Abstract
Background
Optimal management of cancer‐associated thrombosis (CAT) in patients with thrombocytopenia remains difficult given competing risks of recurrent thrombosis and increased bleeding. We determine the impact of the ISTH Scientific and Standardization Committee (SCC) guidance on CAT management and thrombocytopenia on platelet transfusion, bleeding, and recurrent thrombosis.
Methods
A retrospective review was performed of patients with CAT and thrombocytopenia who required anticoagulation for VTE for 11 months before and after implementation of the ISTH SCC guidance. Medical records were reviewed to identify the type of VTE event, number of platelet transfusions, incidence of bleeding, and VTE recurrence within pre‐ and postintervention time periods.
Results
A total of 41 and 80 cases were included in the preintervention and postintervention periods, respectively. The preintervention group showed a trend toward less acute VTE events (39% vs 55%; P = .05). The postintervention period had an increased per‐patient platelet transfusion (median, 2.5 vs 4; P = .05). Nonmajor bleeding was increased in the postintervention group (2% vs 16%; P = 0.03) and included all six (8%) major hemorrhages (P = .09). There was numerically less recurrent thrombosis in the postintervention group (20% vs 8%; P = .07), which was not significantly different when accounting for acuity of VTE. Management adherence was strong, at 91%, in the postintervention group.
Conclusion
The ISTH guidance on management of cancer‐associated thrombosis in patients with thrombocytopenia was successfully implemented in an academic medical center. There was no significant difference in bleeding or recurrent thrombosis outcomes after adjusting for acuity of VTE.
Keywords: anticoagulants, hemorrhage, platelet count, platelet transfusion, thrombocytopenia, thrombosis
Essentials.
Blood clots in patients with cancer are difficult to treat with low platelets.
A standardized approach to treating these clots was implemented at an academic medical center.
There was no significant difference in bleeding or recurrent clot after adjusting for clot acuity.
More research on the best treatment for blood clots in patients with low platelets is needed.
1. BACKGROUND
Thrombosis is the second‐leading cause of death for patients with cancer undergoing chemotherapy and contributes to significant morbidity. 1 Compared to patients without cancer, individuals with cancer‐associated thrombosis (CAT) have increased fatal pulmonary embolism (PE), in‐hospital mortality, and higher‐risk deep venous thrombosis (DVT) than those without cancer. 2 , 3 Mortality is also higher in patients with cancer with venous thromboembolism (VTE) with rates of 64.5% and 88.1% at 1 and 10 years, respectively. 4 The underlying mechanisms for hypercoagulability in patients with cancer are multifactorial and often involve release of procoagulant factors from tumor cells, monocytes, or macrophages, as well as chemotherapy, insertion of catheters, and infections. 5 , 6 , 7 Despite a procoagulant state, patients with cancer also have a higher risk of bleeding, especially if they have thrombocytopenia from chemotherapy or their underlying disease. 8 , 9
Optimal management of VTE to reduce associated morbidity in the setting of the competing risks of thrombocytopenia and increased bleeding risk is difficult. Generally, two strategies are used: (1) full‐dose anticoagulation with supportive platelet transfusions to maintain platelet count >50 × 103/µL or (2) reduced‐dose anticoagulation while platelets are <50 × 103/µL. 10 , 11 As noted by Carrier et al, 12 the highest risk of recurrence of VTE is within the first month, providing rationale to use full‐dose anticoagulation with an increased transfusion threshold to platelets of 50 × 103/µL during this time period. Anticoagulation in patients with platelet counts >50 × 103/µL has been associated with a low bleeding risk. 13
The ISTH Scientific and Standardization Committee (SCC) released an updated guidance statement in 2018 on management of anticoagulation in patients with CAT and thrombocytopenia. 10 To summarize, the ISTH SCC recommended full‐dose anticoagulation for acute VTE if the platelet count is >50 × 103/µL. For patients with acute CAT and high‐risk features, it is recommended to give therapeutic anticoagulation with transfusion support to maintain platelet counts >40 to 50 × 103/µL. High‐risk features include symptomatic segmental or more proximal PE, proximal DVT, or history of recurrent/progressive thrombosis. 10 In those with lower‐risk events (distal DVT of the lower extremities, incidental subsegmental PE, or catheter‐related VTE) or subacute VTE, a dose‐adjusted strategy for platelet counts between 25 and 50 × 103/µL and holding anticoagulation when platelet counts are <25 × 103/µL is recommended. While several different sets of guidelines and statements exist, few studies have reported outcomes of patients managed using these protocols.
We aimed to evaluate hospitalized patients with cancer diagnosed with VTE and thrombocytopenia receiving therapeutic anticoagulation before and after implementation of the ISTH SCC guidelines to determine impacts of the guidance statement as a standardized treatment approach on platelet transfusion utilization, bleeding, and recurrent thrombosis.
2. METHODS
This study was performed as part of a quality improvement initiative for management of anticoagulation in hospitalized patients with cancer with VTE and thrombocytopenia at our institution. A treatment algorithm based on the 2018 ISTH SCC guidance statement (Figure 1) was created. 10 Distribution of the algorithm occurred by email, presentation at division meetings, and posting copies in provider team rooms, as well as creating a standing reference on our institution’s intranet website. A search of the electronic medical record was used to identify patients with malignancy, thrombocytopenia (defined as platelets <50 × 103/µL), and administration of an anticoagulant. The preintervention time period included cases between July 2017 and May 2018. During this time, antithrombotic management was based on clinical judgment regarding anticoagulant choice, anticoagulant dosing, and administration of platelet transfusions. Postintervention cases included those from July 2018 through May 2019. Anticoagulant choice was determined on the basis of clinical judgement, but dosing and platelet transfusion decisions were guided using the algorithm. Data were collected during the postintervention period to assess compliance with the algorithm. Adherence to platelet transfusion was measured by evaluating platelet counts before transfusion, and whether transfusion was indicated on the basis of acuity of VTE event. Adherence to anticoagulation management was measured by confirming the dose administered in the context of a patient’s platelet count and VTE risk and timing. Cases were excluded from analysis if patients received therapeutic anticoagulation for an indication other than VTE (ie, atrial fibrillation, mechanical heart valve, etc), received prophylactic dosing of anticoagulation without a VTE event, or if on chart review did not receive an anticoagulant dose while in the hospital.
FIGURE 1.
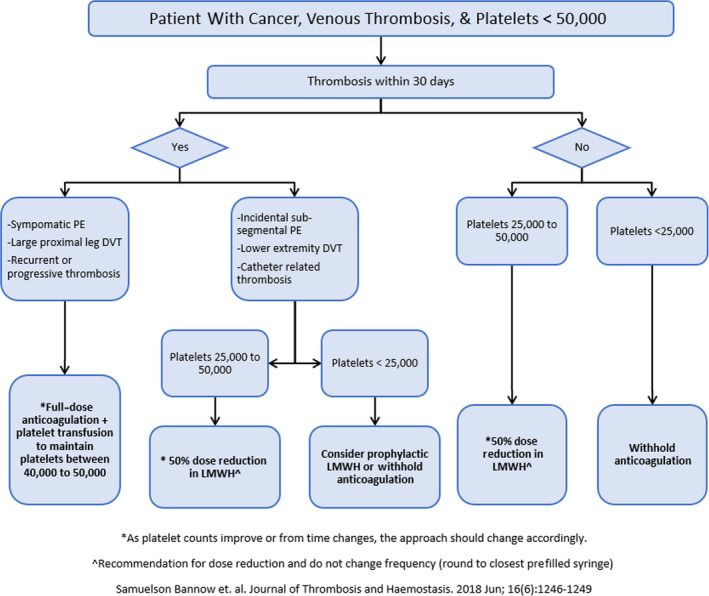
Treatment algorithm developed and implemented based on the ISTH Scientific Standardization Committee guidance statement for management of cancer‐associated thrombosis in thrombocytopenia. DVT, deep venous thrombosis; LMWH, low‐molecular‐weight heparin; PE, pulmonary embolism
Charts were reviewed to identify type of VTE event and acuity, number of platelet transfusions while being treated for VTE, bleeding, and VTE recurrence. VTE events included PE (including subsegmental), proximal DVT, catheter‐associated DVT, and distal DVT of the lower extremities. Patients with only superficial thrombosis were excluded. An acute VTE was defined as a new VTE event occurring within 30 days of thrombocytopenia and therapeutic anticoagulation was administered. Patients were followed from the date of anticoagulant administration up until the end date of the evaluated time period.
Major bleeding was defined on the basis of ISTH criteria (fatal bleeding and/or symptomatic bleeding into a critical area or organ and/or bleeding causing a fall in hemoglobin of ≥2 g/dL or that requires transfusion of ≥2 units of whole blood or red cells). 14 Clinically relevant nonmajor bleeding was considered if treatment was needed but the episode did not meet the criteria for major bleeding. Bleeding episodes were counted if they occurred while the patient was receiving anticoagulation at the time of bleeding. Recurrence of thrombosis was defined by extension of an existing VTE or VTE at a new site identified on an imaging study. Time to recurrence was the number of days between reduction or discontinuation of anticoagulation and evidence of a new or progressive VTE.
Mortality between the preintervention and postintervention groups was also evaluated. Mortality was considered if the patient died from any cause within 30 or 90 days of the admission date from the index hospitalization. Summary statistics were calculated, and chi‐square and Fisher’s exact tests were used to compare categorical variables. Continuous variables were compared using the Mann‐Whitney test. A P value of <0.05 was considered statistically significant.
3. RESULTS
The electronic search identified 56 cases in the preintervention group and 105 cases in the postintervention group (Figure 2). On further chart review, 15 and 25 cases were excluded from analysis in these groups, respectively. The most common reason for exclusion was atrial fibrillation, with 11 cases in the preintervention group and 17 in the postintervention group. In sum, 41 cases were analyzed in the preintervention group and 80 cases in the postintervention group. Average follow‐up time was slightly longer in the preintervention group compared to the postintervention group (6 months [standard deviation (SD), 2.19] vs 5 months [SD, 3.04], respectively; P = .004). Most patients were men (66% and 55%), and the median age was 62 and 64 years old (Table 1). Solid tumors accounted for 27% and 19% of cases in the pre and postintervention groups, respectively. The proportion of patients with hematologic malignancy was similar between the preintervention and postintervention groups, except for patients with acute myeloid leukemia and myelodysplastic syndrome (AML/MDS), which was higher in the postintervention group (12% vs 28%). Of the patients with hematologic malignancy, approximately half were recipients of a bone marrow transplant. There was no statistically significant difference in the age, sex, ethnicity/race, or cancer type between each group.
FIGURE 2.

Cases excluded from analysis. VTE, venous thromboembolism
TABLE 1.
Patient demographics and categorization of VTE events between preintervention and postintervention groups
| Before ISTH Guidance, N = 41 | After ISTH Guidance, N = 80 | P value | |
|---|---|---|---|
| Age, y, median (IQR) | 62 (56‐65) | 64 (57‐70) | .18 |
| Male sex, n (%) | 27 (66) | 44 (55) | .25 |
| Follow‐up time, mo, mean (SD) | 6 (2.19) | 5 (3.04) | .004 |
| Race/Ethnicity, n (%) | |||
| White/Caucasian | 38 (93) | 72 (90) | .89 |
| Black/African American | 2 (5) | 4 (5) | |
| Asian | 1 (2) | 1 (1) | |
| Hispanic | 0 (0) | 1 (1) | |
| Unknown | 0 (0) | 2 (3) | |
| Cancer type, n (%) | |||
| AML/MDS | 5 (12) | 22 (28) | .42 |
| ALL | 4 (10) | 6 (8) | |
| Plasma cell disorder | 11 (27) | 20 (25) | |
| Lymphoma | 10 (24) | 18 (23) | |
| Solid tumor/other | 11 (27) | 15 (19) | |
| Bone marrow transplant, n (%) | 20 (49) | 43 (54) | .60 |
| Thrombosis type, n (%) | |||
| Within 30 days | 16 (39) | 44 (55) | .05 |
| PE/proximal DVT | 8 (20) | 24 (30) | |
| CRT/distal DVT | 8 (20) | 20 (25) | |
| VTE >30 days | 25 (61) | 36 (45) | |
| PE/proximal DVT | 23 (56) | 32 (40) | |
| CRT/distal DVT | 2 (5) | 4 (5) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CRT, catheter‐related thrombosis; DVT, deep vein thrombosis; IQR, interquartile range; MDS, myelodysplastic syndrome; PE, pulmonary embolism; SD, standard deviation; VTE, venous thromboembolism.
Management in the preintervention group was heterogenous regardless of VTE acuity. In the entire preintervention group, anticoagulation doses were held in most cases (n = 24; 59%) for platelet counts <50 × 103/µL (n = 17) or 30 × 103/µL (n = 7). Dose reduction for platelets <50 × 103/µL was followed in 7 (17%) cases. In 11 cases (27%), anticoagulation was continued, and platelets were transfused to thresholds of 30 × 103/µL (n = 3) or 50 × 103/µL (n = 8). Postintervention management was more concise, with an adherence rate of 91% to the algorithm.
In the pre‐intervention group, there were 16 (39%) acute VTE events, of which 8 (20%) were high‐risk thromboses. There were 25 (61%) index VTE events that occurred >30 days from the time of thrombocytopenia, most of which (n = 23; 56%) were high risk (Table 1). The median number of platelet transfusions per patient was 2.5 (interquartile range [IQR], 1‐6). Anticoagulation doses were held in 24 (59%) of cases in the preintervention group, the majority of which were related to thrombocytopenia, and bleeding in one case (2%).
In the preintervention group, there were fewer bleeding events than recurrent thrombosis. There was one case (2%) of nonmajor bleeding identified in the preintervention group, and no (0%) cases of major bleeding (Table 2). The platelet count at the time of bleeding was 37 × 103/µL, and the patient was being treated with apixaban when bleeding occurred. There were eight (20%) cases of recurrent thrombosis in the preintervention group. The median time to recurrence was 20 days (IQR, 10‐37 days) and the median platelet count at time of recurrence was 63 × 103/µL (IQR, 28‐97 × 103/µL; Table 2). Six of the eight recurrent VTE events happened in patients with an index VTE event >30 days prior. Only one of the eight patients experiencing a recurrent VTE event was treated with anticoagulation at the time of recurrence, and anticoagulation was withheld in the other patients due to thrombocytopenia. Three of the eight cases had platelet counts <50 × 103/µL at the time of VTE recurrence, and all but two cases had platelet counts <100 × 103/µL. Recurrence occurred predominantly in patients with hematologic malignancies (n = 5) compared to solid tumors (n = 3).
TABLE 2.
Platelet transfusion, bleeding, management practices, recurrent thrombosis data, and mortality outcome between preintervention and postintervention groups
| Before ISTH Guidance, N = 41 | After ISTH Guidance, N = 80 | P value | |
|---|---|---|---|
| Platelet transfusion per person, median (IQR) | 2.5 (1‐6) | 4 (2‐11) | 0.05 |
| Interruption or reduction in anticoagulation, n (%) | 24 (59) | 43 (54) | 0.70 |
| Hemorrhage, n (%) | 1 (2) | 13 (16) | 0.03 |
| Index VTE event <30 days | 0 (0) | 8 (18) | 0.10 |
| Index VTE event >30 days | 1 (2) | 5 (14) | 0.39 |
| Major bleed, a n (%) | 0 (0) | 6 (8) | 0.09 |
| Index VTE event <30 days | 0 (0) | 5 (14) | 0.31 |
| Index VTE event >30 days | 0 (0) | 1 (3) | >.99 |
| Platelet count (×103/µL) at time of hemorrhage, median (IQR) | 37 (‐) | 52 (35‐65) | … |
| Index VTE event <30 days | 0 (–) | 61 (39‐93) | … |
| Index VTE event >30 days | 37 (–) | 46 (12‐54) | … |
| Recurrent thrombosis, n (%) | 8 (20) | 6 (8) | 0.07 |
| Index VTE event <30 days | 2 (5) | 1 (1) | 0.17 |
| Index VTE event >30 days | 6 (15) | 5 (6) | 0.50 |
| Type of recurrence | 6–acute DVT | 2–acute DVT | … |
| 2–DVT progression | 1–DVT progression | ||
| 2–CRT | |||
| 1–VTE progression | |||
| Median platelet count at time of recurrence, median (IQR) | 63 (28‐97) | 76 (26‐176) | 0.75 |
| Time to recurrence [days (median, IQR)] | 20 (10‐37) | 40 (21‐110) | 0.17 |
| All‐cause mortality, n (%) | |||
| 30‐day | 5 (12) | 9 (11) | >.99 |
| 90 day | 8 (19) | 14 (18) | 0.81 |
Abbreviations: CRT, catheter‐related thrombosis; DVT, deep venous thrombosis; IQR, interquartile range; VTE, venous thromboembolism.
Major bleed = fatal bleeding and/or symptomatic bleeding into a critical area or organ, and/or bleeding causing a fall in hemoglobin of ≥2 g/dL or that requires transfusion of ≥2 units of whole blood or red cells.
In the postintervention group, there were more acute VTE events identified compared to the preintervention group (n = 44; 55%; P = .05). Of the postintervention group events, more were high‐risk thromboses (PE or proximal DVT) compared to low‐risk thromboses (n = 24 vs 20, respectively). There was also a trend toward increased per‐patient platelet transfusion in this group (median, 4; P = .05; Table 2). Anticoagulation was held in 43 (54%) of cases, mostly related to thrombocytopenia per the algorithm but was related to bleeding in 7 cases (9%).
There was no significant difference in recurrent thrombosis in the postintervention time period compared to the preintervention time period (8% vs 20%; P = .07; Table 2). The median time to recurrence was 40 days in the postintervention group (IQR, 21‐110 days), and the median platelet count was 76 × 103/µL (IQR, 26‐176 × 103/µL; P = .75; Table 2). Like the preintervention group, only one patient with recurrent VTE was on anticoagulation at the time of recurrence, and all but one case of recurrence happened in patients with index VTE >30 days prior. Most cases had a platelet count <100 × 103/µL at the time of recurrence (n = 4; 5%), with two cases having platelet counts <50 × 103/µL. Recurrent VTE in the postintervention time period happened predominantly in hematologic malignancy patients, with only two of six cases identified in patients with solid tumors.
After adjustment for acuity of VTE (>30 days vs <30 days), there was no significant difference in bleeding in the postintervention group compared to the preintervention group (Table 2). Thirteen (16%) cases of bleeding were identified. Eight (10%) of these occurred in patients diagnosed with an acute VTE event, five (6%) of which were considered major bleeds (Table 3). No major bleeds were fatal. The median platelet count at the time of bleeding in this group was 61 (IQR, 39‐93; Table 2). All cases of bleeding happened while being treated with anticoagulation. Enoxaparin was the most prescribed anticoagulant, used in 9 of the 13 cases of bleeding, followed by apixaban in 2 cases and unfractionated heparin and warfarin in 1 case each.
TABLE 3.
Details of bleeding events, stratified by risk and timing of VTE event
| Number | Type of bleed | Malignancy | Platelet count at time of bleed (×103/µL) | Days since last platelet transfusion | Anticoagulant used at time of bleed |
|---|---|---|---|---|---|
| Preintervention group | |||||
| 1 | Epistaxis, gingival bleeding | Solid Tumor | 37 | >30 | Apixaban |
| Postintervention, high‐risk VTE <30 days | |||||
| 1 | Retroperitoneal hematoma a | Lymphoma | 24 | >30 | Unfractionated heparin |
| 2 | GI Bleed a | Lymphoma | 58 | >30 | Enoxaparin |
| 3 | Tracheostomy a | AML/MDS | 35 | 1 | Enoxaparin |
| 4 | Subdural Hematoma a | AML/MDS | 64 | 1 | Enoxaparin |
| 5 | GI bleed | AML/MDS | 50 | >30 | Apixaban |
| Postintervention, low‐risk VTE <30 days | |||||
| 6 | Retroperitoneal Hematoma | Lymphoma | 123 | 1 | Enoxaparin |
| 7 | Renal subcapsular hematoma a | Myeloma, BMT | 101 | >30 | Enoxaparin |
| 8 | Hematochezia | Myeloma | 69 | 1 | Enoxaparin |
| Postintervention, high‐risk VTE >30 days | |||||
| 9 | Pelvic wall hematoma | Lymphoma | 56 | >30 | Warfarin |
| 10 | Hemoptysis | Solid Tumor | 15 | >30 | Enoxaparin |
| 11 | Epistaxis | Solid Tumor | 9 | 19 | Enoxaparin |
| 12 | Subarachnoid hemorrhage a | Lymphoma | 46 | 6 | Apixaban |
| Postintervention, low‐risk VTE >30 days | |||||
| 13 | GI bleed | Solid tumor | 52 | 2 | Enoxaparin |
Abbreviations: AML, acute myeloid leukemia; BMT, bone marrow transplant; GI, gastrointestinal; MDS, myelodysplastic syndrome; VTE, venous thromboembolism.
Denotes bleed was considered a major bleed.
Finally, most patient deaths were related to cancer progression or other acute illness. In the preintervention group, 5 patients (12%) were deceased at 30 days, with an additional 3 patients (n = 8; 19%) deceased at 90 days. Only one of these deaths could be attributed to an acute PE at the time of admission. In the post‐intervention group, 9 patients (11%) and 14 patients (18%) were deceased at 30 and 90 days, respectively. No deaths were directly related to bleeding events or recurrent thrombosis in either group.
4. DISCUSSION
We successfully implemented the ISTH SCC guidance statement on CAT in patients with thrombocytopenia with good adherence to the algorithm. After implementing the guidance, bleeding and recurrent thrombosis outcomes were similar when acuity of VTE was accounted for. Most cases of VTE with identified thrombocytopenia were patients with hematologic malignancies. These results highlight the continued challenges with balancing risks of anticoagulation and thrombocytopenia in patients with CAT and raise awareness to the importance of accounting for timing in VTE course to appropriately assess the difference in outcomes in this population.
There were notable differences between the preintervention and postintervention groups in terms of the number of cases identified as well as the acuity of VTE. The exact reasoning for this is unclear but may have to do with fewer patients receiving any anticoagulation during the preintervention period based on individualized practices, and therefore, were not captured in the initial case identification. The higher number of acute VTE cases in the postintervention group may be accounted for by random variation, changes in patient population with growth of the transplant program, and/or an increased awareness of treatment during thrombocytopenia and impetus to evaluate for VTE in this patient population.
Likely secondary to the difference in acuity of thrombosis between intervention groups, there was an increase in median number of platelet transfusions per patient in the postintervention group. Despite this, our data did not suggest that this translated to an increased risk of recurrent thrombosis. These findings are similar to a 2020 study that showed platelet counts were inversely proportional to platelet transfusion needs, with a majority of patients on full‐dose enoxaparin requiring transfusion. That same study also did not find any increase in recurrent VTE in those patients with thrombocytopenia while on anticoagulation. 15
The risk of thrombosis versus the risk of bleeding must be balanced in patients with cancer‐associated thrombosis and thrombocytopenia. As highlighted by Al‐Samkari and Connors, an analysis from the RIETE (Computerized Registry of Patients With Venous Thrombosis) registry found that 2.6% of patients with CAT developed fatal PE in the first 3 months of treatment despite anticoagulation, but fatal bleeding occurred in only 1%. 2 , 16 One study from 2017 reported no recurrences of VTE in patients treated with a similar algorithm, while a second study evaluating patients with acute leukemia reported two VTE recurrences out of 74 total patients (3%) between groups treated with anticoagulation, inferior vena cava filters or observation only, and a third that noted a recurrence rate of VTE in 2% of patients with leukemia treated with anticoagulation compared to 15% when anticoagulation was held. 17 , 18 , 19 In the second study, patients treated with anticoagulation for VTE had a significantly better overall survival (hazard ratio, 0.38; P = .003) compared to those patients who were observed only. 18 Likewise, our results showed numerically lower recurrent thrombosis in groups treated with anticoagulation, but likely due to the small population included in our cohort, statistical significance was not reached.
Our findings provide some insight that treatment with anticoagulation should be considered to reduce the risk of recurrent VTE. As shown by Cohen et al, 4 the risk of VTE recurrence is greatest in the first 180 days, supporting the rationale that full‐dose anticoagulation should be given with transfusion support if safely possible. In our cohort, all but three VTE occurrences occurred within 60 days of lowering the dose or withholding anticoagulation, and all but two cases occurred when patients were not receiving any form of anticoagulation, despite having ongoing cancer or active cancer treatment. This raises the question of whether anticoagulation, even dose‐reduced or prophylactic dosing, should be considered in nonbleeding patients with thrombocytopenia in this population. The longer median time to recurrence in the postintervention group is likely related to the small number of events with a wider range of days to recurrence (IQR, 10‐37 in the preintervention group, 21‐110 in the postintervention group).
Not surprisingly, there was a non–statistically significant increased risk of bleeding in the postintervention group. Our bleeding rates were similar to findings reported in a 2020 study that described acute blood loss in 13 of 99 patients (13.1%), with 12 of those episodes (12.1%) meeting criteria for major bleeding. 15 However, our rates were lower than those reported by a 2017 retrospective study by Samuelson Bannow et al, 20 which noted bleeding in 37% of cases over a 5‐year period in patients with prolonged thrombocytopenia and an acute VTE event. Most of the bleeding events occurred in patients with acute thrombosis, which aligns with previous data showing approximately one‐third of bleeding events occurring with initiation of anticoagulation. 21 , 22 , 23 The increased risk of bleeding with anticoagulation initiation combined with the greater number of acute events in the post intervention group may explain part of the reason for a greater number of hemorrhages in this group. Further, the higher number of patients with AML/MDS in the postintervention group may have also influenced bleeding risk, as this patient population generally has longer periods of thrombocytopenia, greater transfusion dependence, and coagulopathy inherent to their disease. 24 It is also notable that all major bleeds occurred in patients with hematologic malignancies, namely AML/MDS and lymphoma. These observations suggest that additional attention to bleeding risk at initiation of anticoagulation and certain cancer types should be considered and discussed with patients undergoing treatment for CAT.
Our study has several limitations. As a retrospective chart review, data collection was subject to only information listed in progress notes, lab results, and imaging reports. Further, because we evaluated only those patients with CAT and thrombocytopenia treated with anticoagulation, we cannot report the outcomes of patients with CAT who did not receive anticoagulation, an important area for further study. Specifically, this limited scope hindered our ability to assess baseline bleeding risk associated with thrombocytopenia alone and added the possibility that exclusion of patients who were not anticoagulated did not allow for adequate comparison between pre‐ and postintervention groups regarding bleeding complications on anticoagulation and recurrent thrombosis off anticoagulation. Adding to this is the statistically significant variation in follow‐up in the postintervention group, in which some bleeding or recurrent thrombosis episodes may not have been accounted for. Additionally, the sample sizes of cases included in both the pre‐ and postintervention groups were relatively small, thus limiting the power to detect differences between the groups and fully elucidate effect size of accounting for VTE acuity. Future studies should focus on larger groups of patients as well as consider expansion to additional institutions to assess feasibility of algorithm implementation on a larger scale.
Despite these limitations, our research provides a preliminary view of potential outcomes when a standardized approach is used to treat CAT in patients with thrombocytopenia. Protocol adherence was high (91%), which allowed incidence of VTE recurrence and bleeding to be assessed compared to those treated with anticoagulation at the physician’s discretion. Additionally, patients in the pre‐ and postintervention groups were identified using the same method of electronic medical record capture, minimizing selection bias. If our institution can maintain adequate compliance with the treatment protocol and other institutions are able to adopt this practice, further evaluation over time will allow more precise estimates of VTE recurrence and bleeding.
5. CONCLUSIONS
Implementation of the ISTH SCC guidance statement on anticoagulation management of CAT in patients with thrombocytopenia was feasible with good adherence. After adopting the guidance, there was no significant difference in bleeding or recurrent thrombosis outcomes after adjusting for acuity of VTE. It also continues to highlight the need for prospective studies in this population and that risks and benefits of these treatments should be cautiously weighed.
AUTHOR CONTRIBUTIONS
NH contributed to the acquisition, analysis, and verification of the data and wrote the first manuscript draft. BJ contributed to the study design and data acquisition and provided critical revision of the manuscript draft. LBK developed and supervised all aspects of the project, including data acquisition, analysis, and verification, as well as contributed to the drafting and critical revision of the manuscript. All authors provided approval for submission of the manuscript draft.
RELATIONSHIP DISCLOSURE
This article includes original work that was read and approved for submission by the authors. The authors note no financial conflicts of interest relevant to the study.
Held N, Jung B, Baumann Kreuziger L. Management of cancer‐associated thrombosis with thrombocytopenia: Impact of the ISTH guidance statement. Res Pract Thromb Haemost. 2022;6:e12726. doi: 10.1002/rth2.12726
Handling Editor: Dr Lana Castellucci
Funding information
No funding to declare.
Contributor Information
Nicole Held, Email: nheld@mcw.edu.
Benjamin Jung, @wyldsmurf.
REFERENCES
- 1. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632‐634. [DOI] [PubMed] [Google Scholar]
- 2. Monreal M, Falga C, Valdes M, et al. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2006;4:1950‐1956. [DOI] [PubMed] [Google Scholar]
- 3. Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res. 2018;164(suppl 1):S112‐S118. [DOI] [PubMed] [Google Scholar]
- 4. Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population‐based cohort study. Thromb Haemost. 2017;117:57‐65. [DOI] [PubMed] [Google Scholar]
- 5. Bick RL. Cancer‐associated thrombosis. N Engl J Med. 2003;349:109‐111. [DOI] [PubMed] [Google Scholar]
- 6. Del Principe MI, Del Principe D, Venditti A. Thrombosis in adult patients with acute leukemia. Curr Opin Oncol. 2017;29:448‐454. [DOI] [PubMed] [Google Scholar]
- 7. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117:1334‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamphuisen PW, Beyer‐Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49‐55. [DOI] [PubMed] [Google Scholar]
- 10. Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer‐associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1246‐1249. [DOI] [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network . Cancer‐associated venous thromboembolic disease (version 1.2019). Updated February 28, 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [DOI] [PubMed]
- 12. Carrier M, Prandoni P. Controversies in the management of cancer‐associated thrombosis. Expert Rev Hematol. 2017;10:15‐22. [DOI] [PubMed] [Google Scholar]
- 13. Khanal N, Bociek RG, Chen B, et al. Venous thromboembolism in patients with hematologic malignancy and thrombocytopenia. Am J Hematol. 2016;91:E468‐E472. [DOI] [PubMed] [Google Scholar]
- 14. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 15. Golightly LK, Simendinger BA, Kiser TH. Cancer‐associated thromboembolism: antithrombotic management of hospitalized patients. J Thromb Thrombolysis. 2020;49:59‐66. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019;3:3770‐3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantha S, Miao Y, Wills J, Parameswaran R, Soff GA. Enoxaparin dose reduction for thrombocytopenia in patients with cancer: a quality assessment study. J Thromb Thrombolysis. 2017;43:514‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan M, Cox TM, Nassif M, et al. Comparative outcomes of thrombocytopenic acute leukemic patients with venous thromboembolism at a comprehensive cancer center. J Thromb Thrombolysis. 2018;45:377‐385. [DOI] [PubMed] [Google Scholar]
- 19. Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leuk Lymphoma. 2017;58:2573‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samuelson Bannow BT, Walter RB, Gernsheimer TB, Garcia DA. Patients treated for acute VTE during periods of treatment‐related thrombocytopenia have high rates of recurrent thrombosis and transfusion‐related adverse outcomes. J Thromb Thrombolysis. 2017;44:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hull RD, Pineo GF, Brant RF, et al. Long‐term low‐molecular‐weight heparin versus usual care in proximal‐vein thrombosis patients with cancer. Am J Med. 2006;119:1062‐1072. [DOI] [PubMed] [Google Scholar]
- 22. Lee AY, Levine MN, Baker RI, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146‐153. [DOI] [PubMed] [Google Scholar]
- 23. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484‐3488. [DOI] [PubMed] [Google Scholar]
- 24. Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133(suppl 2):S63‐S69. [DOI] [PubMed] [Google Scholar]


