Abstract
Derosa et al.1 demonstrated that intestinal Akkermansia muciniphila predicts vigorous response to immunotherapy in non-small-cell lung cancer. Baseline level of this microbe has better value than PD-L1 expression and represents a unique approach for stratifying patients that can benefit from immunotherapy.
Derosa et al.1 demonstrated that intestinal Akkermansia muciniphila predicts vigorous response to immunotherapy in non-small-cell lung cancer. Baseline level of this microbe has better value than PD-L1 expression and represents a unique approach for stratifying patients that can benefit from immunotherapy.
Main text
Immune checkpoint inhibitors (ICIs) have revolutionized the therapeutic landscape for several cancer types and have been approved as a first-line therapy choice for individuals with non-small-cell lung cancer (NSCLC) advanced disease and high PD-L1 expression. Despite the predictive effects of PD-L1 status on stratifying ICIs response, drug failure still remains. Understanding the resistance mechanisms will unravel promising biomarkers to predict ICIs responses that would be relevant to clinical trial design and actual clinical practice. Gut microbiota can enhance the efficacy of ICIs and modulate its toxicity. It alters host nutrient metabolites, maintains the gut mucosa barrier, and participates in immunomodulation, ultimately affecting immune responses at the tumor sites.2
Akkermansia muciniphila is a common member of the human gut microorganisms, accounting for 3% to 5% of the microbial community. It potentiates anti-tumor efficacy with chemotherapy or immunotherapy and connects long-term survival across cancer types.3,4 In a recent multicentric study, Derosa et al. prospectively validated and further delineated the value of A. muciniphila in determining responses to ICI.1 A. muciniphila-positive individuals exhibited higher objective response rate (ORR; 28% versus 18%) and longer overall survival (OS; 18.8 versus 15.4 months) compared with A. muciniphila-negative patients. These differences were even more marked in the individuals that received immunotherapy alone as first-line therapy (ORR of 41% versus 19%). Besides, the relative abundance of A. muciniphila presented higher predictive efficacy for OS than PD-L1 expression.
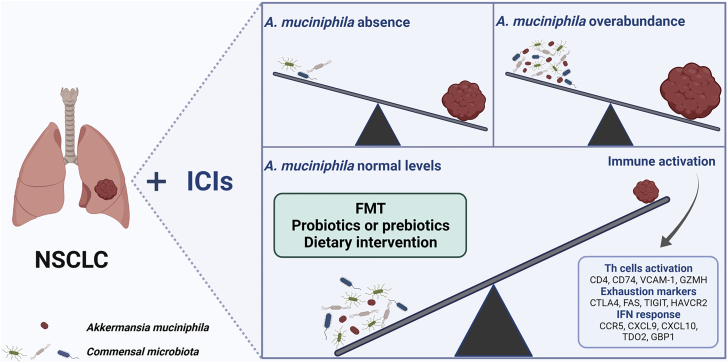
Unexpectedly, the relative abundance of A. muciniphila was found to be more influential on survival than its mere presence or absence when stratifying clinical outcomes. Affected individuals benefit from a “normal” abundance of A. muciniphila (below 4.8%), exhibiting the longest median OS (27.2 months), while those with high levels (above 4.8%) had the shortest survival (7.8 months) compared to individuals who lacked A. muciniphila (15.5 months). From immunomodulatory perspective, affected individuals colonized with A. muciniphila had stronger CD4+ T helper cells activation, increased exhaustion markers and IFN-related genes’ response in tumor biopsies, all associated with ICI responses. Microbiome profiling revealed that the A. muciniphila-positive group is associated with increased gut microbial diversity and enriched with Eubacterium hallii, B. adolescentis, and C. innocuum, and combination with this collateral composition improves prediction of ICIs response (Figure 1).
Figure 1.
The relative abundance of A. muciniphila stratifies clinical outcomes in NSCLC affected individuals
The current study oversets the concept that higher abundance of a beneficial bacteria would result in better outcomes. There are very important translational consequences because repletion of A. muciniphila should be carefully regulated to avoid impairing responses to ICIs.
This impactful study now triggers further questions. Because the findings are related to NSCLC, the efficacy of A. muciniphila to predict ICIs response in other cancer types needs to be assessed. Furthermore, the mechanisms behind the dichotomous opposite effect of low versus high levels of Akkermansia are not yet fully elucidated, with the possibility that patients with high levels represent a subset with gut injury and ongoing repair. Not less important, gut microbes can affect intratumoral bacteria, which in turn can also directly affect local immune responses.5 Thus, the mechanisms of A. muciniphila and related bacteria modulating anti-tumor responses remain to be deeply understood. Because A. muciniphila co-colonized composition reshapes the gut ecosystem and rebuilds bacterial diversity, it will be worthwhile to explore the roles of those bacteria on enhancing anti-tumor responses in mouse models.
Because the relative abundance of A. muciniphila presents striking differential responses to ICI, how can it be finely tuned? A couple of tools to manipulate A. muciniphila in gut microbiota have been described, including fecal microbiota transplantation (FMT), probiotics, prebiotics, and dietary interventions. Although FMT has been proposed as a strategy to overcome resistance to ICIs, the criteria for donor selection have to be further dissected. Would A. muciniphila-enriched healthy donors or ICI responders represent the ideal donor? Recent pilot clinical trials have reflected optimistic results in response to reintroduction of ICIs with FMT in the context of immunotherapy-resistant melanoma.6,7 Several ongoing studies (NCT04924374, NCT05286294, NCT05251389) will hopefully provide more evidence of FMT affecting ICIs response as well as immune and transcriptome changes in gut and tumor. Studies will reveal whether targeting particular microbes or global changes in commensal microbiota may represent better approaches to target ICIs resistance. A. muciniphila-based probiotic or prebiotic supplementation accompanied with its symbiont may recruit beneficial microbe to enhance ICI response but, if present at over-abundant levels, may be counterproductive. The type of diet (calorie-restriction diet, high-fiber diet) could also change gut microbial composition and increase A. muciniphila abundance, although at a less predictable rate, representing another approach of easy access to improving ICI efficacy with likely low-potential toxicity.8,9 A crucial point to take away from this study is that antibiotics significantly decreased the OS in NSCLC, independently from A. muciniphila status, which limits their potential utility to fine-tune A. muciniphila levels. The impact of gut microbiota shifted by antibiotics exposure should be further explored, and the dominant subtypes of antibiotics that dramatically eliminate A. muciniphila and its co-colonization should be studied in the context of ICI.3
In conclusion, individual baseline gut microbiota profiling may represent promising biomarkers to predict ICIs responses as well as immune-related adverse effects.7,10 Understanding and addressing the specific bacteria and roles in contributing to better outcomes should be prioritized.
Acknowledgments
F.M. is supported by NCI (1R37CA237384–01A1), CPRIT (RP200173), V Foundation Translational Award, Andrew Sabin Family Fellowship, and MD Anderson Philanthropic Moonshot Program (MDACC).
Declaration of interests
F.M. is a scientific advisory board member at Neologics Bio.
References
- 1.Derosa L., Routy B., Thomas A.M., Iebba V., Zalcman G., Friard S., Mazieres J., Audigier-Valette C., Moro-Sibilot D., Goldwasser F., et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022;28:315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M., Agdashian D., Terabe M., Berzofsky J.A., Fako V., et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 4.Lee K.A., Thomas A.M., Bolte L.A., Björk J.R., de Ruijter L.K., Armanini F., Asnicar F., Blanco-Miguez A., Board R., Calbet-Llopart N., et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022;28:535–544. doi: 10.1038/s41591-022-01695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riquelme E., Zhang Y., Zhang L., Montiel M., Zoltan M., Dong W., et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 7.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Schwartzenberg R.J., Bisanz J.E., Lyalina S., Spanogiannopoulos P., Ang Q.Y., Cai J., Dickmann S., Friedrich M., Liu S.Y., Collins S.L., et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature. 2021;595:272–277. doi: 10.1038/s41586-021-03663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam K.C., Araya R.E., Huang A., Chen Q., Di Modica M., Rodrigues R.R., Lopès A., Johnson S.B., Schwarz B., Bohrnsen E., et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–5356.e21. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews M.C., Duong C.P.M., Gopalakrishnan V., Iebba V., Chen W.S., Derosa L., Khan M.A.W., Cogdill A.P., White M.G., Wong M.C., et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021;27:1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]