Abstract
Purpose
Hyaluronate gel has been injected as a spacer into the rectovaginal fossa and vesicouterine fossa during brachytherapy for patients with cervical cancer at our institution. The effect of hyaluronate gel injection (HGI) on dose-volume parameters was investigated in this study.
Methods and Materials
Between July 2008 to January 2020, a total of 104 patients (non-HGI group: 52 patients; HGI group: 52 patients) who underwent curative radiation therapy for cervical cancer were selected. The total doses of external beam radiation therapy and brachytherapy for high-risk clinical target volume (CTVHR) D90, bladder D2cc, and rectal D2cc were converted to the equivalent dose in 2 Gy fractions (EQD2) and were analyzed for association with HGI.
Results
Median CTVHR D90 (EQD2) in the non-HGI group was 76.0 Gy (63.7-99.5 Gy), and in the HGI group it was 79.4 Gy (52.6-97.5 Gy) (P = .017). The median bladder D2cc and rectal D2cc (EQD2) were 62.9 Gy and 56.0 Gy in the non-HGI group and 63.7 Gy and 54.8 Gy in the HGI group, which had no significant difference.
Conclusions
In cases with HGI, a significant CTVHR D90 dose increase was obtained with sufficient bladder and rectal doses suppression.
Introduction
Brachytherapy (BT) plays an essential role in radiation therapy for uterine cervical cancer, delivering a substantial dose to the tumor. It is important to reduce the dose of organs at risk (OARs) in the rectum and bladder while ensuring the dose of the high-risk clinical target volume (CTVHR). The transition from 2-dimensional BT to 3-dimensional BT allowed the improvement of high dose delivery to the clinical target volume (CTV) while reducing radiation doses to OARs.1
Groupe Européen de Curiethérapie–European Society for Radiotherapy and Oncology (GEC-ESTRO) guidelines recommend at least 85 Gy in the equivalent dose in 2 Gy fractions (EQD2) for CTVHR D90, and several studies showed the improvement of local control.2, 3, 4
However, even with image-guided brachytherapy (IGBT), sometimes it is challenging to deliver CTVHR D90 >85 Gy while maintaining dose constraints for organs at risk in patients with large asymmetrical tumors or with little fat tissue around the tumor.
Since 2013, we have been injecting hyaluronic acid gel as a spacer into the rectovaginal fossa and vesicouterine fossa before every brachytherapy under the transrectal ultrasound (TRUS) guidance. In the BT treatment of multiple tumor sites, hyaluronic acid gel injection (HGI) has been proven to safely enhance the distance between OARs and target volumes, resulting in considerable OARs dose reduction.5,6 Moreover, a phase 3 clinical trial found that SpaceOAR (Boston Scientific Corporation, Marlborough, MA), the polymerized type of polyethylene-glycol hydrogel, effectively reduced the rectal dose in patients with prostate cancer.7
Our research has previously reported on the efficacy of HGI in dose reduction for rectum and bladder and the incidence of rectal bleeding.8, 9, 10 However, the association between HGI and CTV has not yet been reported. This study aims to report the effect of HGI on dose-volume parameters, especially on CTV, for patients with cervical cancer. We performed propensity score matching to select 2 groups—a non-HGI group and an HGI group—for additional analysis.
Methods and Materials
A total of 163 patients who underwent curative radiation therapy for cervical cancer from July 2008 to January 2020 were included in the study. All patients underwent body computed tomography (CT), pelvic magnetic resonance image (MRI), and biopsy of the cervix for staging. The International Federation of Gynaecology and Obstetrics (2008) stage IB to IVA patients were included, and those with distant metastases were excluded except para-aortic lymph node metastasis. Concurrent weekly CDDP (40 mg/m2) was administered for regional node-positive patients or those with a >4 cm tumor diameter, except for those >80 years of age and with impaired renal function.
According to the Japan Society of Gynecologic Oncology Guidelines,11 20 to 50 Gy of whole pelvic irradiation (WPIR) was followed by 10 to 30 Gy WPIR with a central shield (CS) using a 4-cm width block to reduce the dose to the rectum and bladder until the pelvic sidewall received 50 Gy with 2 Gy per fraction. Since 2017, intensity modulated radiation therapy has been used for WPIR with no CS, which was delivered 45 Gy in 25 fractions. After WPIR, BT was administered using tandem and ovoid or cylinder with or without extra interstitial needles for large or irregularly shaped tumors (intracavitary and interstitial brachytherapy [IC/IS]).
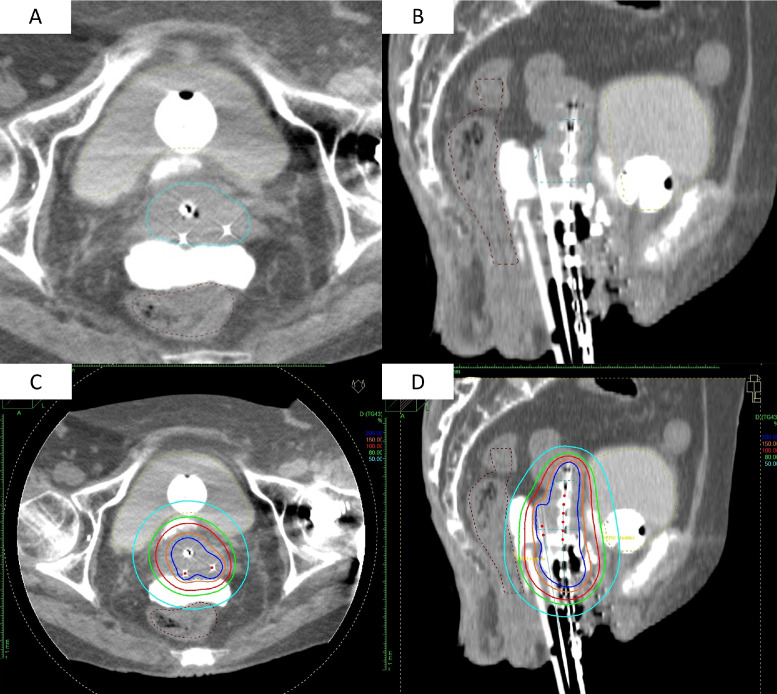
Sacral anesthesia was administered in the treatment room before brachytherapy, and afterward, fentanyl and midazolam were used for analgesia and sedation. Before inserting the applicators, under the TRUS guidance, the 19 G needle (disposable ultrasonography compatible puncture needle; Create Medic Co, Ltd, Kanagawa, Japan) was advanced to the rectovaginal fossa and vesicouterine fossa through the anterior and posterior vaginal wall, respectively, while confirming the position of the needle tip with the sagittal image of the TRUS. After the final confirmation of the needle with the axial image, hyaluronate gel was injected in the spaces. Hyaluronate gel consists of 5 vials of Suvenyl (Chugai Pharmaceutical Co, Tokyo, Japan), 5 mL of saline, and 2 to 4 mL of contrast enhancement agent (Oiparomin 370; Fuji Pharmaceutical Company, Toyama, Japan). The gel was placed for every fraction as it was absorbed in 2 to 3 days Figure 1. shows the case of CT images with HGI. We did not inject gel for patients with bladder or rectal invasion.
Fig. 1.
(A, B) Axial and sagittal computed tomography image with hyaluronate gel injection in the rectovaginal fossa and vesicouterine fossa. Tandem and ovoid are used with 2 interstitial needles for dorsal invasion of the tumor. (C, D) Distribution of the same case. The 100% isodose line (6 Gy) is indicated by the red line. The red line covers the high-risk clinical target volume (blue dotted line) as it avoids extending into the bladder and rectum.
In all cases, the prescribed reference dose per fraction was 6 Gy. After the applicators were placed, CT imaging was obtained with the patients in the lithotomy position, and planning was performed using the software Oncentra (Elekta, Veenendaal, The Netherlands). The CTVHR contouring was based on the Japanese Radiation Oncology Study Group guidelines,12 which describe the contouring of the CT-based high-risk clinical target volume (CTVHR), and we also referred to GEC-ESTRO guidelines.2,13 The plan was optimized to ensure that the 6 Gy isodose cover HRCTV while keeping OAR dose restrictions within guidelines. Our dosimetric constraints for OARs are as follows: <75 Gy for rectum D2cc and <85 Gy for bladder D2cc (EQD2). In the HGI group, dose-escalation was encouraged if the OARs doses were within the limit of the dose constraints, especially for patients with poor response.
To obtain the combined dose of EBRT and brachytherapy, the EQD2 was calculated according to the linear-quadrate model by the following formula, and the EQD2 of CTVHR D90, bladder D2cc and rectal D2cc were obtained:
Here, D is the total dose, d is the dose per fraction, α/β = 10 Gy for CTVHR, and α/β = 3 Gy for OARs.
Although many studies have not included the dose of CS, we referred to the report by Tamaki et al14 and considered that 22% of the CS dose for CTVHR, 15% of the CS dose for bladder, and 5% of the CS dose for rectum contributed to the total dose.
The time to local recurrence was defined as the time interval between the day when radiation therapy started and the date of local relapse or recurrence, and the local control rate of each group were compared. The Common Terminology Criteria for Adverse Events, version 5.0 was used to assess late genitourinary (GU) and gastrointestinal (GI) events, including rectum hemorrhage, cystitis, stenosis or stricture, and fistula.
A retrospective analysis of 163 patients with cervical cancer treated with RT showed a significant difference in the distribution of the use of IC/IS between the non-HGI and HGI groups. It is possible that the discrepancy was because of the fact that the non-HGI group was primarily treated before 2013. Therefore, to extract confounding factors, CTVHR D90, age, International Federation of Gynaecology and Obstetrics stage, the initial size of CTVHR, the total dose of BT, the total dose of external beam radiation, the use of chemotherapy, and the use of IC/IS were subjected to multiple regression analysis. The results showed that 3 covariates were associated with CTVHR D90: initial size (P = .0480), total BT dose (P < .0001), and use of IC/IS (P < .0001). Based on these three factors, 1:1 propensity score matching was performed to select non-HGI and HGI groups patients for further analysis.
The characteristics of the patients and each dosimetric parameter were compared between the non-HGI group and HGI group with the Fisher exact test for categorical variables and the Wilcoxon rank-sum test or Student t test for continuous variables. All tests were performed with JMP Pro 16.0 (SAS Institute, Cary, NC). P values <.05 were considered statistically significant.
The National Cancer Center Hospital's institutional review board approved this retrospective study (approval number 2017-091) following the Declaration of Helsinki's ethical principles.
Results
Between July 2008 and January 2020, according to 1:1 propensity score matching (confounding factors: initial size of tumor, total BT dose, the use of IC/IS), 104 patients (non-HGI group: 52 patients; HGI group: 52 patients) were included. Patients’ characteristics are summarized in Table 1. In both groups, the median dose of EBRT was 50 Gy, and the median dose of BT was 24 Gy. IC/IS was used in 43 cases in the non-HGI group and 40 cases in the HGI group, about 80% of the entire group. There were no differences in baseline tumor characteristics, dose prescription, and the use of interstitial brachytherapy across groups. A significant difference in age and use of chemotherapy was observed, but it was not considered to affect the assessment of dose-volume parameters. The median follow-up period for all the patients was 32.6 months (4.6-147.2 months). Since the use of HGI was started in 2015, the observation period was also significantly longer in the non-HGI group (50.4 months vs 25.4 months,P < .001).
Table 1.
Patient characteristics
| Total | Non-HGI group | HGI group | P value | |
|---|---|---|---|---|
| Number of patients | 104 | 52 | 52 | |
| Age, median (range) | 60 (23-88) | 55 (23-85) | 66 (35-88) | .032* |
| FIGO (2008) | .966 | |||
| IB1 | 5 | 2 | 3 | |
| IB2 | 10 | 6 | 4 | |
| IIA2 | 3 | 1 | 2 | |
| IIB | 31 | 15 | 16 | |
| IIIA | 2 | 1 | 1 | |
| IIIB | 43 | 21 | 22 | |
| IVA | 10 | 6 | 4 | |
| Initial tumor size (cm), median (range) | 5.5 (1.5-9.2) | 5.5 (1.5-8.9) | 5.5 (2.3-9.2) | .834 |
| Histology | .473 | |||
| Squamous cell carcinoma | 94 | 47 | 47 | |
| Adenocarcinoma | 9 | 4 | 5 | |
| Adenosquamous cell carcinoma | 1 | 1 | 0 | |
| EBRT dose (Gy), median (range) | 50 (40-50.4) | 50 (40-50.4) | 50 (42-50.4) | .191 |
| BT dose (Gy), median (range) | 24 (12-36) | 24 (12-30) | 24 (12-36) | .296 |
| Type of BT | .463 | |||
| Intracavitary brachytherapy | 9 | 12 | ||
| IC/IS | 43 | 40 | ||
| Chemotherapy | <.002* | |||
| Concomitant chemotherapy | 74 | 45 | 29 | |
| Nonchemotherapy | 30 | 7 | 23 | |
| Follow-up time (mo), median (range) | 32.6 (4.6-147.2) | 50.4 (9.7-147.2) | 25.4 (4.6-52.7) | <.001* |
Abbreviations: BT = brachytherapy; EBRT = external beam radiation therapy; FIGO = International Federation of Gynaecology and Obstetrics; HGI = hyaluronate gel injection; IC/IS = intracavitary and interstitial brachytherapy.
Statistically significant.
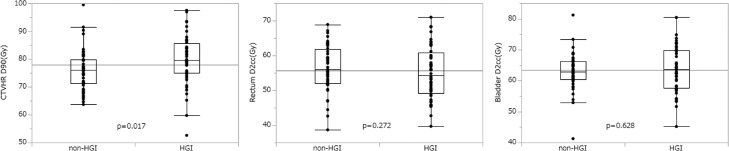
Table 2 and Figure 2 show the dose-volume parameters of CTVHR, rectum, and bladder. The median doses for CTVHR D90 were 76.0 Gy (range, 63.7-99.5) in the non-HGI group and 79.4 Gy (range, 52.6-97.5) in the HGI group. CTVHR D90 was significantly higher in the HGI group (P = .017; 95% confidence interval [CI], 0.69-6.82). The median rectal dose in the non-HGI group was 56.0 Gy (range, 38.7-68.9) and 54.8 Gy (range, 39.7-71.0) in the HGI group (P = .628; 95% CI, −1.96 to 3.23), and the median bladder dose in the non-HGI group was 62.9 Gy (range, 41.3-81.2) and 63.7 Gy (range, 45.2-80.4) in the HGI group (P = .272; 95% CI, −4.22 to 1.20), which showed no significant difference.
Table 2.
Dose-volume parameters of CTVHR, rectum, and bladder by treatment groups
| Non-HGI group | HGI group | P value | |
|---|---|---|---|
| Median CTVHR D90, Gy (range) | 76.0 (63.7-99.5) | 79.4 (52.6-97.5) | .017* |
| Median rectal D2cc, Gy (range) | 56.0 (38.7-68.9) | 54.8 (39.7-71.0) | .272 |
| Median bladder D2cc, Gy (range) | 62.9 (41.3-81.2) | 63.7 (45.2-80.4) | .628 |
Abbreviations: CTVHR = high-risk clinical target volume; HGI = hyaluronate gel injection.
Statistically significant.
Fig. 2.
A box plot shows the dose-volume histogram parameters of the high-risk clinical target volume (CTVHR), rectum, and bladder. The 50% interquartile range, median, and range of data are displayed. All cases were divided into 2 groups: a group without hyaluronate gel injection (non-HGI) and a group with hyaluronate gel injection (HGI).
The 2-year local control rate was 85.9% in the non-HGI group and 91.9% in the HGI group, which was not significantly different (P = .313; hazard ratio, 1.98; 95% CI, 0.51-7.71). There were no adverse events related to HGI, such as bleeding, hematuria, bladder wall injury, or urethral injury requiring hospitalization. One patient (nephritis) in the non-HGI group and 1 patient (vesicovaginal fistula) in the HGI group had late adverse events of GU ≥grade 3. Five patients (rectal bleeding: 2, rectovaginal fistula: 2, ileus:1) in the non-HGI group and 2 patients (rectovaginal fistula:1, ileus:1) in the HGI group experienced late adverse events of GI grade ≥3. None of the adverse events were caused by the tumor, but by the treatment. There were no significant differences in morbidity between these 2 groups (GU: P = .65; 95% CI, −0.01 to 0.06; GI: P = .29; 95% CI, −0.16 to 0.05).
Discussion
In this study, we have shown that HGI into the rectovaginal fossa and vesicouterine fossa resulted in a significant increase in CTVHR D90 without an increase in the dose of OARs in uterine cervical cancer brachytherapy. It has been shown that spacers can reduce the dose of OARs, but no one has mentioned the dose of the target volume.15,16 To the best of our knowledge, this is the first study concerning the dose parameters of CTV when spacers are used.
IGBT is vital in the curative treatment of locally advanced cervical cancer and in combination with external beam radiation and chemotherapy can obtain favorable local control. The outcome of the EMBRACE-I, which used MRI-based IGBT, showed that the median dose of CTVHR D90 was 90 Gy (range, 85-94 Gy) and 5-year local control was 92% (95% CI, 90%-93%).17 In comparison, the median dose of CTVHR D90 in Japan has been reported to be 65 to 74 Gy, a relatively lower dose.18, 19, 20 The median dose of the HGI group in our study was 79.4 Gy, which seems to be relatively high in Japan. However, it did not achieve CTVHR D90 >85 Gy recommended by the GEC-ESTRO.
There are several possible reasons for the low dose of CTVHR D90 in Japan, including the results of this study. First, the cumulative dose schedule used in Japan is lower than those of the United States and Europe. This is because the prospective study in Japan has shown that the clinical outcomes of this schedule are comparable with those of global dose schedule while adverse events were low.21 Second, the uncertainty of CS in evaluating doses is a concerning issue. In general practice, the CS dose is not included in the total dose calculation, and subsequently, the dose of CTVHR may have been underestimated. Tamaki et al reported that when the CS is 4 cm width, 13% to 35% of the CS dose contributes to the total dose of CTVHR.14 Therefore, although it is not precise and contains inherent uncertainty, dose contribution from CS was included in this study to obtain a cumulative dose as accurate as possible. Of course, such calculations have not been widely used; additional discussion is needed in the community which uses CS. Lastly, the combined IC/IS technique has not been used in many facilities in Japan. When the tumor is massive or has an irregular form, it is difficult to cover the dose to CTV with ICBT alone; thus, combining interstitial needles can improve coverage of the CTVHR while adhering to OAR dose constraints.22 In the present study, IC/IS was used in 80% of cases, and HGI was also used, resulting in a significant increase in CTVHR dose compared with the Japanese standards but still lower than 85 Gy.
Although the dose goal is lower than 85 Gy, several Japanese reports demonstrated favorable clinical outcomes.19,23,24 In our previous study, the LC rate was more than 90% even if the dose did not reach 85 Gy in the following low-risk cases: squamous cell carcinoma, reduction ratio before brachytherapy ≥29%, tumor size before brachytherapy ≤4 cm, and total treatment time <9 weeks.25 Risk stratification may be necessary to identify cases requiring more than 85 Gy for CTVHR (eg, large tumor size, adenocarcinoma), and should strive to increase the dose of CTVHR in such cases, in which HGI may be a helpful tool. Reviewing the results of our study, we were overly concerned with the OARs dose, which was low enough. The CTVHR dose may have been increased much further, but we cannot simply increase the dose because we sometimes face severe radiation-related toxicity even in cases with low CTVHR doses.
While the incidence of late adverse events was sufficiently low, we could not accomplish a significant dose reduction of OARs in the HGI group because of our prioritization of increasing the dose in the CTVHR within the OARs dose constraints. Due to the lower dose schedule and use of CS, the incidence of late adverse events in Japan are lower than in other countries. Pötter et al17 reported that actuarial cumulative 5-year incidence of grade 3 to 5 morbidity was 6.8% (95% CI, 5.4-8.6) for GU events, 8.5% (6.9-10.6) for GI events in the EMBRACE-I study. In comparison, even though the follow-up period was short in the present study, rates of late adverse events were low (GU: 1.9%; GI: 3.8%) in the HGI group, and HGI is expected to reduce the dose of OARs and adverse events further by widening the physical space between OARs and CTV.
The limitations of this study are that the study was retrospective from single institution study with inherent information and selection bias, the presence of confounding factors and the difference of follow-up period between the 2 groups. Although patients did not complain of any pain or discomfort caused by HGI during follow-up visits, patient-reported toxicities were not evaluated in this article. Therefore, prospective studies are required to validate its effectiveness in cervical cancer brachytherapy, through which we would also like to promote the technique of HGI to other facilities. Long-term observation is also needed to analyze the relationship between CTVHR D90 and local control rate. Even with those limitations, HGI enables a safe increase in the CTVHR D90 dose and also be helpful for institutions where the total dose already is >85 Gy in contributing to the reduction of late severe rectal or bladder side effects.
Conclusion
Using HGI in the rectovaginal fossa and vesicouterine fossa for patients with cervical cancer increased the dose of CTVHR D90 while the quantity of rectum and bladder D2cc was within the constraints.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Igaki reports grants and personal fees from HekaBio, grants from CICS, grants from Elekta KK, personal fees from AstraZeneca, personal fees from Itochu, personal fees from HIMEDIC, and personal fees from Varian, outside the submitted work. Dr Kashihara reports personal fees from AstraZeneca, outside the submitted work. Dr Nakayama reports grants and personal fees from Pfizer and personal fees from AstraZeneca, outside the submitted work. All other authors have no disclosures to declare.
Data for these analyses are included in the supplementary information files.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.100918.
Appendix. Supplementary materials
References
- 1.Derks K, Steenhuijsen JLG, Van Den Berg HA, et al. Impact of brachytherapy technique (2D versus 3D) on outcome following radiotherapy of cervical cancer. J Contemp Brachytherapy. 2018;10:17–25. doi: 10.5114/jcb.2018.73955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos JCA, Lang S, Kirisits C, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63. doi: 10.1016/j.ijrobp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Kishi K, Sato M, Shirai S, Sonomura T, Yamama R. Reirradiation of prostate cancer with rectum preservation: Eradicative high-dose-rate brachytherapy with natural type hyaluronate injection. Brachytherapy. 2012;11:144–148. doi: 10.1016/j.brachy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Manir KS, Basu A, Ghosh K, Dip RP. Rectal separation using hydroxypropyl methylcellulose in intracavitary brachytherapy of cervical cancer: An innovative approach. J Contemp Brachytherapy. 2016;8:1–5. doi: 10.5114/jcb.2016.62951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Radiat Oncol Biol. 2015;92:971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Kashihara T, Murakami N, Tselis N, et al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: initial Japanese experience. J Radiat Res. 2019;60:501–508. doi: 10.1093/jrr/rrz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami N, Shima S, Kashihara T, et al. Hyaluronic gel injection into the vesicovaginal septum for high-dose-rate brachytherapy of uterine cervical cancer: an effective approach for bladder dose reduction. J Contemp Brachytherapy. 2019;11:1–7. doi: 10.5114/jcb.2019.82612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami N, Nakamura S, Kashihara T, et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy. 2020;19:154–161. doi: 10.1016/j.brachy.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Ebina Y, Mikami M, Nagase S, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24:1–19. doi: 10.1007/s10147-018-1351-y. [DOI] [PubMed] [Google Scholar]
- 12.Ohno T, Wakatsuki M, Toita T, et al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res. 2017;58:341–350. doi: 10.1093/jrr/rrw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahantshetty U, Poetter R, Beriwal S, et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother Oncol. 2021;160:273–284. doi: 10.1016/j.radonc.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamaki T, ei Noda S, Ohno T, Kumazaki Y, Kato S, Nakano T. Dose-volume histogram analysis of composite EQD2 dose distributions using the central shielding technique in cervical cancer radiotherapy. Brachytherapy. 2016;15:598–606. doi: 10.1016/j.brachy.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Damato AL, Kassick M, Viswanathan AN. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound. Brachytherapy. 2017;16:949–955. doi: 10.1016/j.brachy.2017.04.236. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan Akila N., Damato Antonio L., PLN Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol. 2013;31:446–447. doi: 10.1200/JCO.2012.47.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pötter R, Tanderup K, Schmid MP, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021;22:538–547. doi: 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- 18.Otani Y, Ohno T, Ando K, et al. Dosimetric feasibility of computed tomography-based image-guided brachytherapy in locally advanced cervical cancer: a Japanese prospective multi-institutional study. J Radiat Res. 2021;62:502–510. doi: 10.1093/jrr/rraa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusada T, Toita T, Ariga T, et al. Definitive radiotherapy consisting of whole pelvic radiotherapy with no central shielding and CT-based intracavitary brachytherapy for cervical cancer: Feasibility, toxicity, and oncologic outcomes in Japanese patients. Int J Clin Oncol. 2020;25:1977–1984. doi: 10.1007/s10147-020-01736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami N, Kasamatsu T, Wakita A, et al. CT based three dimensional dose-volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer. 2014;14:1–7. doi: 10.1186/1471-2407-14-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toita T, Kitagawa R, Hamano T, et al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: Efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol. 2012;126:211–216. doi: 10.1016/j.ygyno.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Fokdal L, Sturdza A, Mazeron R, et al. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: Analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Murakami N, Kobayashi K, Shima S, et al. A hybrid technique of intracavitary and interstitial brachytherapy for locally advanced cervical cancer: initial outcomes of a single-institute experience. BMC Cancer. 2019;19:221. doi: 10.1186/s12885-019-5430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno T, Noda SE, Okonogi N, et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: Results of a 5-year retrospective study. J Radiat Res. 2017;58:543–551. doi: 10.1093/jrr/rrw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami N, Ando K, Murata M, et al. Why not de-intensification for uterine cervical cancer? Gynecol Oncol. 2021;163:1–5. doi: 10.1016/j.ygyno.2021.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.