliance with the therapist, perceived accessibility of care and the client's belief that the treatment will help (24). This is even more an issue for individuals with concurrent substance abuse disorder and/or addiction, with high drop-out rates across treatments (21). Dropping out of psychosocial treatments is associated with a number of clinical, social and economic consequences, as well as higher risk of relapse, re-hospitalization and poorer prognosis. A previous meta-analysis from our team (25) on the drop-out rates from psychosocial treatments among individuals with a psychotic disorder indicated that ~13% (of the 4,374 participants) dropped out prior to, or during, the treatment. Similar dropout rates have been found by Bighelli et al. (26). The authors suggest that these results may be an underestimation of the actual dropout rate due to publication bias in favor of studies presenting lower drop-out rates, as well as the exclusion from the meta-analysis of trials involving patients with psychosis and substance use disorders. This meta-analysis of 74 trials also revealed that drop-out rates were influenced by age, gender, duration of illness, duration of treatment and treatment setting.
Studies that have evaluated the efficacy of psychosocial interventions for comorbid substance misuse disorders have often based their results on the final sample of participants who completed the intervention. These results rarely account for the initial sample approached nor for dropout rates during the study. As a result, high drop-out rates can lessen the statistical power and the generalizability of those studies, and therefore reduce the possibility of detecting significant effects. If calculations of treatment outcomes and success rates are solely based on the small proportion of participants who complete the study, the results could only reflect the outcomes of those who have better prognostic factors and might not be representative of the population of individuals with comorbid severe mental disorder and substance misuse. It is therefore possible that the success rates reported do not represent the treatment reality of individuals with comorbid severe mental illness and substance misuse, given that those with worse prognostic factors will have likely dropped out of the treatment or study.
Recently, Hunt et al. (4) conducted a meta-analysis on the efficacy of existing interventions and programs for comorbid presentation of severe mental disorders and substance misuse. They covered many psychosocial interventions and programs and included 41 trials for a total of 4,024 participants. In sum, the review reported a lack of quality evidence to support any one psychosocial treatment/program over standard care, and they encountered methodological difficulties, which hindered pooling and the interpretation of results. The meta-analysis did not, however, measure drop-out rates, preferring to exclude studies when these rates were too high.
The objective for the present review is to determine the drop-out rates in studies on psychosocial interventions for people with comorbid severe mental disorders and substance misuse (4), for both the experimental and control conditions. As a secondary objective, we will examine the influence of population (e.g., age, gender, diagnosis and substances used) and trial characteristics (e.g., duration of treatment, type of intervention) on drop-out rates.
Methods
Eligibility Criteria
This meta-analysis included all RCTs with or without blind randomization which included a comparison between psychosocial intervention aiming at substance abuse reduction and a standard treatment in people with serious mental illness. Quasi-randomized studies were excluded. We opted for RCTs, considering that randomization was our minimal quality criterion, and that our previous meta-analysis on drop-out rates included only RCTs (25). Studies with missing data were excluded. We included participants diagnosed with both a diagnosis of substance misuse and severe mental illness, focusing primarily on psychotic spectrum disorders. Studies that included a vast spectrum of disorders were included only if the majority (e.g., ≥50%) of participants had a diagnosis of severe mental illness. We only included studies published in English or French.
Data Collection and Literature Search
We searched Prospero and the existing literature and no meta-analysis on drop-out rates during psychosocial intervention in dual-diagnosis was found. The current meta-analysis included all the articles from Hunt et al. (4) as well as new articles published since. In their Crochrane review, Hunt et al. (4) proceeded to search electronic databases using (*{PSY}* in Intervention) AND (*Substance Use* in Healthcare Condition) of STUDY in a study-based register that is compiled by systematic searches of majors resources (AMED, BIOSIS, CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their updates. They also searched other resources, such as references lists, journal databases, trials registries, and personal contact. They then proceeded to select the studies by inspecting all citations and identified relevant abstracts, articles, and trials using their inclusion criteria, which have been inspected furthermore to ensure reliability (4). On the 41 articles retained by these authors, 33 were retained in the present article. Of the 8 excluded, 3 were not RCTs, 1 was excluded because of language barrier and 4 were duplicates. Because drop-out rates are the main focus of the present research, we also considered the articles rejected by this Cochrane review and proceeded to recuperate 10 articles that were excluded for high attrition rates by Hunt et al. (4). Two of these 10 articles were excluded because they were not RCTs. We also searched Psychinfo, Embase and PubMed databases using PRISMA criteria for new articles published between 2018 and 2021 using: “Psychotic*” OR “psychos*” OR “schiz*” OR “Severe mental illness” AND “substance use” OR “substance abuse” OR “substance misuse” OR “drug use” OR “Drug abuse” OR “Drug usage” OR “Substance related disorder*” OR “drug addiction” AND “Treatment” OR “intervention” OR “psychosocial” OR “program.” We found 454 articles, and 4 new articles were retained based on title, abstract and full-text reads. In sum, we retained a total of 44 articles for analysis. Five other articles have been excluded during data extraction due to missing data. The Flow chart of the selection of studies is shown in Figure 1. Interventions were divided into four categories: Intervention (including CBT, Skills training, MI and CM), Specialized Integrated Services (Integrated treatments for dual disorders), Integrated services with outreach (e.g., assertive community treatment) and Support interventions (e.g., AA). The characteristics of the studies included in the meta-analysis are described in Table 1.
Figure 1.
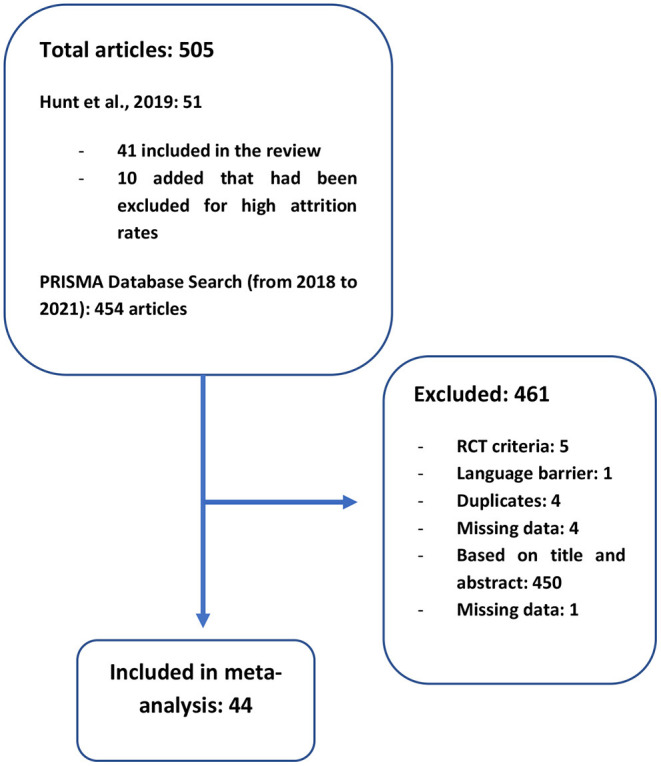
Flow diagram: articles selection process.
Table 1.
Details of included studies.
| Sample characteristics | Intervention details | Study details | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age (interventions) | Mean age (controls) | % males | % psychotic spectrum | % cannabis | % stimulants | % Alcohol | N baseline | Intervention category * | Duration | Comparator | Study quality (/6) | |
| Baker et al. (27) | 31.71 | 30.05 | 75 | 37 | 46.80 | 22.80 | 60.80 | 160 | Int. | One session (30–45 min) | TAU | 4 |
| Baker et al. (28) | 28.83 | 28.83 | 78.20 | 86.60 | 73.10 | 42 | 67.30 | 130 | Int. | 10 sessions (1 per week) | Routine treatment | 4 |
| Barrowclough et al. (29) | 31.1 | 31.1 | 92 | 100 | 61.11 | 66.67 | 61.11 | 36 | Integrated | Over 9 months | TAU | 4 |
| Barrowclough et al. (30) | 37.4 | 38.3 | 86.54 | 100 | 25.08 | NS | 47 | 327 | Integrated | Up to 26 sessions over 12 months | TAU | 6 |
| Bellack et al. (31) | 43.8 | 41.6 | 66.40 | 39.50 | 1.64 | 72.10 | 21.30 | 175 | Integrated | 2 times a week over 6 months | Standard care | 4 |
| Bogenschutz et al. (32) | 42.74 | 41.09 | 52.13 | 18.20 | NS | NS | 100 | 121 | Support Int. | 12 weeks | TAU | 2 |
| Bond et al. (33) | 31.5 | 31.5 | 79 | 70 | NS | NS | 61 | 97 | With outreach | 18 months | TAU | 0 |
| Bonsack et al. (34) | 25 | 25.5 | 87.10 | 100 | 83.70 | NS | NS | 62 | Int. | 4–6 sessions over 6 months | TAU | 2 |
| Burnam et al. (35) | 37 | 37 | 84 | 45 | NS | NS | 73.46 | 276 | Integrated | 3 months | Controls | 2 |
| Cather et al. (36) | 23.2 | 23.1 | 72.50 | 100 | 11.20 | NS | 7.20 | 404 | Integrated | 2 years | Usual care | 6 |
| Chandler and Spicer (37) | 43 | 43 | 71.98 | 54.30 | 11.70 | 30.10 | 31.10 | 182 | Integrated | 2.5 years | TAU | 2 |
| Drake et al. (38) | 32.2 | 32.2 | 76.20 | 100 | 48.10 | 14 | 83 | 130 | With outreach | 3 years | TAU | 4 |
| Eack et al. (39) | 39.68 | 34.67 | 71 | 100 | 73 | NS | 81.80 | 28 | Int. | 18 months | TAU | 4 |
| Edwards et al. (40) | 20.9 | 21.3 | 72.30 | 100 | 48.90 | NS | 2.20 | 47 | Integrated | 3 months weekly sessions | Psychoeducation | 4 |
| Essock et al. (41) | 36.4 | 36.6 | 72 | 76 | NS | NS | 73 | 198 | With outreach | 3 years | Standard care | 2 |
| Gaughran et al. (42) | 43.76 | 44.65 | 57.64 | 100 | NS | NS | NS | 406 | Integrated | 9 months | 6 | |
| Gouzoulis-Mayfrank et al. (43) | 31.14 | 30.8 | 84 | 100 | 72 | 12 | 12 | 100 | Integrated | 18 months | TAU | 2 |
| Graeber et al. (44) | 42.87 | 45 | 96.67 | 100 | 86 | 71 | 100 | 30 | Int. | One session per week over 3–4 weeks | Educational treatment | 2 |
| Graham et al. (45) | 39.5 | 37.69 | 84.75 | 71.19 | 46.70 | 3.30 | 40 | 59 | Int. | 4 to 7 sessions over 2 weeks | TAU | 6 |
| Hellerstein et al. (46) | 31.9 | 31.9 | 76.60 | 100 | 76.60 | 87.20 | 91.50 | 47 | Integrated | 2 session per week over 8 monts | Non-integrated treatment | 2 |
| Herman et al. (47) | 33.2 | 33.2 | 73.90 | 28.10 | 22.70 | 60.20 | 73.40 | 485 | Integrated | 18 months | standard treatment | 2 |
| Hjorthoj et al. (48) | 26.6 | 27.1 | 75.73 | 82.52 | 100 | NS | NS | 103 | Int. | 1–2 sessions per week for the first month. and then one weekly over 6 months | TAU | 6 |
| Jerrell et al. (49) | NS | NS | NS | NS | NS | NS | NS | 98 | Integrated | 12 months | Standard care | 2 |
| Johnson et al. (50) | 24 | 25 | 86.75 | 88.36 | 72 | NS | 77 | 551 | Int. | 12 weeks | TAU | 4 |
| Kavanagh et al. (51) | 22.6 | 22.6 | 60 | 100 | 76 | 24 | 88 | 25 | Int. | 6–9 sessions within 7–10 days. | Standard care | 4 |
| Kemp et al. (52) | 20.6 | 20.8 | 81.25 | 100 | NS | NS | NS | 19 | Int. | 4–6 sessions | TAU | 2 |
| Kikkert et al. (53) | 45.9 | 45.9 | 80.40 | 81.80 | NS | NS | NS | 154 | Integrated | 12 months | TAU | 2 |
| Lehman et al. (54) | 31 | 30 | 74.07 | 68.52 | 50 | 35 | 79 | 54 | Integrated | 12 months | usual community mental health center (CMHC) and psychosocial rehabilitation service | 2 |
| Madigan et al. (55) | 27.6 | 28.2 | 78.41 | 77.27 | 100 | NS | NS | 88 | Int. | Once per week for 12 weeks (3 months) | TAU | 4 |
| Mangrum et al. (56) | 36.5 | 36.6 | 49.07 | 20.93 | NS | NS | NS | 216 | Integrated | 12 months | TAU | 0 |
| Martino et al. (57) | 35.35 | 35.35 | 65 | 51 | 35 | 64 | 82 | 23 | Int. | One session | standard preadmission interview | 2 |
| Martino et al. (58) | 29.71 | 34.1 | 72.70 | 100 | 45.80 | 70.80 | 41.70 | 44 | Integrated | Two sessions | two-session standard psychiatric interview | 2 |
| McDonell et al. (59) | 43.01 | 42.45 | 65.34 | 39.20 | NS | 96 | 47 | 176 | Int. | 3 months | TAU | 2 |
| McDonell et al. (60) | 44.55 | 46.23 | 63.29 | 30.38 | NS | NS | 100 | 79 | Int. | 12 weeks | Noncontingent control group (reinforcers regardless of EtG results and treatment attendance) | 2 |
| Morse et al. (61) | 40 | 40 | 80 | 80 | 19 | NS | 82 | 149 | With outreach | 24 months | Standard care | 2 |
| Mowbray et al. (62) | 33.4 | 33.4 | 74 | 28 | NS | 21.67 | NS | 467 | Integrated | Minimum 28 day stay in the ward | standard inpatient psychiatric treatment | 2 |
| Naeem et al. (63) | 40.47 | 40.47 | 77.01 | 100 | NS | NS | NS | 105 | Int. | 6 sessions over 3 months | Standard care | 4 |
| Nagel et al. (64) | 33.4 & 32.2 | 33 | 57 | 49 | 65 | NS | 63 | 49 | Int. | From 2 to 6 months | Standard care | 2 |
| O'Connell et al. (65) | 37.7 & 36.8 | 30.1 | 66 | 100 | NS | NS | NS | 137 | Int. | 3 months | Standard care | 2 |
| Petry et al. (66) | 41.7 | 41.7 | 58 | 16 | 15.80 | 100 | 36.80 | 19 | Int. | 8 weeks | TAU | 2 |
| Rosenblum et al. (67) | 42 | 44 | 68 | 30 | NS | NS | NS | 349 | Support Int. | 3–6 months | Waiting list control group | 2 |
| Swanson et al. (68) | 32.85 | 34.87 | 63.63 | 44.63 | NS | NS | NS | 93 | Int. | 15 minutes of feedback and a 1 h session | Standard care | 2 |
| Tracy et al. (69) | NS | NS | 50 | NS | NS | NS | NS | 30 | Int. | 4 weeks | Assessment only | 2 |
| Xie et al. (70) | 32.4 | 32.4 | 77.60 | 100 | 45 | 15.20 | 82.70 | 223 | Integrated | 3 years | 4 | |
TAU, treatment as usual; Int., Intervention (e.g. CBT, contingency management, motivational interviewing). Integrated: Specialized integrated services; With outreach, integrated community treatment with outreach (e.g. assertive community treatment); Support Int, support interventions (e.g., 12–steps).
Data Extraction and Quantitative Data Synthesis
For drop-out rates, the number of participants suffering from a severe psychiatric disorder prior to treatment and at the end of treatment, respectively, was extracted from each study. Data on age (average age in terms of years), sex ratio (percentage of males and females), duration of treatment (number of weeks), treatment modality (interventions such MI and/or CBT, specialized integrated services, intervention with outreach, and support intervention), and percentage of patients with alcohol, cannabis and stimulant use disorders were also gathered. Data extraction was verified by two authors of this article. The Comprehensive Meta-Analysis-2 software (71) was used to conduct analyses of effect size, which corresponds to the drop-out rate (e.g., event rate), which represents the loss of participants prior or during treatment among those who agreed to undergo the treatment. Heterogeneity among effect size estimates was assessed with the Q statistics (72), with magnitude of heterogeneity being evaluated with the I2 index (73). As the database was characterized by high heterogeneity (see below), we aggregated event rates across studies using random-effects models, which are more conservative than fixed-effect models, and seem to better address heterogeneity between studies and study populations (74). The possibility of publication bias was examined with Egger's test and visual inspection of funnel plot (75). Sub-analyses were conducted on treatment modality (e.g., intervention, specialized integrated services, integrated services with outreach and support interventions). Meta-regression analyses were used to examine the effects on drop-out rates of continuous variables, namely age, sex ratio, percentage of psychotic patients, duration of treatment, study quality and percentage of specific SUDs (e.g., alcohol, cannabis and stimulants). Finally, using event rates as the effect size, we calculated consent rates, which represent the number of patients who consented to participate in the study relative to those who were approached by the research team.
Data Analysis
Hunt et al. (4) appraised study quality, and evidence was rated as low or very low quality. They report a high or unclear risk of bias because of poor or inadequately reported trial methods, imprecision due to sample sizes, low event rates and wide confidence intervals. We also assessed study quality for the RTCs that were retained for the present article, using Jadad criteria (76). Random allocation, allocation concealment and blindness were the three criteria used, and we adapted the scale for the present research by excluding poor ratings for withdrawals and drop-outs because it was what interested us for the present study. To ensure validity, we conducted two quality evaluation by two researchers to validate and verify Jadad scores. Studies were of low to moderate quality, primarily because of missing data and absence of allocation concealment. Blindness was also not reported or described in a large proportion of the studies included. Study quality for each trial, as determined using Jadad criteria, is detailed in Table 1.
Results
Drop-Out Rates
In the 42 treatment arms, the composite drop-out rate was 27.2% (CI, 95%: 21.0–34.3%) (Table 2). In the case of treatment-as-usual (TAU), the aggregation of 32 studies produced a composite drop-out rate of 20.5% (CI, 95%: 14.2–28.6%) (Table 2). As illustrated in Figure 2, a publication bias was present (Kendall's Tau = −0.309; p = 0.004; Egger's test: t = 3.197; p = 0.003). For both experimental treatment and TAU, results across treatment arms were characterized by very high levels of heterogeneity (I2 = 90% and 90.1%, respectively) (Table 2).
Table 2.
Primary and secondary analyses: drop-out rates across interventions.
| Analysis | Number of treatment arms | Rate (%) | p -value | Confidence interval | Heterogeneity |
|---|---|---|---|---|---|
| Main analysis | |||||
| Experimental treatment | 42 | 27.2 | 0.0001 | (21.0–34.3) | Q = 409.3; p = 0.0001; I2 = 90% |
| TAU | 32 | 20.5 | 0.0001 | (14.2–28.6) | Q = 312.5; p = 0.0001; I2 = 90.1% |
| Sub-analyses (for experimental treatment arms only) | |||||
| Intervention * | 20 | 28.7 | 0.0001 | (19.5–40.2) | Q = 79.2; p = 0.0001; I2 = 76% |
| Specialized Integrated Service | 16 | 27.3 | 0.001 | (17.3–40.3) | Q = 269.7; p = 0.0001; I2 = 94.4% |
| Outreach | 4 | 11.1 | 0.002 | (3.3–31.5) | Q = 14.5; p = 0.002; I2 = 79.4% |
| Support therapy | 2 | 28.3 | 0.209 | (8.4–62.8) | Q = 30.6; p = 0.0001; I2 = 96.7% |
TAU, treatment-as-usual;
Intervention, motivational interviewing and/or cognitive behavioral therapy.
Figure 2.
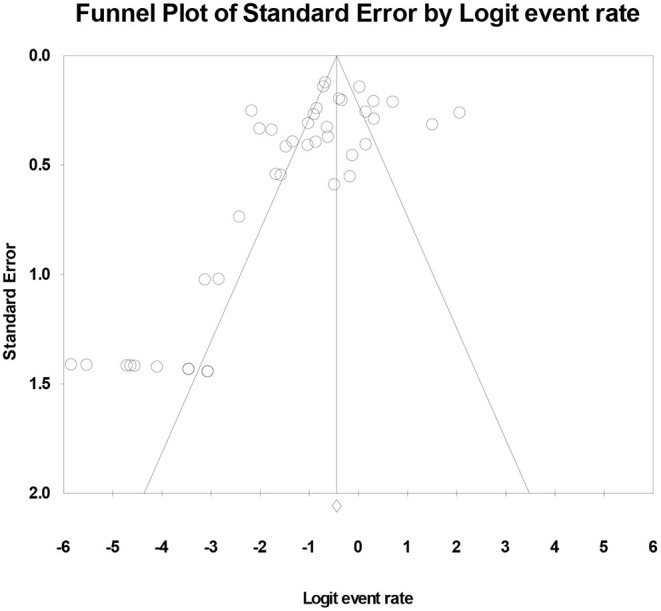
Publication bias for the composite analysis on experimental treatments.
Secondary Analyses
A sub-analysis on treatment modality showed that drop-out rates were fairly similar across interventions (28.7%; 20 treatment arms), specialized integrated services (27.3%; 16 treatment arms) and support therapies (28.3%; 2 treatment arms), but that drop-out rates were lower in trials on interventions with outreach (11.1%; 4 treatment arms) (Table 2). Within each treatment modality, results were characterized by high levels of heterogeneity (between 76 and 96.7%).
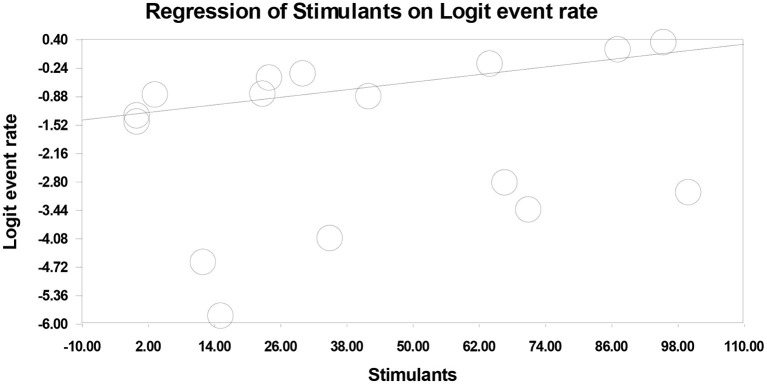
Meta-regression analyses on the experimental treatment arms showed a positive association between stimulant use disorder (StUD) and drop-out rates [16 experimental treatment arms; slope (β) = 0.014; p = 0.0001] (Table 3; Figure 3). That is, the highest drop-out rates were observed in trials including the highest proportion of patients with as StUD. Conversely, age (p = 0.530), sex ratio (p = 0.561), percentage of psychotic patients (p = 0.119), duration of treatment (p = 0.129), study quality (p = 0.967), percentage of patients with alcohol use disorder (p = 0.464) and percentage of patients with cannabis use disorder (p = 0.091) had no significant influence on drop-out rates across trials (Table 3).
Table 3.
Predictors of drop-out rates for experimental treatments.
| Predictor | Number of experimental treatment arms | Slope |
|---|---|---|
| Age | 39 | β = 0.021; p = 0.530 |
| Sex ratio | 41 | β = 0.012; p = 0.561 |
| Duration of treatment (in weeks) | 40 | β = −0.010; p = 0.129 |
| % of patients with psychosis | 40 | β = −0.013; p = 0.119 |
| % of patients with alcohol use disorder | 30 | β = 0.008; p = 0.464 |
| % of patients with cannabis use disorder | 27 | β = 0.015; p = 0.091 |
| % of patients with stimulant use disorder | 16 | β = 0.014; p = 0.0001 |
| Study quality | 42 | β = −0.006; p = 0.967 |
Figure 3.
The influence of percentage of patients with stimulant use disorder on drop-out rates across experimental treatments.
Consent Rates
In the 29 studies offering this information, we found that the composite consent rate was 44.4% (CI, 95%: 0.365–0.526; p = 0.178). Across studies, results were highly heterogeneous (Q = 2,088.2; p = 0.0001; I2 = 98.7%).
Discussion
The objectives of the present research were to determine dropout rates in studies on psychosocial interventions for people with a dual diagnosis of severe mental illness and substance abuse. We also wanted to examine the influence of population (e.g., age, gender, diagnosis and substances used) and trial characteristics (e.g., duration of treatment, type of intervention) on drop-out rates.
The dropout rate of 27.2% for the experimental arm and of 20.5% for TAU suggest that, on average, close to one third of participants in treatment studies never complete the treatment. Furthermore, the publication bias found suggest that studies under report their drop-out rates, which brings us to suppose that the actual drop-out rates might be even higher. Dropout rates results had high heterogeneity (I2 ranging from 76 to 96,7%). This variability might be explained by publication bias, high differences in outcomes measured in studies, and differing ways in which psychosocial interventions were delivered.
Villeneuve et al. (25) found a dropout rate of 13% in their meta-analysis on dropout from psychosocial treatment among individuals with schizophrenia spectrum disorder, which is less than half of what we found in our experimental arm. One of the major differences between their study and ours is that we included persons with both schizophrenia spectrum disorder and substance use disorder. Differences in dropout rates could partly be explained by stimulants use, since the dropout rates appeared worse in those with stimulant use. This difference in results could also possibly be explained by higher severity of symptoms, impulsivity, and lower motivation in our clinical population, although these were not specifically analyzed here. Individuals with a dual diagnosis of psychotic spectrum disorder and substance use disorder in general present with higher symptom severity and more relapses, as well as more deficits in executive functions like planning and thinking before acting, more impulsivity and less motivation in general (77, 78). Another factor that could explain the drop-out rates result is a poor therapeutic alliance (23, 24), and demanding requirements for certain interventions (for example, many interventions required participants to come to clinics regularly, in person) even though motivation is often an issue with this population.
Research on dual diagnoses focus on developing and replicating studies on specialized treatments to demonstrate their efficacity. However, these different interventions and treatment programs present few similarities and vary greatly on the type of interventions they include, the objectives, and the symptoms targeted (79). This brings us to wonder if the research focus should shift away from a focus on the efficacy of specialized treatments, since there is a high dropout rate form experimental treatments and, therefore, the results might only represent the small portion that accept to participate and complete the treatment (25).
Our results also showed a lower drop-out rate, of 11,1%, for intensive programs like assertive community treatments. Although this rate was based on the aggregation of only 4 trials, it is noteworthy that this rate was half of the rate found in more specific treatments in this review. Such programs focus on engaging the participant, are long-term, and do not specifically aim on obtaining results regarding substance misuse (80, 81). In their meta-analysis, Hunt et al. (4) found no difference between treatments in terms of improved outcomes in terms of lost to treatment, death, alcohol or substance used, global functioning and general satisfaction, suggesting that intensive treatment programs like assertive community treatments did not fare better or worse than the other treatments or services analyzed. Most studies search for gains in terms of outcomes (decreased substance use, improved symptoms and global functioning for example), yet, with complex clinical populations such as people with comorbid substance misuse and psychotic disorders, the evolution in terms of clinical outcomes can be slow, suggesting a need for long-term treatments that focus on engaging the person and developing a strong therapeutic alliance. It is also important to consider the complexity of this clinical population. There is often history of abuse and trauma (82, 83), emotional self-regulation issues (84), frequent comorbidity with personality disorders (85), and with anxiety disorders (86), and important cognitive and functional deficits. There is also medication to consider, which can lead to a multitude of side effects depending on dose and type of medication, that can interact with substance misuse. These issues can be a challenge to work with since there are many parameters to account for, and can make it difficult to develop a strong and good therapeutic alliance both from the client and the clinician's perspective (87–89). Interventions should perhaps focus more on engaging participants by developing a strong therapeutic alliance, with the hope of eventually motivating them in working on their substance misuse problem. As discussed, many reasons for dropout reported by participants in studies were related to engagement with the therapist or team (23, 24). This suggests that a more engaging approach to treatment, with outreach such as in assertive community treatment teams, might be more successful in the long run in keeping clients into treatment. Having intensive treatment teams trained to work with psychotic spectrum and substance use disorders (and more specifically in stimulant use disorders) while targeting the development of a good alliance with the participant appears promising to prevent dropout rates.
Although we did not find a significant association between study quality and drop-out rates, it must be pointed that studies included in the current meta-analysis were in vast majority of low to moderate quality. This was mostly due to the absence of allocation concealment most trials and due to the fact that blindness was also not reported or described in most cases. At face value, this may seem to be a limitation, as there were few high quality to analyze. However, one may argue that the access to low / moderate quality trials may be better suited for the assessment of drop-out rates. This can be explained by the fact that higher quality studies often have more resources at their disposition to conduct their research, and thus are more able to invest in research teams and labs that can either follow the participants more closely or pay them more for their participation. Thus, higher the quality trials may, in theory, be more biased in the assessment of dropouts. On the other hand, low quality studies might in fact be more accurate in assessing the clinical reality of offering interventions to people with a severe mental illness and concurrent substance abuse. As such, having more low-quality studies in the context of this research topic is advised as these are perhaps more ecologically valid than high quality studies. In the future, it will be important to collect, in a systematic manner, data on drop-out rates during psychosocial interventions delivered in non-randomized trials. Although, as described by Hunt et al. (4), treatment outcomes are not impressive with this population, future studies should also investigate how drop-out rates affect actual treatment effect sizes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
MB conducted the review and wrote the article. TL co-supervised MB, edited the article. BC conducted the interrater agreements. JH-R formatted the article, references and helped with the data search, and SP co-supervised MB and conducted the analyses. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
SP is holder of the Eli lilly Canada Chair on schizophrenia research.
References
- 1.Lesage A. ≪ The adult severely mentally ill ≫: 144-169, dans J. Cairney et D L Streiner (sous la dir), Mental disorder in Canada An epidemiological perspective. Toronto: University of Toronto Press. (2010). [Google Scholar]
- 2.Friedman MB, Nestadt PS, Furst L, Williams KA. Meeting the mental health challenges of the elder of the boom. In: Rosenberg J, Rosenberg S, editors. Community Mental Health. Challenges for the 21st Century. New York, NY: Routledge; (2013). p. 109–32. [Google Scholar]
- 3.Hunt GE, Large M, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings: systematic review and meta-analysis. Drug Alcohol Depend. (2018) 191:234–58. 10.1016/j.drugalcdep.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Hunt GE, Siegfried N, Morley K, Brooke-Sumner C, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev. (2019) 12:CD001088. 10.1002/14651858.CD001088.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt GE, Malhi GS, Cleary M, Lai HMX, Sitharthan T. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990–2015: systematic review and meta-analysis. J Affect Disord. (2016) 206:331–49. 10.1016/j.jad.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 6.Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990-2014: a systematic review and meta-analysis. Drug Alcohol Depend. (2015) 154:1–13. 10.1016/j.drugalcdep.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 7.Batel P. Addiction and schizophrenia. Eur Psychiatry. (2000) 15:115–22. 10.1016/S0924-9338(00)00203-0 [DOI] [PubMed] [Google Scholar]
- 8.Drake RE, Mueser KT. Psychosocial approaches to dual diagnosis. Schizophr Bull. (2000) 26:105–18. 10.1093/oxfordjournals.schbul.a033429 [DOI] [PubMed] [Google Scholar]
- 9.Brady A, McCallum AE, Glick SD, O'Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. (2008) 200:205–15. 10.1007/s00213-008-1195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poullot P. Trouble Mentaux Graves, Toxicomanie et Violence: Étude Qualitative Du Point De Vue Des Personnes Contrevenantes (Doctoral thesis). Université de Montréal, Montreal, QC, Canada: (2011). Available online at: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/5058 [Google Scholar]
- 11.Sayers SL, Campbell C, Kondrick J, Mann SC, Cornish J, O'Brien C, et al. Cocaine abuse in schizophrenic patients treated with olanzapine versus haloperidol. J Nerv Ment Dis. (2005) 193:379–86. 10.1097/01.nmd.0000165089.14736.bf [DOI] [PubMed] [Google Scholar]
- 12.Helzer JD, Pryzbeck TR. The co-occurence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol Drugs. (1988) 49:219–24. 10.15288/jsa.1988.49.219 [DOI] [PubMed] [Google Scholar]
- 13.Leduc P, Mittleman G. Schizophrenia and psychostimulant abuse: a review and re-analysis of clinical evidence. Psychopharmacology. (1995) 121:407–27. 10.1007/BF02246489 [DOI] [PubMed] [Google Scholar]
- 14.Mueser KT, Bellack AS, Blanchard JJ. Comorbidity of schizophrenia and substance abuse: implications for treatment. J Consult Clin Psychol. (1996) 60:845–56. 10.1037/0022-006X.60.6.845 [DOI] [PubMed] [Google Scholar]
- 15.Tremblay C. Les interventions psychosociales destinées aux familles dont un membre présente un trouble mental grave: un état de la question. Intervention. (2019) 149:33–48. [Google Scholar]
- 16.Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. (2006) 67:5–9. 10.4088/JCP.0706e01 [DOI] [PubMed] [Google Scholar]
- 17.Mueser KT, Deavers F, Penn DL, Cassisi JE. Psychosocial treatments for schizophrenia. Annu Rev Clin Psychol. (2013) 9:465–97. 10.1146/annurev-clinpsy-050212-185620 [DOI] [PubMed] [Google Scholar]
- 18.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW, et al. meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. (2008) 165:179–87. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- 19.Miller WR, Rollnick S. Applications of Motivational Interviewing. 3rd ed. New York, NY: The Guildford Press; (2013). [Google Scholar]
- 20.Rector NA. Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Mental Dis. (2012) 200:832–9. 10.1097/NMD.0b013e31826dd9af [DOI] [PubMed] [Google Scholar]
- 21.Reif S, Stewart MT, Torres ME, Davis MT, Dana BM, Ritter GA. Effectivenesss of value-based purchasing for substance use treatment engagement and retention. J Subst Abuse Treat. (2021) 122:108217. 10.1016/j.jsat.2020.108217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centorrino F, Hernan MA, Drago-Ferrante G, Rendall M, Apicella A, Langar G, et al. Factors associated with noncompliance with psychiatric outpatient visits. Psychiatr Serv. (2001) 52:378–80. 10.1176/appi.ps.52.3.378 [DOI] [PubMed] [Google Scholar]
- 23.Kreyenbuhl J, Nossel IL, Dixon LB. Disengagement from mental health treatment among individuals with schizophrenia and strategies for facilitating connections to care: a review of literature. Schizophr Bull. (2009) 35:696–703. 10.1093/schbul/sbp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. (2016) 15:13–20. 10.1002/wps.20306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villeneuve K, Potvin S, Lesage A, Nicole L. Meta-analysis of rates of drop-out from psychosocial treatment among persons with schizophrenia spectrum disorder. Schizophr Res. (2010) 121:266–70. 10.1016/j.schres.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. (2018) 17:316–29. 10.1002/wps.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker A, Lewin T, Reichler H, Clancy R, Carr V, Garrett R, et al. Evaluation of a motivational interview for substance use within psychiatric in-patient services. Addiction. (2002) 97:1329–37. 10.1046/j.1360-0443.2002.00178.x [DOI] [PubMed] [Google Scholar]
- 28.Baker A, Bucci S, Lewin TJ, Kay-Lambkin F, Constable PM, Carr VJ. Cognitive-behavioural therapy for substance use disorders in people with psychotic disorders: randomised controlled trial. Br J Psychiatry. (2006) 188:439–48. 10.1192/bjp.188.5.439 [DOI] [PubMed] [Google Scholar]
- 29.Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O'Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. Am J Psychiatry. (2001) 158:1706–13. 10.1176/appi.ajp.158.10.1706 [DOI] [PubMed] [Google Scholar]
- 30.Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomized controlled trial. BMJ. (2010) 341:c6325. 10.1136/bmj.c6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry. (2006) 63:426–32. 10.1001/archpsyc.63.4.426 [DOI] [PubMed] [Google Scholar]
- 32.Bogenschutz MP, Rice SL, Tonigan JS, Vogel HS, Nowinski J, Hume D, et al. (2014). 12-step facilitation for the dually diagnosed: A randomized clinical trial. J Subst Abuse Treat. 46:403–411. 10.1016/j.jsat.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond GR, McDonel EC, Miller LD, Pensec M. Assertive community treatment and reference groups: An evaluation of their effectiveness for young adults with serious mental illness and substance abuse problems. Psychosoc Rehabil J. (1991) 15:31–43. 10.1037/h0095785 [DOI] [Google Scholar]
- 34.Bonsack C, Manetti SG, Favrod J, Montagrin Y, Besson J, Bovet P, et al. Motivational intervention to reduce cannabis use in young people with psychosis: a randomized controlled trial. Psychother Psychosom. (2011) 80:287–97. 10.1159/000323466 [DOI] [PubMed] [Google Scholar]
- 35.Burnam MA, Morton SC, McGlynn EA, Petersen LP, Stecher BM, Hayes C, et al. An experimental evaluation of residential and nonresidential treatment for dually diagnosed homeless adults. J Addict Dis. (1996) 14:111–34. 10.1300/J069v14n04_07 [DOI] [PubMed] [Google Scholar]
- 36.Cather C, Brunette MF, Mueser KT, Babbin SF, Rosenheck R, Correll CU, et al. Impact of comprehensive treatment for first episode psychosis on substance use outcomes: a randomized controlled trial. Psychiatry Res. (2018) 268:303–11. 10.1016/j.psychres.2018.06.055 [DOI] [PubMed] [Google Scholar]
- 37.Chandler DW, Spicer G. Integrated treatment for jail recidivists with co-occurring psychiatric and substance use disorders. Commun Ment Health J. (2006) 42:405–25. 10.1007/s10597-006-9055-6 [DOI] [PubMed] [Google Scholar]
- 38.Drake RE, McHugo GJ, Xie H, Fox M, Packard J, Helmstetter B. Ten-year recovery outcomes for clients with co-occurring schizophrenia and substance use disorders. Schizophrenia Bull. (2006) 32:464–73. 10.1093/schbul/sbj064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eack SM, Hogarty SS, Greenwald DP, Litschge MY, McKnight SA, Bangalore SS, et al. Cognitive enhancement therapy in substance misusing schizophrenia: results of an 18-month feasibility trial. Schizophrenia Res. (2015) 161:478–83. 10.1016/j.schres.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, et al. Randomized controlled trial of a cannabis?focused intervention for young people with first-episode psychosis. Acta Psychiatrica Scandinavica. (2006) 114:109–17. 10.1111/j.1600-0447.2006.00783.x [DOI] [PubMed] [Google Scholar]
- 41.Essock SM, Mueser KT, Drake RE, Covell NH, McHugo GJ, Frisman LK, et al. Comparison of ACT and standard case management for delivering integrated treatment for co-occurring disorders. Psychiat Serv. (2006) 57:185–96. 10.1176/appi.ps.57.2.185 [DOI] [PubMed] [Google Scholar]
- 42.Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiat. (2017) 17:1–14. 10.1186/s12888-017-1571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gouzoulis-Mayfrank E, König S, Koebke S, Schnell T, Schmitz-Buhl M, Daumann J. Trans-sector integrated treatment in psychosis and addiction: A randomized controlled study of a motivational, cognitive behavioral therapy program under standard hospital treatment conditions. Deutsches Ärzteblatt Int. (2015) 112:683. 10.3238/arztebl.2015.0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graeber DA, Moyers TB, Griffith G, Guajardo E, Tonigan S. A pilot study comparing motivational interviewing and an educational intervention in patients with schizophrenia and alcohol use disorders. Commun Ment Health J. (2003) 39:189–202. 10.1023/A:1023371705506 [DOI] [PubMed] [Google Scholar]
- 45.Graham HL, Copello A, Griffith E, Freemantle N, McCrone P, Clarke L, et al. Pilot randomised trial of a brief intervention for comorbid substance misuse in psychiatric in?patient settings. Acta Psychiatrica Scandinavica. (2016) 133:298–309. 10.1111/acps.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellerstein DJ, Rosenthal RN, Miner CR. A prospective study of integrated outpatient treatment for substance-abusing schizophrenic patients. Am J Addict. (1995) 4:33–42. 10.3109/10550499508997421 [DOI] [Google Scholar]
- 47.Herman SE, Frank KA, Mowbray CT, Ribisl KM, Davidson WS. Longitudinal effects of integrated treatment on alcohol use for persons with serious mental illness and substance use disorders. J Behav Health Serv Res. (2000) 27:286–302. 10.1007/BF02291740 [DOI] [PubMed] [Google Scholar]
- 48.Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol Med. (2013) 43:1499–510. 10.1017/S0033291712002255 [DOI] [PubMed] [Google Scholar]
- 49.Jerrell JM, Wilson JL, Hiller DC. Issues and outcomes in integrated treatment programs for dual disorders. J Behav Health Serv Res. (2000) 27:303–13. 10.1007/BF02291741 [DOI] [PubMed] [Google Scholar]
- 50.Johnson S, Rains LS, Marwaha S, Strang J, Craig T, Weaver T, et al. A contingency management intervention to reduce cannabis use and time to relapse in early psychosis: the CIRCLE RCT. Health Technol Access. (2019) 23:1–108. 10.3310/hta23450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavanagh DJ, Young R, White A, Saunders JB, Wallis J, Shockley N, et al. A brief motivational intervention for substance misuse in recent?onset psychosis. Drug Alcohol Rev. (2004) 23:151–5. 10.1080/09595230410001704127 [DOI] [PubMed] [Google Scholar]
- 52.Kemp R, Harris A, Vurel E, Sitharthan T. Stop using stuff: trial of a drug and alcohol intervention for young people with comorbid mental illness and drug and alcohol problems. Aust Psychiatry. (2007) 15:490–3. 10.1080/10398560701439665 [DOI] [PubMed] [Google Scholar]
- 53.Kikkert M, Goudriaan A, De Waal M, Peen J, Dekker J. Effectiveness of Integrated Dual Diagnosis Treatment (IDDT) in severe mental illness outpatients with a co-occurring substance use disorder. J Subst Abuse Treat. (2018) 95:35–42. 10.1016/j.jsat.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 54.Lehman AF, Herron JD, Schwartz RP, Myers CP. Rehabilitation for adults with severe mental illness and substance use disorders: A clinical trial. J Nerv Ment Dis. (1993) 181:86–90. 10.1097/00005053-199302000-00003 [DOI] [PubMed] [Google Scholar]
- 55.Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O'Connor JJ, et al. A multi-center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophrenia Res. (2013) 143:138–42. 10.1016/j.schres.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 56.Mangrum LF, Spence RT, Lopez M. Integrated versus parallel treatment of co-occurring psychiatric and substance use disorders. J Subst Abuse Treat. (2006) 30:79–84. 10.1016/j.jsat.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 57.Martino S, Carroll KM, O'Malley SS, Rounsaville BJ. Motivational interviewing with psychiatrically ill substance abusing patients. Am J Addict. (2000) 9:88–91. 10.1080/10550490050172263 [DOI] [PubMed] [Google Scholar]
- 58.Martino S, Carroll KM, Nich C, Rounsaville BJ. A randomized controlled pilot study of motivational interviewing for patients with psychotic and drug use disorders. Addiction. (2006) 101:1479–92. 10.1111/j.1360-0443.2006.01554.x [DOI] [PubMed] [Google Scholar]
- 59.McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiatry. (2013) 170:94–101. 10.1176/appi.ajp.2012.11121831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonell MG, Leickly E, McPherson S, Skalisky J, Srebnik D, Angelo F, et al. A randomized controlled trial of ethyl glucuronide-based contingency management for outpatients with co-occurring alcohol use disorders and serious mental illness. Am J Psychiatry. (2017) 174:370–7. 10.1176/appi.ajp.2016.16050627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morse GA, Calsyn RJ, Klinkenberg WD, Helminiak TW, Wolff N, Drake RE, et al. Treating homeless clients with severe mental illness and substance use disorders: costs and outcomes. Commun Ment Health J. (2006) 42:377–404. 10.1007/s10597-006-9050-y [DOI] [PubMed] [Google Scholar]
- 62.Mowbray CT, Jordan LC, Ribisl KM, Kewalramani A, Luke D, Herman S, et al. Analysis of postdischarge change in a dual diagnosis population. Health Soc Work. (1999) 24:91–101. 10.1093/hsw/24.2.91 [DOI] [PubMed] [Google Scholar]
- 63.Naeem F, Kingdon D, Turkington D. Cognitive behaviour therapy for schizophrenia in patients with mild to moderate substance misuse problems. Cogn Behav Ther. (2005) 34:207–15. 10.1080/16506070510010684 [DOI] [PubMed] [Google Scholar]
- 64.Nagel T, Robinson G, Condon J, Trauer T. Approach to treatment of mental illness and substance dependence in remote Indigenous communities: results of a mixed methods study. Aust J Rural Health. (2009) 17:174–82. 10.1111/j.1440-1584.2009.01060.x [DOI] [PubMed] [Google Scholar]
- 65.O'Connell MJ, Flanagan EH, Delphin-Rittmon ME, Davidson L. Enhancing outcomes for persons with co-occurring disorders through skills training and peer recovery support. J Ment Health. (2020) 29:6–11. 10.1080/09638237.2017.1294733 [DOI] [PubMed] [Google Scholar]
- 66.Petry NM, Alessi SM, Rash CJ. A randomized study of contingency management in cocaine-dependent patients with severe and persistent mental health disorders. Drug Alcohol Depent. (2013) 130:234–7. 10.1016/j.drugalcdep.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenblum A, Matusow H, Fong C, Vogel H, Uttaro T, Moore TL, et al. Efficacy of dual focus mutual aid for persons with mental illness and substance misuse. Drug Alcohol Depent. (2014) 135:78–87. 10.1016/j.drugalcdep.2013.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. J Nerv Ment Dis. (1999) 187:630–5. [DOI] [PubMed] [Google Scholar]
- 69.Tracy K, Babuscio T, Nich C, Kiluk B, Carroll KM, Petry NM, et al. Contingency management to reduce substance use in individuals who are homeless with co-occurring psychiatric disorders. Am J Drug Alcohol Abuse. (2007) 33:253–8. 10.1080/00952990601174931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie H, McHugo GJ, Helmstetter BS, Drake RE. Three-year recovery outcomes for long-term patients with co-occurring schizophrenic and substance use disorders. Schizophrenia Res. (2005) 75:337–48. 10.1016/j.schres.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 71.Borenstein M, Rothstein H. Comprehensive Meta-Analysis: A Computer Program for Research Synthesis. Englewood Cliffs, NJ: Biostat; (1999). [Google Scholar]
- 72.Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. (2010) 303:1961–9. 10.1001/jama.2010.605 [DOI] [PubMed] [Google Scholar]
- 73.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications Inc. (2001). [Google Scholar]
- 74.Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-Analysis. New York, NY: Russell Sage Foundation; (2009). [Google Scholar]
- 75.Egger M, Smith GD, Philips AN. Meta-analysis: principles and procedures. BMJ. (1997) 315:1533–7. 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. (2009) 4:79–88. 10.2174/157488709788186021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibtion in schizophrenia. Psychol Med. (2010) 21:1–9. 10.1017/S0033291710000796 [DOI] [PubMed] [Google Scholar]
- 78.Ulhmann A, Fouche J, Lederer K, Meintjes EM, Wilson D, Stein DJ. White Matter Microstructure and impulsivity in methamphetamine dependence with and without a history of psychosis. Hum Brain Mapp. (2016) 37:2055–67. 10.1002/hbm.23159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Witte NAJ, Crunelle CL, Sabbe B, Moggi F, Dom G. Treatment for outpatients with comorbid schizophrenia and substance use disorders: a review. Eur Addict Res. (2013) 20:105–14. 10.1159/000355267 [DOI] [PubMed] [Google Scholar]
- 80.Drake RE, McHugo GJ, Clark RE, Teague GB, Xie H, Miles K, et al. Assertive community treatment for patients wth co-occuring severe mental illness and substance use disorder: a clinical rial. Am J Orthopsychiatry. (1998) 68:201–15. 10.1037/h0080330 [DOI] [PubMed] [Google Scholar]
- 81.Penzenstadler L, Khazaal Y, Fleury MJ. Editorial: community-based outreach treatment for addictions and concomitant disorders: time for a change of paradigm. Front Psychiatry. (2020) 11:e2. 10.3389/fpsyt.2020.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lysaker PH, LaRocco VA. The prevalence and correlates of trauma-related symptoms in schizophrenia spectrum disorder. Compr Psychiatry. (2008) 49:330–4. 10.1016/j.comppsych.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 83.Morkved N, Winje D, Dovran A, Arefjord K, Johnsen E, Kroken RA, et al. Childhood trauma in schizophrenia spectrum disorders as compared to substance abuse disorders. Psychiatry Res. (2018) 261:481–7. 10.1016/j.psychres.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 84.Uhlmann A, Fouche JP, Koen N, Meintjes EM, Wilson D, Stein DJ. Fronto-temporal alterations and affect regulation in methamphetamine dependence with and without a history of psychosis. Psychiatry Res Neuroimaging. (2016) 248:30–8. 10.1016/j.pscychresns.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 85.Volavka J. Comorbid personality disorders and violent behavior in psychotic patients. Psychiatric Q. (2014) 85:65–78. 10.1007/s11126-013-9273-3 [DOI] [PubMed] [Google Scholar]
- 86.Achim AM, Ouellet R, Lavoie MA, Vallieres C, Jackson PL, Roy MA. Impact of social anxiety on social cognition and functioning in patients with recent-onset schizophrenia spectrum disorders. Schizophr Res. (2013) 145:75–81. 10.1016/j.schres.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 87.Andrews M, Baker AL, Halpin SA, Lewin TJ, Richmond R, Kay-Lambkin FJ, et al. Early therapeutic alliance, treatment retention, and 12-month outcomes in a healthy lifestyle intervention for people with psychotic disorders. J Nerv Ment Dis. (2016) 204:894–902. 10.1097/NMD.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 88.Avery J, Zerbo E, Ross S. Improving psychiatrists' attitudes towards individuals with psychotic disorders and co-occurring substance use disorders. Acad Psychiatry. (2015) 40:520–2. 10.1007/s40596-015-0361-6 [DOI] [PubMed] [Google Scholar]
- 89.Wolfe S, Kay-Lambkin F, Bowman J, Childs S. To enforce or engage: the relationship between coercion, treatment motivation and therapeutic alliance within community-based drug and alcohol clients. Addict Behav. (2013) 38:2187–95. 10.1016/j.addbeh.2013.01.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.