Abstract
Using a multimodal approach toward developing a new CD70-targeted Chimeric antigen receptor (CAR) T cell in acute myeloid leukemia, Leick et al.1 report on their synergetic strategy, which incorporates both CAR T cell construct modifications with enhancement of leukemia antigen expression to improve CAR T cell functionality.
Using a multimodal approach toward developing a new CD70-targeted Chimeric antigen receptor (CAR) T cell in acute myeloid leukemia, Leick et al.1 report on their synergetic strategy, which incorporates both CAR T cell construct modifications with enhancement of leukemia antigen expression to improve CAR T cell functionality.
Main text
The development of effective therapies for those with relapsed/refractory (r/r) acute myeloid leukemia (AML) remains challenging, warranting a need for novel approaches. Chimeric antigen receptor (CAR) T cells represent one such strategy. Based on the impressive outcomes with CAR T cells in B cell malignancies and targetable antigens in AML, extending the therapeutic index of CAR T cells to AML holds promise.
A major limitation in targeting AML, however, is that on-target, off-tumor toxicities may not be tolerable, particularly because antigens expressed on AML (e.g., CD123 and CD33) are often expressed on normal hematopoietic progenitors and CAR T cell targeting may impair subsequent hematopoiesis. Additionally, the early experience with CAR T cells in AML has been largely disappointing,2 collectively necessitating further study in identifying more optimal targets and ways to improve efficacy.
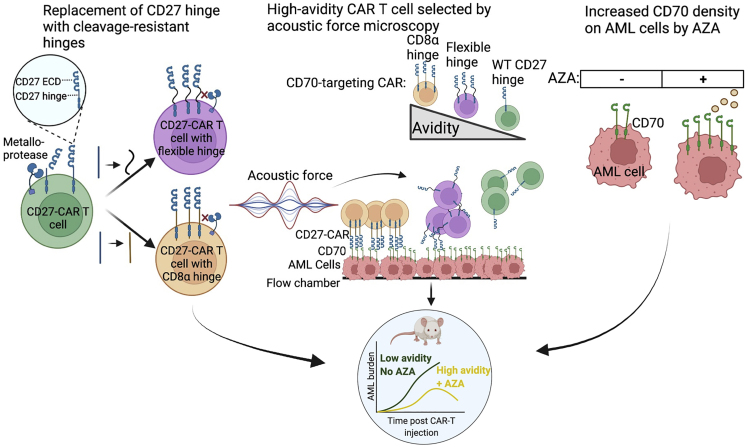
To address the challenge of CAR T cells in AML, Leick and colleagues1 targeted the CD70-CD27 axis, specifically because CD70 is both absent on normal hematopoietic cells and highly expressed on AML blasts,3 and the experience with antibody-based approaches targeting CD70 has been promising.4 They subsequently used an orthogonal approach to both rationally design CAR T cells with improved efficacy and incorporate pharmacodynamic regulation of leukemia antigen expression to synergize with CAR T cell function (Figure 1).
Figure 1.
Engineering CD70-targeting CAR T cells to enhance in vivo activity against AML
CD70 is targeted through a CAR bearing its ligand, CD27. To prevent metalloprotease-mediated cleavage of CD27, its hinge was replaced with a panel of hinges. Leick et al. found that a CD27 CAR with both a flexible hinge and a CD8α hinge had cytotoxic potential, but notably, the avidities of the two CARs differed significantly, as measured by acoustic force microscopy. This higher avidity CD27-CD8α-CAR exhibited stronger in vivo activity when CD70 antigen density on the AML cells was increased by AZA. These modifications contributed to durable and predictable in vivo results.
Focusing first on CAR T cell design, they homed in on the hinge region, which was once regarded simply as a linker between the CAR binding and transmembrane domains. However, the CAR hinge has recently been subject to intense investigation as it can modulate CAR responsiveness against antigenlow tumors.5 Specifically, a CD19 CAR T cell containing a CD28-derived hinge exhibited greater cytotoxic potential against CD19low tumor compared to CARs with a CD8α hinge.6 Moreover, increasing CD8α hinge length in a CD19 CAR T cell decreased cytokine production while maintaining in vivo cytotoxicity in a murine model.7
Thus, utilizing truncated CD27 as the CAR antigen-binding domain, which may have improved functionality over alternate scFvs,3 Leick and colleagues1 incorporated a CD8α hinge to CD70-targeting CAR T cells. Additionally, because CD27 can be proteolytically cleaved from the cell membrane by matrix metalloproteases,3 potentially limiting its efficacy, an in silico tool to approximate the location of the cleavage site to the CD27 hinge8 was used to generate a panel of CARs with cleavage-resistant hinges. Using these strategies, there was augmented avidity, resulting in improved CAR T cell expansion and enhanced efficacy, thereby sustaining durable in vivo responses in mice.1 Furthermore, despite equal in vitro cytotoxic potential, in vivo treatment of AML-bearing mice with CD8α hinge-containing CAR T cells resulted in improved anti-leukemia efficacy and a significantly increased probability of survival.
This important distinction––differentiating in vitro assays from in vivo responses––is critical to the field, but how these results translate to or predict human response remains uncertain. In the present study, cleavage-resistant constructs harboring a flexible linker as compared to the CD8α hinge could not be differentiated by conventional cytotoxicity assays. However, acoustic force microscopy, ranking the avidity of CAR T cells by their ability to resist increasing acoustic forces and remain bound to plate-bound leukemic cells,9 provided a good projection of in vivo responses. Furthermore, using a microfluidics platform, they were able to directly evaluate CAR-ligand interactions, highlighting the importance of biophysical approaches in evaluating CAR T cell function.9 Thus, the authors highlight potentially important strategies for future preclinical testing of novel CAR T cell constructs.
Lastly, with recognition that sole dependence on CAR T cells may not be sufficient for efficacy in AML and that antigen-dim escape can result in treatment failure, pharmacologic modulation aimed at enhancing CAR T cell functionality by augmenting tumor antigen expression was pursued. By increasing CD70 expression on AML through the hypomethylation of the CD70 promoter,4 pre-treatment with azacytidine (AZA) enhanced CD70 expression and anti-CD70 CAR T cell activity in preclinical in vivo models, supporting the utilization of AZA with a CD70-targeted CAR T cell therapy. Supported by the positive results from the combination of AZA with cusatuzumab, an anti-CD70 antibody,4 and the widespread use of AZA in r/r patients with AML to control disease, AZA may also serve as a useful therapeutic in bridging patients with r/r AML to CD70-targeted CAR T cells.
Although the adage of “form follows function” typically applies to the field of design, this premise has broad applicability in CAR T cell development. While significant work delineating the parameters of the hinge that influence CAR avidity and immune synapse formation remains, CAR T cell construction has to be configured in the context of eradicating the disease that it is targeting. How other factors influencing avidity (e.g., cytoskeleton, co-receptor binding, and cell-cell adhesion) can be leveraged to further enhance CAR activity warrants further study. Importantly, the work by Leick et al.1 represents an important step forward in addressing the roadblocks faced by CAR T cell therapeutics for AML. The strategic design, together with the use of AZA to increase CD70 expression on leukemia, represents a synergistic approach toward testing a new therapeutic option in patients with r/r AML.
Acknowledgments
Authors would like to thank Dr. Naomi Taylor, Pediatric Oncology Branch, National Cancer Institute for her insightful review of this submission. This work was supported in part by the Intramural Research Program, Center of Cancer Research, National Cancer Institute, National Institutes of Health (ZIA BC 011927, N.N.S.). The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services or imply endorsement by the US Government.
Declaration of interests
The authors declare no competing interests.
References
- 1.Leick M.B., Silva H., Scarfo I., Larson R., Choi B.D., Bouffard A.A., Gallagher K., Schmidts A., Bailey S.R., Kann M.C., et al. Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia. Cancer Cell. 2022;40:494–508. doi: 10.1016/j.ccell.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maucher M., Srour M., Danhof S., Einsele H., Hudecek M., Yakoub-Agha I. Current limitations and perspectives of chimeric antigen receptor-T-cells in acute myeloid leukemia. Cancers (Basel) 2021;13:6157. doi: 10.3390/cancers13246157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer T., Parikh K., Sharma S., Omer B., Sedloev D., Chen Q., Angenendt L., Schliemann C., Schmitt M., Muller-Tidow C., et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. 2021;138:318–330. doi: 10.1182/blood.2020008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riether C., Pabst T., Hopner S., Bacher U., Hinterbrandner M., Banz Y., Muller R., Manz M.G., Gharib W.H., Francisco D., et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020;26:1459–1467. doi: 10.1038/s41591-020-0910-8. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K., Tsunei A., Kusabuka H., Ogaki E., Tachibana M., Okada N. Hinge and transmembrane domains of chimeric antigen receptor regulate receptor expression and signaling threshold. Cells. 2020;9:1182. doi: 10.3390/cells9051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majzner R.G., Rietberg S.P., Sotillo E., Dong R., Vachharajani V.T., Labanieh L., Myklebust J.H., Kadapakkam M., Weber E.W., Tousley A.M., et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.cd-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alabanza L., Pegues M., Geldres C., Shi V., Wiltzius J.J., Sievers S.A., Yang S., Kochenderfer J.N. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol. Ther. 2017;25:2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieplak P., Strongin A.Y. Matrix metalloproteinases - from the cleavage data to the prediction tools and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1952–1963. doi: 10.1016/j.bbamcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitters G., Kamsma D., Thalhammer G., Ritsch-Marte M., Peterman E.J.G., Wuite G.J.L. Acoustic force spectroscopy. Nat. Methods. 2015;12:47–50. doi: 10.1038/nmeth.3183. [DOI] [PubMed] [Google Scholar]