Abstract
Background
Population studies show that the use of swimming pools is associated with the risk of asthma and allergic diseases among children. Our objective was to explore the associations between blood trihalomethane (THM) concentrations and asthma among US adolescents, and assess to what extent the association is modified by active tobacco smoke exposure.
Methods
We included 2359 adolescents aged 12–19 years with measured blood concentrations of chloroform (trichloromethane (TCM)), bromodichloromethane (BDCM), dibromochloromethane (DBCM) and bromoform (tribromomethane (TBM)) from the National Health and Nutrition Examination Survey 2005–2012. Logistic regression models were fitted to assess the odds ratios for the association of blood THM concentrations (three or four categories) with the risk of self-reported current and ever (lifetime) asthma.
Results
Blood DBCM concentrations were associated with a higher risk of ever asthma among all adolescents (OR 1.54 (95% CI 1.07–2.21), comparing the extreme exposure categories). The relationship was stronger among adolescents exposed to tobacco smoke (OR 3.96 (95% CI 1.89–8.30), comparing the extreme exposure categories). We also found positive relationships between blood brominated THM concentrations (sum of BDCM, DBCM and TBM) and risk of ever asthma and between blood DBCM and brominated THM concentrations and risk of current asthma among adolescents with tobacco smoke exposure. The relative excess risk of ever asthma due to the interaction between high blood DBCM and brominated THM concentrations and tobacco smoke exposure was 1.87 (95% CI 0.30–3.43) and 0.78 (95% CI 0.07–1.49), respectively.
Conclusions
Exposure to THMs is associated with a higher risk of asthma in adolescents, particularly among those exposed to tobacco smoke.
Short abstract
Among a representative sample of 2359 US adolescents, we found that exposure to THMs was associated with a greater risk of asthma, particularly among those who were co-exposed to tobacco smoke https://bit.ly/3mpHxgq
Introduction
Asthma, characterised by repeated episodes of wheezing, breathlessness, chest tightness and coughing, is the most common chronic lung disease during childhood. Globally, approximately 14% of children have asthma [1]. Growing evidence shows that asthma predisposes children and adolescents to a myriad of long-term sequelae, such as irrecoverable loss of lung function and chronic obstructive pulmonary disease, making it chiefly important to identify potentially modifiable risk factors to improve prevention strategies. The International Study of Asthma and Allergies in Childhood has revealed that the prevalence of allergic diseases including asthma varies greatly between regions, countries and centres within a city or country, suggesting the potential role of local environmental factors [2].
Disinfection byproducts are a class of chemicals formed when disinfectants react with organic matter in source waters. Because disinfection of water is globally used to kill disease-causing microbes in the distribution system, humans are ubiquitously exposed to disinfection byproducts through ingestion, inhalation and dermal absorption during daily consumption and water-use activities. Several population studies have shown that chlorinated swimming pool attendance is associated with a higher risk of asthma and allergic diseases among children and adolescents [3–8]. However, some studies have reported conflicting results [9–11]. Most previous studies assessed disinfection byproduct exposures based on reports of swimming frequency from questionnaires or environmental monitoring data, which were prone to result in exposure misclassification because they did not account for factors that could influence individual intake and disinfection byproduct metabolism. More importantly, while the strong association of tobacco use with asthma risk is well documented [12], no study has explored whether tobacco smoke exposure modifies the association between disinfection byproducts and asthma.
Trihalomethanes (THMs) are the most common species of disinfection byproducts in chlorinated water, accounting for 66% of disinfection byproduct compounds. Blood concentrations represent integrative measures of exposure from multiple routes and are sensitive to low levels of exposure [13]. Although blood THM concentrations decrease within minutes to hours after exposure, they are believed to be relatively stable due to the high frequency of daily water-use activities and slower partitioning out of adipose tissue [14]. In this study, we explored blood THM concentrations in relation to asthma among a representative sample of civilian, noninstitutionalised US adolescents and assessed to what extent the association was modified by tobacco smoke exposure.
Methods
Study population
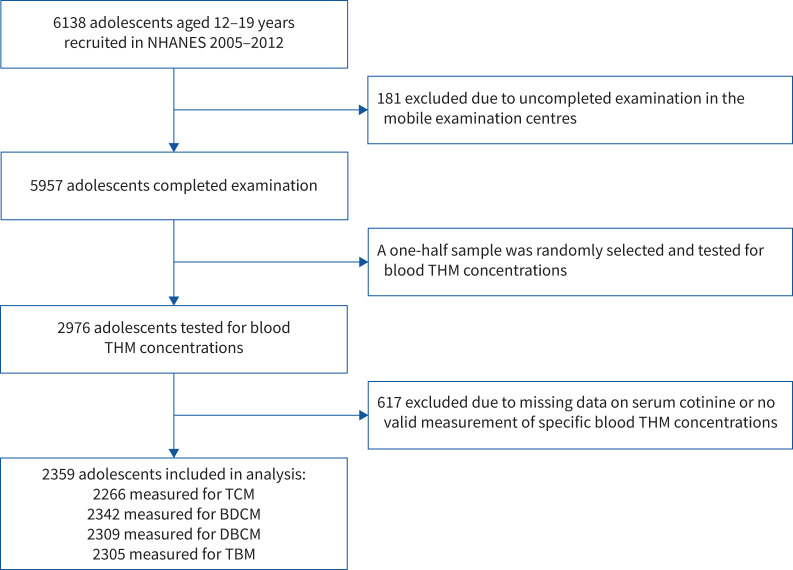
Data were pooled from four independent cycles (2005–2006, 2007–2008, 2009–2010 and 2011–2012) of the National Health and Nutrition Examination Survey (NHANES), a cross-sectional population-based survey designed to assess the health and nutritional status in the noninstitutionalised US population. The collection of NHANES data has been described in detail elsewhere [15]. A randomly sampled subset (one-half) of adolescents aged 12–19 years who participated in NHANES 2005–2012 was tested for blood THM concentrations (n=2976). To be included in our current study, participants had to provide at least one valid measurement of specific blood THM concentrations and had no missing data on tobacco smoke exposure (i.e. serum cotinine). Finally, 2359 adolescents were eligible for inclusion (figure 1). Most demographic and lifestyle characteristics were similar between included and excluded adolescents, indicating that the participants in our analytical sample were representative of the broader NHANES population (supplementary table S1). NHANES was approved by the Ethics Review Board of the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC). All participants provided informed consent before participation.
FIGURE 1.
Study population flowchart. NHANES: National Health and Nutrition Examination Survey; THM: trihalomethane; TCM: trichloromethane (chloroform); BDCM: bromodichloromethane; DBCM: dibromochloromethane; TBM: tribromomethane (bromoform).
Measurement of blood THMs
Procedures for peripheral blood sampling, processing and determination have been described elsewhere [16]. In brief, blood concentrations of four specific THMs (i.e. chloroform (trichloromethane (TCM)), bromodichloromethane (BDCM), dibromochloromethane (DBCM) and bromoform (tribromomethane (TBM)) were measured via solid-phase microextraction gas chromatography and mass spectrometry. Blood brominated trihalomethanes (Br-THMs) were the total concentrations of BDCM, DBCM and TBM, and blood total trihalomethanes (TTHMs) were the summarised concentrations of TCM and Br-THMs. For samples with values lower than the limit of detection (LOD), data were replaced with LOD/√2.
Definition of asthma
Data on asthma and any related symptoms were collected by trained interviewers using a standardised questionnaire. According to the recommendation from a pooled European birth cohort study [17], lifetime or ever asthma was defined by respondents giving an affirmative response to the question, “Has a doctor or other health professional ever told you that you have asthma?”. Current asthma was defined as participants further affirmatively replying to the question “In the past 12 months have you had wheezing or whistling in your chest?”. Adolescents without physician-diagnosed asthma were treated as the comparison.
Tobacco smoke exposure ascertainment
Tobacco smoke exposure was ascertained by serum cotinine and a questionnaire regarding recent tobacco use. Serum cotinine is the leading metabolite of nicotine and can thus be used as a marker for tobacco smoke exposure [18]. Serum cotinine was measured by isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionisation tandem mass spectrometry. For participants aged ≥12 years, they were also asked to report if they had consumed tobacco or nicotine products (e.g. cigarettes, pipes, cigars, chewing tobacco, snuff, nicotine patches and nicotine gum) in the past 5 days. Adolescents were considered exposed to tobacco smoke if their serum cotinine concentrations were >10 ng·mL−1 or they had self-reported consumption of tobacco or nicotine products in the past 5 days [19].
Covariates
Information on age, sex, race/ethnicity, family history of asthma, physical activity, sampling season, current allergic symptoms (i.e. hay fever, rhinitis, allergy, itchy rash and wheeze in the past year), family income and water-use activities (e.g. swimming, showering and bathing) were collected at enrolment. Height and weight were measured and body mass index (BMI) was calculated (kg·m−2). Age-specific BMI z-scores were calculated to obtain standardised values according to the growth charts for the US developed by the Centers for Disease Control and Prevention [20]. Family income was assessed with the income to poverty ratio, which is a ratio of family income to poverty threshold specific to family size, year and state. Leisure-time physical activity was defined as the total hours of moderate-to-vigorous activity during leisure-time per week. Missing data on BMI z-scores (n=31 (1.31%)), family income to poverty ratio (n=154 (6.52%)) and physical activity (n=10 (0.4%)) were imputed with median values.
Statistical analysis
Because tobacco smoke exposure showed a strong association with asthma among adolescents from NHANES [18], the analyses were first conducted among all participants and then performed separately among adolescents with and without tobacco smoke exposure, with adjustment for complex, multistage sampling survey designs (e.g. sampling weights, stratification and clusters). Descriptive statistics were performed to describe participants’ demographic characteristics and distribution of blood THM concentrations according to tobacco smoke exposure. The difference in demographic characteristics between subgroups was assessed using Rao–Scott Chi-squared tests for categorical variables and t-tests for continuous variables.
Both crude (unadjusted) and adjusted logistic regression models were fit to assess the odds ratios and 95% confidence intervals for the association of blood THM concentrations with the risk of ever or current asthma. Adolescents were assigned to quartiles for TCM, Br-THMs and TTHMs, whereas three groups were created for BDCM (tertiles), DBCM (<50th, 50–75th and >75th) and TBM (<75th, 75–87.5th and >87.5th), given that a relatively relevant proportion of observations were below the LOD. Tests for linear trends were conducted by modelling categories of THM concentrations as ordinal variables using the median values within each category. Because tobacco use is strongly associated with asthma [12], we also examined the interaction between blood THM concentrations and tobacco smoke exposure on both multiplicative and additive scales. The multiplicative interaction was assessed using the likelihood ratio test by comparing the fit of models with and without the interaction term between each THM and tobacco smoke exposure. We calculated the relative excess risk due to interaction (RERI) to assess the additive interaction by substituting odds ratios for the relative risks in the RERI equation [21]. Because a higher prevalence of allergy was reported among male swimmers than females [22], we also conducted a stratified analysis to explore potential modification by sex.
Covariates were selected a priori and were then included in multivariable models if their inclusion changed the age-adjusted odds ratios by ≥5%. Final logistic regression models were adjusted for age (continuous), sex (male versus female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American and Other), BMI z-scores (continuous), family income to poverty ratio (0–1.0, 1.1–3.0 or >3.0), family history of asthma (yes versus no), swimming/hot tub/steam room use within 72 h (yes versus no) and survey cycles (2005–2006, 2007–2008, 2009–2010 or 2011–2012).
Several sensitivity analyses of the association between blood THMs and ever asthma were conducted. First, we excluded adolescents who had missing data on BMI z-scores or family income to poverty ratio to assess the influence of the imputation method. Second, we assessed the potential influence of peak exposures by 1) excluding adolescents who spent any time at a swimming pool/hot tub/steam room in the past 72 h, and 2) additionally including the timing of examination session (morning, afternoon and evening), sampling season (November through April versus May through October) and time interval since the last shower/bath (≤2, 3–6, 7–14 and >14 h) as covariates in the adjusted models. Finally, we tested whether recent allergy and physical activity affected our findings by additionally including current allergic symptoms (yes versus no) and leisure-time physical activity (<3, 3–7 and >7 h) in the adjusted models. All data analyses were performed using the PROC SURVEY procedure with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Participant characteristics
Among 2359 adolescents aged 12–19 years (mean 15.5 (95% CI 15.3–15.6) years), 441 (20.4%) were ever diagnosed with asthma and 196 (9.1%) reported current asthma (table 1). Compared with adolescents without tobacco smoke exposure, adolescents who were exposed to tobacco smoke tended to be older (mean 17.1 (95% CI 16.8–17.3) versus 15.2 (95% CI 15.0–15.3) years) and were more likely to be male (240 (60.6%) versus 962 (49.6%)), Non-Hispanic White (144 (67.3%) versus 497 (56.7%)) and have a family income to poverty ratio <1.0 (162 (34.9%) versus 572 (21.3%)) (table 1). However, they were less likely to spend any time in a swimming pool/hot tub/steam room in the past 72 h (15 (5.1%) versus 116 (9.7%)).
TABLE 1.
Characteristics according to tobacco smoke exposure for study participants in the National Health and Nutrition Examination Survey 2005–2012
| All (n=2359) | Tobacco smoke exposure | p-value | ||
| Yes (n=378) | No (n=1981) | |||
| Survey cycle | 0.29 | |||
| 2005–2006 | 882 (24.6) | 156 (28.0) | 726 (24.0) | |
| 2007–2008 | 457 (24.5) | 67 (25.4) | 390 (24.3) | |
| 2009–2010 | 526 (22.7) | 88 (23.6) | 438 (22.5) | |
| 2011–2012 | 494 (28.3) | 67 (23.0) | 427 (29.3) | |
| Age (years) | 15.5 (15.3–15.6) | 17.1 (16.8–17.3) | 15.2 (15.0–15.3) | <0.0001 |
| BMI (z-score) | 0.62 (0.57–0.68) | 0.57 (0.41–0.73) | 0.63 (0.56–0.70) | 0.52 |
| Sex | 0.001 | |||
| Male | 1202 (51.4) | 240 (60.6) | 962 (49.6) | |
| Female | 1157 (48.6) | 138 (39.4) | 1019 (50.4) | |
| Race/ethnicity | 0.002 | |||
| Non-Hispanic White | 641 (58.4) | 144 (67.3) | 497 (56.7) | |
| Non-Hispanic Black | 684 (14.8) | 112 (13.8) | 572 (15.0) | |
| Mexican American | 646 (13.5) | 70 (8.3) | 576 (14.6) | |
| Other | 388 (13.2) | 52 (10.6) | 336 (13.7) | |
| Family income to poverty ratio | 0.0004 | |||
| 0–1.0 | 734 (23.6) | 162 (34.9) | 572 (21.3) | |
| 1.1–3.0 | 859 (35.6) | 128 (37.8) | 731 (35.1) | |
| >3.0 | 612 (40.9) | 70 (27.3) | 542 (43.6) | |
| Leisure-time physical activity (h per week) | 0.07 | |||
| <3 | 1032 (39.3) | 176 (46.7) | 856 (37.8) | |
| 3–7 | 539 (25.1) | 75 (21.6) | 464 (25.8) | |
| >7 | 778 (35.6) | 124 (31.7) | 654 (36.4) | |
| Current allergic conditions | 0.34 | |||
| Yes | 630 (27.3) | 28 (8.5) | 171 (10.4) | |
| No | 1729 (72.7) | 350 (91.5) | 1810 (89.6) | |
| Examination session | 0.71 | |||
| Morning | 1174 (50.3) | 194 (51.3) | 980 (50.1) | |
| Afternoon | 777 (31.7) | 124 (33.0) | 653 (31.5) | |
| Evening | 408 (18.0) | 60 (15.6) | 348 (18.4) | |
| Time interval since last shower/bath (h) | 0.11 | |||
| ≤2 | 312 (14.2) | 56 (16.3) | 256 (13.8) | |
| 3–6 | 578 (23.2) | 102 (24.9) | 476 (22.9) | |
| 7–14 | 584 (25.0) | 105 (28.2) | 479 (24.3) | |
| >14 | 885 (37.6) | 115 (30.6) | 770 (39.0) | |
| Sampling season | 0.09 | |||
| November–April | 1222 (42.8) | 176 (37.8) | 1046 (43.8) | |
| May–October | 1137 (57.2) | 202 (62.2) | 935 (56.2) | |
| Swimming pool/hot tub/steam room use within 72 h | 0.02 | |||
| Yes | 131 (8.9) | 15 (5.1) | 116 (9.7) | |
| No | 2228 (91.1) | 363 (94.9) | 1865 (90.3) | |
| Family history of asthma | 0.48 | |||
| Yes | 455 (22.3) | 80 (24.1) | 375 (21.9) | |
| No | 1904 (77.7) | 298 (75.9) | 1606 (78.1) | |
| Ever (lifetime) asthma | 0.11 | |||
| Yes | 441 (20.4) | 93 (24.6) | 348 (19.6) | |
| Never | 1918 (79.6) | 285 (75.4) | 1633 (80.4) | |
| Current asthma | 0.26 | |||
| Yes | 196 (9.1) | 45 (11.0) | 151 (8.7) | |
| No | 2163 (90.9) | 333 (89.0) | 1830 (91.3) | |
Data are presented as n (%) or mean (95% CI), unless otherwise stated; accounting for complex, multistage sampling survey designs (e.g. sampling weights, stratification and clusters) to ensure nationally representative estimation. 31, 154 and 10 participants had missing information on body mass index (BMI) z-score, family income to poverty ratio and levels of leisure-time physical activity, respectively. p-value was calculated by the Rao–Scott Chi-squared test and t-test for categorical and continuous variables, respectively.
Distribution of blood THMs
TCM, BDCM, DBCM and TBM were detected in 90.0%, 71.1%, 51.5% and 27.8% of the total study population, respectively (table 2). The median blood concentrations of TCM, BDCM, DBCM, TBM, Br-THMs and TTHMs were 6.6, 1.1, 0.4, 0.7, 2.7 and 10.6 pg·mL−1, respectively. The median blood THM concentrations were similar according to tobacco smoke exposure, although the detection rate of TCM, BDCM, DBCM and TBM was slightly higher among adolescents without tobacco exposure. Participants’ demographic characteristics and lifestyle factors were mostly similar across quartiles of blood TTHM concentrations, except for survey cycle, race/ethnicity, time interval since last shower/bath and swimming pool/hot tub/steam room use within 72 h (supplementary table S2).
TABLE 2.
Distribution of blood trihalomethane (THM) concentrations according to tobacco smoke exposure (National Health and Nutrition Examination Survey 2005–2012)
| TCM (pg·mL−1) | BDCM (pg·mL−1) | DBCM (pg·mL−1) | TBM (pg·mL−1) | Br-THMs (pg·mL−1) | TTHMs (pg·mL−1) | |
| All (n=2359) | ||||||
| n | 2266 | 2342 | 2309 | 2305 | 2253 | 2161 |
| % >LOD | 90.0 | 71.1 | 51.5 | 27.8 | NA | NA |
| GM | 7.0 | 1.3 | 0.9 | 1.0 | 3.6 | 11.6 |
| Median (IQR) | 6.6 (3.1–14.0) | 1.1 (0.4–2.9) | 0.4 (0.4–1.6) | 0.7 (0.7–1.0) | 2.7 (1.6–5.8) | 10.6 (5.6–22.2) |
| Tobacco smoke exposure (n=378) | ||||||
| n | 366 | 374 | 366 | 373 | 361 | 349 |
| % >LOD | 86.6 | 67.7 | 47.0 | 24.9 | NA | NA |
| GM | 6.8 | 1.3 | 0.9 | 0.9 | 3.5 | 11.6 |
| Median (IQR) | 7.0 (2.6–13.9) | 1.0 (0.4–2.9) | 0.4 (0.4–1.6) | 0.7 (0.7–0.7) | 2.6 (1.6–5.4) | 10.6 (5.0–22.3) |
| No tobacco smoke exposure (n=1981) | ||||||
| n | 1900 | 1968 | 1943 | 1932 | 1892 | 1812 |
| % >LOD | 90.6 | 71.8 | 52.4 | 28.4 | NA | NA |
| GM | 7.0 | 1.3 | 0.9 | 1.0 | 3.6 | 11.6 |
| Median (IQR) | 6.5 (3.2–14.0) | 1.1 (0.4–2.9) | 0.4 (0.4–1.6) | 0.7 (0.7–0.7) | 2.7 (1.6–5.8) | 10.6 (5.6–22.2) |
TCM: trichloromethane (chloroform); BDCM: bromodichloromethane; DBCM: dibromochloromethane; TBM: tribromomethane (bromoform); Br-THMs: sum of BDCM, DBCM and TBM; TTHMs: sum of TCM and Br-THMs; LOD: limit of detection; GM: geometric mean; IQR: interquartile range; NA: not applicable.
Blood THMs and asthma
In the unadjusted models, blood THM concentrations were not associated with the risk of ever asthma among adolescents (table 3). After adjusting for tobacco smoke exposure and other confounders, however, blood DBCM and Br-THM concentrations were associated with a higher risk of ever asthma among all adolescents (OR 1.54 (95% CI 1.07–2.21) and 1.46 (95% CI 0.98–2.17), respectively, comparing the extreme exposure categories). When the analysis was stratified by tobacco smoke exposure, we observed a higher risk of ever asthma across the categories of blood DBCM and Br-THM concentrations among adolescents who had tobacco smoke exposure both in the unadjusted and adjusted logistic regression models (all ptrend<0.05). In the adjusted models for smokers, adolescents in the highest exposure category of DBCM and Br-THMs had ORs for ever asthma of 3.96 (95% CI 1.89–8.30) and 3.28 (95% CI 1.43–7.53), respectively, compared with adolescents in the lowest exposure category (table 3). These associations, however, were not observed among adolescents without tobacco smoke exposure. The multiplicative and additive interaction results suggested that the associations of blood DBCM and Br-THM concentrations with ever asthma were modified by tobacco smoke exposure (all pinteraction<0.05) (tables 3 and 4). A strong relative excess risk of ever asthma due to the interaction of high blood DBCM and Br-THM concentrations with tobacco smoke exposure was observed: 1.87 (95% CI 0.30–3.43) and 0.78 (95% CI 0.07–1.49), respectively (table 4).
TABLE 3.
Odds ratios of ever asthma in relation to blood trihalomethane (THM) concentrations among US adolescents according to tobacco smoke exposure (National Health and Nutrition Examination Survey 2005–2012)
| Blood THM (pg·mL−1) | All (n=2359) | Tobacco smoke exposure (n=378) | No tobacco smoke exposure (n=1981) | pinteraction-value for adjusted model | ||||||
| n/N # | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) ¶ | n/N # | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) + | n/N # | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) + | ||
| TCM | 0.32 | |||||||||
| Q1 (1.48–4.11) | 109/567 | Reference | Reference | 22/95 | Reference | Reference | 87/472 | Reference | Reference | |
| Q2 (4.12–8.34) | 106/566 | 0.73 (0.53–1.01) | 0.76 (0.57–1.03) | 22/86 | 0.89 (0.41–1.94) | 0.82 (0.40–1.73) | 84/480 | 0.71 (0.49–1.01) | 0.73 (0.52–1.03) | |
| Q3 (8.35–16.90) | 106/561 | 0.74 (0.50–1.09) | 0.79 (0.51–1.23) | 23/99 | 0.66 (0.27–1.59) | 0.54 (0.22–1.33) | 83/462 | 0.75 (0.51–1.11) | 0.86 (0.52–1.41) | |
| Q4 (>16.90) | 103/572 | 0.79 (0.53–1.19) | 0.80 (0.53–1.20) | 24/86 | 1.36 (0.52–3.55) | 1.32 (0.53–3.32) | 79/486 | 0.69 (0.47–1.02) | 0.72 (0.48–1.08) | |
| ptrend-value§ | 0.45 | 0.48 | 0.46 | 0.43 | 0.17 | 0.26 | ||||
| BDCM | 0.05 | |||||||||
| T1 (0.44–0.75) | 159/778 | Reference | Reference | 30/136 | Reference | Reference | 129/642 | Reference | Reference | |
| T2 (0.76–2.50) | 148/784 | 0.88 (0.61–1.28) | 0.83 (0.57–1.21) | 26/120 | 1.16 (0.55–2.44) | 0.99 (0.40–2.41) | 122/664 | 0.84 (0.56–1.24) | 0.78 (0.51–1.19) | |
| T3 (>2.50) | 132/780 | 0.94 (0.69–1.29) | 0.94 (0.69–1.29) | 37/118 | 1.74 (0.86–3.55) | 1.66 (0.77–3.57) | 95/662 | 0.68 (0.48–0.94) | 0.82 (0.59–1.14) | |
| ptrend-value§ | 0.25 | 0.95 | 0.12 | 0.14 | 0.02 | 0.35 | ||||
| DBCM | 0.03 | |||||||||
| <50th (0.44–0.65) | 221/1155 | Reference | Reference | 34/198 | Reference | Reference | 187/957 | Reference | Reference | |
| 50–75th (0.66–1.87) | 105/578 | 0.88 (0.59–1.31) | 1.00 (0.65–1.54) | 27/91 | 1.65 (0.80–3.41) | 1.61 (0.77–3.36) | 78/487 | 0.78 (0.50–1.22) | 0.90 (0.54–1.49) | |
| >75th (>1.87) | 107/576 | 1.26 (0.89–1.79) | 1.54 (1.07–2.21) | 29/77 | 3.34 (1.54–7.24) | 3.96 (1.89–8.30) | 78/499 | 1.01 (0.71–1.44) | 1.28 (0.85–1.93) | |
| ptrend-value§ | 0.15 | 0.01 | 0.003 | <0.001 | 0.79 | 0.15 | ||||
| TBM | 0.99 | |||||||||
| <75th (0.71–1.13) | 324/1730 | Reference | Reference | 70/291 | Reference | Reference | 254/1439 | Reference | Reference | |
| 75–87.5th (1.14–2.10) | 58/287 | 1.35 (0.86–2.12) | 1.43 (0.92–2.24) | 13/52 | 1.30 (0.49–3.48) | 1.69 (0.70–4.09) | 45/235 | 1.35 (0.82–2.21) | 1.44 (0.85–2.44) | |
| >87.5th (>2.10) | 48/288 | 1.03 (0.63–1.71) | 1.23 (0.71–2.15) | 9/30 | 1.29 (0.44–3.79) | 1.39 (0.48–4.00) | 39/258 | 1.01 (0.61–1.67) | 1.25 (0.72–2.17) | |
| ptrend-value§ | 0.78 | 0.38 | 0.54 | 0.34 | 0.89 | 0.36 | ||||
| Br-THMs | 0.02 | |||||||||
| Q1 (1.58–1.78) | 105/563 | Reference | Reference | Reference | Reference | 87/462 | Reference | Reference | ||
| Q2 (1.79–3.15) | 116/563 | 1.12 (0.74–1.72) | 1.04 (0.68–1.58) | 18/101 | 1.44 (0.67–3.09) | 1.39 (0.57–3.38) | 95/474 | 1.07 (0.67–1.72) | 0.91 (0.54–1.53) | |
| Q3 (3.16–6.69) | 100/564 | 0.87 (0.60–1.27) | 0.92 (0.62–1.35) | 21/89 | 1.31 (0.48–3.59) | 1.21 (0.41–3.60) | 78/471 | 0.79 (0.54–1.16) | 0.86 (0.55–1.36) | |
| Q4 (>6.69) | 101/563 | 1.23 (0.83–1.85) | 1.46 (0.98–2.17) | 22/93 | 3.08 (1.38–6.84) | 3.28 (1.43–7.53) | 72/485 | 0.99 (0.66–1.51) | 1.19 (0.77–1.82) | |
| ptrend-value§ | 0.34 | 0.04 | 29/78 | 0.005 | 0.003 | 0.87 | 0.25 | |||
| TTHMs | 0.20 | |||||||||
| Q1 (3.07–6.99) | 108/540 | Reference | Reference | 21/85 | Reference | Reference | 87/455 | Reference | Reference | |
| Q2 (7.00–12.97) | 99/541 | 0.57 (0.38–0.84) | 0.58 (0.38–0.88) | 22/91 | 0.67 (0.31–1.47) | 0.52 (0.25–1.10) | 77/449 | 0.55 (0.36–0.83) | 0.57 (0.37–0.90) | |
| Q3 (12.98–25.63) | 99/540 | 0.69 (0.45–1.05) | 0.76 (0.49–1.18) | 19/92 | 0.57 (0.22–1.44) | 0.49 (0.20–1.19) | 80/449 | 0.72 (0.47–1.10) | 0.87 (0.54–1.40) | |
| Q4 (>25.63) | 99/540 | 0.86 (0.58–1.29) | 0.92 (0.59–1.42) | 26/81 | 1.66 (0.72–3.83) | 1.42 (0.65–3.12) | 73/459 | 0.73 (0.50–1.06) | 0.83 (0.54–1.29) | |
| ptrend-value§ | 0.91 | 0.80 | 0.13 | 0.14 | 0.36 | 0.85 | ||||
TCM: trichloromethane (chloroform); Q: quartile; BDCM: bromodichloromethane; T: tertile; DBCM: dibromochloromethane; TBM: tribromomethane (bromoform); Br-THMs: sum of BDCM, DBCM and TBM; TTHMs: sum of TCM and Br-THMs. #: proportions of adolescents with ever diagnosed asthma are absolute, unweighted values; ¶: adjusted for age, sex, race/ethnicity, body mass index (BMI) z-score, family income to poverty ratio, family history of asthma, swimming pool/hot tub/steam room use within 72 h, survey cycle and tobacco smoke exposure; +: adjusted for age, sex, race/ethnicity, BMI z-score, family income to poverty ratio, family history of asthma, swimming pool/hot tub/steam room use within 72 h and survey cycle; §: tests for linear trend were conducted by modelling categories of THM concentrations as ordinal variables using the median values within each category.
TABLE 4.
Additive scale interactions between blood dibromochloromethane (DBCM) and brominated trihalomethanes (Br-THMs; sum of bromodichloromethane, dibromochloromethane and tribromomethane (bromoform)) concentrations and tobacco smoke exposure and the risk of asthma#
| Blood THM | Tobacco smoke exposure ¶ | Ever asthma | Current asthma | ||
| n | OR (95% CI) | n | OR (95% CI) | ||
| DBCM | |||||
| Low DBCM (<75th) | No | 1444 | 1.00 (reference) | 1292 | 1.00 (reference) |
| Yes | 289 | 1.20 (0.85–1.70) | 259 | 1.44 (0.90–2.31) | |
| High DBCM (≥75th) | No | 499 | 0.98 (0.73–1.31) | 457 | 1.05 (0.70–1.59) |
| Yes | 77 | 3.04 (1.80–5.13) | 61 | 3.51 (1.73–7.12) | |
| RERI (95% CI) | 1.87 (0.30–3.43)* | 2.01 (−0.42–4.44) | |||
| Br-THMs | |||||
| Low Br-THMs (<50th) | No | 817 | 1.00 (reference) | 715 | 1.00 (reference) |
| Yes | 166 | 1.08 (0.69–1.69) | 145 | 1.15 (0.59–2.27) | |
| High Br-THMs (≥50th) | No | 1075 | 0.81 (0.63–1.04) | 990 | 1.11 (0.77–1.59) |
| Yes | 195 | 1.67 (1.13–2.48) | 170 | 2.59 (1.52–4.39) | |
| RERI (95% CI) | 0.78 (0.07–1.49)* | 1.32 (0.01–2.64)* | |||
RERI: relative excess risk due to interaction. #: all models were adjusted for age, sex, race/ethnicity, body mass index z-score, family income to poverty ratio, family history of asthma, swimming pool/hot tub/steam room use within 72 h and survey cycle; ¶: participants were considered exposed to tobacco smoke if their serum cotinine concentrations were >10 ng·mL−1 or they had self-reported consumption of tobacco or nicotine products in the past 5 days. *: p<0.05.
Similar results were observed when the associations between blood THM concentrations and current asthma risk were explored (table 5). In multivariable models, adolescents in the highest versus lowest exposure category of DBCM and Br-THMs had ORs for current asthma of 3.72 (95% CI 1.40–9.89) and 3.79 (95% CI 1.22–11.79), respectively. Again, these associations between DBCM and Br-THM concentrations and current asthma were not apparent among adolescents without tobacco smoke exposure (table 5). There was no evidence of multiplicative interaction between blood DBCM and Br-THM concentrations and tobacco smoke exposure on the risk of current asthma (table 5). However, we found a strong excess risk of current asthma due to the additive interaction between high blood Br-THM concentrations and tobacco smoke exposure (RERI 1.32 (95% CI 0.01–2.64)) (table 4).
TABLE 5.
Odds ratios of current asthma in relation to blood trihalomethane (THM) concentrations among US adolescents according to tobacco smoke exposure (National Health and Nutrition Examination Survey 2005–2012)#
| Blood THM (pg·mL−1) | All (n=2114) | Tobacco smoke exposure (n=330) | No tobacco smoke exposure (n=1784) | pinteraction-value for adjusted model | ||||||
| n/N ¶ | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) + | n/N ¶ | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) § | n/N ¶ | Unadjusted model OR (95% CI) | Adjusted model OR (95% CI) § | ||
| TCM | 0.32 | |||||||||
| Q1 (1.48–4.11) | 45/503 | Reference | Reference | 7/80 | Reference | Reference | 38/423 | Reference | Reference | |
| Q2 (4.12–8.34) | 40/500 | 0.67 (0.40–1.12) | 0.66 (0.39–1.13) | 11/75 | 1.28 (0.34–4.85) | 1.07 (0.31–13.75) | 29/425 | 0.58 (0.32–1.07) | 0.56 (0.30–1.08) | |
| Q3 (8.35–16.90) | 56/511 | 0.92 (0.58–1.46) | 0.96 (0.57–1.63) | 14/90 | 1.54 (0.43–5.54) | 1.20 (0.35–4.15) | 42/421 | 0.81 (0.50–1.32) | 0.89 (0.49–1.61) | |
| Q4 (>16.90) | 46/515 | 0.81 (0.47–1.40) | 0.78 (0.44–1.41) | 12/74 | 2.09 (0.52–8.43) | 1.67 (0.53–5.25) | 34/441 | 0.65 (0.35–1.19) | 0.62 (0.33–1.19) | |
| ptrend-value## | 0.73 | 0.68 | 0.23 | 0.29 | 0.34 | 0.33 | ||||
| BDCM | 0.05 | |||||||||
| T1 (0.44–0.75) | 63/682 | Reference | Reference | 9/115 | Reference | Reference | 54/567 | Reference | Reference | |
| T2 (0.76–2.50) | 66/702 | 0.99 (0.59–1.65) | 0.99 (0.60–1.64) | 16/110 | 1.94 (0.74–5.03) | 1.39 (0.54–3.59) | 50/592 | 0.87 (0.51–1.48) | 0.86 (0.49–1.51) | |
| T3 (>2.50) | 66/714 | 0.95 (0.57–1.59) | 1.15 (0.67–1.96) | 20/101 | 3.01 (1.02–8.82) | 2.58 (0.96–6.93) | 46/613 | 0.72 (0.43–1.21) | 0.92 (0.54–1.57) | |
| ptrend-value## | 0.85 | 0.57 | 0.05 | 0.06 | 0.22 | 0.83 | ||||
| DBCM | 0.03 | |||||||||
| < 50th (0.44–0.65) | 93/1027 | Reference | Reference | 15/179 | Reference | Reference | 78/848 | Reference | Reference | |
| 50–75th (0.66–1.87) | 51/524 | 1.00 (0.61–1.65) | 1.26 (0.74–2.16) | 16/80 | 2.51 (1.01–6.24) | 2.28 (0.93–5.59) | 35/444 | 0.81 (0.45–1.45) | 1.06 (0.56–2.00) | |
| >75th (>1.87) | 49/518 | 1.26 (0.72–2.19) | 1.63 (0.90–2.98) | 13/61 | 2.51 (0.98–6.45) | 3.72 (1.40–9.89) | 36/457 | 1.10 (0.62–1.96) | 1.46 (0.79–2.68) | |
| ptrend-value## | 0.40 | 0.12 | 0.06 | 0.01 | 0.66 | 0.21 | ||||
| TBM | 0.99 | |||||||||
| < 75th (0.71–1.13) | 138/1544 | Reference | Reference | 34/255 | Reference | Reference | 104/1289 | Reference | Reference | |
| 75–87.5th (1.14–2.10) | 26/255 | 1.12 (0.55–2.26) | 1.16 (0.59–2.28) | 5/44 | 0.33 (0.11–0.95) | 0.43 (0.14–1.30) | 21/211 | 1.39 (0.74–2.60) | 1.37 (0.67,2.70) | |
| >87.5th (>2.10) | 27/267 | 1.38 (0.67–2.83) | 1.63 (0.75–3.52) | 5/26 | 0.96 (0.25–3.72) | 1.12 (0.28–4.57) | 22/241 | 1.51 (0.73–3.12) | 1.87 (0.87–4.01) | |
| ptrend-value## | 0.37 | 0.21 | 0.66 | 0.92 | 0.25 | 0.10 | ||||
| Br-THMs | 0.02 | |||||||||
| Q1 (1.58–1.78) | 41/499 | Reference | Reference | 7/90 | Reference | Reference | 34/409 | Reference | Reference | |
| Q2 (1.79–3.15) | 48/495 | 1.04 (0.56–1.94) | 0.98 (0.53–1.82) | 9/77 | 1.66 (0.46–5.92) | 1.24 (0.33–4.67) | 39/418 | 0.94 (0.52–1.71) | 0.81 (0.43–1.54) | |
| Q3 (3.16–6.69) | 51/515 | 1.08 (0.57–2.07) | 1.28 (0.65–2.51) | 13/84 | 1.52 (0.47–4.88) | 1.15 (0.35–3.76) | 38/431 | 1.01 (0.53–1.94) | 1.24 (0.61–2.56) | |
| Q4 (>6.69) | 49/511 | 1.36 (0.73–2.53) | 1.64 (0.87–3.11) | 15/64 | 3.68 (1.10–12.34) | 3.79 (1.22–11.79) | 34/447 | 1.07 (0.56–2.05) | 1.30 (0.68–2.46) | |
| ptrend-value## | 0.28 | 0.08 | 0.02 | 0.01 | 0.75 | 0.26 | ||||
| TTHMs | 0.20 | |||||||||
| Q1 (3.07–6.99) | 40/472 | Reference | Reference | 6/70 | Reference | Reference | 34/402 | Reference | Reference | |
| Q2 (7.00–12.97) | 44/485 | 0.57 (0.32–1.02) | 0.60 (0.32–1.10) | 10/79 | 0.90 (0.25–3.31) | 0.62 (0.16–2.45) | 34/406 | 0.53 (0.29–0.96) | 0.56 (0.29–1.08) | |
| Q3 (12.98–25.63) | 51/493 | 0.91 (0.50–1.65) | 0.98 (0.51–1.86) | 14/87 | 1.64 (0.44–6.08) | 1.46 (0.45–4.72) | 37/406 | 0.77 (0.42–1.43) | 0.89 (0.44–1.80) | |
| Q4 (>25.63) | 45/486 | 0.98 (0.55–1.74) | 1.00 (0.54–1.84) | 13/68 | 2.49 (0.69–9.04) | 1.91 (0.63–5.77) | 32/418 | 0.79 (0.43–1.47) | 0.84 (0.44–1.61) | |
| ptrend-value## | 0.65 | 0.62 | 0.06 | 0.10 | 0.79 | 0.96 | ||||
TCM: trichloromethane (chloroform); Q: quartile; BDCM: bromodichloromethane; T: tertile; DBCM: dibromochloromethane; TBM: tribromomethane (bromoform); Br-THMs: sum of BDCM, DBCM and TBM; TTHMs: sum of TCM and Br-THMs. #: participants who ever received a diagnosis of asthma but with no wheezing or whistling in the past year were excluded from this analysis (n=245); ¶: proportions of adolescents with ever diagnosed asthma are absolute, unweighted values; +: adjusted for age, sex, race/ethnicity, body mass index (BMI) z-score, family income to poverty ratio, family history of asthma, swimming pool/hot tub/steam room use within 72 h, survey cycle, and tobacco smoke exposure; §: adjusted for age, sex, race/ethnicity, BMI z-score, family income to poverty ratio, family history of asthma, swimming pool/hot tub/steam room use within 72 h, and survey cycle; ##: tests for linear trend were conducted by modelling categories of THM concentrations as ordinal variables using the median values within each category.
We did not find any evidence of effect modification by sex for the associations of blood DBCM and Br-THM concentrations and risk of ever or current asthma (pinteraction>0.05) (supplementary table S3). However, we found positive associations between blood TCM and TTHM concentrations and risk of current asthma only among males with tobacco smoke exposure, which were modified by sex (both pinteraction<0.05) (supplementary table S3), although the concentrations of blood THMs and serum cotinine were similar among males and females (supplementary figures S1 and S2). The elevated risk of ever asthma comparing the extreme exposure categories of blood DBCM and Br-THM concentrations among adolescents exposed to tobacco smoke persisted when we excluded adolescents who had missing data on BMI z-scores or family income to poverty ratio (supplementary table S4), when we additionally included specific covariates related to THM exposures, leisure-time physical activity or allergic symptoms in the adjusted models (supplementary table S5 and S6) and when we excluded adolescents who spent any time at a swimming pool/hot tub/steam room in the past 72 h (supplementary table S7).
Discussion
This cross-sectional analysis of a representative sample of adolescents from the US population showed that higher blood DBCM and Br-THM concentrations were associated with a greater risk of ever or current asthma among adolescents exposed to tobacco smoke. These associations, however, were not observed in adolescents without tobacco smoke exposure. The joint effects of high blood DBCM and Br-THM concentrations and tobacco smoke exposure on ever or current asthma were greater than the summed effects due to each individual exposure. We also found some evidence of modification by sex for the positive associations between blood TCM and TTHM concentrations and risk of current asthma, which was observed only among males with tobacco smoke exposure.
Previous population studies have shown that early-life exposure to chlorinated swimming pool environments was associated with a higher risk of asthma, especially among young adolescents and those with an atopic predisposition [3–8, 23]. Furthermore, chlorinated swimming pool attendance has been associated with an increased prevalence of allergic diseases (e.g. conjunctivitis, rhinitis and laryngitis), bronchial hyperreactivity and respiratory damage [23, 24]. However, conflicting results are also reported [9–11]. Prior research mostly assessed disinfection byproduct exposures based on reports of swimming frequency from self-reported questionnaires, which is prone to exposure misclassification due to the multiple exposure routes and sources and inter- and intra-individual physiological differences in absorption and metabolism of disinfection byproducts. Internal exposure biomarkers reflect integrative measures of exposure to disinfection byproducts from all routes and sources, providing a more accurate exposure assessment [13]. In an early study conducted among 133 indoor swimming pool workers, Fantuzzi et al. [25] reported that employees with THM alveolar air values >21 µg·m−3 experienced higher risks of dyspnoea, asthma, red eyes and blocked nose than participants with lower exposure levels.
Most mechanistic studies suggest that THMs may increase asthma risk by inducing perturbations of the immune system. In animal studies, Munson et al. [26] reported depressed humoral and cellular immunity in mice after administration of BDCM or DBCM. Auttachoatet et al. [27] observed decreased numbers of blood circulating neutrophils in female B6C3F1 mice when TCM exposure occurred via drinking water. Exposure to TCM by inhalation at 20 ppm was also reported to produce a greater number of inflammatory and goblet cells in the lungs and increased blood levels of IgE in mice [28]. A recent population study showed that THM levels in exhaled breath after swimming in a chlorinated pool were associated with acute changes in serum immune markers [29]. Other proposed mechanisms for disinfection byproduct exposure related to asthma and respiratory damage include oxidative stress and hyperpermeability of the lung epithelium [28]. In support of these hypotheses, Varraso et al. [30] revealed that exposure to THMs was associated with higher levels of oxidative stress markers in 21 male swimmers; Font-Ribera et al. [28] studied 50 healthy and nonsmoker adults, reporting that THM concentrations in exhaled breath after swimming in a chlorinated pool were positively associated with serum club cell secretory protein 16 (CC16), a marker of lung epithelium permeability and integrity.
To the best of our knowledge, this present study is the first to assess the THM–asthma association among adolescents. We found positive associations between blood BDCM and DBCM concentrations and risk of ever or current asthma among adolescents who were exposed to tobacco smoke exposure. The strong additive interaction between high blood BDCM and DBCM concentrations and tobacco smoke exposure suggests that tobacco use might interact synergistically with THM exposure to further accelerate the development of asthma. This is not surprising given the well-documented association between tobacco use and asthma risk [12], as well as shared mechanisms of tobacco smoke and THMs [31]. Substantial evidence from animal models and clinical studies has shown that the imbalance between oxidants and antioxidants resulting from exposure to tobacco smoke is associated with oxidative stress, increased mucosal inflammation and increased expression of inflammatory cytokines, which eventually promote the development of asthma and respiratory diseases [32]. Co-exposure to THMs and tobacco smoke may also exert their synergistic effects through shared metabolic pathways. For instance, exposure to tobacco smoke increases the activity of enzymes that metabolise cigarette toxins (e.g. cytochrome P450) and reduces the activity of enzymes that detoxify these compounds (e.g. glutathione S-transferase enzymes) [33], which are also involved in the metabolism and activation of THMs [34]. Interestingly, we found positive associations between blood TCM and TTHMs and the risk of current asthma among males with tobacco smoke exposure, indicating some evidence of effect modification by sex given the similar levels of blood THM and cotinine in males and females. In support of these findings, previous studies have revealed a higher prevalence of allergy among male swimmers than females [22]. A stronger association of THM exposure with other health outcomes, such as neonatal neurobehavioral development and child cognition, was also reported in boys compared with girls [35, 36]. However, more studies are needed to explore the underlying mechanisms of any sex-specific associations and multiple testing concerns cannot be ruled out.
The drinking water concentrations of TTHMs (median 25.3 μg·L−1) in the NHANES population were among the medium environmental exposure levels measured in previous studies from the UK (mean 26.5 μg·L−1), Spain (median 23.5 μg·L−1), Italy (median 1.5 μg·L−1), Greece (mean 29.8 μg·L−1) and China (median 10.53 μg·L−1) [37, 38]. In this study, the excess risk of asthma due to high blood DBCM and Br-THM concentrations and tobacco smoke exposure was higher than the summed risk associated with each individual factor, suggesting that the previously well-established associations of tobacco smoke exposure with asthma could be further exacerbated by high blood BDCM and DBCM concentrations. Our novel findings strengthen the growing evidence that local environmental factors play an important role in the development/exacerbation of asthma and emphasise the importance of preventing adolescents’ asthma by reducing exposure to both THMs and tobacco smoke. The Disinfectants and Disinfection Byproducts Rule issued by the US Environmental Protection Agency in 1998 and 2006 has resulted in continued lower blood THM concentrations in the NHANES population from 2001 to 2012 [39], supporting the effectiveness of setting maximum contaminant levels in household water as a policy for reducing exposure to THMs and ultimately preventing THM-related health effects. Given that few countries have measured blood THM concentrations in the general population, an improved and continued worldwide surveillance of THM levels in drinking water and humans is needed.
The major strength of this study is its nationally representative and large sample of US adolescents. In addition, we used direct measurements of internal exposure biomarkers for THMs and tobacco smoke exposure, which reduced exposure misclassification. Our study also has several limitations. First, despite the strong biological plausibility of THM exposure on asthma and allergic diseases, the exposure biomarkers were collected after asthma incidence occurred, which precluded inferences of causality due to the potential for reverse causation. However, we are not aware of any plausible mechanism through which asthma might affect THM exposure or excretion. In support of this point, we found that participants’ water-use activities (swimming pool/hot tub/steam room use) within 72 h were similar according to current asthma status (supplementary table S8). This suggests that asthma history did not influence exposure conditions and thus minimises the potential for reverse causation. Second, although blood concentrations are reliable biomarkers of THM exposure and are believed to reflect steady-state exposure levels due to the high frequency of daily water-use activities and slower partitioning out of adipose tissue [14], exposure misclassification cannot be fully excluded given that the measured THM concentrations may vary over time and may not provide accurate estimates of the etiologically relevant exposure that precedes the outcome. Nevertheless, such nondifferential misclassification would tend to bias effect estimates towards the null. Third, despite our control for multiple potential confounders, the possibility of residual uncontrolled confounding (e.g. tobacco polycyclic aromatic hydrocarbons and metabolic genotypes) cannot be ruled out. Fourth, the detection rate of blood DBCM and TBM concentrations was relatively low (51.5% and 27.8%, respectively), which may result in biased risk estimation. Fifth, causality cannot be inferred due to the observational nature of the cross-sectional study design. Finally, physician-diagnosed asthma and wheezing symptoms were self-reported, which were subject to potential errors in reporting by the study participants, although previous studies have demonstrated the reliability of self-reported asthma [40].
The results of this cross-sectional analysis among a representative sample of US adolescents suggest that exposure to THMs is associated with a greater risk of asthma, particularly among those who are co-exposed to tobacco smoke. Our results refine and extend previous evidence showing that swimming pool attendance is associated with a higher risk of developing asthma and allergic diseases, and emphasise the importance of preventing adolescents’ asthma by reducing exposure to both THMs and tobacco smoke.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01440-2021.Supplement (895.8KB, pdf)
Shareable PDF
Footnotes
Author contributions: Y. Sun analysed the data. Y. Sun and Y-X. Wang drafted the manuscript. Y-X. Wang and C. Messerlian led the study conception, study design, analysis plan and interpretation of findings. P-F. Xia validated the accuracy of data analysis with a technical review. Y. Sun, P-F. Xia, J. Xie, V. Mustieles, Y. Zhang, Y-X. Wang and C. Messerlian interpreted the results and critically appraised the manuscript for important intellectual content.
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: Y. Sun has nothing to disclose.
Conflict of interest: P-F. Xia has nothing to disclose.
Conflict of interest: J. Xie has nothing to disclose.
Conflict of interest: V. Mustieles has nothing to disclose.
Conflict of interest: Y. Zhang has nothing to disclose.
Conflict of interest: Y-X. Wang has nothing to disclose.
Conflict of interest: C. Messerlian has nothing to disclose.
Support statement: This study was supported by the National Natural Science Foundation of China (81903281). C. Messerlian is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (R01ES031657). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Pearce N, Ait-Khaled N, Beasley R, et al. . Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007; 62: 758–766. doi: 10.1136/thx.2006.070169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbank AJ, Sood AK, Kesic MJ, et al. . Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol 2017; 140: 1–12. doi: 10.1016/j.jaci.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard A, Carbonnelle S, de Burbure C, et al. . Chlorinated pool attendance, atopy, and the risk of asthma during childhood. Environ Health Perspect 2006; 114: 1567–1573. doi: 10.1289/ehp.8461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard A, Carbonnelle S, Dumont X, et al. . Infant swimming practice, pulmonary epithelium integrity, and the risk of allergic and respiratory diseases later in childhood. Pediatrics 2007; 119: 1095–1103. doi: 10.1542/peds.2006-3333 [DOI] [PubMed] [Google Scholar]
- 5.Bernard A, Nickmilder M, Voisin C, et al. . Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics 2009; 124: 1110–1118. doi: 10.1542/peds.2009-0032 [DOI] [PubMed] [Google Scholar]
- 6.Bernard A, Nickmilder M, Voisin C. Outdoor swimming pools and the risks of asthma and allergies during adolescence. Eur Respir J 2008; 32: 979–988. doi: 10.1183/09031936.00114807 [DOI] [PubMed] [Google Scholar]
- 7.Andersson M, Hedman L, Nordberg G, et al. . Swimming pool attendance is related to asthma among atopic school children: a population-based study. Environ Health 2015; 14: 37. doi: 10.1186/s12940-015-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voisin C, Sardella A, Marcucci F, et al. . Infant swimming in chlorinated pools and the risks of bronchiolitis, asthma and allergy. Eur Respir J 2010; 36: 41–47. doi: 10.1183/09031936.00118009 [DOI] [PubMed] [Google Scholar]
- 9.Font-Ribera L, Kogevinas M, Zock JP, et al. . Swimming pool attendance and risk of asthma and allergic symptoms in children. Eur Respir J 2009; 34: 1304–1310. doi: 10.1183/09031936.00180608 [DOI] [PubMed] [Google Scholar]
- 10.Font-Ribera L, Villanueva CM, Nieuwenhuijsen MJ, et al. . Swimming pool attendance, asthma, allergies, and lung function in the Avon Longitudinal Study of Parents and Children cohort. Am J Respir Crit Care Med 2011; 183: 582–588. doi: 10.1164/rccm.201005-0761OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JH, Fuertes E, Krop EJ, et al. . Swimming pool attendance and respiratory symptoms and allergies among Dutch children. Occup Environ Med 2012; 69: 823–830. doi: 10.1136/oemed-2011-100621 [DOI] [PubMed] [Google Scholar]
- 12.Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J 2013; 41: 716–726. doi: 10.1183/09031936.00073312 [DOI] [PubMed] [Google Scholar]
- 13.Weisel CP, Kim H, Haltmeier P, et al. . Exposure estimates to disinfection by-products of chlorinated drinking water. Environ Health Perspect 1999; 107: 103–110. doi: 10.1289/ehp.99107103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blount B, Backer L, Aylward L, et al. . Human exposure assessment for DBPs: factors influencing blood trihalomethane levels. In: Nriagu JO, ed. Encyclopedia of Environmental Health. Oxford, Elsevier, 2011; pp. 100–107. [Google Scholar]
- 15.National Center for Health Statistics . National Health and Nutrition Examination Survey. 2021. Date last accessed: 7 July 2021. www.cdc.gov/nchs/nhanes/index.htm
- 16.National Center for Health Statistics . Laboratory Procedure Manual. Trihalomethanes/MTBE/Nitromethane. 2015. Date last accessed: 7 July 2021. wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/VOCMWB_G_MET.pdf
- 17.Lodrup Carlsen KC, Roll S, Carlsen KH, et al. . Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One 2012; 7: e43214. doi: 10.1371/journal.pone.0043214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest 2002; 122: 409–415. doi: 10.1378/chest.122.2.409 [DOI] [PubMed] [Google Scholar]
- 19.Kit BK, Simon AE, Brody DJ, et al. . US prevalence and trends in tobacco smoke exposure among children and adolescents with asthma. Pediatrics 2013; 131: 407–414. doi: 10.1542/peds.2012-2328 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . A SAS program for the 2000 CDC Growth Charts (ages 0 to <20 years). 2019. Date last accessed: 7 July 2021. www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 21.Hauser R, Williams P, Altshul L, et al. . Evidence of interaction between polychlorinated biphenyls and phthalates in relation to human sperm motility. Environ Health Perspect 2005; 113: 425–430. doi: 10.1289/ehp.7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paivinen MK, Keskinen KL, Tikkanen HO. Swimming and asthma: differences between women and men. J Allergy 2013; 2013: 520913. doi: 10.1155/2013/520913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florentin A, Hautemaniere A, Hartemann P. Health effects of disinfection by-products in chlorinated swimming pools. Int J Hyg Environ Health 2011; 214: 461–469. doi: 10.1016/j.ijheh.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 24.Paivinen MK, Keskinen KL, Tikkanen HO. Swimming and asthma: factors underlying respiratory symptoms in competitive swimmers. Clin Respir J 2010; 4: 97–103. doi: 10.1111/j.1752-699X.2009.00155.x [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Righi E, Predieri G, et al. . Prevalence of ocular, respiratory and cutaneous symptoms in indoor swimming pool workers and exposure to disinfection by-products (DBPs). Int J Environ Res Public Health 2010; 7: 1379–1391. doi: 10.3390/ijerph7041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munson AE, Sain LE, Sanders VM, et al. . Toxicology of organic drinking water contaminants: trichloromethane, bromodichloromethane, dibromochloromethane and tribromomethane. Environ Health Perspect 1982; 46: 117–126. doi: 10.1289/ehp.8246117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auttachoat W, Germolec DR, Collins BJ, et al. . Immunotoxicological profile of chloroform in female B6C3F1 mice when administered in drinking water. Drug Chem Toxicol 2009; 32: 77–87. doi: 10.1080/01480540802433880 [DOI] [PubMed] [Google Scholar]
- 28.Font-Ribera L, Kogevinas M, Zock JP, et al. . Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ Health Perspect 2010; 118: 1538–1544. doi: 10.1289/ehp.1001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlaanderen J, van Veldhoven K, Font-Ribera L, et al. . Acute changes in serum immune markers due to swimming in a chlorinated pool. Environ Int 2017; 105: 1–11. doi: 10.1016/j.envint.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 30.Varraso R, Massin N, Hery M, et al. . Not only training but also exposure to chlorinated compounds generates a response to oxidative stimuli in swimmers. Toxicol Ind Health 2002; 18: 269–278. doi: 10.1191/0748233702th150oa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danilevičiūtė A. Effects of exposures to drinking water trihalomethanes and tobacco smoke on adverse pregnancy outcomes in relation to glutathione S-transferase T1 and M1 gene polymorphism. 2012. Date last accessed: 16 October 2021. www.vdu.lt/cris/bitstream/20.500.12259/122051/1/asta_danileviciute_dd.pdf
- 32.Strzelak A, Ratajczak A, Adamiec A, et al. . Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health 2018; 15: 1033. doi: 10.3390/ijerph15051033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki S, Sata F, Katoh S, et al. . Adverse birth outcomes associated with maternal smoking and polymorphisms in the N-nitrosamine-metabolizing enzyme genes NQO1 and CYP2E1. Am J Epidemiol 2008; 167: 719–726. doi: 10.1093/aje/kwm360 [DOI] [PubMed] [Google Scholar]
- 34.Levallois P, Giguere Y, Nguile-Makao M, et al. . Disinfection by-products exposure and intra-uterine growth restriction: do genetic polymorphisms of CYP2E1 or deletion of GSTM1 or GSTT1 modify the association? Environ Int 2016; 92–93: 220–231. doi: 10.1016/j.envint.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villanueva CM, Gracia-Lavedan E, Julvez J, et al. . Drinking water disinfection by-products during pregnancy and child neuropsychological development in the INMA Spanish cohort study. Environ Int 2018; 110: 113–122. doi: 10.1016/j.envint.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 36.Chen YJ, Liu C, Huang LL, et al. . First-trimester blood concentrations of drinking water trihalomethanes and neonatal neurobehavioral development in a Chinese birth cohort. J Hazard Mater 2019; 362: 451–457. doi: 10.1016/j.jhazmat.2018.09.040 [DOI] [PubMed] [Google Scholar]
- 37.Ding H, Meng L, Zhang H, et al. . Occurrence, profiling and prioritization of halogenated disinfection by-products in drinking water of China. Environ Sci Process Impacts 2013; 15: 1424–1429. doi: 10.1039/c3em00110e [DOI] [PubMed] [Google Scholar]
- 38.Evlampidou I, Font-Ribera L, Rojas-Rueda D, et al. . Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ Health Perspect 2020; 128: 17001. doi: 10.1289/EHP4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashley DL, Smith MM, Silva LK, et al. . Factors associated with exposure to trihalomethanes, NHANES 2001–2012. Environ Sci Technol 2020; 54: 1066–1074. doi: 10.1021/acs.est.9b05745 [DOI] [PubMed] [Google Scholar]
- 40.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest 1993; 104: 600–608. doi: 10.1378/chest.104.2.600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01440-2021.Supplement (895.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01440-2021.Shareable (284.5KB, pdf)